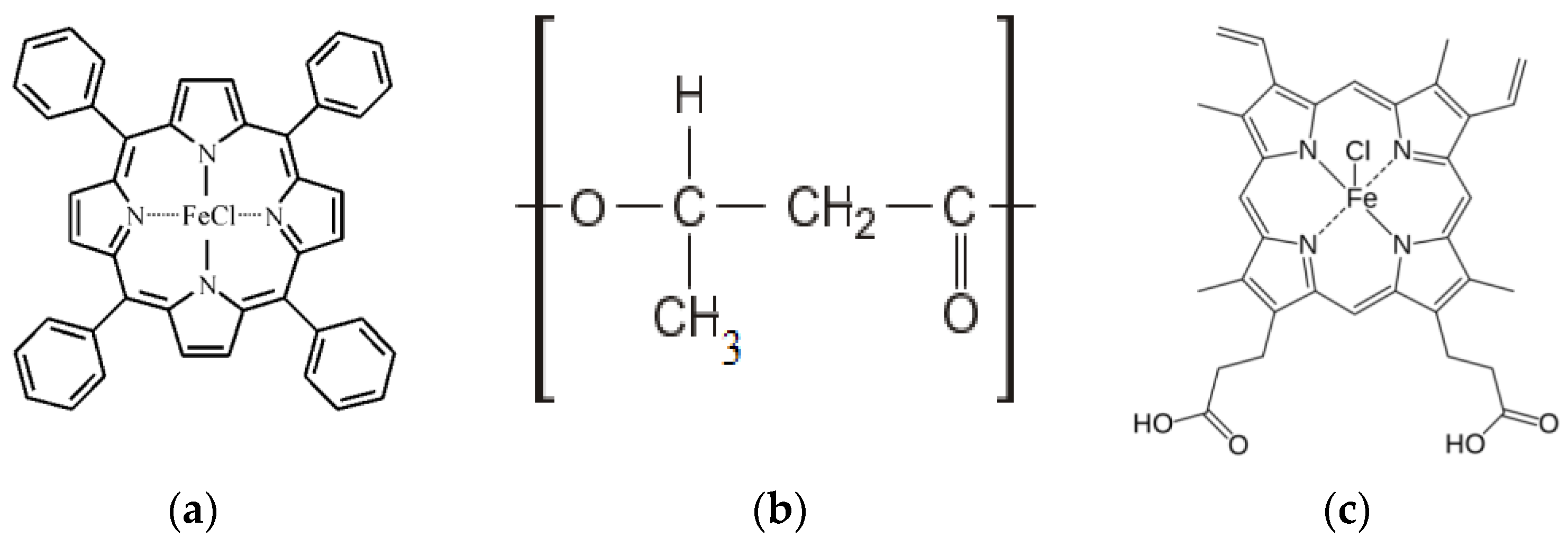
3.1. Effect of Hemin Concentration on the Geometric Parameters of the Fiber
The PHB/hemin materials were obtained by double molding. The essence of the electrospinning process ensured the curing of the fibers of the polymer matrix after complete evaporation of the solvent [
28]. This method made it possible to uniformly arrange additives that occupied the free space between crystallites in the amorphous regions of the polymer [
29]. The introduction of hemin led to the formation of uniform and thin fibers, which might be due to the presence of metal, which increased the electrical conductivity of the spinning solution, and which, of course, improved the quality of the resulting fibers [
30].
The introduction of hemin into the PHB structure accelerated the enzymatic hydrolysis of ultrathin PHB/hemin fibers compared to PHB/FeCl-TFP fibers due to a looser amorphous phase and a lower degree of crystallinity. When porphyrin metal complexes were added to the PHB molding solution, the morphology of the fibrous material changed dramatically. When 1–5% of FeCl-TFP complexes were added, elliptical elements in the fiber structure disappeared completely. When 1% of the FeCl-TFP complex was added, fibers were formed mainly with average diameters of 1.5–2.0; 3.0–4.0 and 5.0–6.0 microns. The presence of thin fibers of less than 3 microns was a consequence of the splitting effect of the primary jet of the molding solution in the field of electrostatic forces. With an increase in the concentration of FeCl-TFP from 3 to 5%, fibers with a diameter of 3 microns predominated.
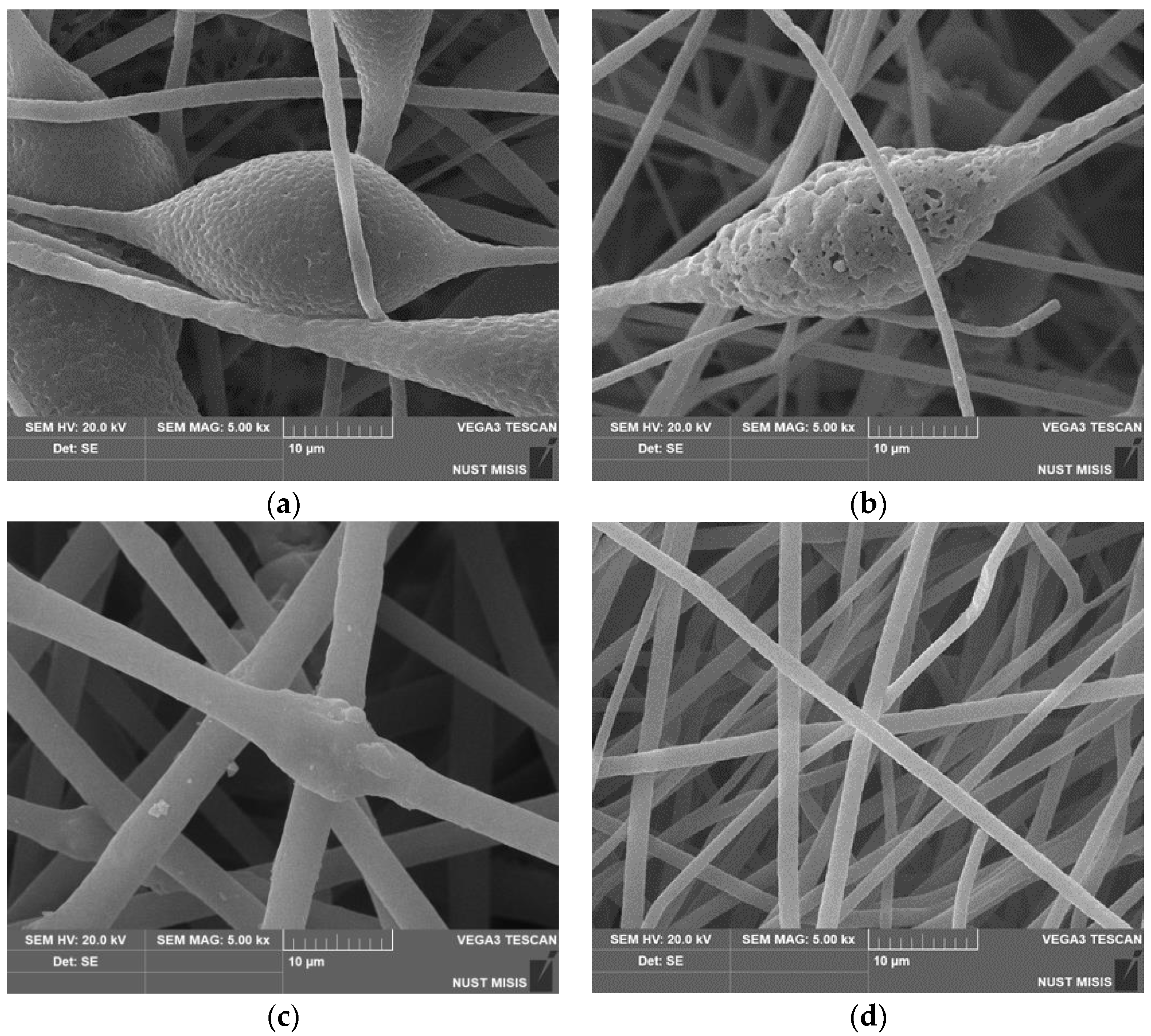
Scanning electron microscopy was used to study in detail the morphology of fibrous materials. The images of the most characteristic parts of the samples are shown in
Figure 1. The structure of the initial fibers of PHB and with a low content of hemin (up to 3%) is heterogeneous. The fibers are characterized by a high degree of tortuosity, adhesions and the presence of large formations in the form of thickenings of an elliptical shape. The average size of these structures is from 20 to 30 microns in the longitudinal direction and 15–25 microns in the transverse direction. An increase in the concentration of hemin leads to a decrease in the diameter and an increase in the uniformity of the fibers in diameter. The microrelief, with characteristic pores (
Figure 2a) changes with the introduction of even 1% additive. Defects in the form of glues and thickenings are extremely rare, the fibers become thinner, the number of defects is reduced, and with the introduction of 5%, defects in the form of thickenings are almost completely absent. Roughness on the surface of the fibers almost completely disappears at 5% addition, as do elliptical thickenings.
The deepening of pores on such a thickening (
Figure 2b) can be explained by more intensive evaporation of the solvent due to the technology of electrospinning using two solvents, which is used, among other things, to obtain highly porous fibers [
30], however, for a 5% hemin content, this factor did not add to the contribution in the form of a more optimal balance of electrical conductivity-viscosity of the casting solution. In all likelihood, the presence of large hemin molecules in the intermolecular space of PHB led to an increase in the free volume of amorphous regions, which increased the rate of solvent desorption. To determine the uniformity of distribution of hemin in the fibers, we used the EDX method.
To ensure that the hemin was indeed incorporated into the fiber structure and not on the surface, an EDX analysis was performed. The results of the analysis of the composition of the material by the energy-dispersive X-ray method showed that hemin in the studied concentration range was distributed fairly evenly in the material, and not only concentrated in inclusions on the surface. Iron and chlorine atoms were chosen as the atom identifying hemin, since they were included in the central part of the tetrapyrrole ring.
3.2. X-ray Diffraction Study of PHB/Hemin Fibers of Different Composition
Along with a change in the geometry of ultrathin fibers with the introduction of an additive, one should also expect changes in the structural and dynamic parameters of the compositions.
It is known that the intermolecular interaction between porphyrin-metal complex particles, as well as between these particles and PHB molecules, depends significantly on the nature of the metal complex. Thus, it was shown in [
31,
32,
33] that porphyrin molecules were attracted and, as a result, rather large particles of porphyrin were formed in PHB fibers. The intermolecular interaction of these particles with PHB macromolecules was extremely small. Although the molecules of the porphyrin complex with FeCl had a strong interaction with PHB macromolecules, the presence of the loride extraligand in the metal complex led to intermolecular repulsion, i.e., the molecules aggregated into smaller particles. Finally, Sn(TPP)Cl
2 molecules were distributed in the system at the molecular level, due to the presence of two chlorine anions, repulsive forces between the complexes acted. The interaction of these complexes was weaker than that of Fe(TPP)Cl complexes with PHB macromolecules.
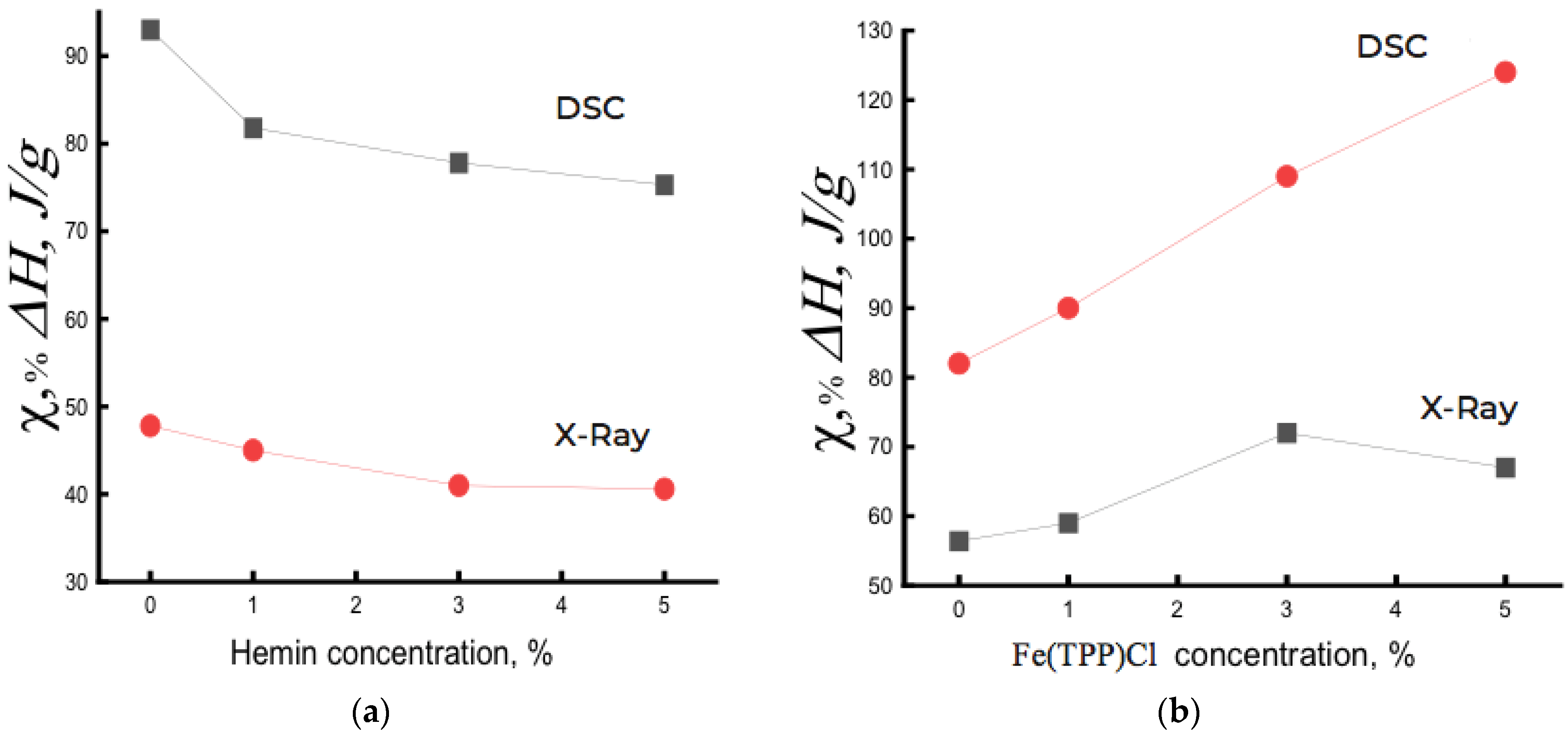
Figure 3a shows the dependence of the degree of crystallinity χ on the composition of the PHB/hemin system. For comparison,
Figure 3b shows such a dependence for the PHB/Fe(TPP)Cl system. As seen, the dependencies point in different directions. Fe(TPP)Cl particles, being crystallization nuclei, caused an increase in the degree of crystallinity, and hemin, aggregating into large particles, prevented the crystallization of the PHB/hemin mixture and, as a result, the fraction of crystallites decreased with increasing hemin concentration in the polymer. In a system with 5% hemin, crystalline hemin particles 55–100 nm in size were formed. If the large period d increased with the addition of Fe(TPP)Cl particles to PHB (from 58 to 63 nm), then this parameter practically did not change in the PHB/hemin system.
It should be noted that with an increase in the concentration of Fe(TPP)Cl from 3% to 5%, a slight decrease in the degree of crystallinity was observed, apparently due to the aggregation of complexes into large particles, which did not lead to a further increase in the proportion of crystallites during fiber spinning.
Thus, X-ray diffraction study indicated a decrease in the degree of crystallinity in the PHB/hemin system, and in PHB/Fe(TPP)Cl fibers, an increase in the longitudinal dimensions (along the fibril) of crystal structures, a large period, and also the degree of crystallinity with increasing concentration of the additive was observed. Along with the presented changes, the transverse sizes of crystallites significantly decreased upon the addition of porphyrin: for example, L020 changed from 20.2 Å in PHB to ≈ 16 Å in PHB/Fe(TPP)Cl, and L110 changed from 20.1 Å in PHB to ≈14.5 Å in PHB with an additive. Therefore, it can be concluded that the addition of porphyrin to PHB fibers caused a decrease in the transverse dimensions of fibrils, and the longitudinal size of crystallites increased significantly. The observed changes, in our opinion, took place due to the straightening of the folded sections of the chains in the crystallites and their partial crystallization during the electrospinning of the fibers. The presence of small Fe(TPP)Cl particles contributed to the processes of intensive plasticization of the polymer, since it was in the PHB/Fe(TPP)Cl samples that the strongest increase in the degree of crystallinity occurred with increasing additive concentration. In PHB/hemin samples, however, the degree of crystallinity decreased significantly with an increase in the additive, which may be due to the formation of large particles, and with an increase in their concentration, the process of crystallite deformation took place to an increasing extent.
The presented data indicate that the addition of Fe(TPP)Cl particles to PHB fibers caused a significant increase in the degree of crystallinity with a higher longitudinal crystallite size (L002). When hemin was added, due to its aggregation into large particles, crystallization processes were inhibited, which led to a significant decrease in the degree of fiber crystallinity.
3.4. Exposure of Ultrathin PHB Fibers in an Aqueous Medium in the Presence of Hemin and Porphyrin Complex Fe(TPP)Cl
Sorption and diffusion transport of water in PHB films were considered in detail earlier in a number of works, in which the diffusion coefficients and water absorption isotherms were estimated, the segmental mobility of PHB molecules was studied, and micrographs of the surface of samples obtained by SEM were presented, as well as their structural characteristics [
15,
35,
36,
37,
38]. It was interesting to study the effect of exposure in an aqueous medium (distilled water) at a temperature of 70 °C on the structural and dynamic parameters of the PHB/hemin mixture composition and compare it with the regularities for the PHB/Fe(TPP)Cl system.
The structure and dynamics of medical materials to a large extent affect the diffusion processes of drugs introduced into polymers and, consequently, the kinetic patterns of drug release into the body’s environment. Since the polymer in the body is in the aquatic environment, it is important to identify changes in its structural and dynamic parameters during swelling. The choice of temperature for processing fibrous materials in water at 70 °C is associated with the need to increase the hydrophilicity of PHB, since after removing most of the water by drying, a smaller part of it remains in the polymer in a bound form (hydrogen bonds). Even a slight increase in the hydrophilicity of polymer matrices affects the diffusion processes of drug transport, which, as a rule, are polar. At room temperature, the diffusion of water into the polymer takes a long time, up to several days. The plasticizing action of water, which is most intense in the amorphous regions of the fibers, in combination with elevated temperature creates favourable conditions for the completion of additional crystallization of the polymer, leads to a more ordered state of the intergranular regions and to a redistribution of the ratio between the “loose” and dense phases. Earlier studies of the effect of an aqueous medium on the structure of polymers were carried out [
12,
14,
36]. At 70 °C, hydrogen bonds are destroyed and the process of additional orientation of straightened chains in the fiber is realized. It was necessary to establish the degree of these structural changes for the system studied in the work.
Crystallites and amorphous regions generally do not correspond to the free energy minimum. The system tends to a minimum of free energy, and this process is facilitated when the intermolecular interaction decreases (for example: the structure loosens at elevated temperature, when additives are introduced into the polymer, etc.). Loose, amorphous regions relax to the state of a supercooled melt, and crystalline regions relax to a minimum of free energy through a change in size and, first of all, longitudinal dimensions (). It should be noted that amorphous regions in polymers are heterogeneous, there are regions with a high degree of straightening and regions with folded macromolecules. Under conditions where steric hindrance is removed (e.g., at elevated temperature), highly straightened chains assume more straightened conformations.
Water molecules act on the polymer structure in two ways: on the one hand, they have a plasticizing effect, which allows the crystallites to increase their size, on the other hand, after the removal of water, hydrated complexes remain in the fiber, loosening the polymer structure. Water molecules, hydrated complexes penetrate into the end surfaces of crystallites and linear structures, partially destroying them and, as a result, the degree of crystallinity decreases, as was shown in [
15]. The accumulation of hydrated complexes in the amorphous component leads to its decompaction. It was necessary to establish the extent of these structural changes. At different exposure times in samples containing the addition of hemin and Fe(TPP)Cl at different concentrations, the intensity of the listed processes is different.
Let us first consider the effect of water treatment at 70 °C on the crystal structure of the PHB/hemin fiber (
Table 1).
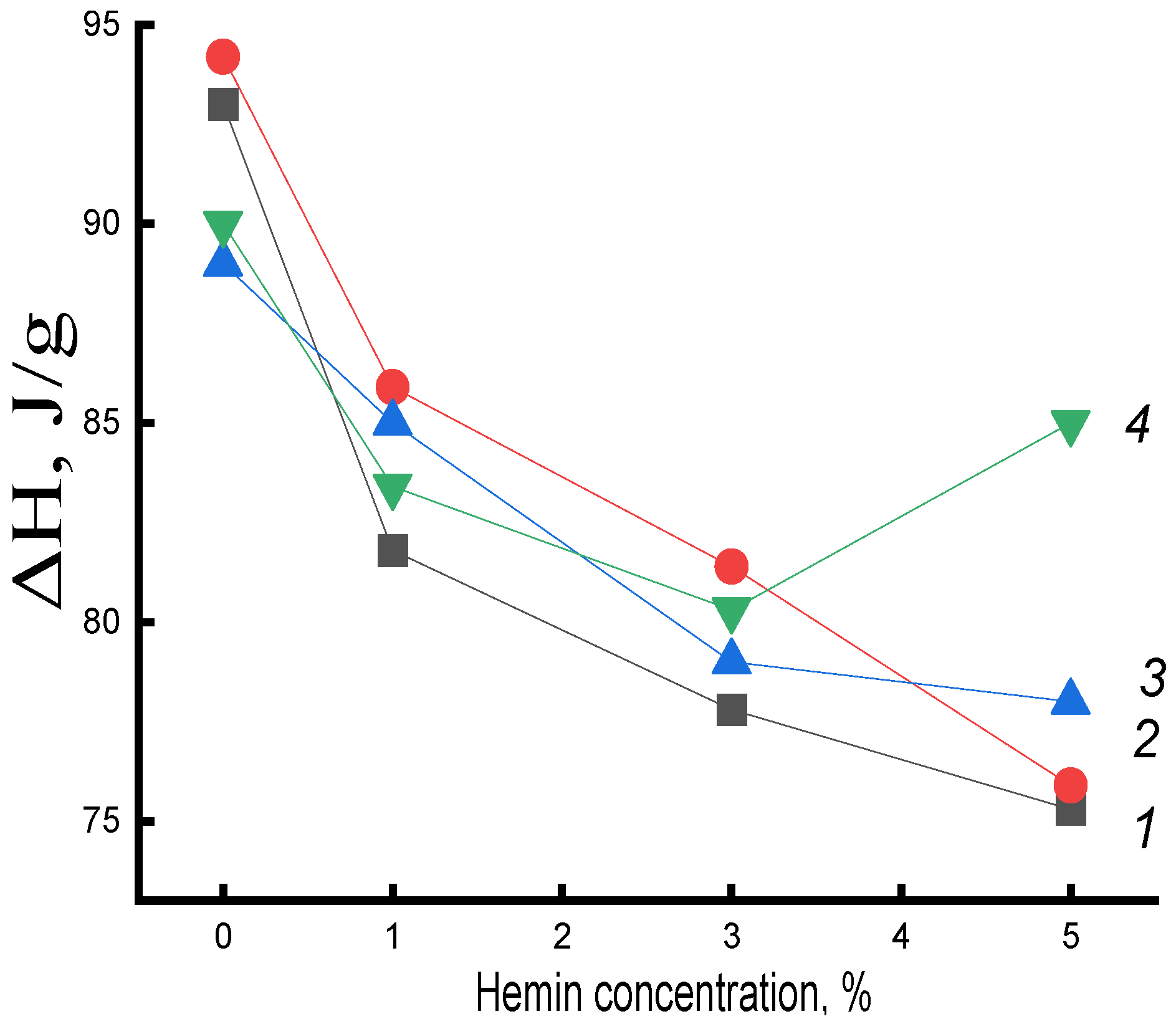
Figure 4 presents the data on the change in the enthalpy of melting of this system on the concentration of hemin at different exposure times in an aqueous medium. An increase in ΔH in mixed compositions compared to the original untreated in an aqueous medium was observed at all studied times of water-temperature exposure to the polymer (with the exception of fibers with 5% hemin). In PHB fibers, the enthalpy of melting decreased. The research results indicated the contradictory nature of the impact of the aquatic environment on the fibers of PHB and PHB with complexes.
As previously noted, water molecules affected the polymer structure in two ways: on the one hand, they had a plasticizing effect, which allowed crystallites to increase in size. On the other hand, after the removal of water, hydrated complexes remained in the fiber, which are formed between the polar groups of PHB and hemin, as well as between hemin molecules, PHB groups. As a result, the fiber structure became looser and the enthalpy of the system decreased.
The increase in ΔH in PHB fibers with hemin after exposure can be explained by the predominance of plasticization processes and, as a consequence, an increase in the proportion of straightened chains in the fiber and, upon longer exposure, the formation of hydrated complexes that loosened the structure of crystalline regions (end surfaces of crystallites, linear structures). Hemin, due to large particle sizes, greatly loosens the structure of PHB, which leads to a significant concentration of water molecules in the fiber, the plasticizing factor also increases and, as a result, ΔH increases. In samples with 5% hemin, loosening was maximal and represented the highest increase in the melting enthalpy in them(
Figure 5) It can be assumed that hemin, due to large particle sizes, does not deform the end surfaces of crystallites and linear systems; therefore, at a 5% additive in the polymer, the maximum opportunity for a certain proportion of macromolecules to straighten is realized; stress relaxation occurs in the nonequilibrium supramolecular structure of the fiber, which leads to additional densification of amorphous intercrystalline regions of their packing into crystallites.
Let us now consider the dynamic characteristics of the amorphous phase of ultrathin PHB/hemin fibers after water-temperature treatment. The plasticizing effect of water, which is most intense in the amorphous regions of the polymer, in combination with elevated temperature, created favorable conditions for the completion of its additional crystallization and for a more ordered state of the intercrystalline regions. After the removal of water, hydrated complexes remained in the fiber, which loosened the amorphous structure to a greater extent than non-hydrated particles; therefore, a sharp decrease in the correlation time was observed.
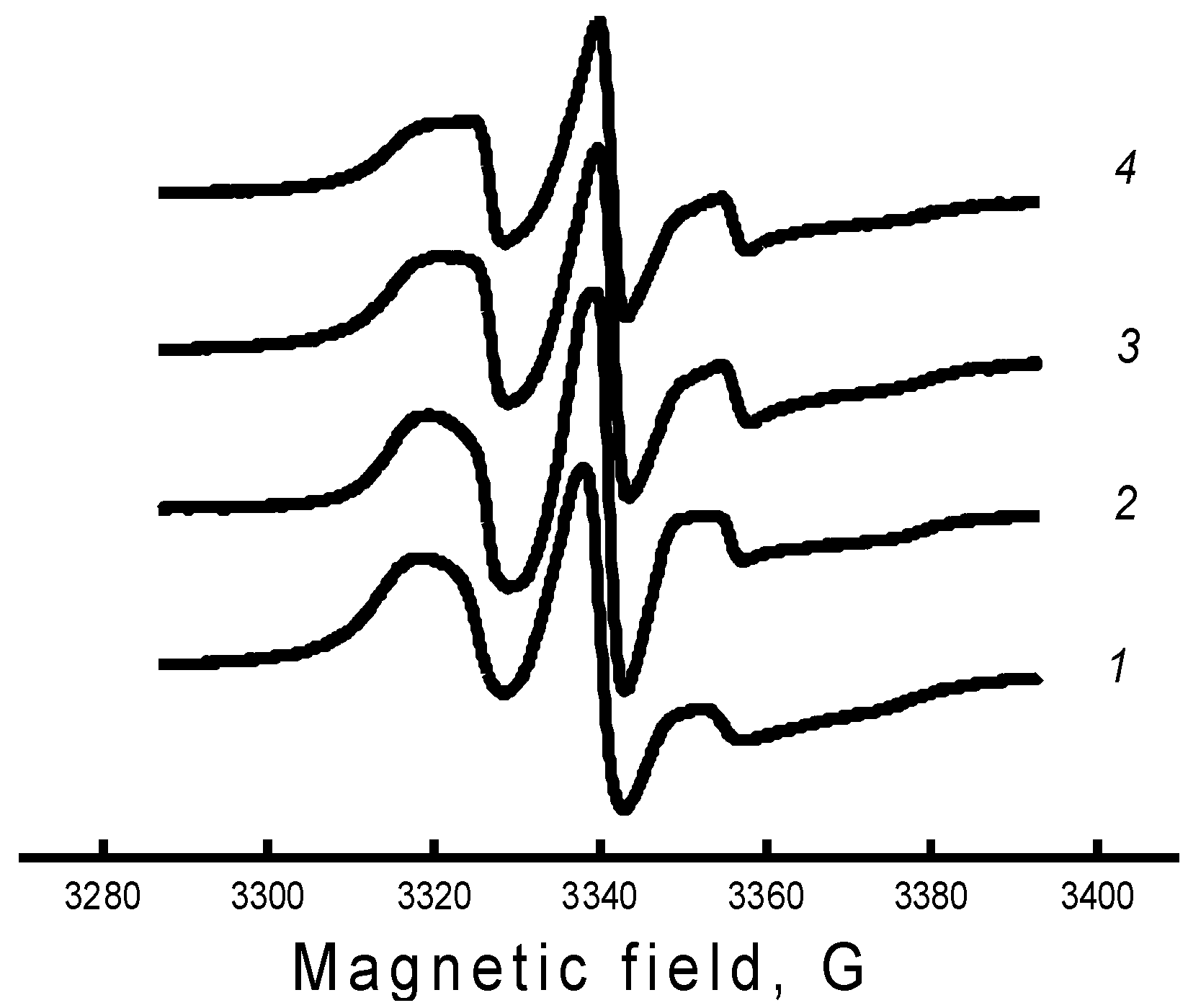
The specificity of this work was associated with a qualitative analysis of changes in the mobility of radicals during one or another treatment of the polymer matrix, and not with the interpretation of the exact values of the parameters of rotational mobility. For this reason, we needed to introduce a parameter that would qualitatively characterize the rotational mobility of radicals, be determined without a numerical analysis of all points of the spectrum, and have sufficient sensitivity to small changes in the shape of the spectrum, such as the changes shown in
Figure 6. It can be seen that the greatest difference in the spectra was observed in the region of the high-field and low-field components; therefore, as the parameter of the spectrum shape, we chose the characteristic rotational correlation time calculated by the well-known formula [
38,
39]. This formula was proposed to calculate the rotational correlation times of nitroxyl radicals of the piperidine series in the case of their isotropic rotation in the region of rotational correlation times 5 × 10
−11 ≤ τ ≤ 1 × 10
−9 s. In this region, the EPR spectrum consisted of three well-resolved components, the width of which was described in terms of the Redfield theory. In our case, this parameter qualitatively characterized the line shape of the EPR spectrum and could be considered as some characteristic correlation time.
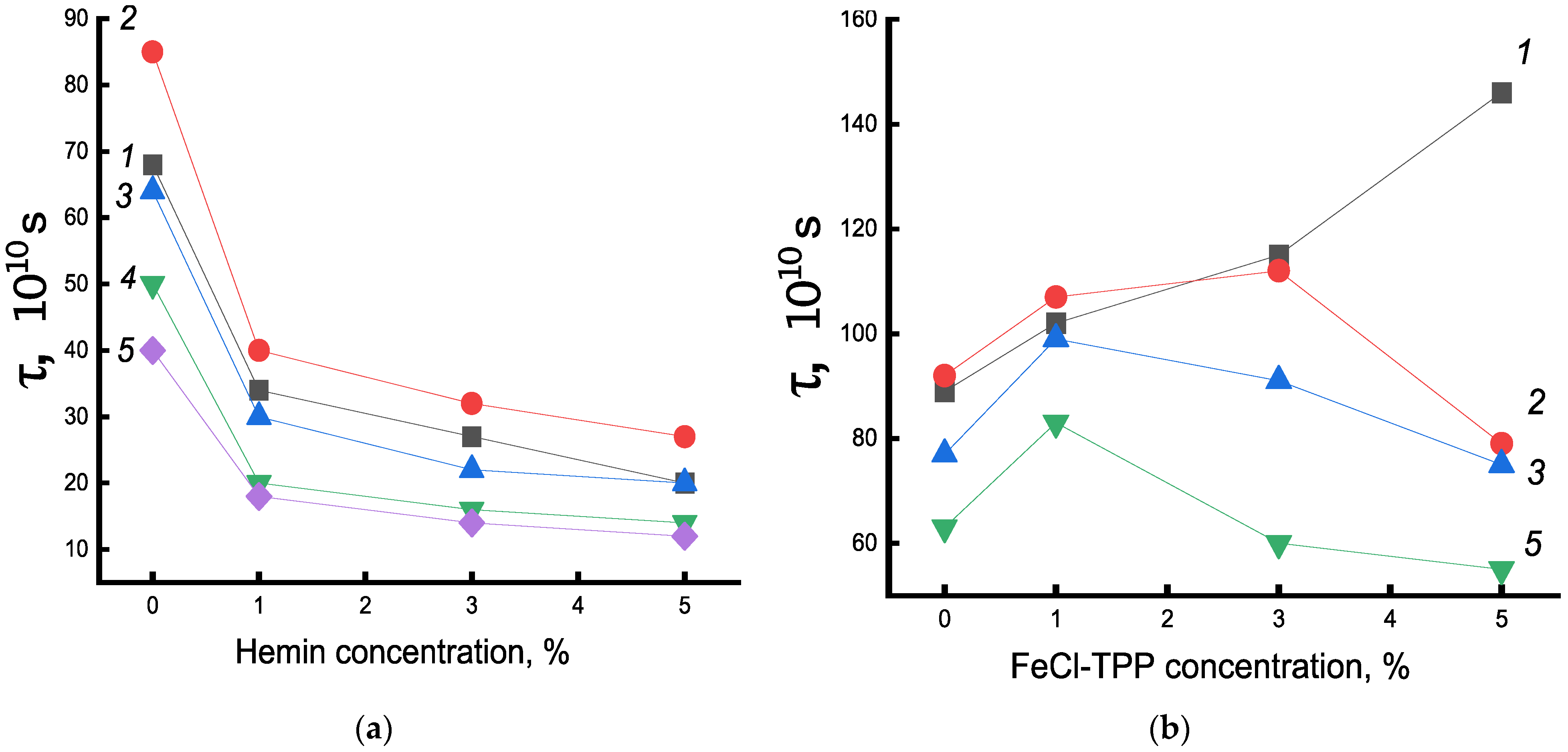
Figure 7a shows the change in the time of rotational correlation of radicals with an increase in the concentration of hemin complexes. The greatest intensity of the decrease in τ in the system was in the concentration up to 1% hemin. A further increase in the concentration of hemin in the fiber also caused an increase in molecular mobility, but not so significant. A similar pattern of change in χ and ΔH was observed with an increase in hemin concentration, which indicated the decompression of amorphous and crystalline regions upon the addition of hemin to the PHB structure.
In contrast to the observed changes in the structure of the amorphous component of the fiber with hemin, in the PHB/Fe(TPP)Cl system, in which the additive particles are small, opposite patterns of change in τ are observed (
Figure 7b). Fe(TPP)Cl particles, being crystallization nuclei, promoted an increase in the degree of crystallinity and, as a result, a significant increase in the proportion of straightened macromolecules, which led to a decrease in molecular dynamics [
12,
35].
Let us now consider the effect of exposure to an aqueous medium at 70 °C on the molecular mobility of the PHB/hemin system. After 30 min exposure in an aqueous medium, τ increased for all systems, which indicated a slowdown in molecular mobility. However, with an increase in the time of water-temperature exposure, the mobility of chains in the amorphous phase increased significantly for all compositions of the system, although the enthalpy of melting remained higher than for untreated fibers. It can be assumed that as the density of amorphous regions (with increasing crystallinity) also increased, the concentration of the radical in them also decreased and, as a result, the molecular mobility increased. Characteristically, the most dramatic change in molecular dynamics and melting enthalpy was observed after the addition of 1% hemin at all times of water-temperature exposure. Apparently, the concentration of hemin increased not so sharply due to the formation of ever larger particles with an increase in the concentration of the additive.
The explanation of the obtained data was the fact that hemin, due to the large particle size, strongly loosened the PHB structure, which led to a significant concentration of water molecules in the fiber and to two opposite processes: not only the plasticizing factor increases, leading to the growth of straightened chains in the fiber, but to an increase in the concentration of hydrated complexes and, as a result, the structure of the amorphous phase was loosened. In our case, at all processing times, as shown by DSC and τ data (up to 30 min), the first factor prevailed. The decrease in the correlation time for all compositions of the system at treatment times of more than 30 min was explained by the ever lower concentration of the radical in the dense regions of the polymer, which were formed under water-temperature exposure, as well as by the loosening of the amorphous phase after the formation of hydrated complexes, the concentration of which increased with the time of fiber treatment. To investigate how the density of the fiber changed, data were obtained on the equilibrium concentration of the radical adsorbed in samples of the studied compositions of the same mass using the software from Bruker (winer).
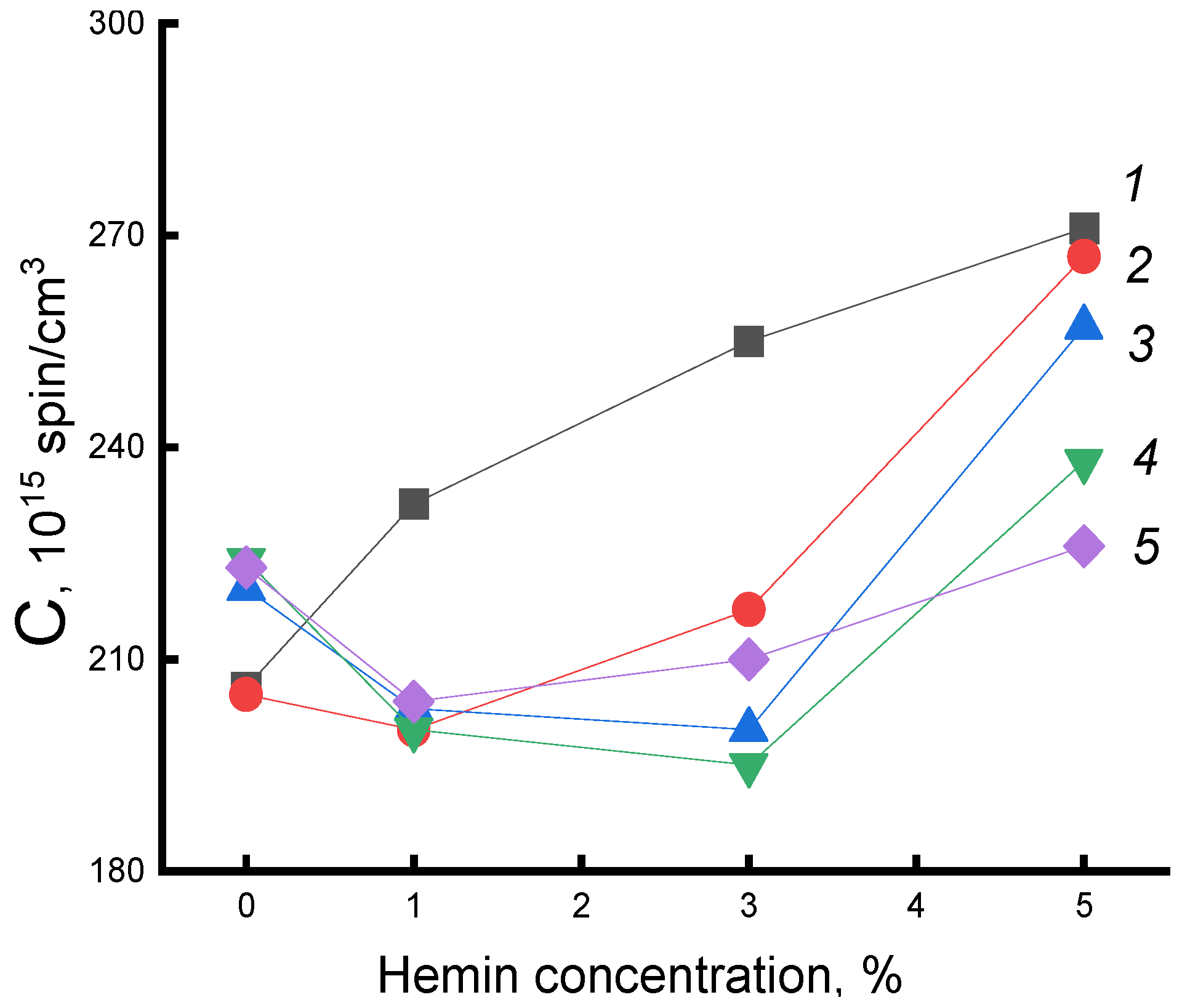
Figure 8 shows the dependences of the concentration of the radical (C) on the composition of the fibers for a series of exposure times. As expected, the concentration of the radical increased with increasing additive. After water-temperature treatment in PHB samples, the concentration increased with increasing exposure time, which was consistent with the increase in the fraction of the amorphous component according to DSC data. In fibers with hemin, the concentration of the radical decreased (deviation 3%). The addition of 5% hemin was accompanied by the highest increase in ΔH with increasing exposure time; the strongest changes in the radical concentration are observed precisely in these fibers. Therefore, it can be argued that despite the formation of hydrated complexes that loosened the polymer structure, the processes of plasticization of the structure leading to an increase in the enthalpy of melting, proceeding with the compaction of the material, prevailed.
Let us now consider the change in molecular dynamics under water-temperature treatment of PHB/Fe(TPP)Cl fibers. The dependence of molecular dynamics on the additive concentration after exposure to an aqueous medium was extreme. At an additive concentration of 1%, there was a sharp increase in τ. It was in these polymers that a high proportion of straightened chains was evidenced by a sharp increase in χ and ΔH with an increase in the concentration of the porphyrin complex. Therefore, at a low concentration of this additive, the plasticizing effect prevailes. As a result, mobility slowed down. At higher concentrations of Fe(TPP)Cl, the processes loosening the structure due to the formation of hydrated complexes began to predominate to an increasing extent, and the molecular mobility increased. Similar processes took place with an increase in the time of water-temperature treatment. The concentration of the radical in the fibers after exposure was significantly lower than in the original samples. For example, in the initial sample of PHB-porphyrin 5% concentration of the radical 9.3 × 1015, and after annealing—3.1 × 1015.
Thus, the hydrophilization of the studied systems depended both on the composition of the composition and on the type of additive in PHB, as well as on the time of water-temperature treatment. The sorption capacity, due to the high affinity of water for Fe(TPP)Cl and hemin molecules, increased sharply compared to PHB, in this case, the diffusion coefficients of water and low molecular weight compounds increased exponentially.
Studies of the structural and dynamic characteristics of fibrous materials PHB/hemin and PHB/FeClTPP after exposure to an aqueous medium at 70 °C showed that:
The enthalpy of melting of PHB/hemin fibers at all times of water-temperature treatment (up to 5 h) and for all concentrations of hemin increased, which was due to the predominance of plasticization processes and structure compaction over loosening processes due to the formation of hydrated complexes in the polymer. The molecular mobility of the probe, and hence the mobility of macromolecules, slowed down after holding the fiber in an aqueous medium for 30 min due to an increase in the enthalpy of melting (according to DSC data). At higher times of such an impact on the system, the molecular dynamics began to increase, which was caused by the predominance of structure loosening processes during the formation of hydrated complexes. The radical was sorbed at a lower and lower concentration in amorphous regions with increasing exposure time in an aqueous medium due to an increase in density.
Opposite regularities are observed when the water-temperature effect iwas applied to the PHB/Fe(TPP)Cl system. The presence of the complex determined the complex dependence of τ on its concentration in the fiber after exposure to water. The segregation of Fe(TPP)Cl molecules into small particles determined the structural and dynamic changes not only with an increase in the concentration of the additive, but also during water-temperature treatment of the polymer.
It is well known that water, penetrating into the polymer matrix, can affect both the physicochemical and mechanical properties of polymers and the flows of the third low molecular weight component (electrolyte, drug substance) [
38].
Additional information on the dynamic behaviour of the PHB/hemin system of various compositions was obtained by studying the temperature dependence of the radical rotation rate and determining the corresponding activation energies Eτ. A characteristic feature of Eτ as a function of the percentage of hemin in PHB was a sharp difference between these values in mixed compositions. This parameter decreased with increasing hemin concentration: 0%—50, 1%—28, 3%—22, 5%—18 kJ/mol. Such a sharp decrease in the activation energy of probe rotation upon the introduction of hemin was associated with a change in the state of the intercrystalline polymer phase; the density of the amorphous phase decreased significantly with an increase in the hemin concentration.
3.5. Effect of Ozonation on the Dynamics of Radical Rotation in PHB/Hemin and PHB/FeClTPP Composition Fibers
When biomedical materials are used, along with mechanical and thermal effects, their structure and segmental mobility are affected by ozone. Two sources of its appearance in the atmosphere should be indicated here. Firstly, ozone is formed during the operation of powerful electrical devices that ensure the vital activity of patients both during surgical operations and during therapy or monitoring in a hospital. Secondly, ozone in some special cases continues to be used in the sterilization of medical devices. Despite the daily contact of this aggressive compound with polymers, its effect on their morphological and dynamic characteristics remains a little-studied area of polymer materials science.
In the process of ozonation, two opposite processes take place that affect the molecular dynamics in the polymer: they are the breakage of macromolecules, which contributes to an increase in chain mobility and the formation of oxygen-containing groups on the side chains of macromolecules, which contributes to an increase in the rigidity of macromolecules and, as a result, a slowdown in molecular dynamics. The loops of macromolecules on the end surfaces of the crystallites are oxidized at the highest rate, since the highest stresses and, consequently, the highest oxidation rate are concentrated in these areas [
12,
35]. The rupture of these loops is accompanied by the straightening of regions of macromolecules, which slows down the molecular mobility in these regions. The formation of oxygen-containing groups in the side chains of macromolecules leads to an increase in intermolecular interaction and, as a result, the molecular mobility of the chains also slows down, whereas the rigidity of the chains increases and the processes of chain reorientation take place, which also leads to a slowdown in their mobility. In parallel, the processes of chain destruction proceed, which, as a result, can cause both the reorientation of chains and their folding into coils, depending on the initial degree of straightening of macromolecules. The tendency of a macromolecule to unfold and reorient itself or to coil into a ball is determined by the conformational criterion
(where h is the average distance between the ends of the chains, L is the contour length of the chain, k is the number of segments). Chains in which
k >
k* tend to reorientate, macromolecules in which
k <
k* tend to take the coil conformation.
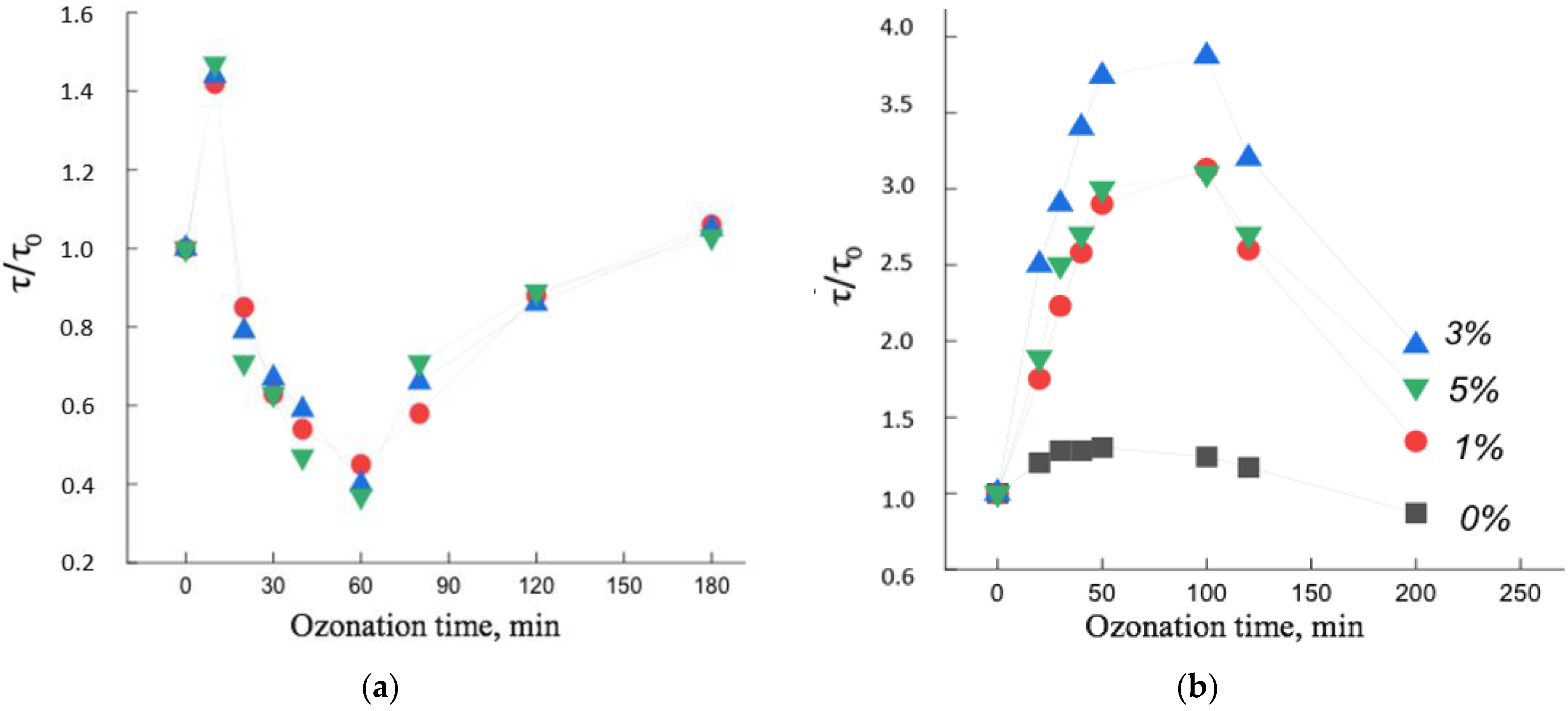
Figure 8 shows the dependences of the rotational correlation time of the spin probe on the time of ozonation of the PHB/hemin and PHB/Fe(TPP)Cl systems. It can be seen that, firstly, the dependences were extreme, and, secondly, the change in the mobility of radicals with increasing ozonation time largely depended on the concentration of the additive and on the time of ozone-oxygen exposure of the polymer.
The extreme nature of the dependencies was due to the following factors. In PHB/hemin fibers (up to 30 min) and in PHB/Fe(TPP)Cl (up to 100 min), processes leading to a slowdown in molecular dynamics predominated. Moreover, if such changes in the PHB/hemin fiber were not so significant, then in the PHB/Fe(TPP)Cl system this effect was much greater, which indicated the predominance of processes leading to an increase in the rigidity of the polymer matrix. Let us consider the reason for the sharp difference in the patterns of change in molecular dynamics from the time of ozonation. The addition of Fe(TPP)Cl, as noted earlier, caused a significant increase in the degree of crystallinity and linear systems (these particles are the nuclei of crystallization), as a result, the proportion of straightened chains in the fiber greatly increased and, with an increase in the proportion of the composite, the χ and ΔH. The accumulation of oxygen-containing groups on such chains was accompanied by a significant increase in their rigidity and an increase in intermolecular interaction, as a result of which the coefficient k* increased and some of the macromolecules straightened, as a result, the molecular dynamics slowed down. Rupture of sufficiently straightened chains, which at the same time reorient themselves and their mobility also decreased. Another aspect leading to an increase in τ was the breaking and straightening of the chains on the end surfaces of the crystallites (due to the highest stresses on the chain folds). All these components caused a sharp increase in τ at the initial stage of ozonation of PHB/Fe(TPP)Cl fibers. With a longer exposure to ozone, destruction processes began to predominate with an increase in molecular mobility. With an increase in the concentration of the additive, the proportion of straightened chains, the proportion of crystallites increased, which caused an increasingly stronger growth effect τ with a change in the concentration of the additive (
Figure 9).
In contrast to the Fe(TPP)Cl additive, hemin loosened the polymer structure to an increasing extent with increasing concentration. Against the background of a loose structure, the growth of intermolecular interaction during the accumulation of oxygen-containing groups did not have such a significant effect, and the fraction of end surfaces of crystallites also decreased with an increase in the fraction of hemin. As a result, a weak growth of τ is observed at the initial stage of ozonation. At deeper oxidation states, the molecular dynamics began to increase, and only after ozonation for more than 60 min did the chain mobility begin to decrease, which was apparently caused by the formation of a high concentration of oxygen-containing groups.
In general, the effect of ozone on the structural and dynamic characteristics of PHB doped with porphyrin metal complexes largely depended on the nature of the additive [
15,
36,
38,
40,
41].
Thus, when comparing the results of ozonation of two polymers PHB/hemin and PHB/Fe(TPP)Cl, it follows that the structure that was formed upon addition of the complex determined to a large extent the ability of the system to change the dynamic parameters from the time of ozonation.