Electrical Stimuli-Responsive Decomposition of Layer-by-Layer Films Composed of Polycations and TEMPO-Modified Poly(acrylic acid)
Abstract
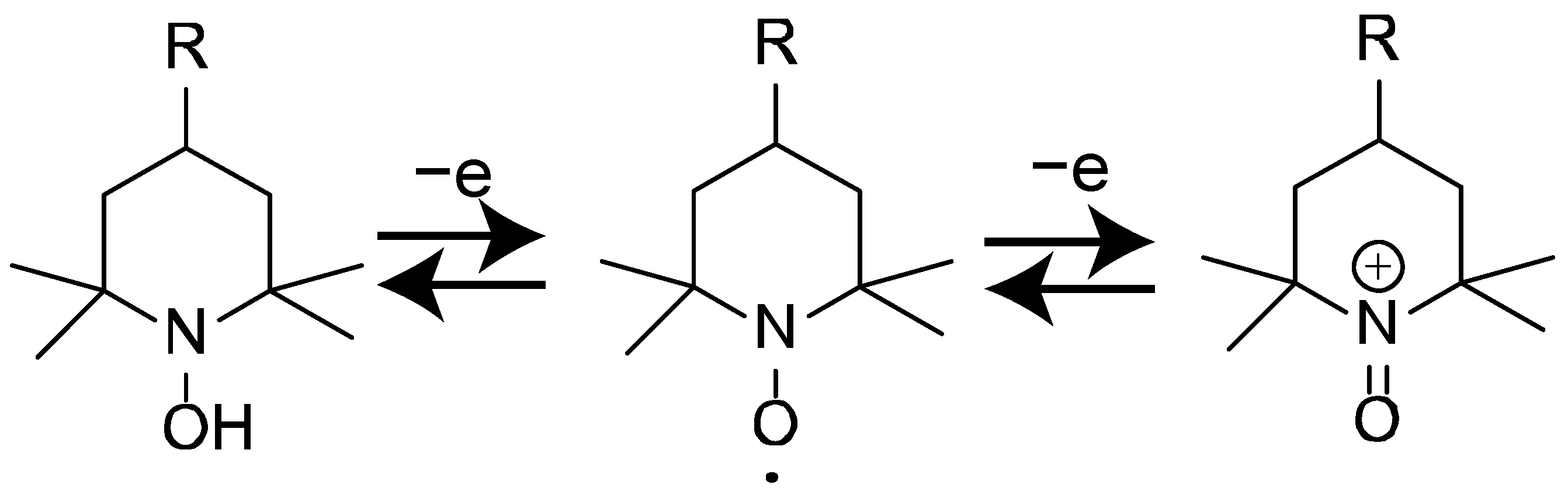
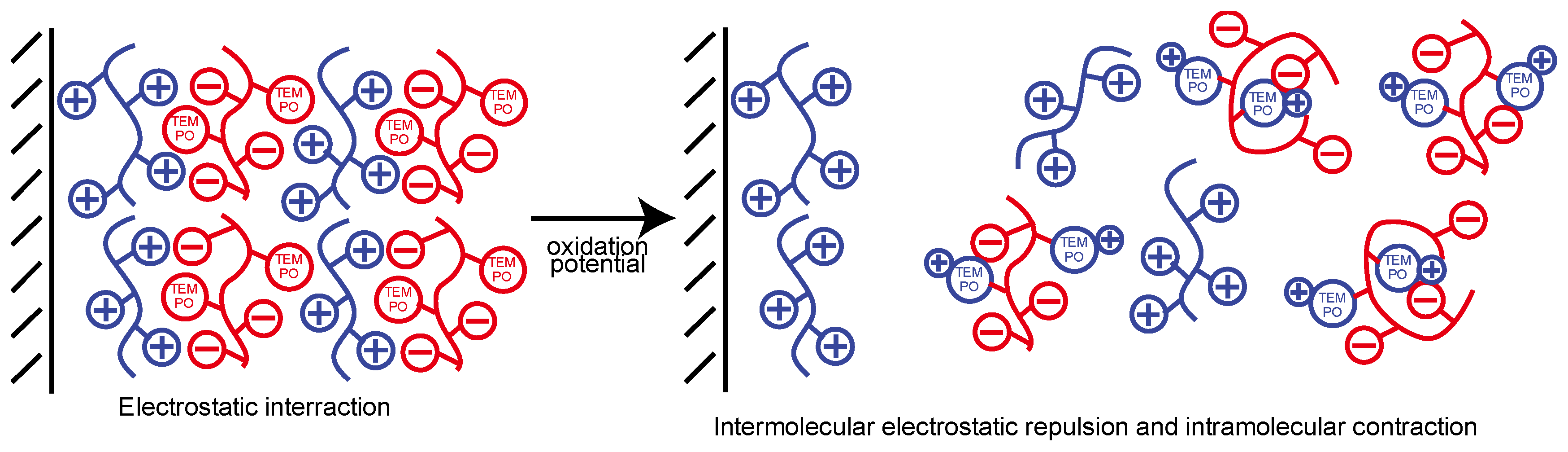
1. Introduction
2. Materials and Methods
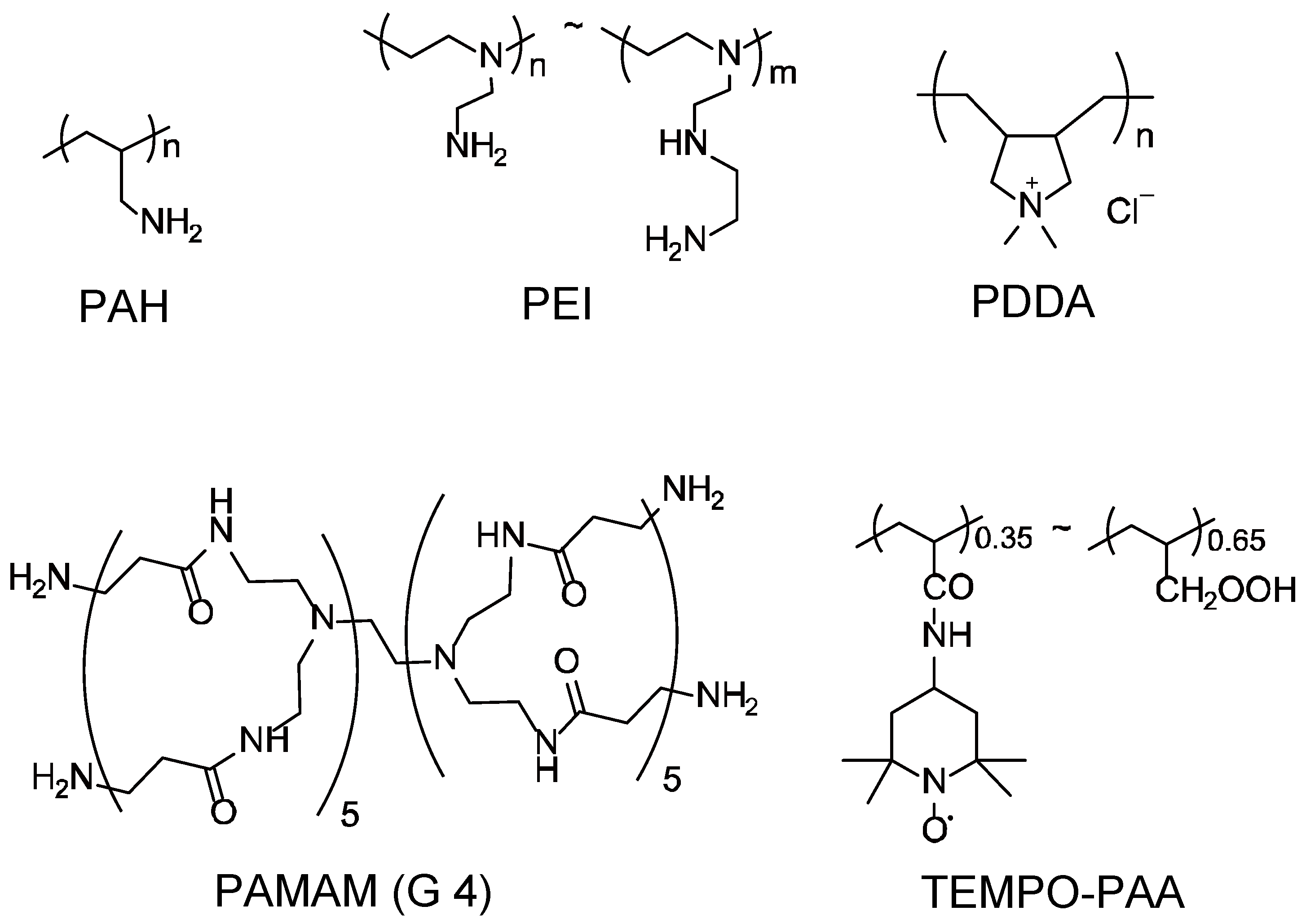
2.1. Materials
2.2. Analyses
2.3. Preparation of LbL Films
2.4. Cyclic Voltammetry and Constant Potential Application
2.5. Release of FITC-Polycation Due to Constant Potential Application Obtained from the (FITC-Polycation/TEMPO-PAA)5 Films-Coated Electrodes
3. Results
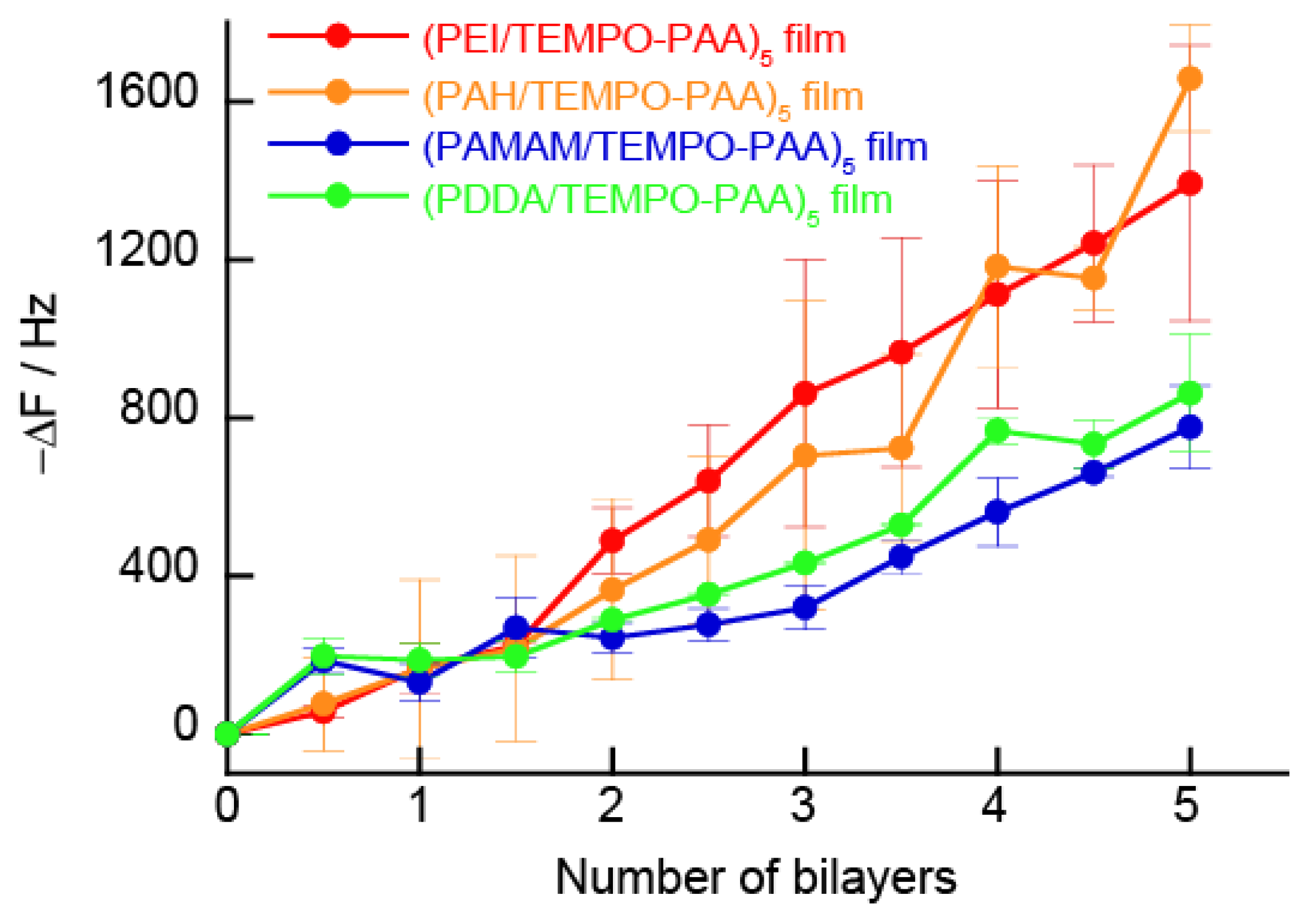
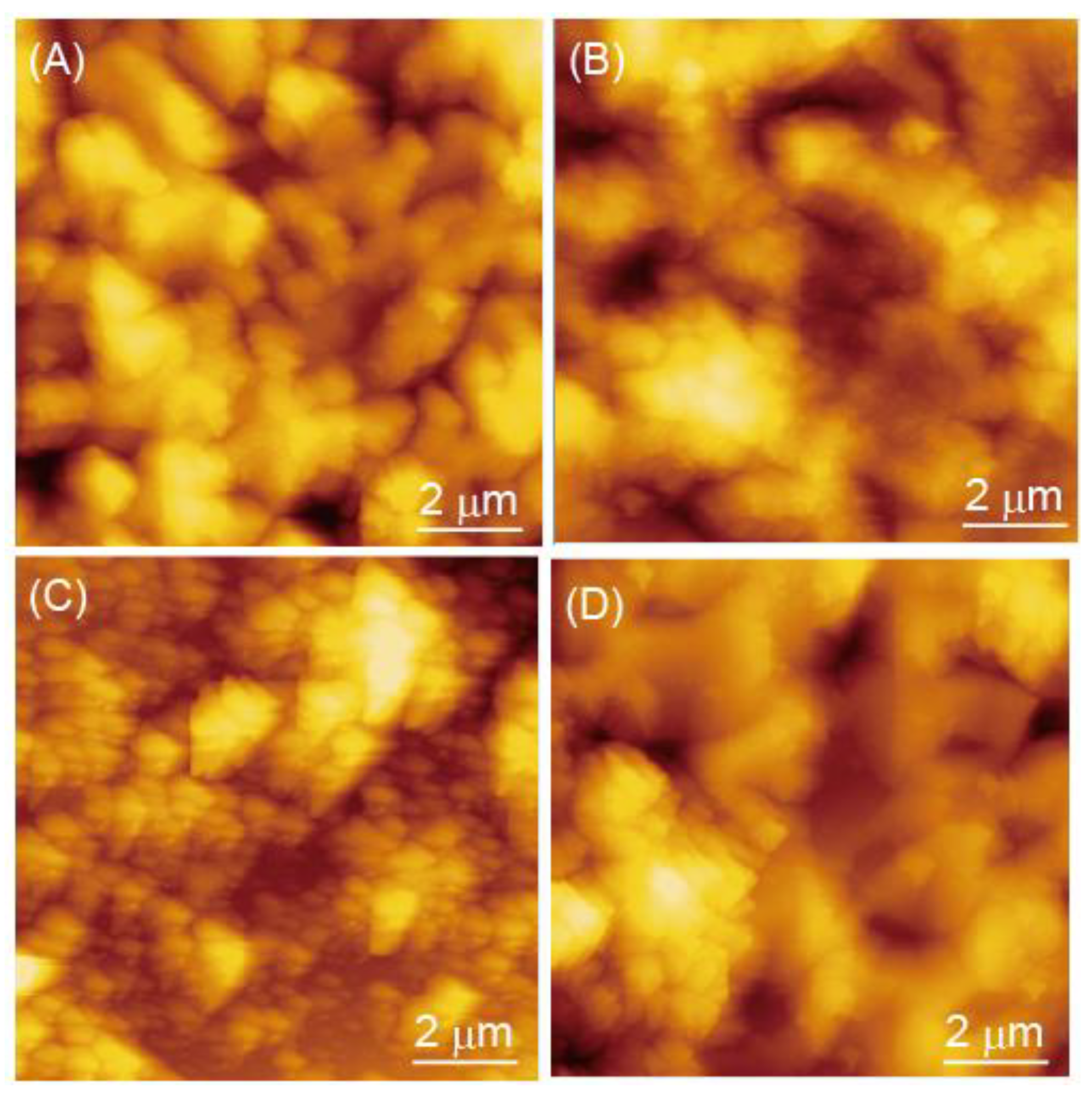
3.1. Preparation of (Polycation/TEMPO-PAA)n Films
3.2. Electrical Stimuli-Responsive Decomposition of (Polycation/TEMPO-PAA)5 Film-Coated Electrodes
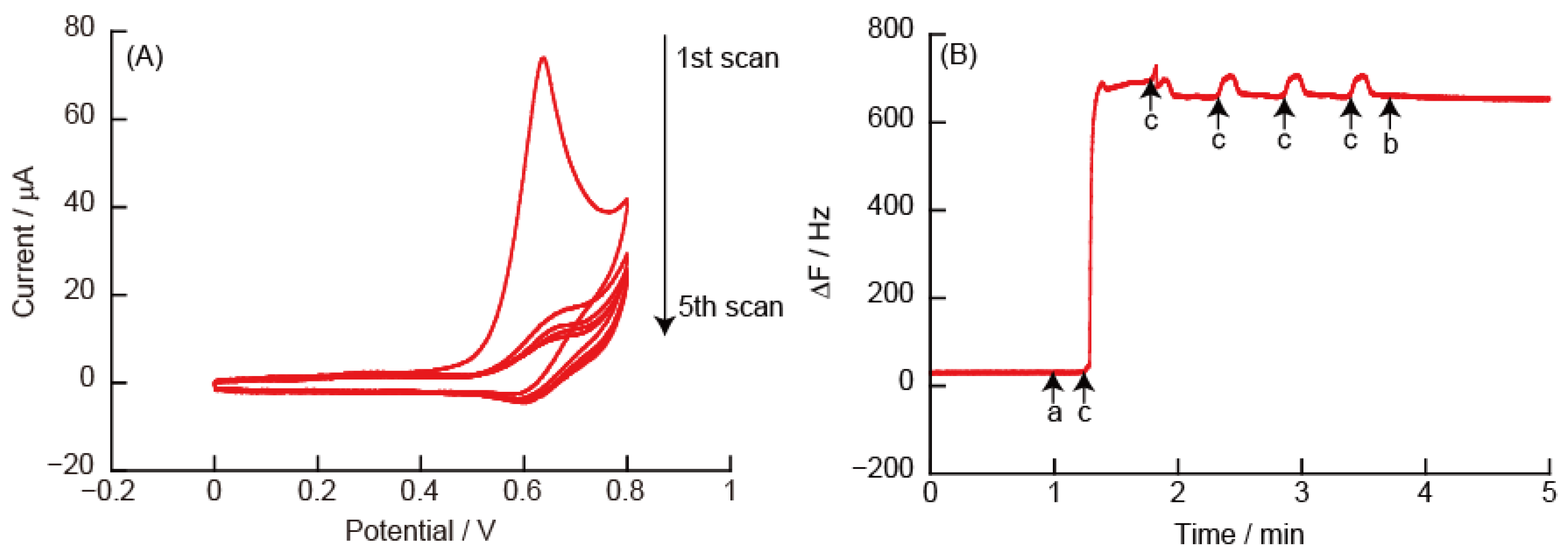
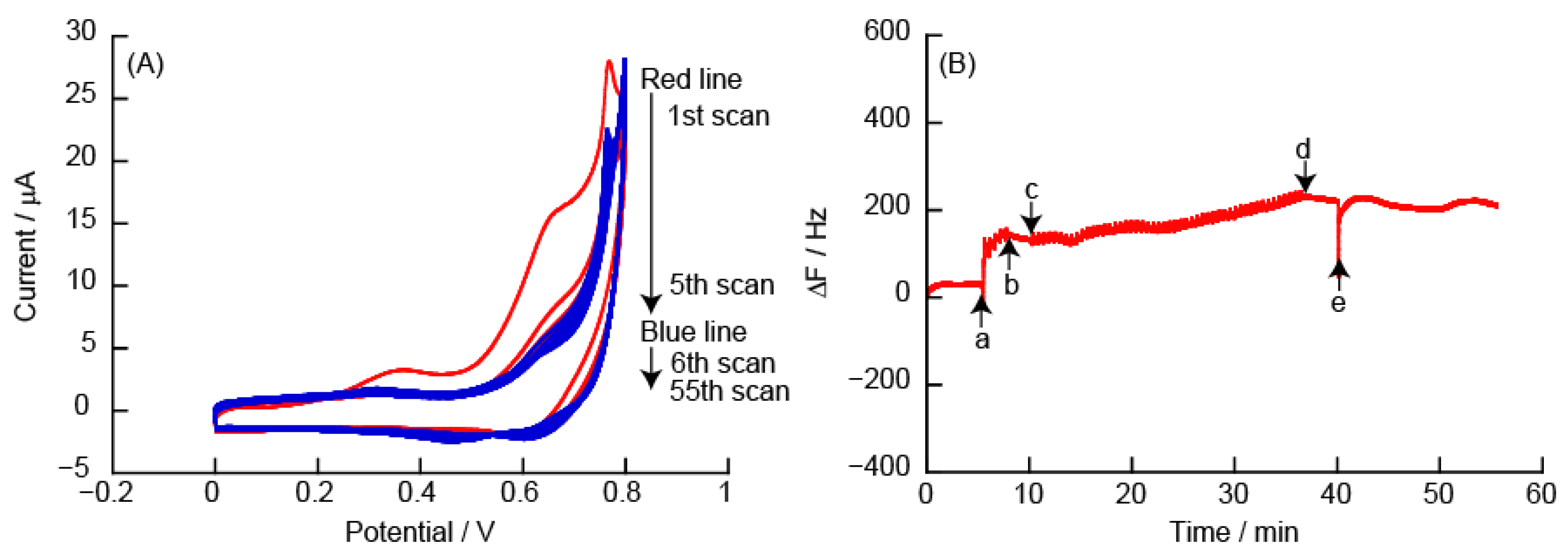
3.2.1. CVs and eQCM Measurements Obtained from (PEI/TEMPO-PAA)5 Film-Coated Electrodes
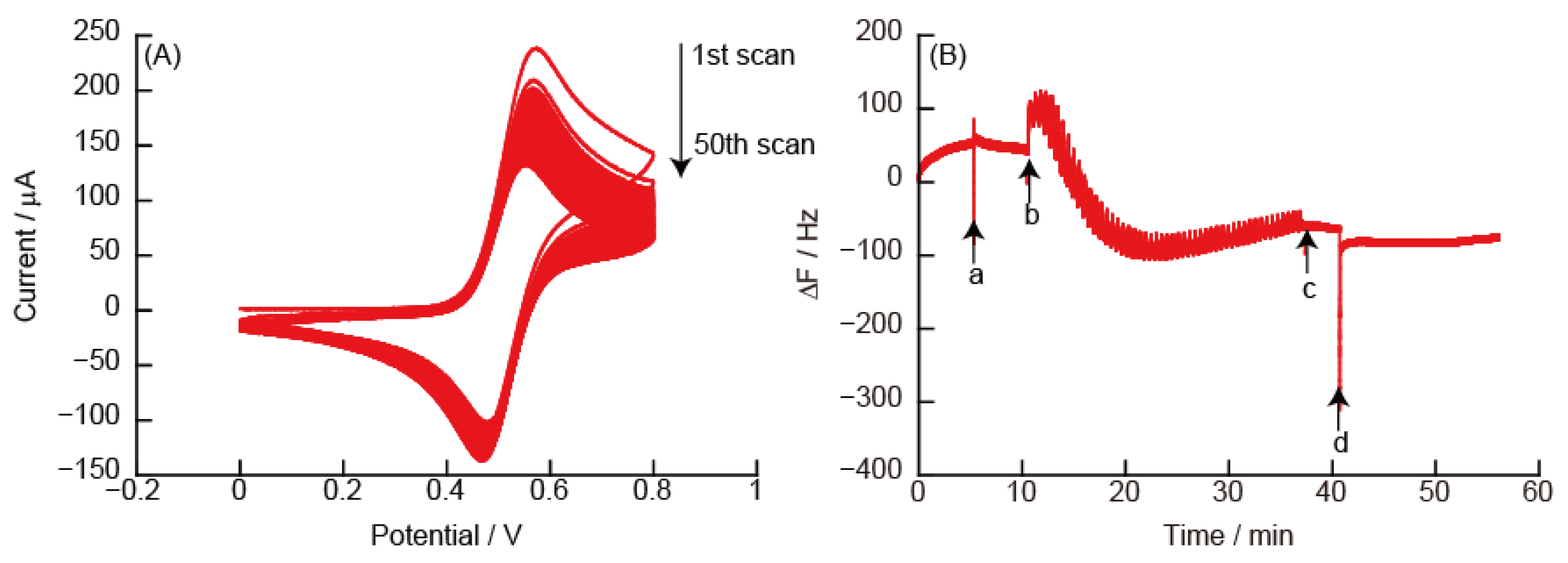
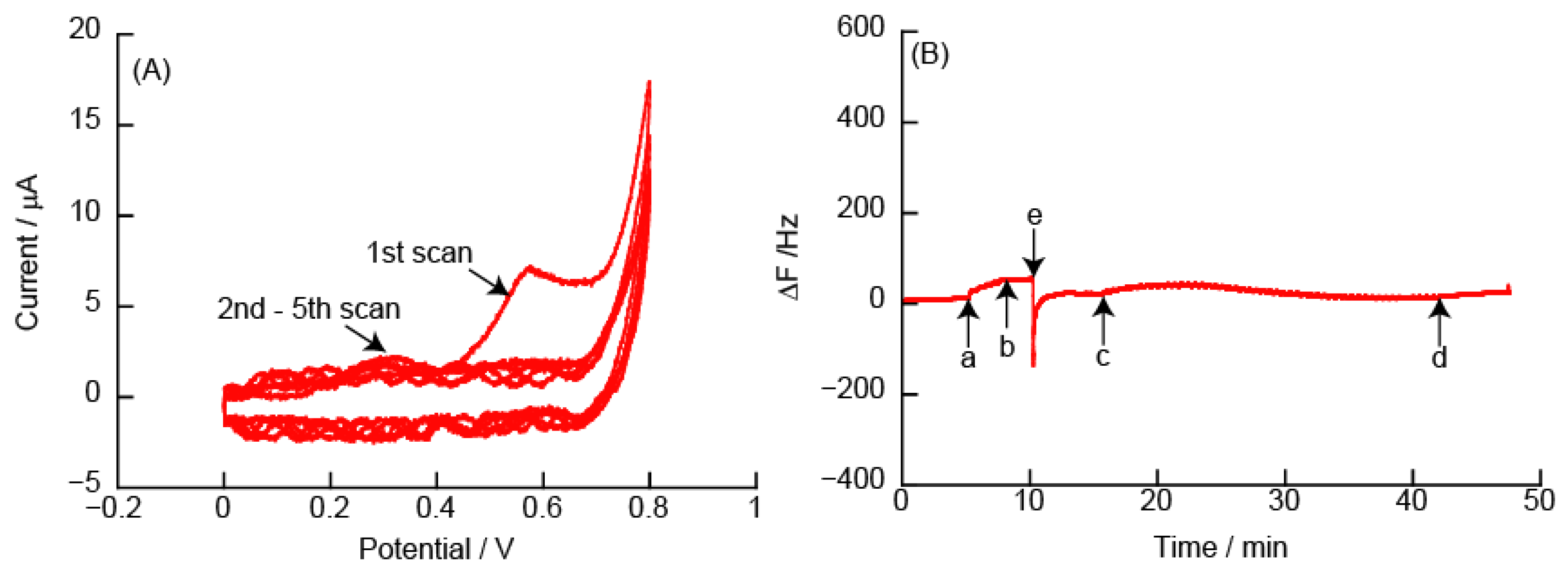
3.2.2. CVs and eQCM Measurements Obtained from (PAH/TEMPO-PAA)5 Film-Coated Electrodes
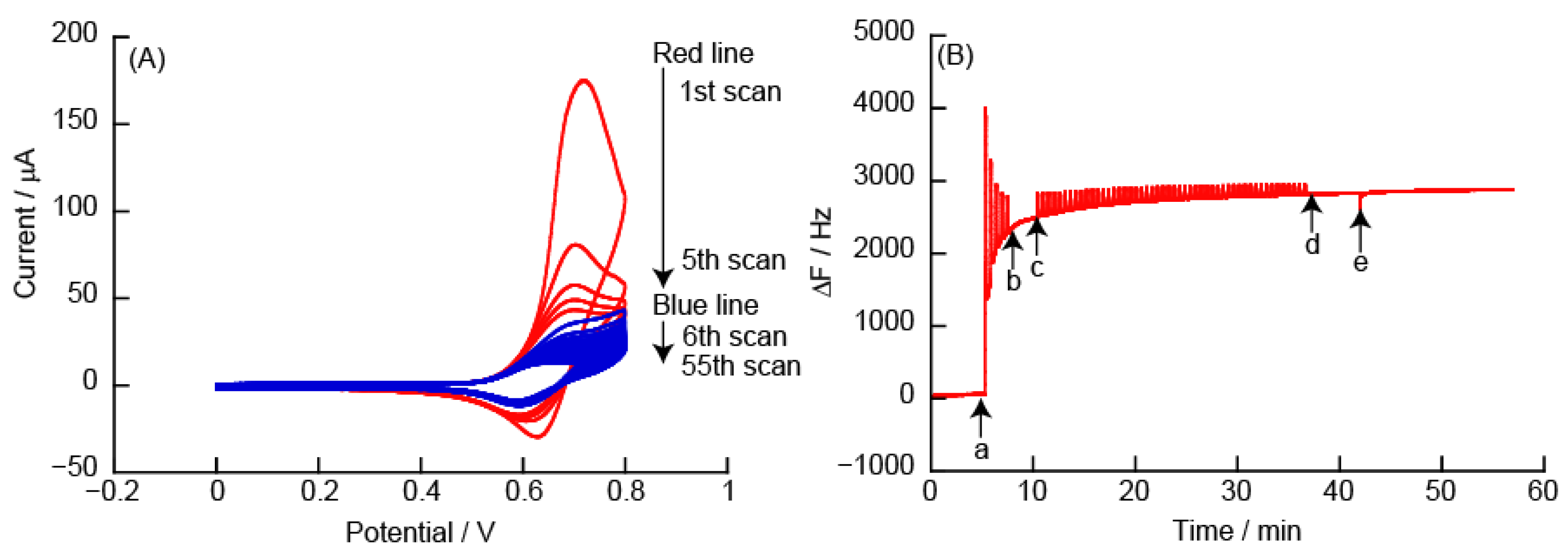
3.2.3. CVs and eQCM Measurements Obtained from (PDDA/TEMPO-PAA)5 Film-Coated Electrodes
3.2.4. CVs and eQCM Measurements Obtained from (PAMAM/TEMPO-PAA)5 Film-Coated Electrodes
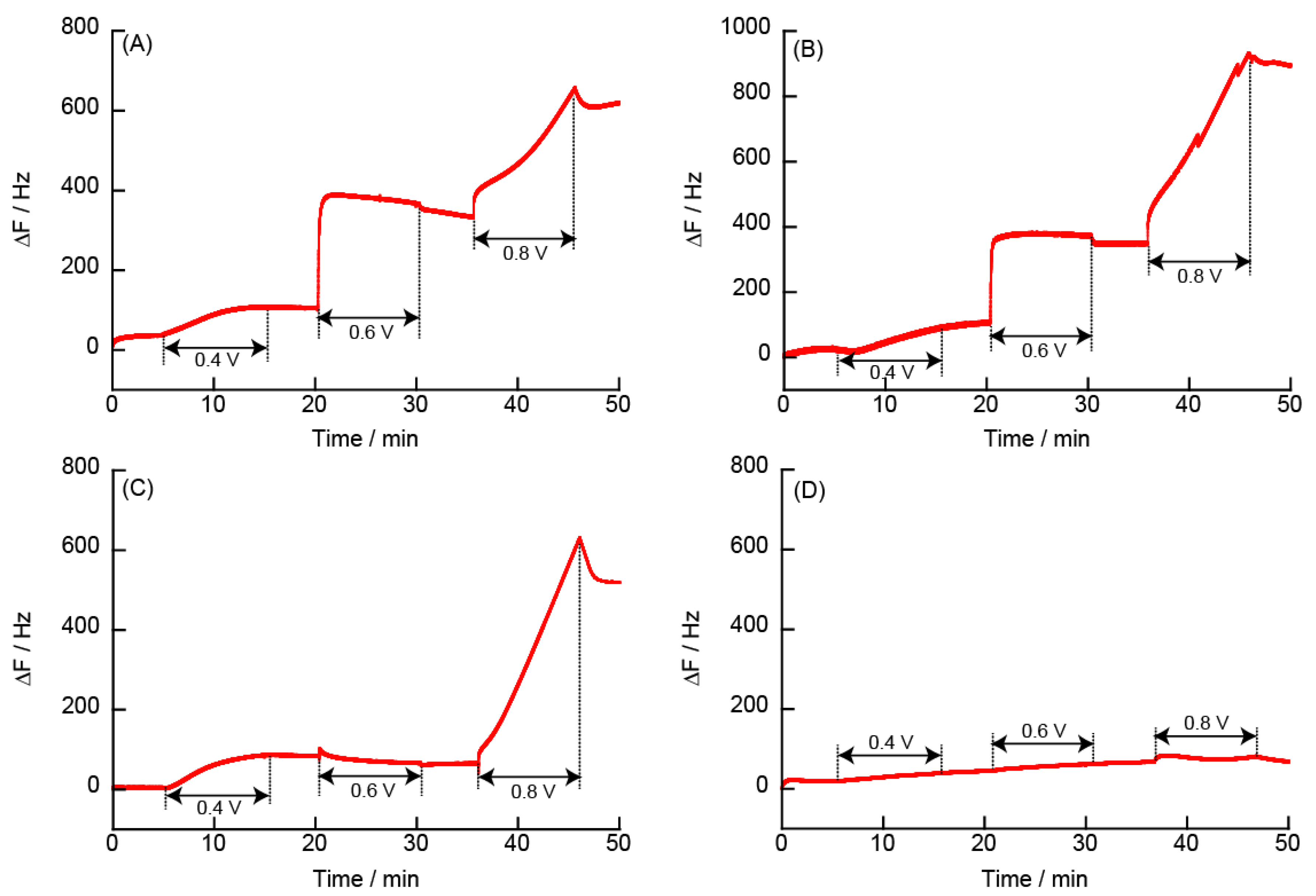
3.2.5. Changes in Resonance Frequency Due to Application of a Constant Potential
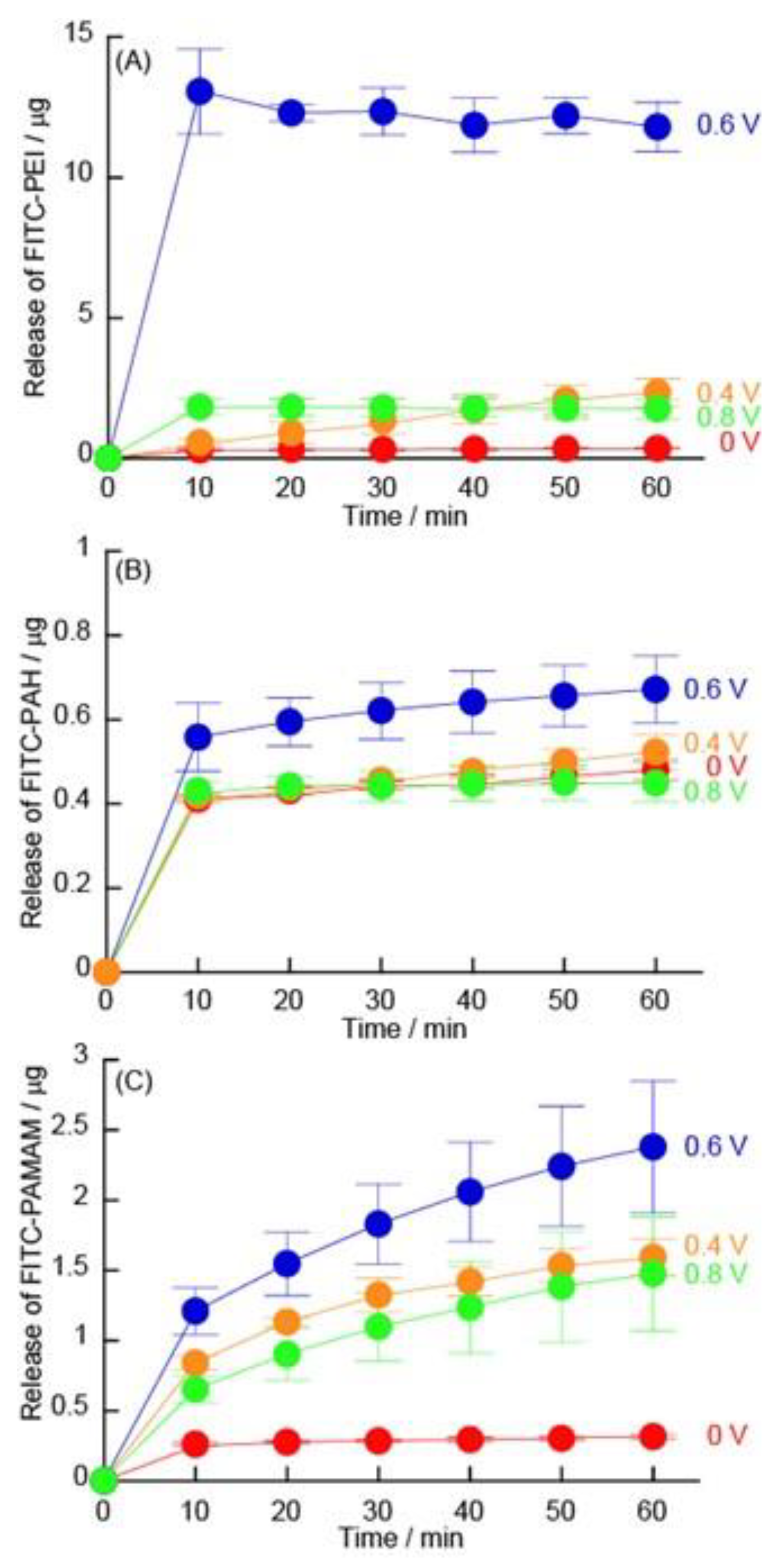
3.3. Release of FITC-Modified Polycations under Constant Potential
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ariga, K. Progress in molecular nanoarchitectonics and materials nanoarchitectonics. Molecules 2021, 26, 1621. [Google Scholar] [CrossRef] [PubMed]
- Ielo, I.; Giacobello, F.; Sfameni, S.; Rando, G.; Galletta, M.; Trovato, V.; Rosace, G.; Rosaria, M.P. Nanostructured surface finishing and coatings: Functional properties and applications. Materials 2021, 14, 2733. [Google Scholar] [CrossRef] [PubMed]
- Gosecka, M.; Gosecki, M. Chemoresponsive polymer systems for selective molecular recognition of organic molecules in biological systems. Acta Biomater. 2020, 116, 32–66. [Google Scholar] [CrossRef] [PubMed]
- Lyu, X.; Gonzalez, R.; Horton, A.; Li, T. Immobilization of enzymes by polymeric materials. Catalysts 2021, 11, 1211. [Google Scholar]
- Maksimkin, A.V.; Dayyoub, T.; Telyshev, D.V.; Gerasimenko, A.Y. Electroactive polymer-Based composites for artificial muscle-like actuators: A review. Nanomaterials 2022, 12, 2272. [Google Scholar] [CrossRef]
- Arnon, H.; Granit, R.; Porat, R.; Poverenov, E. Development of polysaccharides-based edible coatings for citrus fruits: A layer-by-layer approach. Food Chem. 2015, 166, 465–472. [Google Scholar]
- Ghiorghita, C.; Bucatariu, F.; Dragan, E.S. Influence of cross-linking in loading/release applications of polyelectrolyte multilayer assemblies. A review. Mater. Sci. Eng. C 2019, 105, 110050. [Google Scholar] [CrossRef]
- Heuberger, L.; Korpidou, M.; Eggenberger, O.M.; Kyropoulou, M.; Palivan, C.G. Current perspectives on synthetic compartments for biomedical applications. Int. J. Mol. Sci. 2022, 23, 5718. [Google Scholar] [CrossRef]
- He, Y.; Hong, C.; Li, J.; Howard, M.T.; Li, Y.; Turvey, M.E.; Uppu, D.S.S.M.; Martin, J.R.; Zhang, K.; Irvine, D.J.; et al. Synthetic Charge-invertible polymer for rapid and complete implantation of layer-by-layer microneedle drug films for enhanced transdermal vaccination. ACS Nano 2018, 12, 10272–10280. [Google Scholar]
- Dubas, S.T.; Schlenoff, J.B. Swelling and smoothing of polyelectrolyte multilayersby salt. Langmuir 2001, 17, 7725–7727. [Google Scholar] [CrossRef]
- Guzmán, E.; Ortega, F.; Rubio, R.G. Layer-by-layer materials for the fabrication of devices with electrochemical applications. Energies 2022, 15, 3399. [Google Scholar] [CrossRef]
- Robert, S.C.; Kristine, S.E.; Uhrich, K.E. Synthesis and cytotoxicity of salicylate-based poly(anhydride esters). Biomacromolecules 2005, 6, 27–29. [Google Scholar]
- Zhou, J.; Pishko, M.V.; Lutkenhaus, J.L. Thermoresponsive layer-by-layer assemblies for nanoparticle-based drug delivery. Langmuir 2014, 30, 5903–5910. [Google Scholar] [CrossRef]
- Shi, D.; Ran, M.; Zhang, L.; Huang, H.; Li, X.; Chen, M.; Akashi, M. Fabrication of biobased polyelectrolyte capsules and their application for glucose-triggered insulin Delivery. ACS Appl. Mater. Interfaces 2016, 8, 13688–13697. [Google Scholar] [CrossRef]
- Sato, K.; Imoto, Y.; Sugama, J.; Seki, S.; Inoue, H.; Odagiri, T.; Hoshi, T.; Anzai, J. Sugar-induced disintegration of layer-by-layer assemblies composed of concanavalin A and glycogen. Langmuir 2005, 21, 797–799. [Google Scholar] [CrossRef]
- Yoshida, K.; Yamaguchi, A.; Midorikawa, H.; Kamijo, T.; Ono, T.; Dairaku, T.; Sato, T.; Fujimura, T.; Kashiwagi, Y.; Sato, K. Adsorption and release of rose bengal on layer-by-layer films of poly(vinyl alcohol) and poly(amidoamine) dendrimers bearing 4-carboxyphenylboronic acid. Polymers 2020, 12, 1854. [Google Scholar] [CrossRef]
- Tokuda, Y.; Miyagishima, T.; Tomida, K.; Wang, B.; Takahashi, S.; Sato, K.; Anzai, J. Dual pH-sensitive layer-by-layer films containing amphoteric poly(diallylamine-co-maleic acid). J. Colloid Interface Sci. 2013, 399, 26–32. [Google Scholar]
- Yoshida, K.; Kashimura, Y.; Kamijo, T.; Ono, T.; Dairaku, T.; Sato, T.; Kashiwagi, Y.; Sato, K. Decomposition of glucose-sensitive layer-by-layer films using hemin, DNA, and glucose oxidase. Polymers 2020, 12, 319. [Google Scholar] [CrossRef]
- Yoshida, K.; Ono, T.; Dairaku, T.; Kashiwagi, Y.; Sato, K. Preparation of hydrogen peroxide sensitive nanofilms by a layer-by-layer technique. Nanomaterials 2018, 8, 941. [Google Scholar] [CrossRef]
- Yoshida, K.; Awaji, K.; Shimizu, S.; Iwasaki, M.; Oide, Y.; Ito, M.; Dairaku, T.; Ono, T.; Kashiwagi, Y.; Sato, K. Preparation of microparticles capable of glucose-induced insulin release under physiological conditions. Polymers 2018, 10, 1164. [Google Scholar]
- Takahashi, S.; Anzai, J. Recent Progress in Ferrocene-Modified Thin Films and Nanoparticles for Biosensors. Materials 2013, 6, 5742–5762. [Google Scholar] [PubMed]
- Ma, W.; Zhang, Y.; Li, F.; Kou, D.; Lutkenhaus, J.L. Layer-by-layer assembly and electrochemical study of alizarin red s-based thin films. Polymers 2019, 11, 165. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.C.; Guo, S.L.; Chung, K.D.; Hu, W.W. Electrical Field-Assisted Gene Delivery from Polyelectrolyte Multilayers. Polymers 2020, 12, 133. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Parent, K.L.; Buttry, D.A. Electrochemical solid-state phase transformations of silver nanoparticles. J. Am. Chem. Soc. 2012, 13, 5610–5617. [Google Scholar] [CrossRef] [PubMed]
- Ciriminna, R.; Ghahremani, M.; Karimi, B.; Pagliaro, M. Electrochemical alcohol oxidation mediated by TEMPO-like nitroxyl radicals. ChemistryOpen 2017, 6, 5–10. [Google Scholar]
- Poizot, P.; Gaubicher, J.; Renault, S.; Dubois, L.; Liang, Y.; Yao, Y. Opportunities and challenges for organic electrodes in electrochemical energy storage. Chem. Rev. 2020, 120, 6490–6557. [Google Scholar]
- Vereshchagin, A.A.; Kalnin, A.Y.; Volkov, A.I.; Lukyanov, D.A.; Levin, O.V. Key features of TEMPO-containing polymers for energy storage and catalytic systems. Energies 2022, 15, 2699. [Google Scholar]
- Rozantzev, E.G.; Neiman, M.B. Organic radical reactions involving no free valence. Tetrahedron 1964, 20, 131–137. [Google Scholar] [CrossRef]
- Semmelhack, M.F.; Chou, C.S.; Cortes, D.A. Nitroxyl-mediated electrooxidation of alcohols to aldehydes and ketones. J. Am. Chem. Soc. 1983, 105, 4492–4494. [Google Scholar] [CrossRef]
- Takahashi, S.; Aikawa, Y.; Kudo, T.; Ono, T.; Kashiwagi, Y.; Anzai, J. Electrochemical decomposition of layer-by-layer thin films composed of TEMPO-modified poly(acrylic acid) and poly(ethyleneimine). Colloid Polym. Sci. 2014, 292, 771–776. [Google Scholar]
- Watanabe, K.; Sugiyama, K.; Komatsu, S.; Yoshida, K.; Ono, T.; Fujimura, T.; Kashiwagi, Y.; Sato, K. Voltammetric pH measurements using azure A-containing layer-y-layer film immobilized electrodes. Polymers 2020, 12, 2328. [Google Scholar]
- Craig, M.; Holmberg, K.; Ru, E.L.; Etchegoin, P. Polypeptide multilayer self-assembly studied by ellipsometry. J. Drug Deliv. 2014, 2014, 424697. [Google Scholar] [CrossRef]
- Ciejka, J.; Grzybala, M.; Gut, A.; Szuwarzynski, M.; Pyrc, K.; Nowakowska, M.; Szczubiałka, K. Tuning the surface properties of poly(allylamine hydrochloride)-based multilayer films. Materials 2021, 14, 2361. [Google Scholar]
- Chauhan, A.S. Dendrimers for drug delivery. Molecules 2018, 23, 938. [Google Scholar]
- Kheraldine, H.; Rachid, O.; Habib, A.M.; Al Moustafa, A.E.; Benter, I.F.; Akhtar, S. Emerging innate biological properties of nano-drug delivery systems: A focus on PAMAM dendrimers and their clinical potential. Adv. Drug Deliv. Rev. 2021, 178, 113908. [Google Scholar] [CrossRef]
- Yoshida, K.; Hasebe, Y.; Takahashi, S.; Sato, K.; Anzai, J. Layer-by-layer deposited nano- and micro-assemblies for insulin delivery: A review. Mater. Sci. Eng. C 2014, 34, 384–392. [Google Scholar]
- Díez-Pascual, A.M.; Rahdar, A. LbL Nano-assemblies: A versatile tool for biomedical and healthcare applications. Nanomaterials 2022, 12, 949. [Google Scholar] [CrossRef]
- Queiroz, N.L.; Nascimento, J.A.M.; Nascimento, M.L.; Nascimento, V.B.; Oliveira, S.C.B. Oxidation Mechanism of Fluorescein at Glassy Carbon Electrode. Electroanalysis 2017, 29, 489–496. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoshida, K.; Kamijo, T.; Ono, T.; Dairaku, T.; Takahashi, S.; Kashiwagi, Y.; Sato, K. Electrical Stimuli-Responsive Decomposition of Layer-by-Layer Films Composed of Polycations and TEMPO-Modified Poly(acrylic acid). Polymers 2022, 14, 5349. https://doi.org/10.3390/polym14245349
Yoshida K, Kamijo T, Ono T, Dairaku T, Takahashi S, Kashiwagi Y, Sato K. Electrical Stimuli-Responsive Decomposition of Layer-by-Layer Films Composed of Polycations and TEMPO-Modified Poly(acrylic acid). Polymers. 2022; 14(24):5349. https://doi.org/10.3390/polym14245349
Chicago/Turabian StyleYoshida, Kentaro, Toshio Kamijo, Tetsuya Ono, Takenori Dairaku, Shigehiro Takahashi, Yoshitomo Kashiwagi, and Katsuhiko Sato. 2022. "Electrical Stimuli-Responsive Decomposition of Layer-by-Layer Films Composed of Polycations and TEMPO-Modified Poly(acrylic acid)" Polymers 14, no. 24: 5349. https://doi.org/10.3390/polym14245349
APA StyleYoshida, K., Kamijo, T., Ono, T., Dairaku, T., Takahashi, S., Kashiwagi, Y., & Sato, K. (2022). Electrical Stimuli-Responsive Decomposition of Layer-by-Layer Films Composed of Polycations and TEMPO-Modified Poly(acrylic acid). Polymers, 14(24), 5349. https://doi.org/10.3390/polym14245349







