Abstract
Acidic gas penetration through the internal pressure sheath of a flexible riser tends to cause a corrosive environment in the annulus, reducing the service life of the flexible riser. Nanoparticles can act as gas barriers in the polymer matrix to slow down the gas permeation. Herein, we prepared PA11/SiO2 composites by the melt blending method. The effect of adding different amounts of SiO2 to PA11 on its gas barrier properties was investigated by conducting CO2 permeation tests between 20 °C and 90 °C. As the temperature increased, the lowest value of the permeability coefficient that could be achieved for the PA11 with different contents of SiO2 increased. The composites PA/0.5% SiO2 and PA/1.5% SiO2 had the lowest permeation coefficients in the glassy state (20 °C) and rubbery state (≥50 °C). We believe that this easy-to-produce industrial PA/SiO2 composite can be used to develop high-performance flexible riser barrier layers. It is crucial for understanding riser permeation behavior and enhancing barrier qualities.
1. Introduction
Flexible risers are connecting pipelines that transport oil and gas from the seabed to the ground, which plays a key role in oil and gas development [1]. During the operation of the flexible risers, the internal fluid of the risers contains lots of acidic gases (CO2, H2S), as well as gas-phase water, which penetrates from the inner pressure sheath into the area between the inner and outer sheaths, a space known as the permeation annulus (Figure 1). Meanwhile, condensate or seawater can penetrate the annular space due to damage of the outer sheaths during installation. The highly corrosive environment caused by water and acidic gases poses a serious challenge to the selection of long-life internal pressure sheaths materials [2,3,4].
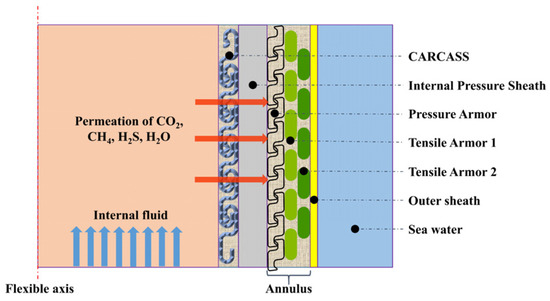
Figure 1.
Permeation in the flexible riser.
Commonly used polymers for internal pressure sheaths include polyamide-11 (PA11), polyvinylidene fluoride (PVDF), high-density polyethylene (HDPE), and cross-linked polyethylene (XLPE), with PA11 being the most used of the numerous materials [5]. Polyamides have good all-around properties and are one of the high-performance engineering plastics [6]. PA11 possesses outstanding mechanical characteristics (such as high fatigue resistance, a low frictional coefficient, and excellent creep resistance) and is widely used as a candidate material for flexible riser liners [7]. It also has high impact strength and remarkable chemical resistance [8], and has been used in multiple applications, including offshore pipelines, cables, automobiles, electroactive polymer actuators, and sensors [7,8,9,10]. However, PA11 needs to be modified to further improve the barrier property and to alleviate the disadvantage of poor heat resistance.
Polymer-based nanocomposites have been extensively explored for the numerous innovative and remarkable features that nanoparticles can provide to polymers [11,12,13,14]. Previous studies have shown that the performance of PA11 can be improved by nanoparticle modification [7,15]. In the flexible riser field, the elastic modulus and the hardness parameters of the PA11 matrix were enhanced by the addition of montmorillonite (MMT) [16]. The concentration of 1.0 wt.% of commercial graphene nanoplatelets (GNP) promoted improvements in the thermal and mechanical properties of PA11 [17]. Among many inorganic nanoparticles, SiO2 has been widely studied, due to its low price, easy availability, and high surface area. The PA11/SiO2 nanocomposite coatings with nominal 15 vol% hydrophobic silica attained an enhancement in scratch resistance by 35% and wear resistance by 67% compared to neat polymer coatings [15]. Although some researchers have studied the effect of different nanoparticles on polymer permeability [18,19,20,21], a single nanoparticle ratio and a single test temperature cannot accurately reflect the mechanism of nanoparticle influence on the barrier behavior of polymer-based nanocomposites.
In this paper, PA11/SiO2 (sphere, 25 nm, 100 m2/g) composites synthesized with different ratios were prepared by melt blending to evaluate their potential application in oil and gas pipelines. The CO2 permeability of the PA11/SiO2 composites at different temperatures was measured to explore the mechanism of the effect of different nanoparticle ratios on the permeation behavior of the polymer matrix. The results of the study are important for understanding riser permeation behavior and improving barrier properties, and can provide a reference for the prediction of the flexible riser annulus under various working circumstances.
2. Materials and Methods
2.1. Materials
Polyamide 11 resin (PA11, Rilsan® BESNO P4) was supplied by Arkema (Suzhou, China). Hydrophobic gas-phase SiO2 powder (sphere) with a particle size of 25 nm and a specific surface area of 100 m2/g was supplied by MACKLIN (Beijing, China).
2.2. Fabrication of PA11/SiO2 Composites
First, the PA11 and SiO2 were vacuum-dried at 70 °C for 24 h, after which they were melt blended at 205 °C and 50 rpm for 10 min at a certain weight ratio using a mixer (Extruder, Brabender GMBH&Co.KG, Duisburg, Germany). The samples were then pre-pressed at 205 °C and 200 bar pressure for 5 min using a flatbed vulcanizing machine (LHHS 20-170919A, Lina Industrial Co., Ltd., Guangdong, China). Eventually, the PA11/x% SiO2 (x = 0.5, 1.0, 1.5, 2.0.) composite samples were obtained by continued cold pressing at 25 °C and 100 bar for 10 min, where x% represents the mass fraction of the SiO2 in PA11.
2.3. Morphology Characterization
Scanning electron microscopy (SEM, SU8010, HITACHI, Hitachi City, Japan) and Transmission Electron Microscopy (TEM, Tecnai G2 F20, FEI, Sarum, OR, USA) were conducted to analyze the morphology of the prepared composites. Ultramicrotome (EM UC6, Leica, Wetzlar, Germany) was used to prepare the specimens with a thickness of ~100 nm for the TEM investigation.
2.4. Structure Characterizations
Infrared spectra of the composites were recorded with a Fourier transform infrared spectrometer (FTIR, Tensor II, Bruker, Billica, MA, USA) between 4000 and 600 cm−1. X-ray diffraction (XRD) analysis was conducted on a Bruker D8 Focus X-ray diffractometer by a solid detector and Cu Ka radiation. The 2θ ranges were from 5° to 40° at a rate of 5°/min.
2.5. Thermal Analysis
The non-isothermal crystallization behavior in the melts of the composites were performed by using a differential scanning calorimeter (DSC, 204F1, Netzsch, Berlin, Germany). To eliminate thermal history, the samples were first heated from 30 to 205 °C at 20 °C min−1, held at the high temperature for 5 min, and then cooled to 20 °C at 10 °C min−1. After this thermal treatment, the samples were heated from 20 °C to 200 °C at 10 °C min−1 for analysis (2nd scan). The crystallization temperature (Tc) and the enthalpy of crystallization (ΔHc) were measured during the cooling scan. The melting temperature (Tm) and the enthalpy of fusion (ΔHm) were measured during the 2nd scanning process. The crystallinity (Xm) was estimated according to Equation (1):
where ΔHm and ΔH0 denote the heats (J/g) of melting of the PA11/SiO2 composites and PA11 crystals of infinite size with a value of 225.9 J/g [22].
Thermogravimetric analysis (TGA) was conducted with a DTA-TG apparatus (DTG-60, SHIMADZU, Kyoto, Japan) ranging from 50 °C to 700 °C with a heating rate of 10 °C/min in a N2 atmosphere (flow rate 100 mL min−1).
2.6. Mechanical Properties
The mechanical performance was obtained with an Instron machine (WDL-5000N, Daochun, Yangzhou, China) at a strain speed of 50 mm min−1. The samples were cut into 15 × 2 mm dumbbell shapes.
Dynamic mechanical analysis (DMA) was performed using a DMA apparatus (Q800, TA INSTRUMENTS, New Castle, PA, USA) over a temperature range of −20 °C to 100 °C (3 °C min−1, 1 Hz), which covers the temperature range of the PA11 used for flexible riser inner sheaths. The tests were carried out in tension mode. The DMA measurement was used to determine the viscoelastic properties of the tested materials, such as storage modulus (E′), loss modulus (E″), and damping parameter (tan δ) [23].
2.7. Gas Barrier Test
The applicable temperature range of PA11 is from −20 °C to90 °C, according to ISO 13628-2017 (Petroleum and natural gas industries—design and operation of subsea production systems Part 11: Flexible pipe systems for subsea and marine applications). The samples with a thickness of approximately 0.5 mm were placed in the permeability unit test apparatus. The low-pressure ends of the composite materials were supported by breathable steel, so that the increase in pressure would not cause deformation failure. The experimental temperature was far lower than the aging temperature of the materials, which will not cause material failure. The input pressure for the test was 4 MPa, and the test temperatures were 20 °C, 30 °C, 50 °C, 70 °C, and 90 °C. Gas permeation tests were conducted according to the corresponding standard GB/T 40260-2021 (Test method for determining gas permeability of polymer membrane materials), and different batches of the samples were used to test the gas permeability.
The permeation of gas molecules into the polymer was divided into three stages [24], as shown in Figure 2. The first stage was the impermeable state, where the gas molecules collided with the polymer surface. After the transient (unsteady) permeation state, the steady-state permeation state was finally reached, where the diffusion was stable, due to the complete saturation of the internal volume. The permeability coefficient (P) and diffusion coefficient (D) were calculated according to the permeability curve through Equations (2) and (3), respectively [25,26,27]:
where A denotes the permeated area, P1 and P2 stand for high-end and low-end pressure, and h and τ refer to the thickness of the samples and lag time, respectively.
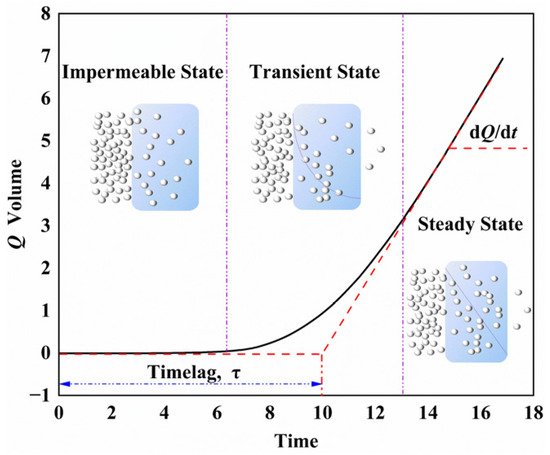
Figure 2.
Gas permeate volume curve with time.
3. Results and Discussion
3.1. Morphology Characterization
SEM images for the pure PA11 and the PA11/SiO2 composite cross-sections are shown in Figure 3. The distribution of the SiO2 in the PA11 matrix was almost uniform with less agglomeration (the red circle in Figure 3c–f). In addition, the SEM image shows that the sample was flat and free of defects, which ensured the reliability of the mechanical properties and gas permeate behavior tests.
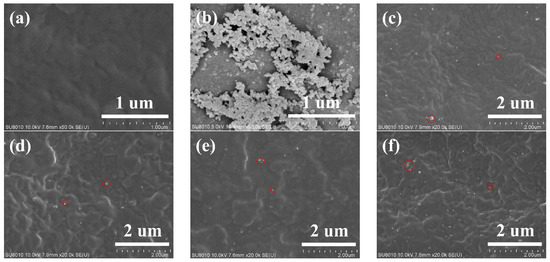
Figure 3.
SEM images of composite with different SiO2 contents: (a) PA11, (b) SiO2, (c) PA11/0.5% SiO2, (d) PA11/1.0% SiO2, (e) PA11/1.5% SiO2, (f) PA11/2.0% SiO2.
TEM was performed to further investigate the distribution morphology of the SiO2. The SiO2 nanoparticles had a round structure and a size of 25–35 nm, as shown in Figure 4a. For the PA11/SiO2 composites, the dispersion of the SiO2 particles in the PA11 matrix was more uniform when the addition amount of nanoparticles was low (below 1.0%), as shown in Figure 4b,c. When the addition was increased to 1.5%, there was a certain degree of nanoparticle agglomeration in the PA11, with a size of about 5 μm, as shown in Figure 4d.

Figure 4.
TEM images PA11/SiO2 composite, (a) SiO2, (b) PA11/0.5% SiO2, (c) PA11/1.0% SiO2, (d) PA11/1.5% SiO2, (e) PA11/2.0% SiO2.
3.2. DSC Characterization
It was assumed that the gas penetration in the polymers occurred in the amorphous zone rather than in the crystalline region, which is a limited region [28]. To investigate the impact of the SiO2 incorporation on the melting and crystallization behaviors of the PA11, DSC tests were performed on the pure PA11 and PA11/SiO2 composites, as shown in Figure 5. As summarized in Table 1, the Tc of the neat PA11 was 153.3 °C. With the addition of the SiO2 nanoparticles, the Tc values of the composites improved, demonstrating that SiO2 acted as a nucleating agent for the PA11 crystallization and increased the crystallization rate [29,30]. Furthermore, the addition of a small amount of SiO2 had no significant effect on the crystallinity of the PA11.
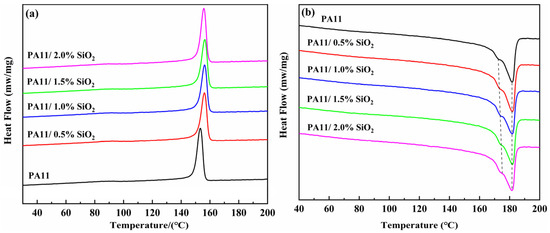
Figure 5.
DSC curves of PA11/SiO2 composites: (a) crystallization curve, (b) melting curve.

Table 1.
DSC thermodynamic parameters of PA11/SiO2 composites.
This was mainly attributed to the potent intrinsic hydrogen bonding network that is typical of polyimides, which stabilized the PA11 amorphous phase [31]. For melting curves, all PA11/SiO2 composite samples showed two melting peaks, which can be attributed to the fusion of different types of crystals in the semi-crystallized PA11. The melting peaks at higher temperatures might be attributed to the melting of the perfect crystals, whereas the melting peaks at lower temperatures could be assigned to the deficient crystals produced by short molecular chains [32,33].
3.3. Structure Characterization
The crystal structures of the PA11 and PA11/SiO2 nanocomposites were characterized by XRD, as presented in Figure 6. The main crystalline peaks in the XRD spectra were observed at 2θ = 7°, 20.5°, and 23.5°, which corresponded to the α-polymorph of the PA11 [34]. Moreover, the addition of the SiO2 did not affect the crystal structure of the PA11.
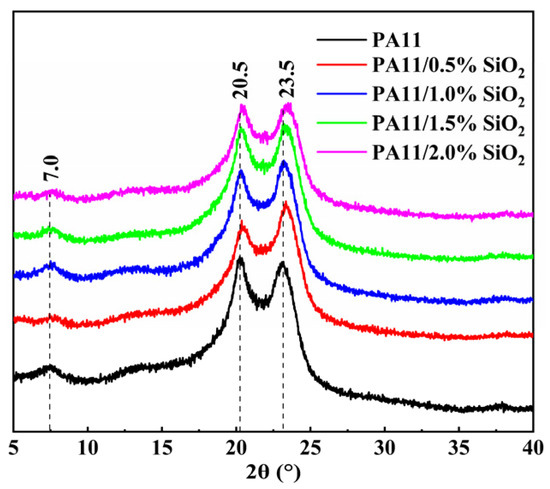
Figure 6.
XRD spectra of PA-11 and PA11/SiO2 composites.
The structures of the PA11, SiO2, and PA11/SiO2 composites were characterized by FTIR (Figure 7). In the curve of the PA11, the bands at 1635 and 1539 cm−1 corresponded to the C=O stretching vibration and N-H bending vibration, respectively [6,35,36,37,38,39,40]. The peaks at 806 cm−1 and 1105 cm−1 indicated the anti-symmetric stretching and asymmetric stretching vibrations of the Si-O-Si, respectively [41,42]. The area ratio of absorption bands at 1091 cm−1 (A1091) and 806 cm−1 (A806) to 1635 cm−1 (A1635) exhibited the relative amounts of Si-O-Si groups in the composites. As shown in Figure 7g, compared with the PA11, the peak area ratios of the A1091/1635, and A806/1635 increased with the SiO2 additive amount, which demonstrated the successful introduction of the SiO2 into the PA11 matrix.
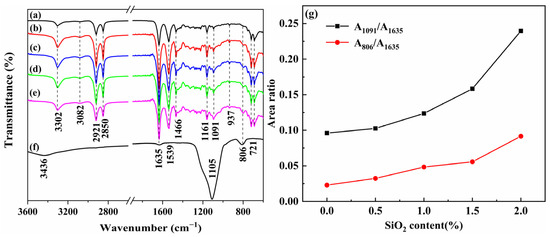
Figure 7.
FTIR spectra of PA11 and PA11/SiO2 composites: (a) PA11, (b) PA11/0.5% SiO2, (c) PA11/1.0% SiO2, (d) PA11/1.5% SiO2, (e) PA11/2.0% SiO2, (f) SiO2, (g) A1091/A1635 and A806/A1635 ratios of FTIR peak areas.
3.4. Mechanical and Thermal Stability Characterization
Figure 8 depicts the stress–strain curves of the PA11 and PA11/SiO2 composites. The mechanical properties determined from the curves are shown in Table 2. The neat PA11 had a tensile strength of 78.4 MPa, an elasticity modulus of 89.9 MPa, and a break elongation of 447.7%. As the SiO2 content increased, the nanoscale SiO2 aggregated and existed in the form of defects in the PA11/SiO2 composites, which reduced the tensile strengths and break elongations of the composites to some extent. When compared to the pristine PA11, the presence of SiO2 in the PA11 resulted in a higher elasticity modulus and a decrease in the break elongation. The findings are consistent with previous research [43,44].
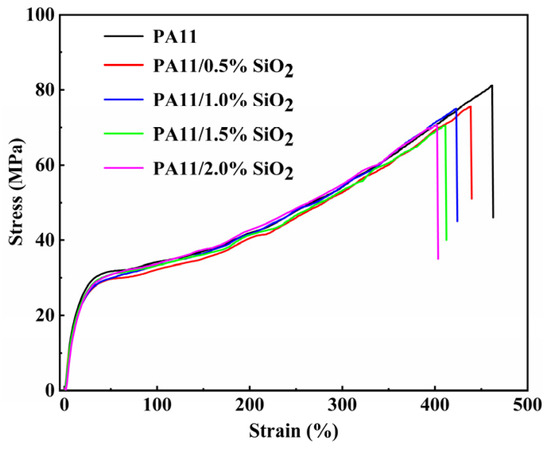
Figure 8.
The stress–strain curves of PA11 and PA11/SiO2 composites.

Table 2.
Mechanical properties of PA11/SiO2 composites.
The mechanical properties of the PA11 and nanocomposites were further studied by dynamic mechanical analysis (DMA). The changes in the energy storage modulus (E′) and loss modulus (E″) with temperature are shown in Figure 9. E′ and E″ increased above or below the glass transition temperature (Tg). Changes below the Tg were greater than those above the Tg. This result was consistent with other studies on polymer nanocomposites reinforced by nanomaterials [30,45].
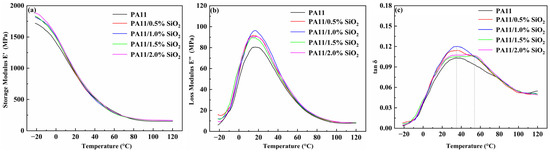
Figure 9.
(a) Storage modulus, (b) loss modulus, and (c) tan δ of neat PA11 along with PA11/SiO2 composites.
When the PA11 was in the glassy state rather than the rubber state, the effect of the nanoparticles was more significant. The relaxation of the PA11 and nanocomposites was studied using tan δ data shown in Figure 9c. For the Tg-associated α relaxation centered at 35–40 °C, there was a transition to a higher temperature in many nanocomposite samples. The SiO2 may have reduced the free volume in the PA11 by hindering the chain movement and conformation change. In addition, at the high SiO2 content in Figure 9c, the tan δ peak became wider, and a second higher Tg was observed, showing that the molecular chain movement of the PA11 was restricted, resulting in a large realistic response of the nanocomposites [46,47,48].
TGA and DTG were performed to test the thermal stability of the neat PA11 and the nanocomposites with nitrogen airflow (Figure 10a,b). All the tested materials showed two main weight losses, at 280 °C and 460 °C. The first degradation step, with a shallow peak in the DTG curve centered at around 280 °C, was attributed to the decarboxylation process [49]. In the second degradation step, a main peak was identified in the DTG curves. On the other hand, the melting temperature of SiO2 was over 1600 °C. Thus, it was inferred that the degradation occurred due to the decomposition of the PA11 backbones.
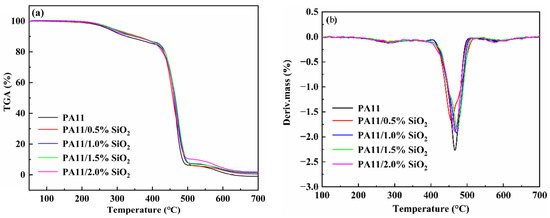
Figure 10.
TGA curves (a) and DTG curves (b) of neat PA11 and PA11/SiO2 composites.
3.5. CO2 Permeability Characterization and Behavior Analysis
For vertical two-phase flow in pipes, the common flow patterns are annular flow, slug flow, bubble flow, and dispersed bubble flow [50]. The multiphase flow pattern has a significant effect on the temperature and pressure distribution of the flexible riser, which in turn affects the rate of gas phase permeation into the annulus within the flexible riser. Flexible risers are usually required for long-term service and therefore require an in-depth study of the steady-state permeation processes. The CO2 permeabilities of the PA11 and PA11/SiO2 composites were measured at 20 °C, 30 °C, 50 °C, 70 °C, and 90 °C. These temperatures were chosen because they are typical values for PA11 applications in flexible risers. In addition, the design of the temperatures involved two states of the PA11, the glassy state and the rubbery state. Figure 11 displays the results of the permeation coefficients for the PA11/SiO2 composites, each averaged over four parallel specimens from different batches. The test data error of different samples in the same batch and the error of different samples was calculated, which can meet the requirement of 5.0% of the standard.
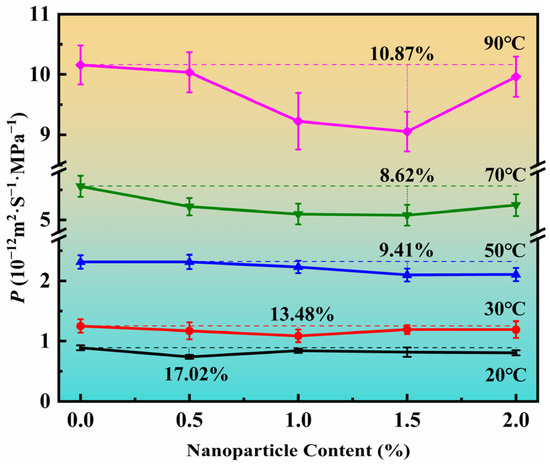
Figure 11.
Permeability coefficient of PA11 and PA11/SiO2 composites at 20 °C, 30 °C, 50 °C, 70 °C, and 90 °C.
The percentage reduction in the permeability coefficient of the PA11/SiO2 composites compared to the pure PA11 varied at different temperatures. The maximum decreases in the permeability coefficients of the PA11/SiO2 composites at 20–90 °C were: 17.02% at 20 °C, 13.48% at 30 °C, 9.41% at 50 °C, 8.62% at 70 °C, and 10.87% at 90 °C. It is evident that the percentage difference between the PA11 and PA11/SiO2 permeability coefficients decreased with increasing temperature. Additionally, as the temperature increased, the increase of nanoparticle content had a greater impact on the permeation behavior of the composites.
To further analyze the influence of the various nanoparticle contents on the behavior of the gas permeation at various temperatures, the activation energies for the diffusion, solubility, and permeation of the composites of PA11/SiO2 were calculated based on the widely used solution-diffusion model [51,52]. The effect of the temperature on gas diffusion and solubility was expressed by the Arrhenius Equations (4) and (5), respectively [28]:
where D0 is the pre-exponential factor and ED is the activation energy of diffusion; ΔHS is the partial molar enthalpy of sorption. T is the temperature (K), and R is the ideal gas constant. Hence, the permeability can be obtained by multiplying the solubility coefficient (a thermodynamic coefficient) by the diffusivity coefficient (a kinetic coefficient), and the gas permeability as a function of temperature was obtained using Equation (6) [53,54,55]:
where P0 is the pre-exponential factor and EP is the activation energy of permeation. In general, permeability increases with increasing temperature. The values of ED and EP were determined for each gas from the slopes of the ln(D) and ln(P) vs. 1000/T curves (Figures S1 and S2. Tables S1 and S2), respectively. The distinction between the EP and ED was represented by ΔHS and is summarized in Table 3.

Table 3.
Apparent activation energies for permeability, diffusion, and heat of solution of CO2 in PA11.
ED increased with the increase in the nanoparticle content, indicating the diffusion of gas molecules was more difficult. ΔHS was negative, showing that gas dissolution was an exothermic process, whereas EP and ED were positive, suggesting that the permeation and diffusion were heat-absorbing processes. As temperature increased, the permeability and diffusion coefficients of the PA11 to CO2 increased, whereas the solubility coefficient exhibited the opposite trend, as shown in Figure S3.
Gas permeation is closely related to the state of motion of the polymer. The free volume theory of gas diffusion in glass polymers proposed by Vrentas and Duda [57,58] assumes the existence of pores, i.e., free volumes, between polymer chain segments. Gas molecules jump from one free volume unit to the next to complete the diffusion process. To achieve this process, gas molecules need to have sufficiently large free volume units in adjacent positions and sufficient energy to overcome the jump resistance. Figure S4 depicts the variation pattern of the free volume with temperature and shows an obvious distinction in the permeation curves of the composites in the glass and rubber states. When the polymer was in the glassy state, the chain segment motion was in a frozen state, and no conformational changes could occur; the chain segment spacing formed during the thermal movement of the molecular chain segments was the main channel for gas diffusion [59].
In the glassy state, gas molecules had high resistance to jumping between free volumes, and the solubility had a large effect on the permeability coefficient. As shown in Figure 4, the 0.5% proportion of nanoparticles efficiently dispersed and occupied the free volume of the PA11, thus reducing the free volume (Figure 12a) and solubility. The uniformly dispersed nanoparticles could also simultaneously extend the diffusion paths of the gas molecules. As a result, the highest reduction in the PA11/0.5% SiO2 composite permeability coefficient occurred at 20 °C below the glass transition temperature. As the nanoparticle content increased, the nanoparticles began to agglomerate and fill the free volume, resulting in gaps and increasing solubility. The ΔHS values in Table 3 showed the same pattern.
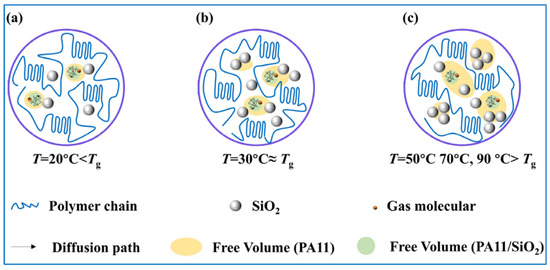
Figure 12.
Diffusion paths of gas molecules and influence of nanoparticles on free volume at different temperatures, (a) PA11/0.5% SiO2, (b) PA11/1.0% SiO2, (c) PA11/1.5% SiO2.
When the temperature gradually increased, the chain segments were unfrozen from the frozen state. In the rubbery state case, the free volume increased, and the gas molecules had low resistance to jumping. The larger free volume meant that more nanoparticles were needed to effectively inhibit the increase in permeability caused by the increase in free volume (Figure 12c). The ED increased as the nanoparticle content rose, indicating that diffusion was difficult, and that the presence of nanoparticles slowed down the movement of the molecular chains while extending the diffusion path. When the content of nanoparticles was greater than 1.5%, the activation energy decreased, due to agglomeration diffusion. Therefore, the PA11/1.5% SiO2 composite exhibited the lowest permeability coefficient in the rubbery state.
4. Conclusions
In this study, the PA11/SiO2 composites were made by vacuum densification and mixing in a range of proportions. The DMA results demonstrated that the presence of nanoparticles impeded the mobility of the molecular chains. It is evident from the CO2 permeation performance tests carried out at 20 °C, 30 °C (the glassy state), 50 °C, 70 °C, and 90 °C (the high elasticity state) that when the temperature rose from 20 °C to 90 °C, the amount of SiO2 that displayed the lowest permeability coefficient rose. When the composites were in a glass state, the solubility had the greatest influence on the permeability behavior. The uniformly dispersed nanoparticles made the molecular diffusion path longer, which had an obvious blocking effect. At 20 °C, the permeability coefficient of the PA11/0.5% SiO2 composite decreased by 17.02% compared with that of the PA11. When the composite was in the rubber state, more nanoparticles were required to inhibit the permeability increase caused by the increase in free volume. However, when the nanoparticles aggregated, the diffusion coefficient increased. Therefore, the permeability coefficient of the PA11/1.5% SiO2 composite in the rubbery state was the lowest. The PA11/SiO2 composites exhibited strong potential for use as flexible risers with high barrier performance.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/polym14204260/s1, Figure S1: Temperature influence on transport coefficients of CO2 in PA11/SiO2 composite, Permeability versus reciprocal temperature; Figure S2: Temperature influence on transport coefficients of CO2 in PA11/SiO2 composite, diffusion versus reciprocal temperature; Figure S3: Permeability coefficient, diffusion coefficient, and solubility of CO2 in PA11 vary with temperature; Figure S4: Permeation curve of PA11 at 20 °C, 30 °C, 50 °C, 70 °C, and 90 °C, diagram of free volume with temperature; Table S1: Intercept and slope of permeability–temperature fitting curve; Table S2: Intercept and slope of diffusion–temperature fitting curve.
Author Contributions
Conceptualization, J.W. and D.H.; methodology, J.W. and X.Z.; validation, Q.Z., Y.L. and X.M.; formal analysis, J.W.; investigation, J.W. and C.C.; resources, D.H.; data curation, X.M. and C.C.; writing—original draft preparation, J.W.; writing—review and editing, X.M. and D.H.; visualization, X.Z.; supervision, X.Y. and Q.Z; project administration, Q.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the science and technology project of CNOOC (China) Co., LTD. “Research on Fluid Penetration Simulation and Control Technology of Deepwater Oil and Gas Transmission Hose”, (Grant No. 2020-YXKJ-013).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Martins, O.D.C.; Souza, R.R.; Lima, R.S.T.; Reguly, A. Development of micromagnetic techniques for stress analysis in flexible risers. Rev. Matéria 2011, 16, 613–620. [Google Scholar]
- Ossai, C.I.; Boswell, B.; Davies, I.J. Pipeline failures in corrosive environments—A conceptual analysis of trends and effects. Eng. Fail. Anal. 2015, 53, 36–58. [Google Scholar] [CrossRef]
- Tiu, B.; Advincula, R.C. Polymeric corrosion inhibitors for the oil and gas industry: Design principles and mechanism. React. Funct. Polym. 2015, 95, 25–45. [Google Scholar] [CrossRef]
- Pottmaier, D.; Melo, C.R.; Sartor, M.N.; Kuester, S.; Amadio, T.M.; Fernandes, C.; Marinha, D.; Alarcon, O.E. The Brazilian energy matrix: From a materials science and engineering perspective. Renew. Sustain. Energy Rev. 2013, 19, 678–691. [Google Scholar] [CrossRef]
- Frederico, G.D.A.; Veiga, A.G.; da CP Gomes, A.P.A.; da Costa, M.F.; Rocco, M.L.M. Using XPS and FTIR spectroscopies to investigate polyamide 11 degradation on aging flexible risers—ScienceDirect. Polym. Degrad. Stab. 2021, 195, 109787. [Google Scholar]
- Simek, J.; Dockalova, V.; Hrdlicka, Z.; Duchacek, V. Effect of liquid butadiene rubber on mechanical properties of polyamide 11/polyamide 12 blends. J. Polym. Eng. 2015, 35, 349–357. [Google Scholar] [CrossRef]
- Liu, T.; Lim, K.P.; Tjiu, W.C.; Pramoda, K.P.; Chen, Z.K. Preparation and characterization of nylon 11/organoclay nanocomposites. Polymer 2003, 44, 3529–3535. [Google Scholar] [CrossRef]
- Wang, B.B.; Hu, G.S.; Zhao, X. Preparation and characterization of nylon 6 11 copolymer. Mater. Lett. 2006, 60, 2715–2717. [Google Scholar] [CrossRef]
- Mago, G.; Kalyon, D.M.; Fisher, F.T. Nanocomposites of polyamide-11 and carbon nanostructures: Development of microstructure and ultimate properties following solution processing. J. Polym. Sci. Part B Polym. Phys. 2011, 49, 1311–1321. [Google Scholar] [CrossRef]
- Moshynets, O.; Bardeau, J.F.; Tarasyuk, O.; Makhno, S.; Cherniavska, T.; Zhuzha, O.D.; Potters, G.; Rogalsky, S. Antibiofilm Activity of Polyamide 11 Modified with Thermally Stable Polymeric Biocide Polyhexamethylene Guanidine 2-Naphtalenesulfonate. Int. J. Mol. Sci. 2019, 20, 348. [Google Scholar] [CrossRef]
- Zhan, H.; Nie, Y.; Chen, Y.; Bell, J.M.; Gu, Y. Thermal transport in 3D nanostructures. Adv. Funct. Mater. 2020, 30, 1903841. [Google Scholar] [CrossRef]
- Naskar, A.K.; Keum, J.K.; Boeman, R.G. Polymer matrix nanocomposites for automotive structural components. Nat. Nanotechnol. 2016, 11, 1026–1030. [Google Scholar] [CrossRef] [PubMed]
- Anguita, J.V.; Smith, C.; Stute, T.; Funke, M.; Silva, S. Publisher Correction: Dimensionally and environmentally ultra-stable polymer composites reinforced with carbon fibres. Nat. Mater. 2020, 19, 317–322. [Google Scholar] [CrossRef] [PubMed]
- Medeiros, D.; Jardim, P.; de Tatagiba, M.; d’Almeida, J. Composites of recycled nylon 11 and titanium based nanofillers. Polym. Test. 2015, 42, 108–114. [Google Scholar] [CrossRef]
- Petrovicova, E.; Knight, R.; Schadler, L.S.; Twardowski, T.E. Nylon 11/silica nanocomposite coatings applied by the HVOF process. II. Mechanical and barrier properties. J. Appl. Polym. Sci. 2015, 78, 2272–2289. [Google Scholar] [CrossRef]
- Rodrigues, A.D.C.; Bastos, I.N.; Kappel, M.A.A.; Nascimento, C.R.; Ferreira, L.S.; da Silva, A.L.N. Micromechanical Property Study of Nylon 11 and Organoclay Systems for Offshore Flexible Pipe. Fibers Polym. 2021, 22, 3172–3182. [Google Scholar] [CrossRef]
- Da Cruz, B.D.S.M.; Tienne, L.G.P.; Gondim, F.F.; Candido, L.d.; Chaves, E.G.; Marques, M.D.F.V.; da Luz, F.S.; Monteiro, S.N. Graphene nanoplatelets reinforced Polyamide-11 nanocomposites thermal stability and aging for application in flexible pipelines. J. Mater. Res. Technol. 2022, 18, 1842–1854. [Google Scholar] [CrossRef]
- Santos, B.P.S.; Arias, J.J.R.; Jorge, F.E.; Santos, R.E.P.D.; Fernandes, B.S.; Candido, L.D.; Peres, A.C.D.; Chaves, E.G.; Marques, M.D.F.V. Preparation, characterization and permeability evaluation of poly (vinylidene fluoride) composites with ZnO particles for flexible pipelines. Polym. Test. 2021, 94, 107064. [Google Scholar] [CrossRef]
- Santos, B.P.S.; Arias, J.J.R.; Jorge, F.E.; Santos, R.E.P.D.; Gondim, F.F.; Fernandes, B.S.; Candido, L.S.; Peres, A.C.C.; Gervasoni, E.; Marques, M.D.V. Synthesis and characterization of poly(vinylidene fuoride) composites with flower-like ZnO particles for flexible pipelines applications. J. Mater. Res. Technol. 2021, 13, 99–110. [Google Scholar] [CrossRef]
- Santos, B.P.S.; Arias, J.J.R.; Jorge, F.E.; Santos, R.E.P.D.; Fernandes, B.S.; Candido, L.S.; Peres, A.C.C.; Chaves, E.G.; Marques, V.M.F. PVDF containing different oxide nanoparticles for application in oil and gas pipelines. Mater. Today Commun. 2021, 26, 101743. [Google Scholar] [CrossRef]
- Gondim, F.F.; Tienne, L.; Cruz, B.; Chaves, E.G.; Marques, M.V. Poly(vinylidene fluoride) with zinc oxide and carbon nanotubes applied to pressure sheath layers in oil and gas pipelines. J. Appl. Polym. Sci. 2020, 138, 50157. [Google Scholar] [CrossRef]
- Wang, B.; Wang, L.; Wang, Y.; Zhou, Z. Unusual toughening effect of graphene oxide on the graphene oxide/nylon 11 composites prepared by in situ melt polycondensation. Compos. Part B Eng. 2013, 55, 215–220. [Google Scholar] [CrossRef]
- Lebaupin, Y.; Chauvin, M.; Hoang, T.-Q.T.; Touchard, F.; Beigbeder, A. Influence of constituents and process parameters on mechanical properties of flax fibre-reinforced polyamide 11 composite. J. Thermoplast. Compos. Mater. 2016, 30, 1503–1521. [Google Scholar] [CrossRef]
- Wijmans, J.G.; Baker, R.W. The solution-diffusion model: A review. J. Membr. Sci. 1995, 107, 1–21. [Google Scholar] [CrossRef]
- Bastani, D.; Esmaeili, N.; Asadollahi, M. Polymeric mixed matrix membranes containing zeolites as a filler for gas separation applications: A review. J. Ind. Eng. Chem. 2013, 19, 375–393. [Google Scholar] [CrossRef]
- Amooghin, A.E.; Shehni, P.M.; Ghadimi, A.; Sadrzadeh, M.; Mohammadi, T. Mathematical modeling of mass transfer in multicomponent gas mixture across the synthesized composite polymeric membrane. J. Ind. Eng. Chem. 2013, 19, 870–885. [Google Scholar] [CrossRef]
- Arjmandi, M.; Pakizeh, M. Mixed matrix membranes incorporated with cubic-MOF-5 for improved polyetherimide gas separation membranes: Theory and experiment. J. Ind. Eng. Chem. 2014, 20, 3857–3868. [Google Scholar] [CrossRef]
- Flaconneche, B.; Martin, J.; Klopffer, M.H. Permeability, Diffusion and Solubility of Gases in Polyethylene, Polyamide 11 and Poly (Vinylidene Fluoride). Oil Gas Sci. Technol. 2001, 56, 261–278. [Google Scholar] [CrossRef]
- Zhang, Q.; Yu, M.; Fu, Q. Crystal morphology and crystallization kinetics of polyamide-11/clay nanocomposites. Polym. Int. 2004, 53, 1941–1949. [Google Scholar] [CrossRef]
- Peng, S.X.; Shrestha, S.; Youngblood, J.P. Crystal structure transformation and induction of shear banding in Polyamide 11 by surface modified Cellulose Nanocrystals. Polymer 2017, 114, 88–102. [Google Scholar] [CrossRef]
- Lonjon, A.; Caffrey, I.; Carponcin, D.; Dantras, E.; Lacabanne, C. High electrically conductive composites of Polyamide 11 filled with silver nanowires: Nanocomposites processing, mechanical and electrical analysis. J. Non-Cryst. Solids 2013, 376, 199–204. [Google Scholar] [CrossRef]
- Hua, Z.; Shi, X.; Chen, Y. Preparation, structure, and property of highly filled polyamide 11/BaTiO3 piezoelectric composites prepared through solid-state mechanochemical method. Polym. Compos. 2017, 40, 177–185. [Google Scholar]
- Wan, J.; Bu, Z.Y.; Li, C.; Hong, F.; Li, B.G. Preparation, melting, glass relaxation and nonisothermal crystallization kinetics of a novel dendritic nylon-11. Thermochim. Acta 2011, 524, 117–127. [Google Scholar] [CrossRef]
- Dechet, M.A.; Goblirsch, A.; Romeis, S.; Zhao, M.; Lanyi, F.J.; Kaschta, J.; Schubert, D.W.; Drummer, D.; Peukert, W.; Schmidt, J. Production of polyamide 11 microparticles for Additive Manufacturing by liquid-liquid phase separation and precipitation. Chem. Eng. Sci. 2019, 197, 11–25. [Google Scholar] [CrossRef]
- Huang, Y.Q.; Dai, D.D.; Li, H.B.; Sun, L.; Runt, J.; Huang, K.S.; Yeh, J.T. Oxygen barrier, free volume, and blending properties of fully bio-based polyamide 11/poly(vinyl alcohol) blends. J. Appl. Polym. Sci. 2019, 137, 48562. [Google Scholar] [CrossRef]
- Domingos, E.; Pereira, T.M.C.; Castro, E.V.R.D.; Romao, W.; Guimaraes, R.C.L. Monitoring the Degradation of Polyamide 11 (PA-11) via Fourier Transform Infrared Spectroscopy (FTIR). Polimeros 2013, 23, 37–41. [Google Scholar] [CrossRef]
- Yang, X.; Wang, H.; Chen, J.; Fu, Z.; Zhao, X.; Li, Y. Copolymers containing two types of reactive groups: New compatibilizer for immiscible PLLA/PA11 polymer blends. Polymer 2019, 177, 139–148. [Google Scholar] [CrossRef]
- Jubinville, D.; Chang, B.P.; Pin, J.-M.; Mohanty, A.K.; Misra, M. Synergistic thermo-oxidative maleation of PA11 as compatibilization strategy for PA6 and PBT blend. Polymer 2019, 179, 121594. [Google Scholar] [CrossRef]
- Halim, K.; Farrell, J.B.; Kennedy, J.E. Preparation and characterisation of polyamide 11/montmorillonite (MMT) nanocomposites for use in angioplasty balloon applications. Mater. Chem. Phys. 2013, 143, 336–348. [Google Scholar] [CrossRef]
- Gaabour, L.H. Influence of silica nanoparticles incorporated with chitosan/polyacrylamide polymer nanocomposites. J. Mater. Res. Technol. 2019, 8, 2157–2163. [Google Scholar] [CrossRef]
- Do, V.-T.; Chun, D.-M. Fabrication of large-scale, flexible, and robust superhydrophobic composite films using hydrophobic fumed silica nanoparticles and polydimethylsiloxane. Polymer 2022, 244, 124630. [Google Scholar] [CrossRef]
- Ramalla, I.; Gupta, R.K.; Bansal, K. Effect on superhydrophobic surfaces on electrical porcelain insulator, improved technique at polluted areas for longer life and reliability. Int. J. Eng. Technol. 2015, 4, 509–519. [Google Scholar] [CrossRef]
- Mancic, L.; Pontón, P.I.; Letichevsky, S.; Costa, A.M.; Marinkovic, B.A.; Rizzo, F.C. Application of silane grafted titanate nanotubes in reinforcing of polyamide 11 composites. Compos. Part B Eng. 2016, 93, 153–162. [Google Scholar] [CrossRef]
- Yamunadevi, V.; Vijayanand, G.; Ganeshan, P.; Sowmiya, S.; Raja, K. Effect on the behaviour of dynamic mechanical analysis for hybrid epoxy nanocomposite. Mater. Today Proc. 2020, 37, 223–227. [Google Scholar] [CrossRef]
- Nicharat, A.; Sapkota, J.; Weder, C.; Foster, E.J. Melt processing of polyamide 12 and cellulose nanocrystals nanocomposites. J. Appl. Polym. Sci. 2015, 132, 42752. [Google Scholar] [CrossRef]
- Aroon, M.A.; Ismail, A.F.; Matsuura, T.; Montazer-Rahmati, M.M. Performance studies of mixed matrix membranes for gas separation: A review. Sep. Purif. Technol. 2010, 75, 229–242. [Google Scholar] [CrossRef]
- Moore, T.T.; Koros, W.J. Non-ideal effects in organic–inorganic materials for gas separation membranes. J. Mol. Struct. 2005, 739, 87–98. [Google Scholar] [CrossRef]
- Chung, T.S.; Lan, Y.J.; Yi, L.; Kulprathipanja, S. Mixed matrix membranes (MMMs) comprising organic polymers with dispersed inorganic fillers for gas separation. Prog. Polym. Sci. 2007, 32, 483–507. [Google Scholar] [CrossRef]
- Bugatti, V.; Bernardo, P.; Clarizia, G.; Viscusi, G.; Vertuccio, L.; Gorrasi, G. Ball Milling to Produce Composites Based of Natural Clinoptilolite as a Carrier of Salicylate in Bio-Based PA11. Polymers 2019, 11, 634. [Google Scholar] [CrossRef]
- Wu, B.; Firouzi, M.; Mitchell, T.; Rufford, T.E.; Leonardi, C.; Towler, B. A critical review of flow maps for gas-liquid flows in vertical pipes and annuli. Chem. Eng. J. 2017, 326, 350–377. [Google Scholar] [CrossRef]
- Klepić, M.; Jansen, J.C.; Fuoco, A.; Esposito, E.; Izák, P.; Petrusová, Z.; Vankelecom, I.F.; Randová, A.; Fíla, V.; Lanč, M. Gas separation performance of carbon dioxide-selective poly (vinyl alcohol)–ionic liquid blend membranes: The effect of temperature, feed pressure and humidity. Sep. Purif. Technol. 2021, 270, 118812. [Google Scholar] [CrossRef]
- Rabiee, H.; Alsadat, S.M.; Soltanieh, M.; Mousavi, S.A.; Ghadimi, A. Gas permeation and sorption properties of poly (amide-12-b-ethyleneoxide)(Pebax1074)/SAPO-34 mixed matrix membrane for CO2/CH4 and CO2/N2 separation. J. Ind. Eng. Chem. 2015, 27, 223–239. [Google Scholar] [CrossRef]
- Salahshoori, I.; Seyfaee, A.; Babapoor, A.; Neville, F.; Moreno-Atanasio, R. Evaluation of the effect of silica nanoparticles, temperature and pressure on the performance of PSF/PEG/SiO2 mixed matrix membranes: A molecular dynamics simulation (MD) and design of experiments (DOE) study. J. Mol. Liq. 2021, 333, 115957. [Google Scholar] [CrossRef]
- Gouveia, A.S.; Yanez, M.; Alves, V.D.; Palomar, J.; Moya, C.; Gorri, D.; Tomé, L.C.; Marrucho, I.M. CO2/H2 separation through poly (ionic liquid)–ionic liquid membranes: The effect of multicomponent gas mixtures, temperature and gas feed pressure. Sep. Purif. Technol. 2021, 259, 118113. [Google Scholar] [CrossRef]
- Zhang, B.; Qiao, J.; Wu, D.; He, X.; Liu, J.; Yi, C.; Qi, S. Enhanced Gas Separation by Free Volume Tuning in a Crown Ether-Containing Polyimide Membrane. Sep. Purif. Technol. 2022, 293, 121116. [Google Scholar] [CrossRef]
- Ash, R.; Barrer, R.M.; Palmer, D.G. Solubility and transport of gases in nylon and polyethylene. Polymer 1970, 11, 421–435. [Google Scholar] [CrossRef]
- Vrentas, J.S.; Duda, J.L. Diffusion in polymer—Solvent systems. I. Reexamination of the free-volume theory. J. Polym. Sci. Polym. Phys. Ed. 1977, 15, 403–416. [Google Scholar] [CrossRef]
- Vrentas, J.S.; Duda, J.L. Diffusion in polymer—Solvent systems. II. A predictive theory for the dependence of diffusion coefficients on temperature, concentration, and molecular weight. J. Polym. Sci. Polym. Phys. Ed. 1977, 15, 417–439. [Google Scholar] [CrossRef]
- Xiao, Y.; Low, B.T.; Hosseini, S.S.; Chung, T.S.; Paul, D.R. The strategies of molecular architecture and modification of polyimide-based membranes for CO2 removal from natural gas—A review. Prog. Polym. Sci. 2009, 34, 561–580. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).