Investigation of Polymer-Assisted CO2 Flooding to Enhance Oil Recovery in Low-Permeability Reservoirs
Abstract
:1. Introduction
2. Material and Method
2.1. Material
2.2. Polymers Static Properties
2.2.1. Viscosity-Increasing Properties
2.2.2. Polymer Rheological Properties
2.2.3. Polymer Reservoir Adaptability
2.3. The Flow Performance of TRPS in Low-Permeability Reservoirs
2.3.1. The Injectivity Ability of TRPS
2.3.2. Resistance-Increasing Performance of TRPS-Assisted CO2 Flooding
2.3.3. Profile Control Performance of TRPS
2.4. EOR Efficiency of TRPS-Assisted CO2 Flooding
3. Result and Discussion
3.1. Static Performance Comparison between XG and TRPS
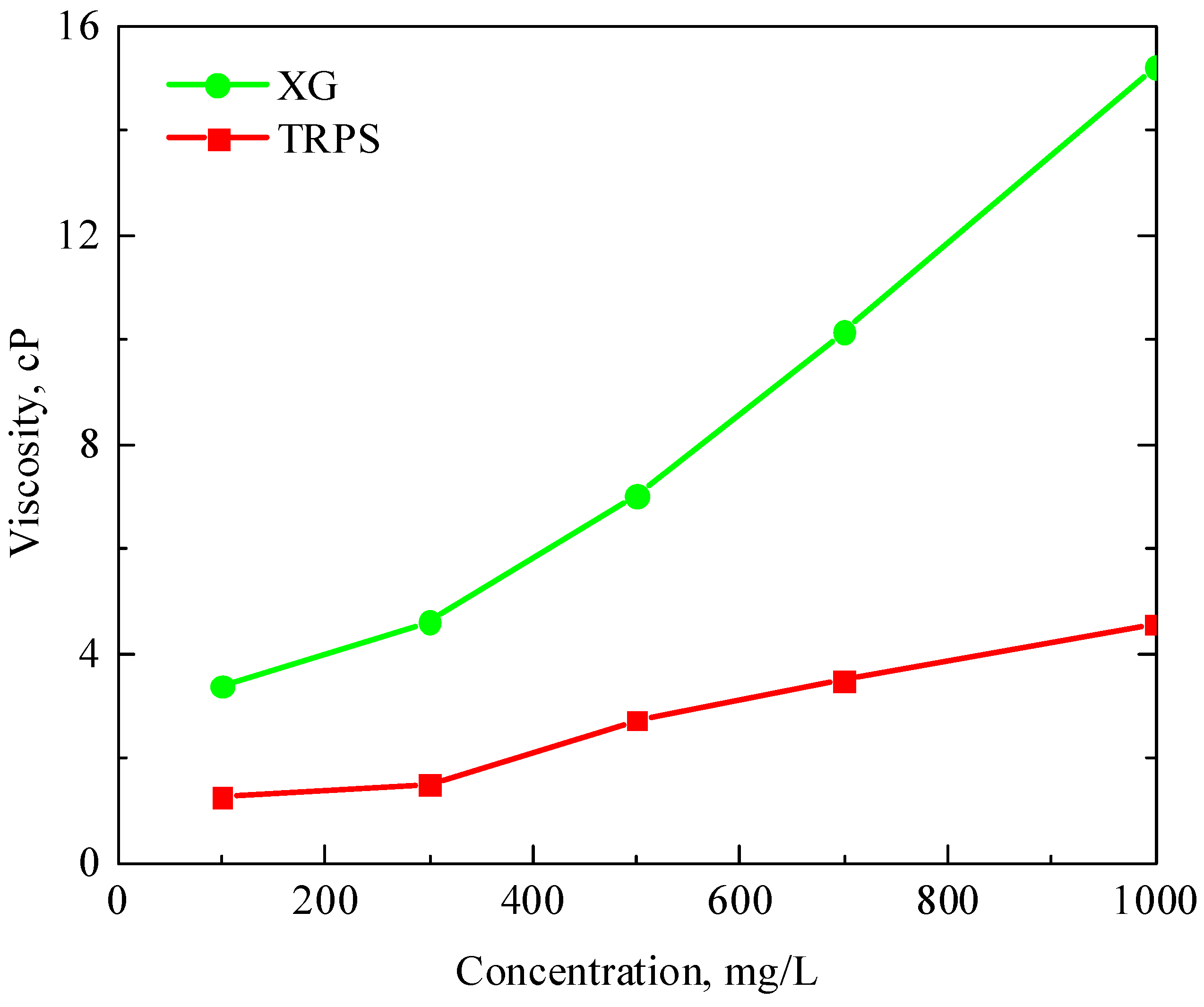
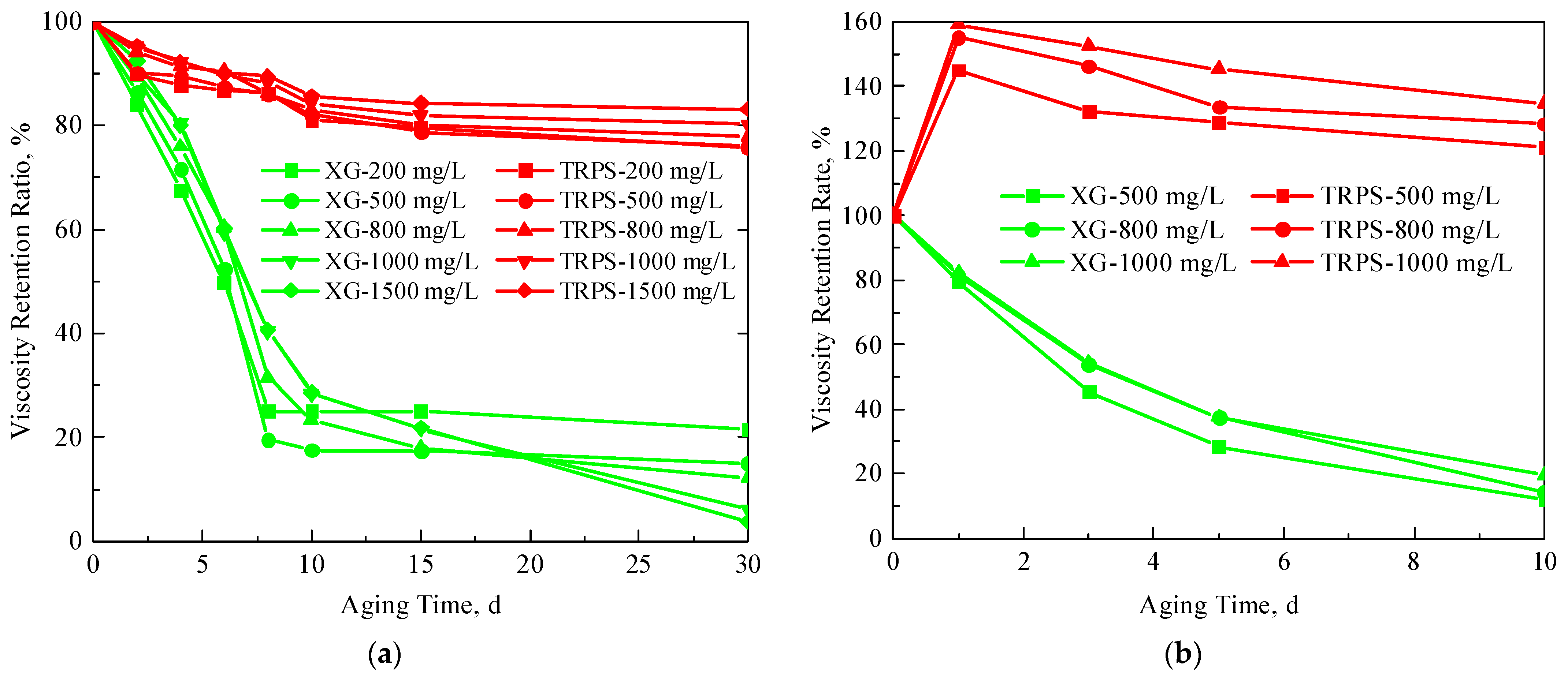
3.1.1. Viscosity-Increasing Performance
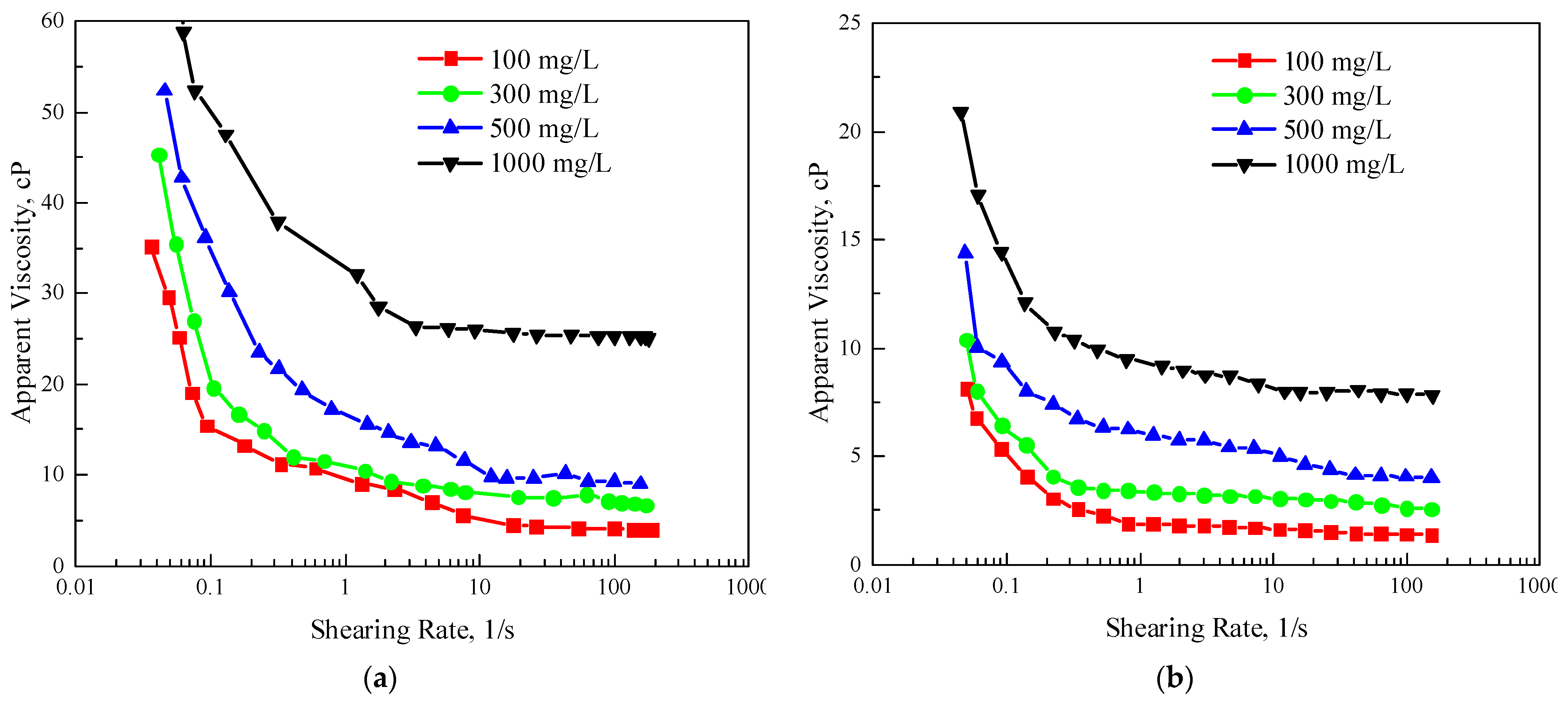
3.1.2. Rheological Properties
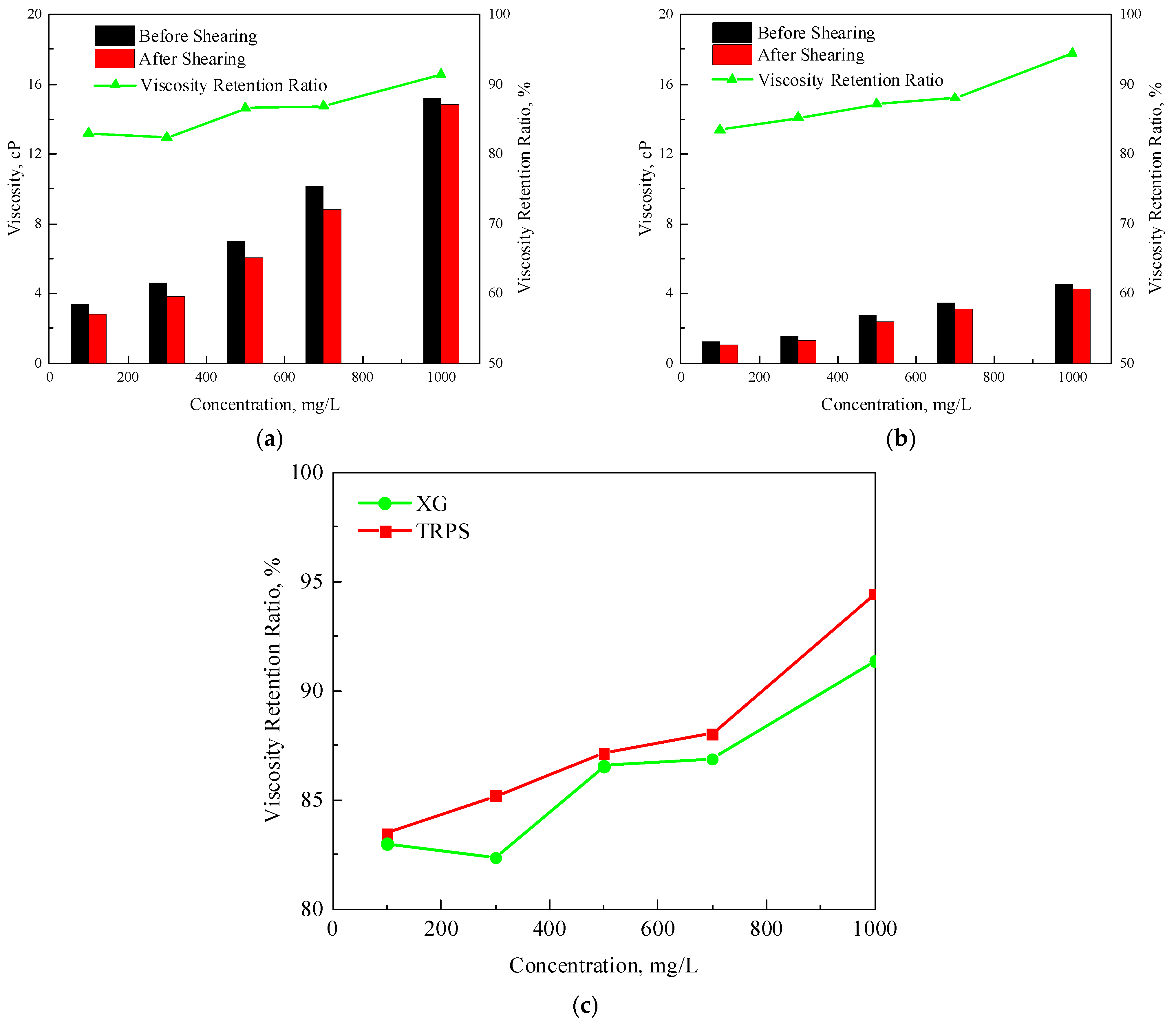
3.1.3. Temperature-Resistance and CO2-Resistance Performance
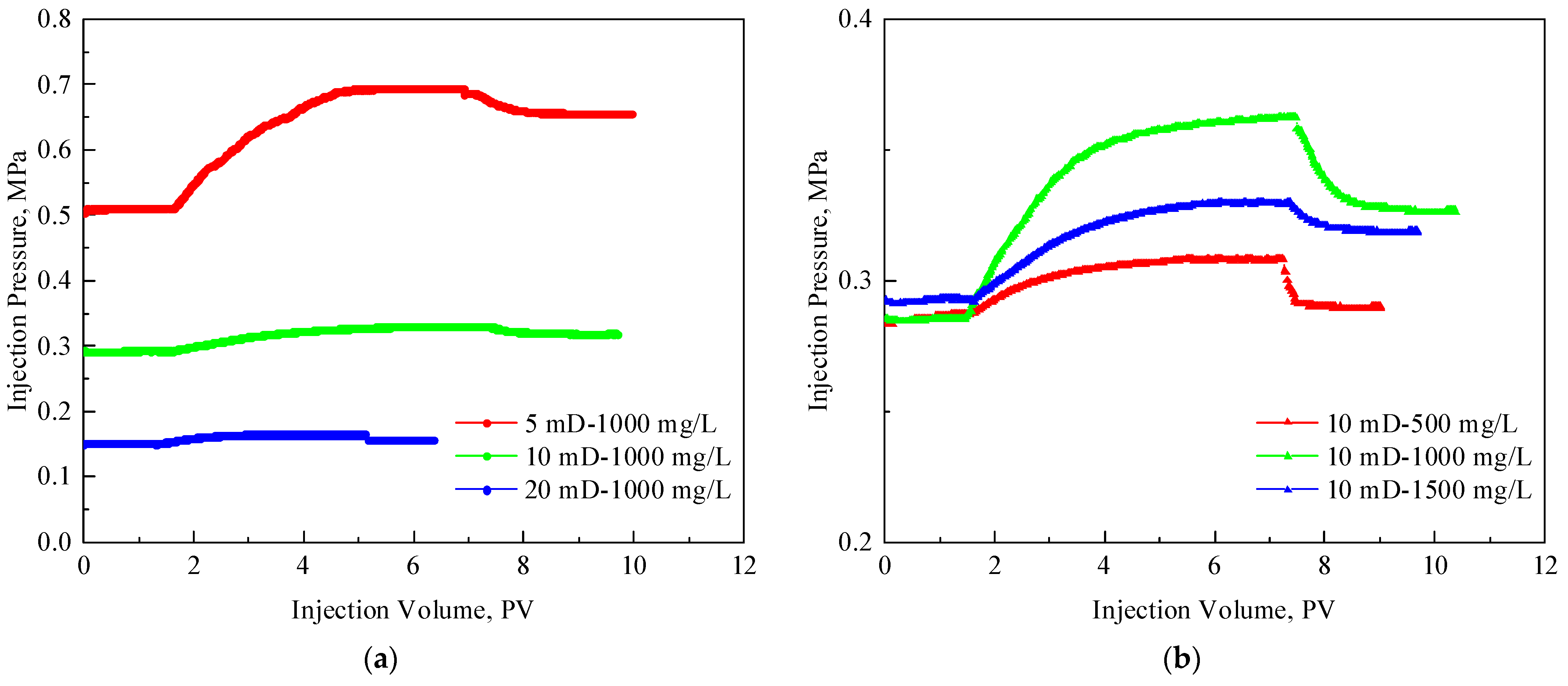
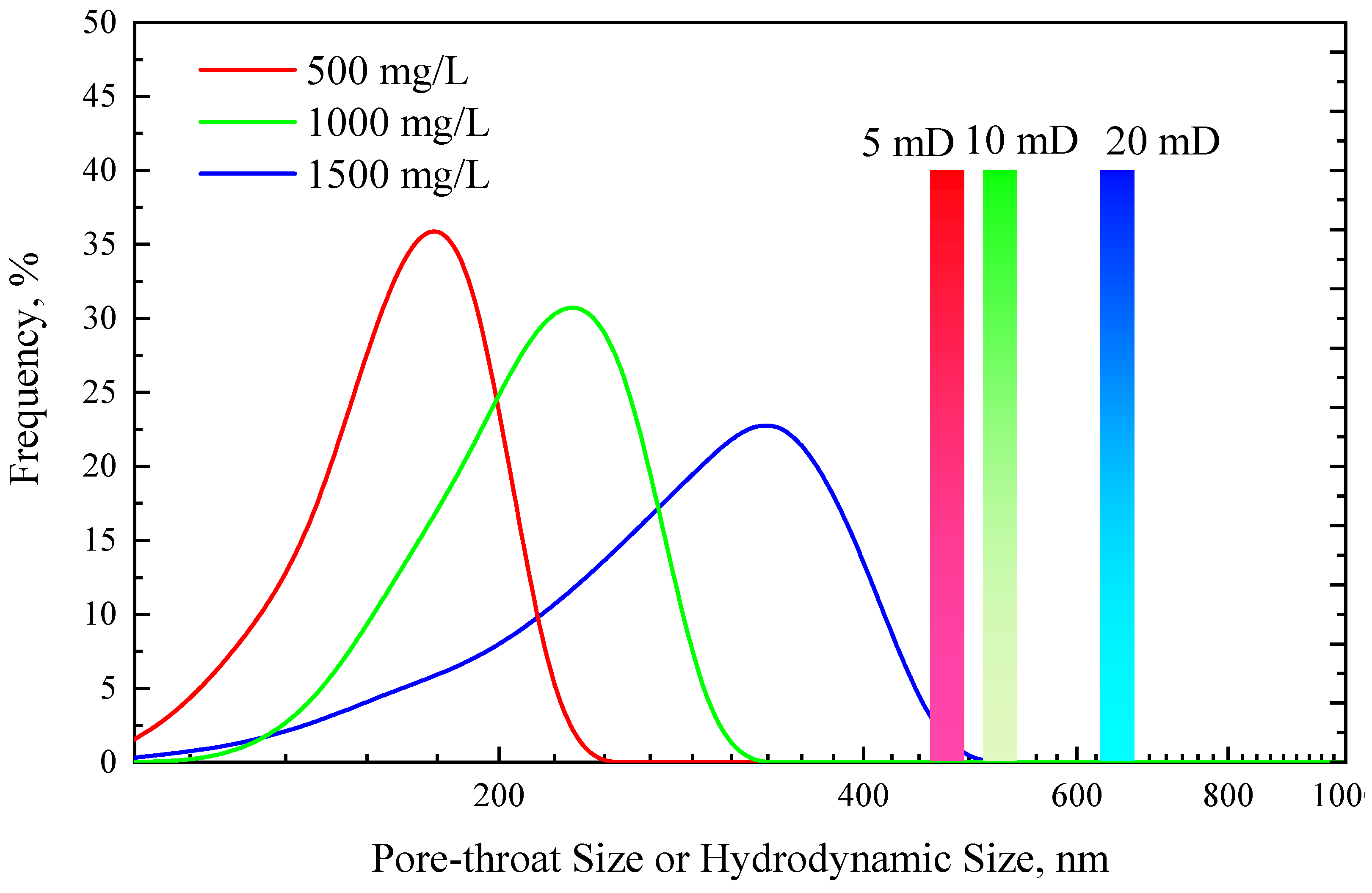
3.2. Injectivity of TRPS
3.3. Resistance Increasing Ablity of TRPS
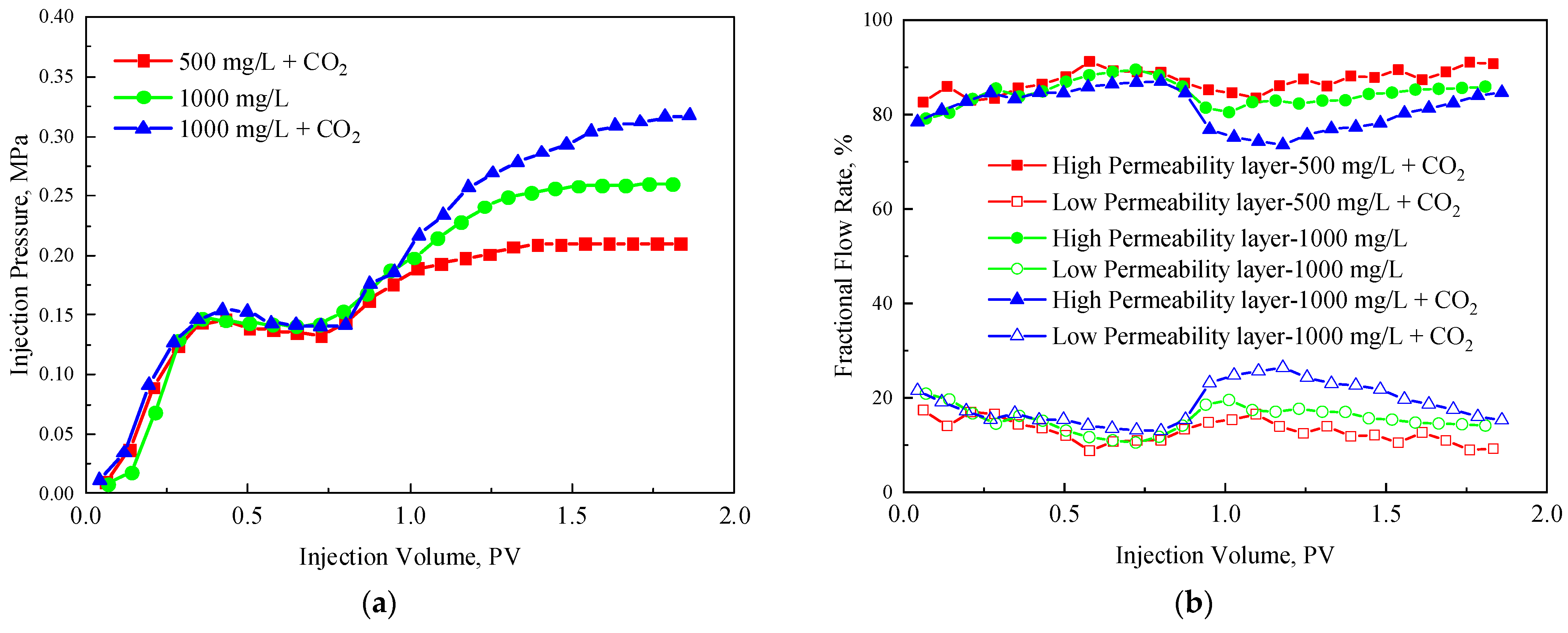
3.4. Profle Control Effect of TRPS
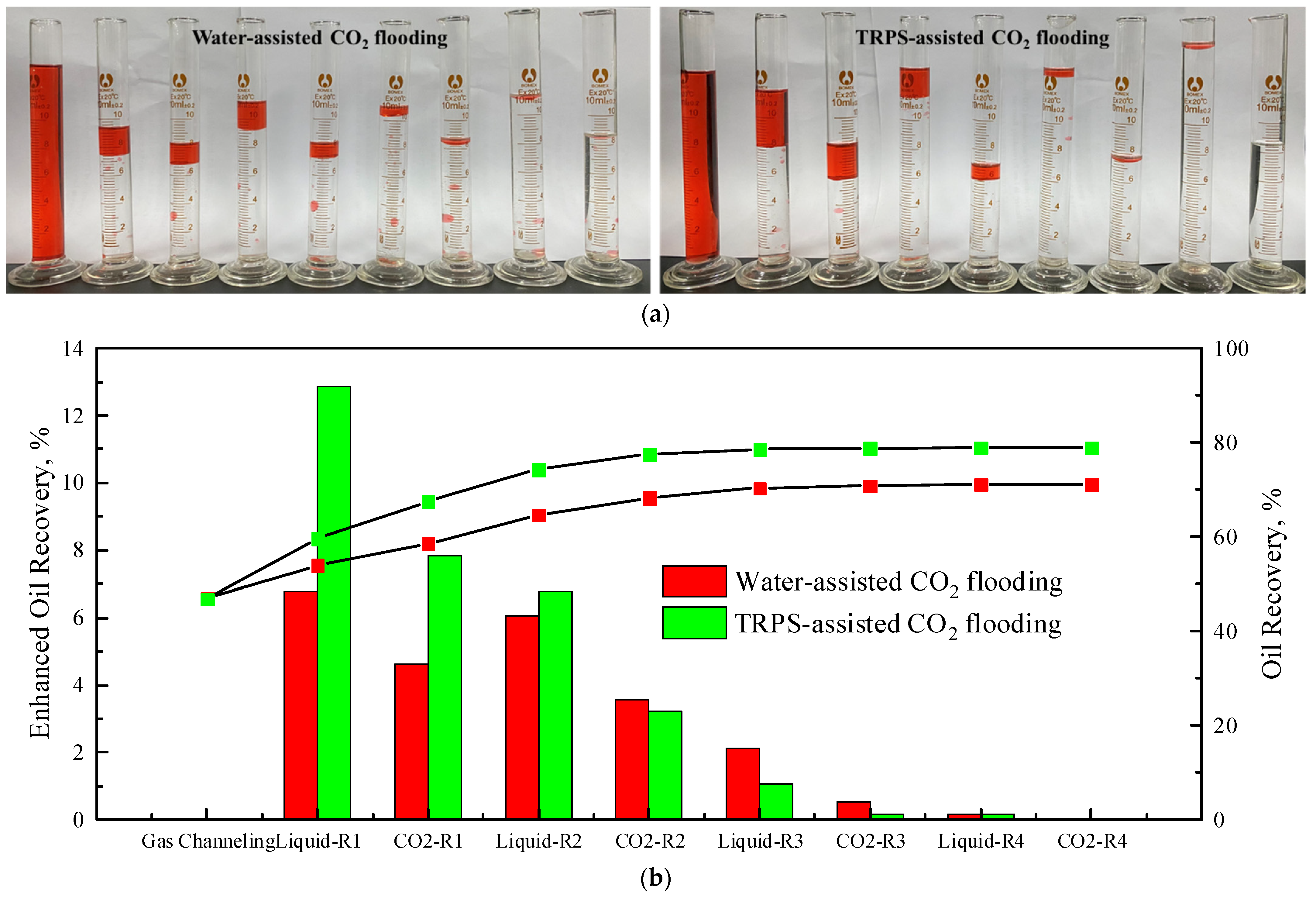
3.5. EOR Effects of TRPS
4. Conclusions
- (1)
- TRPS has a certain viscosity-increasing property, and the viscosity of the solution is 4.57 cP when the concentration is 1000 mg/L. It has good temperature resistance, and its viscosity retention rate is above 80% after aging at 80 °C for 30 days. In addition, TRPS can react with CO2 to increase the size of molecular aggregates significantly, and the viscosity retention rate is about 120% after aging in a CO2 environment for 10 days.
- (2)
- The RF and RFF of the TRPS solution with a concentration of 1000 mg/L in 5 mD cores are 1.36 and 1.28, respectively, showing good injectivity performance. Increasing the concentration of TRPS to 1500 mg/L had little effect on its injectivity performance.
- (3)
- The injection pressure of TRPS and CO2 co-injection is between the injection of HPAM with the same viscosity and the injection of TRPS solution alone, which has good flow performance and resistance-increasing effect. The η and η′ of TRPS-assisted CO2 flooding increase with increased permeability, concentration of TRPS solution, and injection rounds. When the permeability is 5 mD, the base pressure of gas channeling is high, which will reduce the matching system between TRPS and the reservoir, thus affecting the change law of η′.
- (4)
- The effect of TRPS solution on profile improvement: 1000 mg/L TPRS + CO2 > 1000 mg/L TRPS > 500 mg/L TRPS + CO2, considering the η′ and profile improvement effect, the application concentration of TRPS should be 1000 mg/L.
- (5)
- The EOR effect of TPRS-assisted CO2 flooding is 8.21% higher than that of water-assisted CO2 flooding. The EOR effect of TRPS-assisted CO2 displacement is mainly reflected in the first to second rounds, while the EOR effect of water-assisted CO2 displacement is primarily reflected in the first to third rounds. The injection pressure of liquid-assisted CO2 flooding has a cumulative impact of multiple rounds, so the optimal injection round is 3.
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Number | Literature | Contents | Agent | Models | EOR |
|---|---|---|---|---|---|
| 0 | This paper | Polymer-assisted CO2 flooding | CO2-responsive polymer, TRPS | Cores, 5–20 mD | Based on CO2 flooding is 32.93%; based on water-assisted CO2 flooding is 8.21% |
| 1 | Chaturvedi [43] | Polymer-enhanced carbonated water injection | PAM | Sandpack, ~780 mD | Based on water flooding is 16.00% |
| 2 | Zhao [44] | CO2 foam | AOS | Microfludic | Based on water flooding is 13.8–22.4% |
| 3 | Hossein [45] | Ultrasound-assisted CO2 flooding | / | Sandpack | Based on CO2 flooding is less than 15% |
| 4 | Li [42] | Polymer-assisted CO2 flooding | Polymer | Simulation, 1–2000 mD | Based on water flooding ranges from 20–30% |
| 5 | Luo [33] | Polymer-assisted CO2 flooding | Thermo- and CO2-triggered copolymer | Core | Based on water flooding is 21–23% |
| 6 | Yang [46] | Polymer-assisted CO2 flooding in heavy oil reservoir | Polymer | Simulation, 500 mD | Based on WAG is 57% |
| 7 | Gandomkar [47] | Polymer thickening CO2 flooding | Polydimethylsiloxane (PDMS) | Core, 6–8 mD | Based on CO2 flooding is 6–15% |
| 8 | Manan [48] | Polymer/surfactant/nano-particles assisted CO2 flooding | AOS, NPs (TiO2, CuO, SiO2, and Al2O3) | Sandpack | Based on water flooding is about 5.1–15.6% |
| 9 | Zaberi [49] | Polymer-assisted CO2 flooding | Polyfluoroacrylate (PFA) | Berea sandstone, ~31 mD | Based on CO2 flooding is 16% |
| 10 | Liu [5] | Microgel alternate CO2 | Microgel | Core, 2800 mD/780 mD/360 mD | Based on WAG is 12.4% |
References
- Kang, W.-L.; Zhou, B.-B.; Issakhov, M.; Gabdullin, M. Advances in enhanced oil recovery technologies for low permeability reservoirs. Pet. Sci. 2022, 19, 1622–1640. [Google Scholar] [CrossRef]
- Chen, Z.; Su, Y.-L.; Li, L.; Meng, F.-K.; Zhou, X.-M. Characteristics and mechanisms of supercritical CO2 flooding under different factors in low-permeability reservoirs. Pet. Sci. 2022, 19, 1174–1184. [Google Scholar] [CrossRef]
- Zhao, F.; Wang, P.; Huang, S.; Hao, H.; Zhang, M.; Lu, G. Performance and applicable limits of multi-stage gas channeling control system for CO2 flooding in ultra-low permeability reservoirs. J. Pet. Sci. Eng. 2020, 192, 107336. [Google Scholar] [CrossRef]
- Liu, F.; Yue, P.; Wang, Q.; Yu, G.; Zhou, J.; Wang, X.; Fang, Q.; Li, X. Experimental Study of Oil Displacement and Gas Channeling during CO2 Flooding in Ultra—Low Permeability Oil Reservoir. Energies 2022, 15, 5119. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, J.; Li, X.; Xu, C.; Chen, X.; Zhang, B.; Zhao, G.; Zhang, H.; Li, Y. Conformance control by a microgel in a multi-layered heterogeneous reservoir during CO2 enhanced oil recovery process. Chin. J. Chem. Eng. 2022, 43, 324–334. [Google Scholar] [CrossRef]
- Youyi, Z.; Qingfeng, H.; Guoqing, J.; Desheng, M.A.; Zhe, W.A.N.G. Current development and application of chemical combination flooding technique. Petroleum Explor. Develop. 2013, 40, 96–103. [Google Scholar]
- Wang, D.; Zhang, Z.; Cheng, J.; Yang, J.; Gao, S.; Li, L. Pilot Tests of Alkaline/Surfactant/Polymer Flooding in Daqing Oil Field. SPE Reserv. Eng. 1997, 12, 229–233. [Google Scholar] [CrossRef]
- Yupu, W.; He, L. Commercial Success of Polymer Flooding in Daqing Oilfield—Lessons Learned. In Proceedings of the SPE Asia Pacific Oil and Gas Conference and Exhibition, Adelaide, Australia, 11 September 2006. [Google Scholar] [CrossRef]
- Mishra, S.; Bera, A.; Mandal, A. Effect of Polymer Adsorption on Permeability Reduction in Enhanced Oil Recovery. J. Pet. Eng. 2014, 2014, 1–9. [Google Scholar] [CrossRef]
- Kumar, S.; Mandal, A. Rheological properties and performance evaluation of synthesized anionic polymeric surfactant for its application in enhanced oil recovery. Polymer 2017, 120, 30–42. [Google Scholar] [CrossRef]
- Verma, J.; Mandal, A. Potential effective criteria for selection of polymer in enhanced oil recovery. Pet. Sci. Technol. 2021, 40, 879–892. [Google Scholar] [CrossRef]
- Guangzhi, L.I.A.O.; Qiang, W.A.N.G.; Hongzhuang, W.A.N.G.; Weidong, L.; Zhengmao, W. Chemical flooding development status and prospect. Acta Petrolei Sin. 2017, 38, 196. [Google Scholar]
- Li, X.; Zhang, F.; Liu, G. Review on polymer flooding technology. In IOP Conference Series: Earth and Environmental Science; IOP Publishing: Xiamen, China, 2021; p. 012199. [Google Scholar]
- Liu, J.; Zhong, L.; Wang, C.; Li, S.; Yuan, X.; Liu, Y.; Meng, X.; Zou, J.; Wang, Q. Investigation of a high temperature gel system for application in saline oil and gas reservoirs for profile modification. J. Pet. Sci. Eng. 2020, 195, 107852. [Google Scholar] [CrossRef]
- Chen, X.; Li, Y.; Liu, Z.; Zhang, J.; Trivedi, J.; Li, X. Experimental and theoretical investigation of the migration and plugging of the particle in porous media based on elastic properties. Fuel 2023, 332, 126224. [Google Scholar] [CrossRef]
- Chen, X.; Li, Y.; Liu, Z.; Zhang, J.; Chen, C.; Ma, M. Investigation on matching relationship and plugging mechanism of self-adaptive micro-gel (SMG) as a profile control and oil displacement agent. Powder Technol. 2020, 364, 774–784. [Google Scholar] [CrossRef]
- Chen, X.; Li, Y.; Liu, Z.; Li, X.; Zhang, J.; Zhang, H. Core- and pore-scale investigation on the migration and plugging of polymer microspheres in a heterogeneous porous media. J. Pet. Sci. Eng. 2020, 195, 107636. [Google Scholar] [CrossRef]
- Chen, X.; Li, Y.-Q.; Liu, Z.-Y.; Trivedi, J.; Gao, W.-B.; Sui, M.-Y. Experimental investigation on the enhanced oil recovery efficiency of polymeric surfactant: Matching relationship with core and emulsification ability. Pet. Sci. 2023, 20, 619–635. [Google Scholar] [CrossRef]
- Zhong, L.; Liu, J.; Yuan, X.; Wang, C.; Teng, L.; Zhang, S.; Wu, F.; Shen, W.; Jiang, C. Subsurface Sludge Sequestration in Cyclic Steam Stimulated Heavy-Oil Reservoir in Liaohe Oil Field. SPE J. 2020, 25, 1113–1127. [Google Scholar] [CrossRef]
- Hu, J.; Li, A.; Memon, A. Experimental Investigation of Polymer Enhanced Oil Recovery under Different Injection Modes. ACS Omega 2020, 5, 31069–31075. [Google Scholar] [CrossRef]
- Liang, X.; Shi, L.; Cheng, L.; Wang, X.; Ye, Z. Optimization of polymer mobility control for enhanced heavy oil recovery: Based on response surface method. J. Pet. Sci. Eng. 2021, 206, 109065. [Google Scholar] [CrossRef]
- Dann, M.W.; Burnett, D.B.; Hall, L.M. Polymer performance in low permeability reservoirs. In Proceedings of the SPE International Conference on Oilfield Chemistry, Dallas, TX, USA, 25 January 1982. [Google Scholar]
- Ghosh, P.; Sharma, H.; Mohanty, K.K. ASP flooding in tight carbonate rocks. Fuel 2018, 241, 653–668. [Google Scholar] [CrossRef]
- Marliere, C.; Wartenberg, N.; Fleury, M.; Tabary, R.; Dalmazzone, C.; Delamaide, E. Oil Recovery in Low Permeability Sandstone Reservoirs Using Surfactant-Polymer Flooding. In Proceedings of the SPE Latin America and Caribbean Petroleum Engineering Conference, Quito, Ecuador, 19 November 2015; p. D031S030R004. [Google Scholar] [CrossRef]
- Bennetzen, M.V.; Gilani, S.F.; Mogensen, K.; Ghozali, M.; Bounoua, N. Successful polymer flooding of low-permeability, oil-wet, carbonate reservoir cores. In Proceedings of the Abu Dhabi International Petroleum Exhibition and Conference, Abu Dhabi, United Arab Emirates, 10–13 November 2014; p. D021S019R004. [Google Scholar]
- Al-Murayri, M.T.; Kamal, D.S.; Al-Sabah, H.M.; AbdulSalam, T.; Al-Shamali, A.; Quttainah, R.; Glushko, D.; Britton, C.; Delshad, M.; Liyanage, J.; et al. Low-Salinity Polymer Flooding in a High-Temperature Low-Permeability Carbonate Reservoir in West Kuwait. In Proceedings of the SPE Kuwait Oil and Gas Show and Conference, Mishref, Kuwait, 13–16 October 2019. [Google Scholar] [CrossRef]
- Leon, J.M.; Castillo, A.F.; Perez, R.; Jimenez, J.A.; Izadi, M.; Mendez, A.; Castillo, O.P.; Londoño, F.W.; Zapata, J.F.; Chaparro, C.H. A Successful Polymer Flood Pilot at Palogrande-Cebu, A Low Permeability Reservoir in the Upper Magdalena Valley, Colombia. In Proceedings of the SPE Improved Oil Recovery Conference, Oklahoma City, OK, USA, 14–18 April 2018. [Google Scholar] [CrossRef]
- Musevic, I.; Skarabot, M.; Tkalec, U.; Ravnik, M.; Zumer, S. Two-Dimensional Nematic Colloidal Crystals Self-Assembled by Topological Defects. Science 2006, 313, 954–958. [Google Scholar] [CrossRef]
- Su, X.; Cunningham, M.F.; Jessop, P.G. Switchable viscosity triggered by CO2 using smart worm-like micelles. Chem. Commun. 2013, 49, 2655–2657. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Theato, P. CO2-responsive polymers. Macromol. Rapid Commun. 2013, 34, 1118–1133. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Feng, Y.; Wang, J.; He, S.; Guo, Z.; Chu, Z.; Dreiss, C.A. CO2-switchable wormlike micelles. Chem. Commun. 2013, 49, 4902–4904. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Feng, Y.; Wang, Y.; Li, X. CO2-Switchable Viscoelastic Fluids Based on a Pseudogemini Surfactant. Langmuir 2013, 29, 4187–4192. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Zheng, P.; Gao, K.; Wei, B.; Feng, Y. Thermo- and CO2-triggered viscosifying of aqueous copolymer solutions for gas channeling control during water-alternating-CO2 flooding. Fuel 2021, 291, 120171. [Google Scholar] [CrossRef]
- Shen, H.; Yang, Z.; Li, X.; Peng, Y.; Lin, M.; Zhang, J.; Dong, Z. CO2-responsive agent for restraining gas channeling during CO2 flooding in low permeability reservoirs. Fuel 2021, 292, 120306. [Google Scholar] [CrossRef]
- Wu, Y.; Liu, Q.; Liu, D.; Cao, X.P.; Yuan, B.; Zhao, M. CO2 responsive expansion hydrogels with programmable swelling for in-depth CO2 conformance control in porous media. Fuel 2023, 332, 126047. [Google Scholar] [CrossRef]
- Zhang, D.; Li, Y.; Bao, Z. A laboratory experimental study of feasibility of polymer flood for middle-low permeability reservoirs in Daqing. Oilfield Chem. 2005, 22, 78–80. [Google Scholar]
- Pal, N.; Zhang, X.; Ali, M.; Mandal, A.; Hoteit, H. Carbon dioxide thickening: A review of technological aspects, advances and challenges for oilfield application. Fuel 2022, 315, 122947. [Google Scholar] [CrossRef]
- Yang, J.; Dong, H. CO2-responsive aliphatic tertiary amine-modified alginate and its application as a switchable surfactant. Carbohydr. Polym. 2016, 153, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Fan, W.; Tong, X.; Farnia, F.; Yu, B.; Zhao, Y. CO2-Responsive Polymer Single-Chain Nanoparticles and Self-Assembly for Gas-Tunable Nanoreactors. Chem. Mater. 2017, 29, 5693–5701. [Google Scholar] [CrossRef]
- Kumar, S.; Mandal, A. A comprehensive review on chemically enhanced water alternating gas/CO2 (CEWAG) injection for enhanced oil recovery. J. Pet. Sci. Eng. 2017, 157, 696–715. [Google Scholar] [CrossRef]
- Kumar, N.; Sampaio, M.A.; Ojha, K.; Hoteit, H.; Mandal, A. Fundamental aspects, mechanisms and emerging possibilities of CO2 miscible flooding in enhanced oil recovery: A review. Fuel 2022, 330, 125633. [Google Scholar] [CrossRef]
- Li, H.; Zheng, S.; Yang, D. Enhanced swelling effect and viscosity reduction of solvent (s)/CO2/heavy-oil systems. Spe J. 2013, 18, 695–707. [Google Scholar] [CrossRef]
- Chaturvedi, K.R.; Trivedi, J.; Sharma, T. Evaluation of Polymer-Assisted Carbonated Water Injection in Sandstone Reservoir: Absorption Kinetics, Rheology, and Oil Recovery Results. Energy Fuels 2019, 33, 5438–5451. [Google Scholar] [CrossRef]
- Zhao, J.; Torabi, F.; Yang, J. The synergistic role of silica nanoparticle and anionic surfactant on the static and dynamic CO2 foam stability for enhanced heavy oil recovery: An experimental study. Fuel 2020, 287, 119443. [Google Scholar] [CrossRef]
- Hamidi, H.; Haddad, A.S.; Mohammadian, E.; Rafati, R.; Azdarpour, A.; Ghahri, P.; Ombewa, P.; Neuert, T.; Zink, A. Ultrasound-assisted CO2 flooding to improve oil recovery. Ultrason. Sonochem. 2017, 35, 243–250. [Google Scholar] [CrossRef]
- Yang, Y.; Li, W.; Zhou, T.; Dong, Z. Using Polymer Alternating Gas to Enhance Oil Recovery in Heavy Oil. IOP Conf. Ser. Earth Environ. Sci. 2018, 113, 012182. [Google Scholar] [CrossRef]
- Gandomkar, A.; Torabi, F.; Riazi, M. CO2 mobility control by small molecule thickeners during secondary and tertiary enhanced oil recovery. Can. J. Chem. Eng. 2020, 99, 1352–1362. [Google Scholar] [CrossRef]
- Manan, M.A.; Farad, S.; Piroozian, A.; Esmail, M.J.A. Effects of Nanoparticle Types on Carbon Dioxide Foam Flooding in Enhanced Oil Recovery. Pet. Sci. Technol. 2015, 33, 1286–1294. [Google Scholar] [CrossRef]
- Zaberi, H.A.; Lee, J.J.; Enick, R.M.; Beckman, E.J.; Cummings, S.D.; Dailey, C.; Vasilache, M. An experimental feasibility study on the use of CO2-soluble polyfluoroacrylates for CO2 mobility and conformance control applications. J. Pet. Sci. Eng. 2019, 184, 106556. [Google Scholar] [CrossRef]
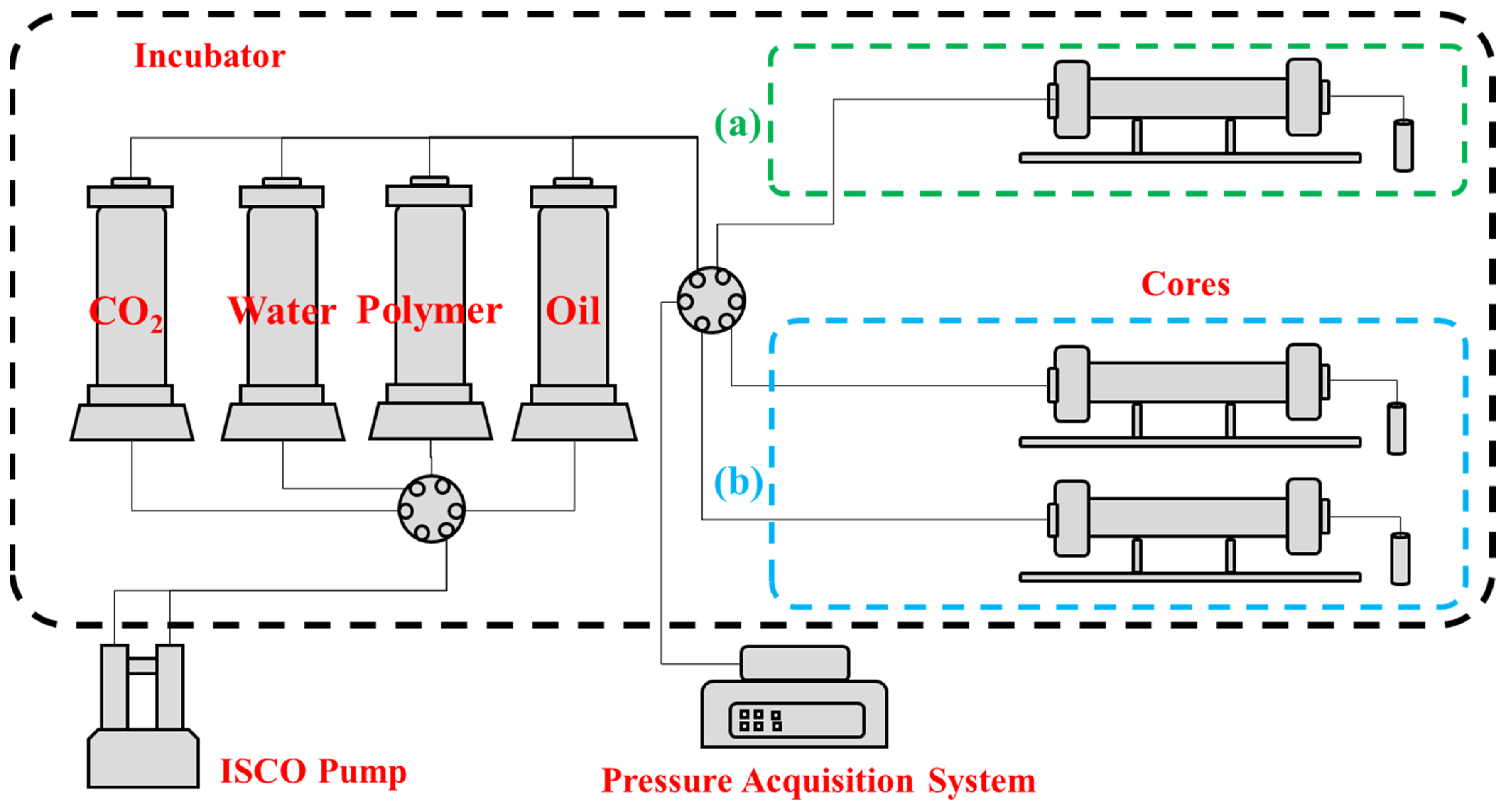




| Polymer Concentration, mg/L | Gas Permeability, mD | Water Permeability, mD | Core Volume, cm3 | Pore Volume, cm3 | Porosity, % |
|---|---|---|---|---|---|
| 1000 | 5 | 2.99 | 226.7 | 24.32 | 10.73 |
| 500 | 10 | 5.36 | 226.7 | 34.28 | 15.12 |
| 1000 | 10 | 5.23 | 226.7 | 35.82 | 15.8 |
| 2000 | 10 | 5.38 | 226.71 | 33.56 | 14.80 |
| 1000 | 20 | 10.12 | 226.71 | 43.80 | 19.32 |
| Solution | Viscosity, cP | Injection CO2 | Gas Permeability, mD | Water Permeability, mD | Porosity, % |
|---|---|---|---|---|---|
| TRPS | 9.3 | Yes | 10 | 5.20 | 15.51 |
| HPAM | 10.1 | No | 10 | 5.36 | 14.98 |
| TRPS | 9.3 | No | 10 | 5.32 | 15.11 |
| Solution | Concentration, mg/L | Injection CO2 | Gas Permeability, mD | Water Permeability, mD |
|---|---|---|---|---|
| TRPS | 500 | Yes | 5/20 | 3.12/9.98 |
| TRPS | 1000 | No | 5/20 | 3.03/10.20 |
| TRPS | 1000 | Yes | 5/20 | 3.21/10.15 |
| Parameters | Round 1 | Round 2 | Round 3 | Round 4 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Permeability, mD | Concentration, mg/L | Gas Channeling Pressure, MPa | η | η′ | η | η′ | η | η′ | η | η′ |
| 5 | 200 | 0.25 | 1.96 | 1.36 | 3.08 | 1.72 | 3.92 | 2.12 | 5.60 | 3.16 |
| 500 | 0.33 | 2.70 | 1.55 | 6.18 | 2.21 | / | / | / | / | |
| 1000 | 0.31 | 6.74 | 6.53 | 11.2 | 10.8 | 16.1 | 15.7 | 22.9 | 22.5 | |
| 10 | 200 | 0.13 | 2.20 | 1.73 | 3.23 | 2.20 | 4.96 | 2.60 | 9.37 | 4.17 |
| 500 | 0.15 | 5.00 | 2.60 | 9.53 | 3.47 | / | / | / | / | |
| 1000 | 0.14 | 8.43 | 6.93 | 12.4 | 7.86 | 20.0 | 10.0 | 28.0 | 13.2 | |
| 20 | 200 | 0.07 | 2.57 | 1.86 | 4.43 | 3.14 | 7.43 | 5.43 | 10.4 | 7.14 |
| 500 | 0.08 | 7.50 | 4.13 | 14.1 | 5.75 | / | / | / | / | |
| 1000 | 0.08 | 8.50 | 5.25 | 18.0 | 7.13 | 29.1 | 14.6 | 42.3 | 17.7 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, X.; Li, Y.; Sun, X.; Liu, Z.; Liu, J.; Liu, S. Investigation of Polymer-Assisted CO2 Flooding to Enhance Oil Recovery in Low-Permeability Reservoirs. Polymers 2023, 15, 3886. https://doi.org/10.3390/polym15193886
Chen X, Li Y, Sun X, Liu Z, Liu J, Liu S. Investigation of Polymer-Assisted CO2 Flooding to Enhance Oil Recovery in Low-Permeability Reservoirs. Polymers. 2023; 15(19):3886. https://doi.org/10.3390/polym15193886
Chicago/Turabian StyleChen, Xin, Yiqiang Li, Xiaoguang Sun, Zheyu Liu, Jianbin Liu, and Shun Liu. 2023. "Investigation of Polymer-Assisted CO2 Flooding to Enhance Oil Recovery in Low-Permeability Reservoirs" Polymers 15, no. 19: 3886. https://doi.org/10.3390/polym15193886
APA StyleChen, X., Li, Y., Sun, X., Liu, Z., Liu, J., & Liu, S. (2023). Investigation of Polymer-Assisted CO2 Flooding to Enhance Oil Recovery in Low-Permeability Reservoirs. Polymers, 15(19), 3886. https://doi.org/10.3390/polym15193886









