Characterization of Synthetic Polymer Coated with Biopolymer Layer with Natural Orange Peel Extract Aimed for Food Packaging
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Preparation of Coated Films
2.2.2. Gas Permeance
2.2.3. Water Vapour Transmission Rate
2.2.4. The Overall Migration
2.2.5. Fourier Transform Infrared Spectrometry
2.2.6. Optical Properties
2.2.7. Antimicrobial Activity
2.3. Statistical Analysis
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Simmonds, G.; Woods, A.T.; Spence, C. ‘Show me the goods’: Assessing the effectiveness of transparent packaging vs. product imagery on product evaluation. Food Qual. Prefer. 2018, 63, 18–27. [Google Scholar] [CrossRef]
- Daniloski, D.; Petkoska, A.T.; Galić, K.; Ščetar, M.; Kurek, M.; Vaskoska, R.; Kalevska, T.; Nedelkoska, D.N. The effect of barrier properties of polymeric films on the shelf-life of vacuum packaged fresh pork meat. Meat Sci. 2019, 158, 107880. [Google Scholar] [CrossRef] [PubMed]
- Galić, K.; Kurek, M.; Ščetar, M. Barrier Properties of Plastic Polymers. In Reference Module in Food Science; Elsevier: Amsterdam, The Netherlands, 2017. [Google Scholar] [CrossRef]
- Siracusa, V. Food Packaging Permeability Behaviour: A Report. Int. J. Polym. Sci. 2012, 2012, 302029. [Google Scholar] [CrossRef]
- Marano, S.; Laudadio, E.; Minnelli, C.; Stipa, P. Tailoring the barrier properties of PLA: A State-of-the-art review for food packaging applications. Polymers 2022, 14, 1626. [Google Scholar] [CrossRef]
- Kurek, M.; Brachais, C.-H.; Ščetar, M.; Voilley, A.; Galić, K.; Couvercelle, J.-P.; Debeaufort, F. Carvacrol affects interfacial, structural and transfer properties of chitosan coatings applied onto polyethylene. Carbohydr. Polym. 2013, 97, 217–225. [Google Scholar] [CrossRef]
- Benbettaïeb, N.; Chambin, O.; Assifaoui, A.; Al-Assaf, S.; Karbowiak, T.; Debeaufort, F. Release of coumarin incorporated into chitosan-gelatin irradiated films. Food Hydrocoll. 2016, 56, 266–276. [Google Scholar] [CrossRef]
- Kurek, M.; Hlupić, L.; Elez Garofulić, I.; Descours, E.; Ščetar, M.; Galić, K. Comparison of protective supports and antioxidative capacity of two bio-based films with revalorised fruit pomaces extracted from blueberry and red grape skin. Food Packag. Shelf Life 2019, 20, 100315. [Google Scholar] [CrossRef]
- Grzelczyk, J.; Oracz, J.; Gałązka-Czarnecka, I. Quality assessment of waste from olive oil production and design of biodegradable packaging. Foods 2022, 11, 3776. [Google Scholar] [CrossRef]
- Samir, A.; Ashour, F.H.; Hakim, A.A.A.; Bassyouni, M. Recent advances in biodegradable polymers for sustainable applications. NPJ Mater. Degrad. 2022, 6, 68. [Google Scholar] [CrossRef]
- Bayer, I.S. Biopolymers in multilayer films for long-lasting protective food packaging: A review. In Sustainable Food Packaging Technology; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2021; pp. 395–426. [Google Scholar]
- Nuruddin, M.; Korani, D.M.; Jo, H.; Chowdhury, R.A.; Montes, F.J.; Howarter, J.A.; Youngblood, J.P. Gas and water vapor barrier performance of cellulose nanocrystal–citric acid-coated polypropylene for flexible packaging. ACS Appl. Polym. Mater. 2020, 2, 4405–4414. [Google Scholar] [CrossRef]
- Rehman, A.; Jafari, S.M.; Aadil, R.M.; Assadpour, E.; Randhawa, M.A.; Mahmood, S. Development of active food packaging via incorporation of biopolymeric nanocarriers containing essential oils. Trends Food Sci. Technol. 2020, 101, 106–121. [Google Scholar] [CrossRef]
- Pabast, M.; Shariatifar, N.; Beikzadeh, S.; Jahed, G. Effects of chitosan coatings incorporating with free or nano-encapsulated Satureja plant essential oil on quality characteristics of lamb meat. Food Control 2018, 91, 185–192. [Google Scholar] [CrossRef]
- Hasheminejad, N.; Khodaiyan, F. The effect of clove essential oil loaded chitosan nanoparticles on the shelf life and quality of pomegranate arils. Food Chem. 2020, 309, 125520. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Núñez, J.R.; Madera-Santana, T.J.; Sánchez-Machado, D.I.; López-Cervantes, J.; Soto Valdez, H. Chitosan/hydrophilic plasticizer-based films: Preparation, physicochemical and antimicrobial properties. J. Polym. Environ. 2013, 22, 41–51. [Google Scholar] [CrossRef]
- Goy, R.C.; Britto, D.D.; Assis, O.B.G. A review of the antimicrobial activity of chitosan. Polímeros 2009, 19, 241–247. [Google Scholar] [CrossRef]
- Yashaswini, P.; Vind, A. Antimicrobial properties of orange (Citrus reticulata var. Kinnow) peel extracts against pathogenic bacteria. Int. J. Curr. Microbiol. Appl. Sci. 2018, 7, 737–746. [Google Scholar] [CrossRef]
- Shehata, M.G.; Awad, T.S.; Asker, D.; El Sohaimy, S.A.; Abd El-Aziz, N.M.; Youssef, M.M. Antioxidant and antimicrobial activities and UPLC-ESI-MS/MS polyphenolic profile of sweet orange peel extracts. Curr. Res. Food Sci. 2021, 4, 326–335. [Google Scholar] [CrossRef]
- Ethos X Application Reports—Microwave Green Extraction of Natural Products. Available online: https://www.milestonesci.com/wp-content/uploads/2017/08/EthosX_NaturalProducts_Applications.pdf (accessed on 7 February 2023).
- München, G. Gas Permeability Testing Manual; Brugger Feinmechanik GmbH: Munich, Germany, 2008. [Google Scholar]
- E96-80; Standard Test Methods for Water Vapor Transmission of Materials. ASTM: West Conshohocken, PA, USA, 1980.
- Debeaufort, F.; Martin-Polo, M.; Voilley, A. Polarity homogeneity and structure affect water vapor permeability of model edible films. J. Food Sci. 1993, 58, 426–429. [Google Scholar] [CrossRef]
- EUR-Lex. Commission Regulation (EU) 2016/1416 of 24 August 2016 Amending and Correcting Regulation (EU) No 10/2011 on Plastic Materials and Articles Intended to Come into Contact with Food. 2016/1416. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=celex:32016R1416 (accessed on 20 May 2023).
- EN 1186-1; Materials and Article in Contact with Foodstuffs—Plastics. Part 1: Guide to the Selection of Conditions and Test Methods for Overall Migration. European Committee for Standardization CEN: Brussels, Belgium, 2002.
- Zuo, G.; Song, X.; Chen, F.; Shen, Z. Physical and structural characterization of edible bilayer films made with zein and corn-wheat starch. J. Saudi Soc. Agric. Sci. 2019, 18, 324–331. [Google Scholar] [CrossRef]
- Celina, M.C.; Quintana, A. Oxygen diffusivity and permeation through polymers at elevated temperature. Polymer 2018, 150, 326–342. [Google Scholar] [CrossRef]
- Mahand, S.N.; Yazdanbakhsh, A.; Tayouri, M.I.; Zarei, A.; Nouranian, S.; Ruckdäschel, H.; Khonakdar, H.A. Theoretical and experimental investigation of selective gas permeability in polystyrene/polyolefin elastomer/nanoclay nanocomposite films. Polym. Test. 2023, 120, 107960. [Google Scholar] [CrossRef]
- Kim, D.; Seo, J. A review: Breathable films for packaging applications. Trends Food Sci. Technol. 2018, 76, 15–27. [Google Scholar] [CrossRef]
- Salehi, A.; Jafari, S.H.; Khonakdar, H.A.; Ebadi-Dehaghani, H. Temperature dependency of gas barrier properties of biodegradable PP/PLA/nanoclay films: Experimental analyses with a molecular dynamics simulation approach. J. Appl. Polym. Sci. 2018, 135, 46665. [Google Scholar] [CrossRef]
- Gajdoš, J.; Galić, K.; Kurtanjek, Ž.; Ciković, N. Gas permeability and DSC characteristics of polymers used in food packaging. Polym. Test. 2000, 20, 49–57. [Google Scholar] [CrossRef]
- Kurek, M.; Klepac, D.; Ščetar, M.; Galić, K.; Valić, S.; Liu, Y.; Yang, W. Gas barrier and morphology characteristics of linear low-density polyethylene and two different polypropylene films. Polym. Bull. 2011, 67, 1293–1309. [Google Scholar] [CrossRef]
- Lin, Y.J.; Dias, P.; Chen, H.Y.; Hiltner, A.; Baer, E. Relationship between biaxial orientation and oxygen permeability of polypropylene film. Polymer 2008, 49, 2578–2586. [Google Scholar] [CrossRef]
- Galus, S.; Mikus, M.; Ciurzyńska, A.; Domian, E.; Kowalska, J.; Marzec, A.; Kowalska, H. The effect of whey protein-based edible coatings incorporated with lemon and lemongrass essential oils on the quality attributes of fresh-cut pears during storage. Coatings 2021, 11, 745. [Google Scholar] [CrossRef]
- Peng, Y.; Li, Y. Combined effects of two kinds of essential oils on physical, mechanical and structural properties of chitosan films. Food Hydrocoll. 2014, 36, 287–293. [Google Scholar] [CrossRef]
- Di Giuseppe, F.A.; Volpe, S.; Cavella, S.; Masi, P.; Torrieri, E. Physical properties of active biopolymer films based on chitosan, sodium caseinate, and rosemary essential oil. Food Packag. Shelf Life 2022, 32, 100817. [Google Scholar] [CrossRef]
- Mahmoudzadeh, M.; Fassihi, A.; Emami, J.; Davies, N.M.; Dorkoosh, F. Physicochemical, pharmaceutical and biological approaches toward designing optimized and efficient hydrophobically modified chitosan-based polymeric micelles as a nanocarrier system for targeted delivery of anticancer drugs. J. Drug Target. 2013, 21, 693–709. [Google Scholar] [CrossRef]
- Bastarrachea, L.; Dhawan, S.; Sablani, S.S. Engineering properties of polymeric-based antimicrobial films for food packaging: A review. Food Eng. Rev. 2011, 3, 79–93. [Google Scholar] [CrossRef]
- Cao, N.; Yang, X.; Fu, Y. Effects of various plasticizers on mechanical and water vapor barrier properties of gelatin films. Food Hydrocoll. 2009, 23, 729–735. [Google Scholar] [CrossRef]
- Donhowe, I.G.; Fennema, O. The effects of plasticizers on crystallinity, permeability, and mechanical properties of methylcellulose films. J. Food Process. Preserv. 1993, 17, 247–257. [Google Scholar] [CrossRef]
- Liang, J.; Ludescher, R.D. Influence of glycerol on molecular mobility and hydrogen bond network in amorphous glucose matrix. Carbohydr. Res. 2012, 361, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Xia, Q.; Wang, S.; Li, J.; Huang, Q.; Ludescher, R.D. Influence of glycerol on the molecular mobility, oxygen permeability and microstructure of amorphous zein films. Food Hydrocoll. 2015, 44, 94–100. [Google Scholar] [CrossRef]
- Caicedo, C.; Díaz-Cruz, C.A.; Jiménez-Regalado, E.J.; Aguirre-Loredo, R.Y. Effect of plasticizer content on mechanical and water vapor permeability of maize starch/PVOH/chitosan composite films. Materials 2022, 15, 1274. [Google Scholar] [CrossRef]
- Aguirre-Loredo, R.Y.; Rodríguez-Hernández, A.I.; Morales-Sánchez, E.; Gómez-Aldapa, C.A.; Velazquez, G. Effect of equilibrium moisture content on barrier, mechanical and thermal properties of chitosan films. Food Chem. 2016, 196, 560–566. [Google Scholar] [CrossRef]
- Ma, X.; Qiao, C.; Wang, X.; Yao, J.; Xu, J. Structural characterization and properties of polyols plasticized chitosan films. Int. J. Biol. Macromol. 2019, 135, 240–245. [Google Scholar] [CrossRef]
- Faust, S.; Foerster, J.; Lindner, M.; Schmid, M. Effect of glycerol and sorbitol on the mechanical and barrier properties of films based on pea protein isolate produced by high-moisture extrusion processing. Polym. Eng. Sci. 2021, 62, 95–102. [Google Scholar] [CrossRef]
- Ballesteros-Mártinez, L.; Pérez-Cervera, C.; Andrade-Pizarro, R. Effect of glycerol and sorbitol concentrations on mechanical, optical, and barrier properties of sweet potato starch film. NFS J. 2020, 20, 1–9. [Google Scholar] [CrossRef]
- Li, Y.; Tang, C.; He, Q. Effect of orange (Citrus sinensis L.) peel essential oil on characteristics of blend films based on chitosan and fish skin gelatin. Food Biosci. 2021, 41, 100927. [Google Scholar] [CrossRef]
- De Oliveira Filho, J.G.; de Deus, I.P.B.; Valadares, A.C.F.; Fernandes, C.C.; Estevam, E.B.B.; Egea, M.B. Chitosan film with citrus limonia essential oil: Physical and morphological properties and antibacterial activity. Colloids Interfaces 2020, 4, 18. [Google Scholar] [CrossRef]
- Šuput, D.; Popović, S.; Hromiš, N.; Bulut, S.; Lazić, V. Biopolymer films properties change affected by essential oils addition. J. Process. Energy Agric. 2019, 23, 61–65. [Google Scholar] [CrossRef]
- Zubair, M.; Shahzad, S.; Hussain, A.; Pradhan, R.A.; Arshad, M.; Ullah, A. Current Trends in the utilization of essential oils for polysaccharide- and protein-derived food packaging materials. Polymers 2022, 14, 1146. [Google Scholar] [CrossRef]
- Gozutok, M.; Basar, A.O.; Sasmazel, H.T. Development of Antibacterial Composite Electrospun Chitosan-Coated Polypropylene Materials. J. Nanosci. Nanotechnol. 2018, 18, 2881–2891. [Google Scholar] [CrossRef]
- Cejudo Bastante, C.; Cran, M.J.; Casas Cardoso, L.; Serrano, C.M.; Bigger, S.W. Mass transfer and optical properties of active PET/PP food-grade films impregnated with olive leaf extract. Polymers 2021, 14, 84. [Google Scholar] [CrossRef]
- Wang, J.; Gardner, D.J.; Stark, N.M.; Bousfield, D.W.; Tajvidi, M.; Cai, Z. Moisture and oxygen barrier properties of cellulose nanomaterial-based films. ACS Sustain. Chem. Eng. 2017, 6, 49–70. [Google Scholar] [CrossRef]
- Fasihnia, S.H.; Peighambardoust, S.H.; Peighambardoust, S.J.; Oromiehie, A.; Soltanzadeh, M.; Peressini, D. Migration analysis, antioxidant, and mechanical characterization of polypropylene-based active food packaging films loaded with BHA, BHT, and TBHQ. J. Food Sci. 2020, 85, 2317–2328. [Google Scholar] [CrossRef]
- Goulas, A. Overall migration from commercial coextruded food packaging multilayer films and plastics containers into official EU food simulants. Eur. Food Res. Technol. 2001, 212, 597–602. [Google Scholar] [CrossRef]
- Glaser, T.K.; Plohl, O.; Vesel, A.; Ajdnik, U.; Ulrih, N.P.; Hrnčič, M.K.; Bren, U.; Fras Zemljič, L. Functionalization of polyethylene (PE) and polypropylene (PP) material using chitosan nanoparticles with incorporated resveratrol as potential active packaging. Materials 2019, 12, 2118. [Google Scholar] [CrossRef]
- Smith, B.C. The Infrared Spectra of Polymers III: Hydrocarbon Polymers. Spectroscopy 2021, 36, 22–25. [Google Scholar] [CrossRef]
- Murashko, O.N.; Lin-Chao, S. Escherichia coli responds to environmental changes using enolasic degradosomes and stabilized DicF sRNA to alter cellular morphology. Proc. Natl. Acad. Sci. USA 2017, 114, E8025–E8034. [Google Scholar] [CrossRef] [PubMed]






| Sample Composition | Chitosan (%, w/v) | Tween 80 (%, w/v) | Glycerol (w/pdw) | OPEO (w/v) | Coating layer Thickness (µm) | Coating Layer Proportion (Mass) |
|---|---|---|---|---|---|---|
| A | 2 | 0.3 | 30 | - | 19 | 20% |
| B | 2 | 0.6 | 30 | - | 25 | 20% |
| C | 2 | 1.2 | 30 | - | 32 | 20% |
| A/OPEO | 2 | 0.3 | 30 | 1% | 20 | 25% |
| B/OPEO | 2 | 0.6 | 30 | 2% | 22 | 25% |
| C/OPEO | 2 | 1.2 | 30 | 4% | 37 | 30% |
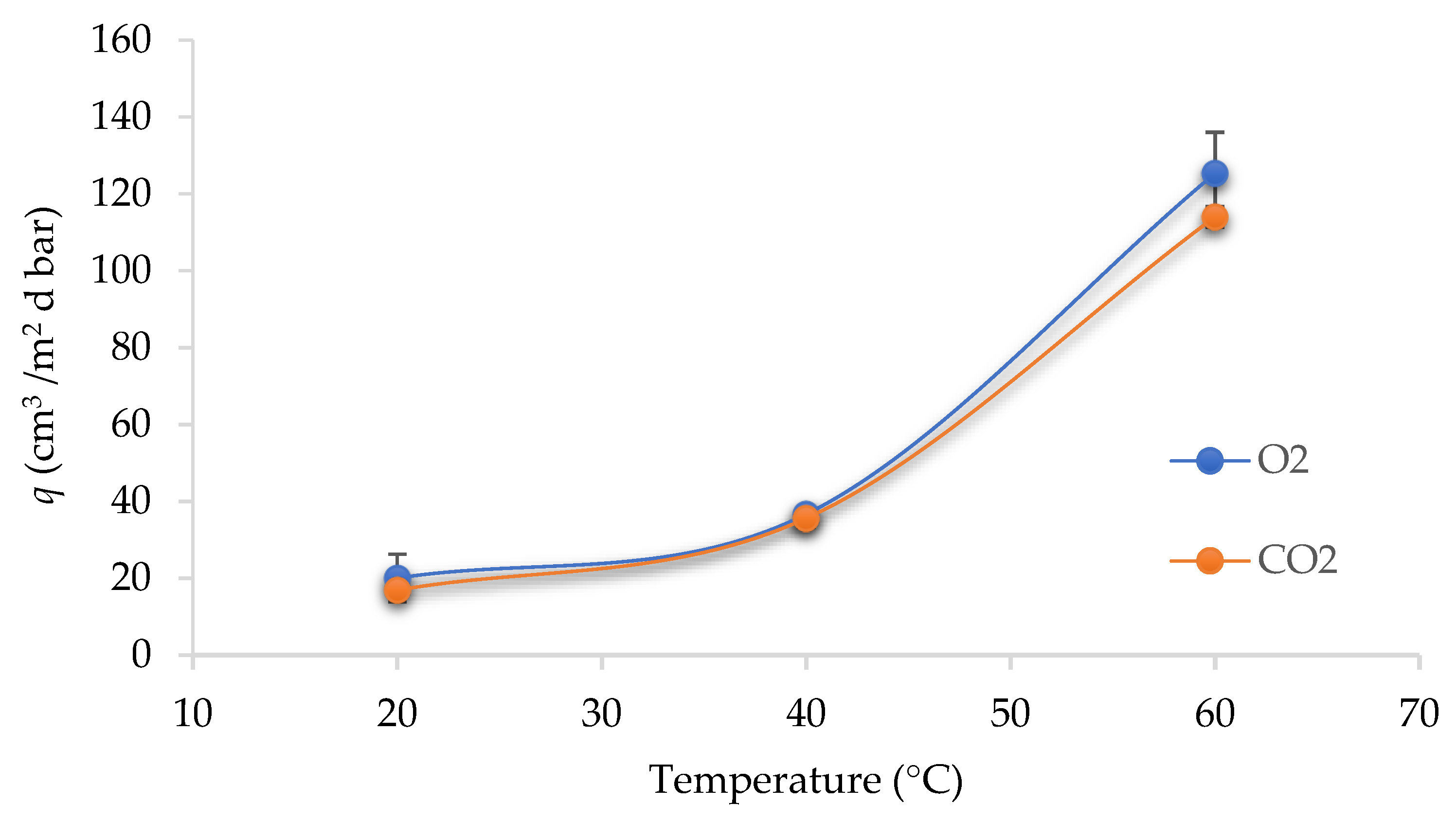
| Sample Composition | q (cm3/m2·d·bar) | |
|---|---|---|
| O2 | CO2 | |
| PET-O/PP | 20.03 ± 6.20 a | 16.90 ± 1.06 a |
| A | 4.53 ± 2.82 b | 8.06 ± 0.00 b |
| C | 6.00 ± 2.12 b | 7.74 ± 0.07 b |
| A/OPEO | 2.25 ± 0.70 b | 2.83 ± 0.14 c |
| C/OPEO | 3.38 ± 1.41 b | 5.07 ± 0.21 c |
| Samples | WVTR ·10−5 (g/m2·s) |
|---|---|
| PET-O/PP | 1.27 ± 0.45 |
| A | 5.89 ± 0.60 |
| B | 3.31 ± 0.34 |
| C | 9.50 ± 6.33 |
| A/OPEO | 3.58 ± 0.58 |
| B/OPEO | 4.09 ± 0.39 |
| C/OPEO | 5.32 ± 0.29 |
| Samples | L* | a* | b* | ΔE | T600 |
|---|---|---|---|---|---|
| PET-O/PP | 87.75 ± 0.10 a | −3.68 ± 0.10 a | −14.47 ± 0.16 c | 0.0±0.0 d | 1.97 |
| A | 87.07 ± 0.21 b,c | −4.60 ± 0.36 b,c | −12.40 ± 0.77 b | 2.4± 0.9 a | 1.61 |
| B | 87.25 ± 0.13 a,b | −4.39 ± 0.19 b | −12.62 ± 0.42 b | 2.0± 0.5 a | 1.41 |
| C | 86.51 ± 0.75 d,e | −5.02 ± 0.46 b,c | −11.53 ± 1.00 a,b | 3.5± 1.2 b,c | 1.31 |
| A/OPEO | 86.92 ± 0.05 b,c,d | −4.83 ± 0.28 b,c | −11.43 ± 0.38 a,b | 3.4± 0.4 a,b | 1.63 |
| B/OPEO | 86.64 ± 0.30 c,d | −4.81 ± 0.42 b,c | −12.44 ± 1.15 b | 2.6±1.2 a,b | 1.56 |
| C/OPEO | 85.98 ± 0.31 e | −5.90 ± 0.39 d | −9.90 ± 1.33 a | 5.2±1.0 c | 1.80 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gabrić, D.; Kurek, M.; Ščetar, M.; Brnčić, M.; Galić, K. Characterization of Synthetic Polymer Coated with Biopolymer Layer with Natural Orange Peel Extract Aimed for Food Packaging. Polymers 2023, 15, 2569. https://doi.org/10.3390/polym15112569
Gabrić D, Kurek M, Ščetar M, Brnčić M, Galić K. Characterization of Synthetic Polymer Coated with Biopolymer Layer with Natural Orange Peel Extract Aimed for Food Packaging. Polymers. 2023; 15(11):2569. https://doi.org/10.3390/polym15112569
Chicago/Turabian StyleGabrić, Domagoj, Mia Kurek, Mario Ščetar, Mladen Brnčić, and Kata Galić. 2023. "Characterization of Synthetic Polymer Coated with Biopolymer Layer with Natural Orange Peel Extract Aimed for Food Packaging" Polymers 15, no. 11: 2569. https://doi.org/10.3390/polym15112569
APA StyleGabrić, D., Kurek, M., Ščetar, M., Brnčić, M., & Galić, K. (2023). Characterization of Synthetic Polymer Coated with Biopolymer Layer with Natural Orange Peel Extract Aimed for Food Packaging. Polymers, 15(11), 2569. https://doi.org/10.3390/polym15112569