Abstract
Consumers are now more concerned about food safety and hygiene following the COVID-19 pandemic. Antimicrobial packaging has attracted increased interest by reducing contamination of food surfaces to deliver quality and safe food while maintaining shelf life. Active packaging materials to reduce contamination or inhibit viral activity in packaged foods and on packaging surfaces are mostly prepared using solvent casting, but very few materials demonstrate antiviral activity on foods of animal origin, which are important in the human diet. Incorporation of silver nanoparticles, essential oils and natural plant extracts as antimicrobial agents in/on polymeric matrices provides improved antifungal, antibacterial and antiviral properties. This paper reviews recent developments in antifungal, antibacterial and antiviral packaging incorporating natural or synthetic compounds using preparation methods including extrusion, solvent casting and surface modification treatment for surface coating and their applications in several foods (i.e., bakery products, fruits and vegetables, meat and meat products, fish and seafood and milk and dairy foods). Findings showed that antimicrobial material as films, coated films, coating and pouches exhibited efficient antimicrobial activity in vitro but lower activity in real food systems. Antimicrobial activity depends on (i) polar or non-polar food components, (ii) interactions between antimicrobial compounds and the polymer materials and (iii) interactions between environmental conditions and active films (i.e., relative humidity, oxygen and water vapor permeability and temperature) that impact the migration or diffusion of active compounds in foods. Knowledge gained from the plethora of existing studies on antimicrobial polymers can be effectively utilized to develop multifunctional antimicrobial materials that can protect food products and packaging surfaces from SARS-CoV-2 contamination.
1. Introduction
According to the latest global estimates from the World Health Organization (WHO), contaminated food results in 600 million cases of foodborne diseases and 420,000 deaths worldwide every year [,,]. Foods are highly susceptible to spoilage, cross-contamination or re-contamination by microorganisms (e.g., bacteria, yeast, molds and viruses) throughout the food chain during food processing, food retail (supermarkets, convenience stores or restaurants) or home storage. Several factors affect quality changes in foods including humidity, oxygen, food chemical components and matrix structures [,]. Some microbial strains produce toxins that are harmful to consumers. Spoilage from yeast and mold leads to major economic losses in the food industry and is also detrimental to consumer health. Pathogenic bacteria responsible for food spoilage, poisoning and toxicity such as Campylobacter spp., Clostridium perfringens, Escherichia coli, Clostridium botulinum, Salmonella spp. and Listeria monocytogenes are major hazards to consumer safety []. Enteric foodborne viruses such as human norovirus (NoV) and hepatitis A virus (HAV) are of great concern for food safety because they cause gastroenteritis and foodborne illnesses in humans [,]. Raw or uncooked foods, fresh produce and ready-to-eat (RTE) foods can easily become cross-contaminated or re-contaminated with foodborne pathogens through contact with food handlers and contact surfaces during processing or packaging. The COVID-19 pandemic is still causing major public health problems in both developed and developing countries. Consumers are increasingly concerned about the possible transmission of SARS-CoV-2 via food and packaging surfaces and its potential effect on food safety. Consumers are also concerned about the safety of synthetic preservatives and their potential health risks. Various technologies have been employed to inactivate or reduce the number of bacteria and viruses including the use of sanitizers or disinfectants, traditional thermal processing technologies and nonthermal processing technologies; however, complete foodborne bacterial removal and viral inactivation of food products are difficult.
Functional packaging material with antifungal, antibacterial or antiviral properties is one alternative to preservative addition in bulk foods. Figure 1 presents different incorporation techniques used for developing antimicrobial activity and their applications on food products. Antifungal and antibacterial packaging developed by embedding or coating antibacterial agents in or on packaging materials extends the lag phase and reduces or inhibits the maximum cell count in the stationary phase of yeast, mold and bacterial growth [,,]. Recent research has investigated the antimicrobial efficacy of metal nanoparticles, organic acids and their salts, essential oils, natural extracts, enzymes and bacteriocins incorporated into polymer matrices via different methods including solvent casting, coating solution, surface modification, blown extrusion and cast extrusion. Polymer materials serve as vehicles for loading antimicrobial agents in food packaging such as films, coated films, edible films or coating, pouches and sachets. According to a report by ‘Credence Research’, the market for biodegradable food packaging is expected to reach USD 7058.8 million by the end of 2023, with a compound annual growth rate (CAGR) of 11% from years 2016 to 2023 []. Antiviral packaging is designed to improve food safety by either preventing cross-contamination on food surfaces or inactivating target-specific foodborne viruses. Antifungal and antibacterial packaging materials have been intensively studied, but scant research has addressed food-grade polymers and biopolymers with antiviral activity for food applications.
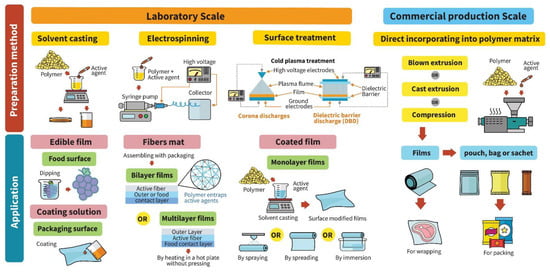
Figure 1.
Different incorporation techniques used for developing antimicrobial packaging and their applications on food products.
This review of antimicrobial food packaging includes literature covering the previous five years with focus on (i) recent preparation methods of packaging material and recent progress in packaging applications including compression molding, blown extrusion, cast extrusion and solvent casting with either direct addition of antimicrobial agents into the polymer matrix or coating onto the material surface to produce wrapping films, pouches and coating, (ii) antimicrobial efficiency in vitro and in vivo and the challenge test for evaluating the efficiency of antimicrobial material to inhibit the growth of certain microorganisms inoculated intentionally on food products for a certain period and storage condition, (iii) antimicrobial mechanisms against specific microbial food spoilage and foodborne pathogens including yeast and mold, bacteria and viruses by either direct or indirect contact assays that may provide alternative strategies to improve antimicrobial packaging material and (iv) antiviral activity of biobased materials functionalized with natural plant extracts and silver nanoparticles in common viruses such as murine norovirus (MNV) and hepatitis A virus (HAV), with specific focus on surfaces or packaging materials for food products. Future challenges, opportunities and perspectives of using existing knowledge on antimicrobial packaging are discussed to develop multifunctional antimicrobial materials to preserve food safety and quality from spoilage by pathogenic microorganisms and protect food products and packaging surfaces from SARS-CoV-2 contamination.
2. Antifungal Food Packaging
2.1. Fungal Spoilage in Food Products
Yeast spoilage occurs on or in foods rich in fermentable carbohydrates, high sugar or salt content, low water activity (aw), low pH and presence or absence of oxygen [,]. Yeasts are found in and cause spoilage of dairy products (cheese and fermented milk), alcoholic beverages (wine and beer), non-alcoholic beverages (soft drinks and fruit juices), fruits, dried and salted meats and fish [,]. Spoilage yeasts commonly found growing in food and beverages include species of Candida, Debaryomyces, Saccharomyces and Zygosaccharomyces [,].
Mold spoilage is favored in food products with high aw, low pH and exposure to headspace oxygen when stored at room temperature or below [,]. A wide range of food products including cereals, bakery items, fresh pasta, dairy (cheese and yogurt), fresh fruits and vegetables and dried or salted meat and fish are susceptible to fungal spoilage [,]. The main mold species that often cause food spoilage include Aspergillus spp., Fusarium spp., Penicillium spp. and Rhizopus spp. []. Molds negatively impact the economy of the food industry and are also a health risk due to mycotoxin production by fungal pathogens.
2.2. Applications of Active Packaging to Control Fungal Spoilage
Active packaging with anti-yeast or anti-mold activities is used as an alternative strategy to inhibit or retard surface fungal growth as well as maintain food quality (e.g., coating, film and sachet) [,]. Antifungal packaging is a feasible option to reduce fungal growth and mycotoxin production. Incorporation of antifungal compounds into the packaging can kill or extend the lag phase or decrease mycelial growth and spore germination during the stationary phase of mold growth, as well as inhibit and/or kill cells in the stationary phase of yeast growth [,]. Spoilage inhibition can be performed either by direct contact between packaging material and foods or by indirect contact, whereby the antimicrobial packaging releases a volatile agent into the headspace of the packaging []. Recent studies have examined the inhibition of yeast and mold spoilage by direct contact, which is a simple modification of the disk diffusion assay (in vitro test) and can be related to direct contact of the films on food surfaces in the in vivo test.
2.2.1. Effect on Yeast
Yeast species from the genera Candida and Rhodotorula commonly cause spoilage as foamy, bottle swelling and yeasty off-flavors in yogurt and dairy products [,]. Natamycin is mostly incorporated as a polymer on the contact surface of dairy products. Due to its low solubility, it inhibits yeast growth by binding specifically to ergosterol in the yeast cells without permeabilizing the yeast plasma membrane [,,]. Natamycin immobilized on the surface of LDPE film treated for 6 min with UV irradiation demonstrated maximum grafting efficiency (81.96%) and anti-yeast activity against Rhodotorula mucilaginosa and Candida parapsilosis in the disk diffusion test with diameters of 10.62 and 8.06 mm, respectively, and also decreased yeast population in contaminated Iranian yogurt drink (Doogh) from day 0 to day 23 by 52.33% compared to the control sample (increased by 37.86%) []. Candida albicans is the most common human fungal pathogen, with causes ranging from mucous membranes to systemic infections []. Candida albicans showed inhibition in the agar spot diffusion test in the presence of carboxymethyl cellulose coating solution containing 0.05 and 0.5% w/v natamycin, but inhibition was weaker than Aspergillus flavus []. Candida is more resistant to natamycin than mold spores. By contrast, Brzezinska et al. (2019) [] observed that PLA, PCL or PHB films incorporated with 1.0% polyhexamethyleneguanidine (PHMG) derivatives had a stronger biocidal effect on cells of Candida albicans than Aspergillus niger and Penicillium chrysogenum due to the inhibited activity of Candida albicans hydrolases (40 to 52% depending on the type of films). Therefore, natamycin or PHMG derivatives introduced into the polymer can be used as a food contact surface material to reduce the growth of spoilage yeasts in dairy products (e.g., fermented milk and cheese). However, the more resistance or sensitivity of yeast cells to each active material compared with mold spores is unclear.
2.2.2. Effect on Rhizopus, Penicillium and Aspergillus Species
Aspergillus, Rhizopus and Penicillium species are most frequently used as models to study food spoilage fungi by direct contact. Table 1 presents a review of current antifungal packaging based on synthetic and natural antifungal agents incorporated with non-biodegradable and biodegradable materials and their applications in food products.
Fasihnia et al. (2018) [] found PP films incorporated with 2 to 6% (w/w) sorbic acid were effective against Aspergillus niger with inhibition zones of 3.20 and 6.70 cm2 after 96 h of incubation, respectively. Da Rocha et al. (2018) [] observed that Argentine anchovy protein films incorporated with 1.50% (w/v) of sorbic acid or benzoic acid had no effect on Rhizopus oryzae after 48 h of incubation. The antimicrobial action of sorbic acid is not well understood but is considered to be based on cytoplasmic acidification and inhibition of the basic metabolic response of the cells []. In theory, the antimicrobial effect increases if lipophilic compounds enter the cell more easily []. Wangprasertkul et al. (2021) [] found that PBAT/TPS blend films containing sodium benzoate and/or potassium sorbate gave different antimicrobial performances between in vitro and fresh rice noodles. The presence of oil on the noodle surface accelerated diffusion and enhanced the release of sodium benzoate and potassium sorbate compared to aqueous matrices of agar media and reduced oxygen permeability of the films, resulting in reduced mold growth. Similarly, Küçük et al. (2020) [] observed that maximum inhibition zone diameters were obtained on zein films with 4000 ppm natamycin at 2.20 and 3.37 cm, while alginate films with 4000 ppm natamycin gave 4.40 and 4.97 cm for Aspergillus niger and Penicillium camamberti, respectively, but alginate films with natamycin were ineffective against Penicillium camamberti in kashar cheese during storage under refrigerated temperature for 45 days compared to in vitro agar medium. Natamycin has low solubility in water with low food penetration but remains on the food surface. Thus, antifungal activity depends on release rate or concentration and composition of food products as well as permeability of the films. Interaction between the polymer and active compounds, as well as miscibility in the polymeric film matrix structure, also reduces water sensitivity, hydrophilicity and solubility that relate to the release of active compounds [,,].
Biological controls using antagonistic yeasts such as Williopsis saturnus var. saturnus can inhibit spore germination of filamentous fungi, damage the cell membrane and alter protein expression. In whey protein concentrate edible film containing 7 and 9 log CFU/cm2, W. saturnus gave more efficient inhibition of Penicillium expansum and Aspergillus niger by 29% and 19%, respectively, after incubation for 5 days on nutritious medium acidified to pH 4.5 and 5.2.
Quantum fillers have recently been introduced as new functional fillers for food packaging biopolymer films. They provide excellent antifungal activity by partially damaging the cell wall structure, penetration and degradation of cellular components []. Alginate films with 3 wt% sulfur quantum dot films exhibited strong fungicidal activity against Aspergillus niger and Penicillium chrysogenum strains by reducing the number and density of conidia and hyphae, with inhibition regions of 14 mm and 18 mm, respectively, after incubation for 3 days, and showed promise by inhibiting mold growth of bread wrapped for 14 days [].
Volatile active substances (such as herbs, spices, essential oils and their constituents) can be applied either in the direct contact phase or in the vapor phase. A direct contact assay was used to examine the antifungal effectiveness against fungal spoilage on single-packed bakery products (such as buns, white bread or butter cakes) using cellulose acetate films containing 1.5%, 2.5% and 3.5% oregano essential oil. Hamburger buns showed delayed growth of filamentous fungi until the 29th day []. PLA/PBSA blend films containing 3 and 6 wt% thymol were able to delay the visible growth of yeast and mold in packed bread by 7 and 9 days, respectively []. P(3HB-co-4HB) film with 30% thyme oil extended shelf life of bread to more than 5 days []. PBAT/PLA films containing 2 and 5% carvacrol prevented mold growth and extended shelf life of bread and butter cake by 2–4 days []. PLA or PBAT blend films containing trans-cinnamaldehyde (2%, 5% and 10%) effectively inhibited the microbial growth of bacteria and fungi for more than 21 days [], while PE film coated with zein containing 0.5% garlic extract and bread aroma maintained bread free of mold infection for 30 days []. The extensive concentration of essential oil flavor and scent in packaging materials had a negative effect on the food acceptability in terms of taste and odor.
By contrast, vapor contact offers an alternative way to reduce the development of undesirable flavors. During storage, essential oils and their constituents are released as vapor from the film matrix into the packaging headspace, and this inhibits or delays fungal growth and sporulation. In an in vivo test, a sachet containing a combination of eugenol and citral essential oils in microcapsules showed stronger inhibitory effects on Penicillium roqueforti than Aspergillus niger in vitro and effectively inhibited colony spots on sliced bread without affecting bread smell or taste (in vapor phase) []. Antifungal activity of films has been investigated in vitro by disk diffusion and vapor phase methods. Growth inhibition of Aspergillus spp. (61.7–80.45% reduction) and Penicillium spp. (55.9–74.1% reduction) in the vapor phase was observed for PLA/PBSA blend film containing 6 wt% thymol, while PLA/PBSA blend film containing 3 wt% thymol showed lower growth reduction of the fungi (15.15–23.16% and 11.49–26.21%, respectively) []. Srisa and Harnkarnsujarit (2020) [] confirmed that essential oils were more effective antifungals on culture media than vapor phases for PLA or PBAT blend films containing trans-cinnamaldehyde. Klinmalai et al. (2021) [] stated that minimal affinity between agar media and film components limited direct diffusion of essential oils into agar media, while the main antifungal mechanism occurred via release of volatile compounds into the headspace, followed by absorption into media matrices.
2.2.3. Effect on Phytopathogenic Fungus
Colletotrichum gloeosporioides and Botrytis cinerea are fungi responsible for the plant disease anthracnose that causes harvest losses. LDPE films with 3.5% w/w lauric acid affected growth of Colletotrichum tamarilloi by decreasing mycelial size and also impacted the cottony characteristic of the fungus in vitro. The presence of disease symptoms, as growth of mycelia in the peduncle or skin breakage in tree tomato, was not observed for about two weeks for the yellow variety and one week for the red variety stored at 27 °C []. Biodegradable films containing carbon quantum dots [] and coffee parchment waste extracts [] exhibited high antifungal activity against Colletotrichum tamarilloi in the in vitro assay. In gaseous contact, PLA films incorporated with 15% R-(−)-carvone and 20% thymol were the most effective at 12 °C in suppressing mycelial growth of avocado and citrus Colletotrichum gloeosporioides isolates, respectively, whereas film incorporated with 20% thymol had the highest antifungal activity against both anthracnose isolates at 25 °C []. In another case, quinoa protein/chitosan coating with 10% dilution of thymol nano-emulsion showed a significant decrease in yeast and mold of 4.7 log CFU/cm2 in artificially inoculated Botrytis cinerea cherry tomatoes after 7 days at 5 °C [], and polyvinyl alcohol/corn starch blends decreased the disease incidence level by up to 27% and by around 40% of infected fruit for Botrytis cinerea and Penicillium expansum for 12 days, respectively. Damage severity was reduced by around 30% and by 33% with respect to the control samples when using carvacrol-loaded coatings for Botrytis cinerea and Penicillium expansum, respectively. Severe damage to the fungal membranes and cell walls led to morphological deformations, collapse and deterioration of the conidia and/or hyphae [].
Trichoderma sp. is a mushroom pathogen causing green mold on the commercially cultivated white button mushroom Agaricus bisporus. Trichoderma sp. isolated from postharvest white mushrooms can be inhibited by sodium alginate films with β-cyclodextrin microencapsulated carvacrol [] and corn starch/polyvinyl alcohol blend films with carvacrol nano-emulsions []. The authors indicated that antifungal activity of the polysaccharide-based films against Trichoderma sp. might be due to inhibition of spore germination and a chemical effect or physical destruction during the action of carvacrol release when the films were directly in contact with agar media.
2.2.4. Effect on Aflatoxigenic Fungi
The most common fungal species associated with aflatoxin production of cereals are Aspergillus flavus and Aspergillus parasiticus. Aspergillus flavus has been investigated by contact methods and was inhibited by film incorporating sorbic acid and benzoic acid [], natamycin [] and carbon quantum dots []. The cationic peptide ε-poly-l-lysine can destroy the integrity of the plasma membrane and/or the cell walls of the fungal spores, leading to reduction of spore germination and damage to the mycelia []. Starch films incorporated with ε-poly-l-lysine at concentrations ranging from 1.6 to 6.5 mg/cm2 were more efficient against Aspergillus parasiticus and Penicillium expansum after incubation for 7 days and extended the shelf life of bread inoculated with Aspergillus parasiticus or Penicillium expansum by 1 and 3 days, respectively []. Interesting, the impact of the interactions between environmental conditions and these active films on reduction of aflatoxigenic fungal growth and aflatoxin production was closely dependent on relative humidity and environmental temperature. The essential oil slowly released in the vapor phase. Mateo et al. (2017) [] proved that EVOH films with at least 0.25% (on wet base) of cinnamaldehyde effectively controlled growth of aerobic aflatoxigenic species Aspergillus flavus and Aspergillus parasiticus and aflatoxin production in maize grains (in vapor phase). The growth rate of both species was higher at 0.99 than at 0.96 aw and at 37 °C than at 25 °C. Higher temperature increased molecular mobility in the film matrix, which increased the diffusion of volatile molecules [,]. Li et al. (2019) [] found that chitosan films with 1.5 and 3.0 μL/cm2 of turmeric essential oil exhibited stronger inhibitory effect on conidial formation and inhibited the growth of Aspergillus flavus at 25 °C, with inactivation of the formation of green fresh spores. Production of aflatoxin was completely inhibited by steam exposure for 7 days (in vapor phase). Antifungal coating with essential oils improved the safety of nuts. Chitosan-based coating with 4% cinnamon essential oil was most effective in restricting Aspergillus flavus and Penicillium citrinum in artificially inoculated peanut kernels to 9.8% and 13.4%, respectively, after 14 days of storage at 25 °C [], while coating solution loaded with thymol significantly inhibited the growth of mold and yeast in chestnuts stored at 0 °C (4.17 log CFU/g on day 180) and maintained the lowest decay rate (5.33%) [].

Table 1.
Investigations on the development of active antifungal packaging during the last five years.
Table 1.
Investigations on the development of active antifungal packaging during the last five years.
| Classification | Antifungal Agents | Polymer Materials | Methods of Preparation | Types of Packaging | Packaged Foods/In Vitro Antimicrobial Test | Observations | References |
|---|---|---|---|---|---|---|---|
| Organic acids and acid salts | Sorbic acid | PP | Extrusion molding | Film |
|
| [] |
| Sorbic acid or benzoic acid | Argentine anchovy protein | Solvent casting | Film |
|
| [] | |
| Sodium benzoate and/or potassium sorbate | PBAT/TPS blends | Blown extrusion | Film |
|
| [] | |
| Essential oils and their constituent | Oregano, carvacrol, cinnamon bark or cinnamaldehyde | EVOH | Solvent casting | Film |
|
| [] |
| Oregano essential oil | Cellulose acetate | Solvent casting | Film |
|
| [] | |
| Eugenol and citral essential oils | Corn porous starch | Osmosis and diffusion | Microcapsules |
|
| [] | |
| Turmeric essential oil | Chitosan | Solvent casting | Film |
|
| [] | |
| Savory or oregano essential oil | Chia mucilage | Solvent casting | Edible film |
|
| [] | |
| Thymol or R-(−)-carvone | PLA | Cast extrusion | Film |
|
| [] | |
| Thymol nano-emulsions | Quinoa protein/chitosan | Solvent casting | Edible film and coating |
|
| [] | |
| Thyme, cinnamon or lemongrass essential oil | Chitosan | Solvent casting | Film and coating |
|
| [] | |
| Thymol | PLA/PBSA blends | Blown extrusion | Film |
|
| [] | |
| Thymol | Chitosan nanoparticles | Coating solution | Coating |
|
| [] | |
| Thyme essential oil | P(3HB-co-4HB) | Solvent casting | Film |
|
| [] | |
| Carvacrol with and without microencapsulated by β-cyclodextrin | Sodium alginate | Solvent casting | Film |
|
| [] | |
| Carvacrol | Polyvinyl alcohol/corn starch blends | Solvent casting | Film and coating |
|
| [] | |
| Carvacrol nano-emulsions | Corn starch/polyvinyl alcohol blends | Solvent casting | Film |
|
| [] | |
| Carvacrol | PLA/PBAT blends | Blown extrusion | Film |
|
| [] | |
| Cinnamaldehyde, eugenol or thymol nano-emulsions | Pullulan | Solvent casting | Film |
|
| [] | |
| Cinnamaldehyde | Pullulan | Solvent casting | Film |
|
| [] | |
| Trans-cinnamaldehyde | PLA/PBAT blends | Cast extrusion | Film |
|
| [] | |
| Natural extracts | Garlic extract and bread aroma (containing 2-acetyl-1-pyrroline) blends | PE, EVOH or zein | Film coating | Coated PE film |
|
| [] |
| Coffee parchment waste extracts | Gellan gum | Solvent casting | Film |
|
| [] | |
| Bacteriocins | Natamycin | PE | Surface modification by spraying | Coated PE film |
|
| [] |
| Natamycin | LDPE | Graft polymerization | Film |
|
| [] | |
| Natamycin | Zein or alginate | Solvent casting | Film |
|
| [] | |
| Natamycin | Carboxymethyl cellulose | Coating solution | Coating |
|
| [] | |
| Cationic peptide | ɛ-Poly-l-lysine | Corn starch | Solvent casting | Film |
|
| [] |
| Cationic polymer | Polyhexamethyleneguanidine (PHMG) derivatives with organic anions: sulfanilic acid salt, stearate and granular polyethylene wax | PLA, PHB or PCL | Extrusion | Flat film |
|
| [] |
| Antagonistic yeasts | Williopsis saturnus var. saturnus | Whey protein concentrate | Solvent casting | Edible film |
|
| [] |
| Quantum fillers | Carbon quantum dots | Chitosan/gelatin blends | Solvent casting | Film and coating |
|
| [] |
| Sulfur quantum dots, sulfur nanoparticles or elemental sulfur | Alginate | Solvent casting | Film |
|
| [] | |
| Fatty acid | Lauric acid | LDPE | Extrusion | Film |
|
| [] |
| Metals | ZnO nanoparticles and chitin nanoparticles blends | Bovine gelatin, gelatin nanocomposite, gelatin emulsion, two layers of gelatin nanocomposite and gelatin emulsion or polyethylene (PE) | Solvent casting | Film |
|
| [] |
| Blends | Potassium sorbate or grapefruit seed extract | Corn starch/chitosan/nano clay blends | Solvent casting | Film |
|
| [] |
3. Antibacterial Food Packaging
3.1. Spoilage and Pathogenic Bacteria in Food Products
Bacteria can utilize food nutrients, obtain energy and grow under different acidity levels (pH), aw, temperature and the presence or absence of oxygen. Based on Gram-stain, chemical and physical properties of their cell wall structure, bacteria are classified into two broad categories as Gram-positive and Gram-negative. Bacteria are also classified according to their temperature preferences into rough categories: psychrophiles (growth range of −5 to 20 °C), psychrotrophic or psychrotolerant (growth range of −5 to 35 °C), mesophiles (growth range of 20 to 45 °C) and thermophiles (growth range of 45 to 70 °C) [,,]. Many different types of bacteria can contaminate and grow in several kinds of foods, causing deterioration, spoilage and food poisoning pathogens. Bacteria of concern for food safety and food quality can be classified into two categories: food spoilage bacteria and food pathogens [,]. Modified atmosphere packaging (MAP) and vacuum packaging are the most popular food preservation and packaging techniques that modify ambient gas atmosphere or reduce O2 concentration to control microbial growth. This extends the product shelf life compared with traditional heat sealing (atmosphere condition) [,]. MAP and vacuum packaging accompanied by chilled storage are popular preservation methods to store fish and meat in markets [,]. However, the bacterial community is very diverse and some strains can grow, albeit more slowly, under oxygen-limiting conditions [].
3.2. Applications of Active Packaging to Control Spoilage and Pathogenic Bacteria
Antibacterial packaging technologies are designed to kill, inhibit or retard the growth of spoilage and pathogenic bacteria that may be contaminated on the surface or within food products. Antibacterial packaging can be achieved by embedding or coating antibacterial agents in or on packaging materials to enhance food safety and quality, extend shelf life and decrease the risk of foodborne pathogens. An overview of the latest antibacterial packaging materials used in food products against both Gram-positive and Gram-negative bacteria is presented in Table 2.
3.2.1. Psychrotrophic Bacteria
Listeria monocytogenes is a psychrotrophic Gram-positive pathogen with a growth range of 0 to 45 °C causing listeriosis infection [], while Staphylococcus aureus is mostly used in tests of antimicrobial packaging. Bacteriocins are mostly incorporated in polymers to control listeria and staphylococcus growth, as they are more active against Gram-positive than Gram-negative bacteria. Aymerich et al. (2022) [] reported that antimicrobial film based on polyvinyl alcohol with enterocin A exerted a strong antilisterial activity in vitro and in dry-cured ham stored at 8 °C, while Woraprayote et al. (2018) [] reported that Bacteriocin 7293 diffusion coated into PLA/sawdust particle blend film was more active against Gram-positive bacteria (Listeria monocytogenes and Staphylococcus aureus) than Gram-negative bacteria (e.g., Aeromonas hydrophila B1, Escherichia coli, Pseudomonas aeruginosa and Salmonella Typhimurium) in vitro and in raw pangasius fish fillet stored at 4 °C. Similar results were found in PLA film containing sophorolipid []. PLA film activated with lysozyme by cold plasma showed a strong antimicrobial effect against Listeria monocytogenes both in vitro and in rice-milk-based smoothie stored at 10 °C [], while PLA film containing ferulic or cinnamic acids did not show a significant antimicrobial action against Listeria monocytogenes due to their limited release into the aqueous culture media in vitro test. This indicated that the release of active compounds from the PLA matrix requires its plasticization, which does not occur when in contact with the culture media [], and also the hydrophobic structure and low water affinity of the matrices may limit water absorption [,] that inhibits the effective release of active compounds.
3.2.2. Mesophilic Bacteria
Escherichia coli is a Gram-negative and mesophilic bacterium with wide-ranging growth at 7 to 50 °C. Escherichia coli has pathogenic strains causing diverse intestinal and extraintestinal infections in humans and animals, while nonpathogenic strains are commonly found in the intestinal flora of most mammals []. Staphylococcus aureus is a Gram-positive, facultative anaerobic typical mesophile with wide-ranging growth at 7 to 48 °C and a causative agent of staphylococcal food poisoning, nausea and vomiting []. Recently, many researchers have used Staphylococcus aureus and Escherichia coli as models for Gram-positive and Gram-negative bacteria in antimicrobial experiments. Chitosan film incorporated with lemongrass essential oil [], chitosan/pullulan blend film containing carvacrol [] and PE films containing thymol or linalool [] was more efficient against Gram-positive Staphylococcus aureus than Gram-negative Escherichia coli or Escherichia coli O157:H7 during in vitro testing. This occurred because Gram-positive bacteria do not have an outer membrane and are surrounded by a thick peptidoglycan wall that is not dense enough to resist small antimicrobial molecules, allowing easy penetration to the cell membrane. Jo et al. (2018) [] reported that LDPE films incorporated with silver nanoparticles were more effective on Staphylococcus aureus than Escherichia coli due to the presence of a thick peptidoglycan layer in Gram-positive bacteria that attracts Ag+ ions, thus resulting in the death of the bacteria, while PP films incorporated with silver nanoparticles were equally effective on both strains. By contrast, Kim et al. (2020) [] found that sulfur nanoparticles capped with chitosan exhibited stronger antimicrobial activity against Escherichia coli (Gram-negative) than Staphylococcus aureus (Gram-positive). Cinnamaldehyde-loaded corn starch/PBAT/PLA blend film most effectively maintained the quality of soy-protein-based meat analogs by inhibiting the growth of Escherichia coli and Staphylococcus aureus during storage at 4 °C []. Similarly, antimicrobial peptide ε-polylysine incorporated into levan/pullulan/chitosan edible films [] and starch/PBAT blend film [] gave a broad spectrum of antibacterial activity against both Gram-positive and Gram-negative pathogens due to the electrostatic attraction between ε-polylysine and the cell wall structure of bacteria, leading to leakage of intracellular components. This facilitated ε-polylysine to enter the cytoplasm, leading to abnormality in gene expressions, disturbance of oxidative stress and even cell death. Gao et al. (2022) [] found that starch/PBAT blend film incorporated with nisin showed lower antimicrobial activity against Escherichia coli because the outer membrane of Gram-negative bacteria was composed of lipopolysaccharides and glycerol phospholipids that created an efficient barrier and prevented nisin from reaching the cytoplasmic membrane.
Salmonella is a Gram-negative mesophilic bacterium with growth in a wide range from 2 to 48 °C that causes salmonellosis in humans as well as typhoid fever, gastroenteritis and septicemia []. Polyvinyl alcohol/montmorillonite K10 clay nanocomposite blend films with in situ generated ginger-extract-mediated silver nanoparticles showed stronger antimicrobial activity against Gram-negative Salmonella Typhimurium than Gram-positive Staphylococcus aureus in vitro []. TPS/PBAT blend films coated with lauric arginate alone or combined with nisin Z dramatically decreased and eliminated Salmonella Typhimurium populations on inoculated raw tiger prawn slices compared to raw bigeye snapper slices at refrigerated or frozen storage []. Some experiments in this review on antimicrobial packaging showed lower effects on Salmonella Typhimurium compared to other Gram-negative or Gram-positive strains [,,].
Similar to pathogens, experiments on antimicrobial active packaging against spoilage bacteria were designed as in vitro or within challenge tests. Some researchers have suggested that plant extracts embedded in certain materials slowed down the release of compounds that affect flavor and reduce the detrimental impact of these plant extracts on food flavor or taste [,]. Gutiérrez-García et al. (2022) [] demonstrated that LDPE film with Yucca baccata butanolic extract decreased the growth of aerobic mesophile bacteria being able to extend the shelf life of ground beef, exposed to air, from 3 to 8 days at 4 °C. Guo et al. (2021) [] demonstrated that starch film packaging containing sea buckthorn pomace extract prolonged the shelf life of beef from 25 to 35 days during super-chilled storage. The combined use of antimicrobial packaging with either modified atmosphere packaging (MAP) or vacuum packaging can enhance the effectiveness of antimicrobials as well as the shelf life of food products during cold storage (i.e., super-chilling, freezing and chilling). Some microbial groups were monitored to verify the general effectiveness of antimicrobial packaging under study, such as total viable count, total coliforms, psychrotrophic bacterial count, yeast and mold, enterobacteria and lactic acid bacteria.
Examples of antimicrobial packaging applied on foods include PBAT/TPS-blended ZnO nanocomposite films [], refrigerated fish fillet PBAT film incorporated with oregano essential oil [], refrigerated Pacific white shrimps packed in PLA blend film incorporated with carvacrol, citral or α-terpineol essential oils [], frozen prawns (Penaeus monodon) packed in cellulose nanoparticles/polyvinyl alcohol blends incorporated with fennel seed oil [] and slices of cooked ham packed in PP films incorporated with oregano essential oil or allium extract stored under vacuum bags at 5 °C [].
Yersinia enterocolitica and Brochothrix thermosphacta were able to grow at temperatures as low as 0 °C, as the major pathogens or spoilage organisms in frozen ground beef [], pork [] and fish and seafood []. Contamination of foods with Yersinia enterocolitica and Brochothrix thermosphacta, especially surfaces of produce items, and their ability to survive at low temperatures during storage are a concern for both food manufacturers and consumers [,]. Previous studies focused on effective antimicrobial activity against spoilage and pathogenic bacteria in vitro or within challenge tests; however, scant research has addressed psychrophilic and psychrotolerant bacteria that can survive and proliferate under super-chilling or frozen conditions.

Table 2.
Antibacterial packaging applications used in food products.
Table 2.
Antibacterial packaging applications used in food products.
| Classification | Antibacterial Agents | Polymer Materials | Methods of Preparation | Types of Packaging | Packaged Foods/In Vitro Antimicrobial Test | Observations | References |
|---|---|---|---|---|---|---|---|
| Essential oils and their constituent | Oregano essential oil | PBAT | Hot melt extrusion | Film |
|
| [] |
| Oregano essential oil or allium extract | PP | Extrusion | Film |
|
| [] | |
| Fennel seed oil | Cellulose nanoparticles/polyvinyl alcohol blends | Solvent casting | Film |
|
| [] | |
| Ginger essential oil emulsion and nano-emulsions | Fish sarcoplasmic protein/chitosan blends | Solvent casting | Film |
|
| [] | |
| Lemongrass essential oil | Chitosan | Solvent casting | Film |
|
| [] | |
| Thymol or linalool | PE | Molding | Film sheet |
|
| [] | |
| Carvacrol, citral or α-terpineol essential oils | PBAT/PLA blends | Blown extrusion | Film |
|
| [] | |
| Carvacrol | Chitosan/pullulan blends | Solvent casting | Film |
|
| [] | |
| Cinnamaldehyde or tea polyphenols | Corn starch/PBAT/PLA blends | Cast extrusion | Film |
|
| [] | |
| Natural extracts | Propolis ethanolic extract | Pullulan | Solvent casting | Film and coating |
|
| [] |
| Propolis ethanolic extract | PLA | Solvent casting | Film |
|
| [] | |
| Yucca baccata butanolic extract | LDPE | Blown extrusion | Film |
|
| [] | |
| Feijoa (Acca sellowiana (Berg) Burret) pulp or husk extract | Brazilian pine seeds starch/citric pectin blends | Solvent casting | Film and coating |
|
| [] | |
| Crude mulberry leaf extract, chlorogenic acid or deoxynojirimycin | Pectin | Solvent casting | Film and coating |
|
| [] | |
| Sea buckthorn pomace extract | Potato starch | Solvent casting | Film |
|
| [] | |
| Phenolic acids | Ferulic or cinnamic acids | PLA | Melt blending and compression molding | Film |
|
| [] |
| Bacteriocins and cationic peptide | Bacteriocin 7293 | PLA/sawdust particle blends | Blown extrusion and diffusion coating | Coated film |
|
| [] |
| Lauric arginate and/or Nisin Z | TPS/PBAT (film)gelatin or pullulan (coating solution) | Blown extrusion and coating solution | Film and coated film |
|
| [] | |
| Ethyl lauroyl arginate | Chitosan/polyvinyl alcohol blends | Solvent casting | Film |
|
| [] | |
| Enterocin A or ethyl lauroyl arginate | Polyvinyl alcohol | Solvent casting | Film |
|
| [] | |
| ε-polylysine | Levan/pullulan/chitosan blends | Solvent casting | Edible films and coating |
|
| [] | |
| ε-polylysine hydrochloride and/or nisin | Starch/PBAT blends | Blown extrusion | Film |
|
| [] | |
| Enzymes | Lysozyme | PLA | Cold plasma treatment | Coated film and pouch |
|
| [] |
| Metals | Silver (Ag) nanoparticles | LDPE | Corona air plasma treatment | Coated film and pouch |
|
| [] |
| Silver (Ag) nanoparticles | LDPE or PP | Extrusion | Film |
|
| [] | |
| Silver (Ag) nanoparticles | LDPE | Blown extrusion | Film |
|
| [] | |
| Zinc oxide (ZnO) nanoparticles | PBAT/TPS blends | Blown extrusion | Film |
|
| [] | |
| Glycolipid biosurfactant | Sophorolipid | PLA | Solvent casting | Film |
|
| [] |
| Blends | Silver (Ag) nanoparticles and/or ginger extract | Polyvinyl alcohol/montmorillonite K10 clay nanocomposite blends | Solvent casting | Film and pouch |
|
| [] |
5. Conclusions and Future Perspectives
Copious research has been conducted on antimicrobial food packaging applications developed from different pure/blends of biopolymers by extrusion or coated film (PBAT, PLA and starch) and solvent casting (chitosan, cellulose, pullulan, pectin, gellan gum and zein). Biobased antimicrobial packaging can reduce the use of chemical preservatives in food products and lower the consumption of plastic-materials-based packaging. Essential oils and their constituents and silver nanoparticles as antimicrobial agents are now gaining extensive attention in the active packaging field to improve the antimicrobial activities of materials against spoilage and pathogens, yeasts, molds, bacteria and viruses. Antifungal packaging efficiency inhibits yeast and mold spoilage by either direct contact or indirect contact. Very low temperatures can reduce antimicrobial agent migration rates from packaging materials, resulting in lower antimicrobial performance than in vitro and challenge test storage at higher temperatures. Antimicrobial material (i.e., films, coated films, coatings and pouches) exhibits highly efficient antimicrobial activity in in vitro assays but lower activity in real food systems that depend on (i) polar or non-polar food components, (ii) interactions between antimicrobial compounds and polymer materials and (iii) interactions between environmental conditions and active films (i.e., relative humidity, oxygen and water vapor permeability and temperature) that closely relate to the migration or diffusion of active compounds in foods. Combining antibacterial packaging with food preservation methods such as modified atmosphere packaging (MAP), vacuum packaging, super-chilling, freezing and chilling enhance the effectiveness of antimicrobials while increasing the shelf life of foods of animal origin. Recent antivirus packaging material for food applications based on biodegradable and natural plant extracts using solvent casting exhibited appropriate antiviral activity to reduce the foodborne viruses murine norovirus (MNV) and hepatitis A virus (HAV) in vitro, and the challenge test was used on coated berries at refrigerated and room temperatures. Further studies on packaging development with antiviral activity via commercial production methods using multifunctional antimicrobial materials, such as mono- or multilayered films consisting of an inner layer with antifungal or antibacterial activity to extend the shelf life of food products and an outer layer with antiviral activity to evaluate package effectiveness against novel coronavirus or related pathogens, are required. All knowledge gained from the multitude of existing studies on antimicrobial polymers can be effectively utilized to develop effective packaging to protect food products from SARS-CoV-2 contamination.
Author Contributions
Conceptualization, A.S. and N.H.; methodology, A.S. and N.H.; formal analysis, A.S. and N.H.; investigation, A.S. and N.H.; writing—original draft preparation, A.S. and N.H.; writing—review and editing, A.S., K.P., H.S., Y.L., P.W., K.W., J.S., T.K., K.T. and N.H.; supervision, N.H.; funding acquisition, N.H. All authors have read and agreed to the published version of the manuscript.
Funding
Kasetsart University Research and Development Institute (KURDI), grant no. FF(KU)25.64.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
This research was supported by Kasetsart University Research and Development Institute (KURDI), grant no. FF(KU)25.64.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Pandey, S.; Sharma, K.; Gundabala, V. Antimicrobial bio-inspired active packaging materials for shelf life and safety development: A review. Food Biosci. 2022, 48, 101730. [Google Scholar] [CrossRef]
- Pires, S.M.; Devleesschauwer, B. Chapter 1-Estimates of global disease burden associated with foodborne pathogens. In Foodborne Infections and Intoxications, 5th ed.; Morris, J.G., Vugia, D.J., Eds.; Academic Press: London, UK, 2021; pp. 3–17. [Google Scholar]
- World Health Organization. WHO Estimates of the Global Burden of Foodborne Diseases: Foodborne Disease Burden Epidemiology Reference Group 2007–2015; World Health Organization: Geneva, Switzerland, 2015. [Google Scholar]
- Harnkarnsujarit, N.; Kawai, K.; Suzuki, T. Effects of Freezing Temperature and Water Activity on Microstructure, Color, and Protein Conformation of Freeze-Dried Bluefin Tuna (Thunnus orientalis). Food Bioprocess Technol. 2014, 8, 916–925. [Google Scholar] [CrossRef]
- Lorenzo, J.M.; Munekata, P.E.; Dominguez, R.; Pateiro, M.; Saraiva, J.A.; Franco, D. Main Groups of Microorganisms of Relevance for Food Safety and Stability. In Innovative Technologies for Food Preservation; Academic Press: London, UK, 2018; pp. 53–107. [Google Scholar]
- Al-Mamun, M.; Chowdhury, T.; Biswas, B.; Absar, N. Food Poisoning and Intoxication: A Global Leading Concern for Human Health. In Food Safety and Preservation; Academic Press: London, UK, 2018; pp. 307–352. [Google Scholar]
- Sánchez, G.; Bosch, A. Survival of Enteric Viruses in the Environment and Food. In Viruses in Foods; Goyal, S.M., Cannon, J.L., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 367–392. [Google Scholar]
- Sair, A.I.; D’Souza, D.H.; Jaykus, L.A. Human Enteric Viruses as Causes of Foodborne Disease. Compr. Rev. Food Sci. Food Saf. 2002, 1, 73–89. [Google Scholar] [CrossRef] [PubMed]
- Srisa, A.; Harnkarnsujarit, N. Antifungal films from trans-cinnamaldehyde incorporated poly(lactic acid) and poly(butylene adipate-co-terephthalate) for bread packaging. Food Chem. 2020, 333, 127537. [Google Scholar] [CrossRef] [PubMed]
- Solano, R.J.; Sierra, C.A.; Murillo, M. Antifungal activity of LDPE/lauric acid films against Colletotrichum tamarilloi. Food Packag. Shelf Life 2020, 24, 100495. [Google Scholar] [CrossRef]
- Panda, P.K.; Sadeghi, K.; Seo, J. Recent advances in poly (vinyl alcohol)/natural polymer based films for food packaging applications: A review. Food Packag. Shelf Life 2022, 33, 100904. [Google Scholar] [CrossRef]
- Chausali, N.; Saxena, J.; Prasad, R. Recent trends in nanotechnology applications of bio-based packaging. J. Agric. Food Res. 2022, 7, 100257. [Google Scholar] [CrossRef]
- Garnier, L.; Valence, F.; Pawtowski, A.; Auhustsinava-Galerne, L.; Frotté, N.; Baroncelli, R.; Deniel, F.; Coton, E.; Mounier, J. Diversity of spoilage fungi associated with various French dairy products. Int. J. Food Microbiol. 2017, 241, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Deak, T. Spoilage yeasts. In Understanding and Measuring the Shelf-Life of Food; Woodhead Publishing: Shaston, UK, 2004; pp. 91–110. [Google Scholar]
- Naderi Bab Anari, H.; Majdinasab, M.; Shaghaghian, S.; Khalesi, M. Development of a natamycin-based non-migratory antimicrobial active packaging for extending shelf-life of yogurt drink (Doogh). Food Chem. 2022, 366, 130606. [Google Scholar] [CrossRef] [PubMed]
- Fleet, G.H. Yeast Spoilage of Foods and Beverages. In The Yeasts; Elsevier: Amsterdam, The Netherlands, 2011; pp. 53–63. [Google Scholar]
- Perricone, M.; Gallo, M.; Corbo, M.R.; Sinigaglia, M.; Bevilacqua, A. Yeasts. In The Microbiological Quality of Food; Woodhead Publishing: Amsterdam, The Netherlands, 2017; pp. 121–131. [Google Scholar]
- Kurtzman, C.P. Detection, identification and enumeration methods for spoilage yeasts. In Food Spoilage Microorganisms; CRC Press LLC: Boca Raton, FL, USA, 2006; pp. 28–54. [Google Scholar]
- Martin, N.H.; Snyder, A.; Wiedmann, M. Spoilage Mold in Dairy Products. In Encyclopedia of Dairy Sciences, 3rd ed.; McSweeney, P.L.H., McNamara, J.P., Eds.; Academic Press: Oxford, UK, 2022; pp. 607–610. [Google Scholar]
- Petruzzi, L.; Corbo, M.R.; Sinigaglia, M.; Bevilacqua, A. Microbial Spoilage of Foods. In The Microbiological Quality of Food; Woodhead Publishing: Amsterdam, The Netherlands, 2017; pp. 1–21. [Google Scholar]
- Cook, F.K.; Johnson, B.L. Microbiological Spoilage of Cereal Products. In Compendium of the Microbiological Spoilage of Foods and Beverages; Sperber, W.H., Doyle, M.P., Eds.; Springer: New York, NY, USA, 2009; pp. 223–244. [Google Scholar]
- Sperber, W.H. Introduction to the Microbiological Spoilage of Foods and Beverages. In Compendium of the Microbiological Spoilage of Foods and Beverages; Sperber, W.H., Doyle, M.P., Eds.; Springer: New York, NY, USA, 2009; pp. 1–40. [Google Scholar]
- Singh, R.P.; Anderson, B.A. The major types of food spoilage: An overview. In Understanding and Measuring the Shelf-Life of Food; Woodhead Publishing: Shaston, UK, 2004; pp. 3–23. [Google Scholar]
- Azhdari, S.; Moradi, M. Application of antimicrobial coating based on carboxymethyl cellulose and natamycin in active packaging of cheese. Int. J. Biol. Macromol. 2022, 209, 2042–2049. [Google Scholar] [CrossRef] [PubMed]
- Ju, J.; Xie, Y.; Yu, H.; Guo, Y.; Cheng, Y.; Qian, H.; Yao, W. A novel method to prolong bread shelf life: Sachets containing essential oils components. LWT 2020, 131, 109744. [Google Scholar] [CrossRef]
- Klinmalai, P.; Srisa, A.; Laorenza, Y.; Katekhong, W.; Harnkarnsujarit, N. Antifungal and plasticization effects of carvacrol in biodegradable poly(lactic acid) and poly(butylene adipate terephthalate) blend films for bakery packaging. LWT 2021, 152, 112356. [Google Scholar] [CrossRef]
- Brzezinska, M.S.; Walczak, M.; Burkowska-But, A.; Chylińska, M.; Kalwasińska, A.; Świątczak, J. Antifungal Activity of Polyhexamethyleneguanidine Derivatives Introduced into Biodegradable Polymers. J. Polym. Environ. 2019, 27, 1760–1769. [Google Scholar] [CrossRef]
- Bahmid, N.A.; Dekker, M.; Fogliano, V.; Heising, J. Development of a moisture-activated antimicrobial film containing ground mustard seeds and its application on meat in active packaging system. Food Packag. Shelf Life 2021, 30, 100753. [Google Scholar] [CrossRef]
- Milanović, V.; Sabbatini, R.; Garofalo, C.; Cardinali, F.; Pasquini, M.; Aquilanti, L.; Osimani, A. Evaluation of the inhibitory activity of essential oils against spoilage yeasts and their potential application in yogurt. Int. J. Food Microbiol. 2021, 341, 109048. [Google Scholar] [CrossRef]
- Afzali, S.; Edalatian Dovom, M.R.; Habibi Najafi, M.B.; Mazaheri Tehrani, M. Determination of the anti-yeast activity of Lactobacillus spp. isolated from traditional Iranian cheeses in vitro and in yogurt drink (Doogh). Sci. Rep. 2020, 10, 6291. [Google Scholar] [CrossRef]
- Meena, M.; Prajapati, P.; Ravichandran, C.; Sehrawat, R. Natamycin: A natural preservative for food applications—A review. Food Sci. Biotechnol. 2021, 30, 1481–1496. [Google Scholar] [CrossRef]
- Te Welscher, Y.M.; ten Napel, H.H.; Balagué, M.M.; Souza, C.M.; Riezman, H.; de Kruijff, B.; Breukink, E. Natamycin Blocks Fungal Growth by Binding Specifically to Ergosterol without Permeabilizing the Membrane. J. Biol. Chem. 2008, 283, 6393–6401. [Google Scholar] [CrossRef]
- Mogavero, S.; Sauer, F.M.; Brunke, S.; Allert, S.; Schulz, D.; Wisgott, S.; Jablonowski, N.; Elshafee, O.; Krüger, T.; Kniemeyer, O.; et al. Candidalysin delivery to the invasion pocket is critical for host epithelial damage induced by Candida albicans. Cell. Microbiol. 2021, 23, e13378. [Google Scholar] [CrossRef] [PubMed]
- Fasihnia, S.H.; Peighambardoust, S.H.; Peighambardoust, S.J.; Oromiehie, A. Development of novel active polypropylene based packaging films containing different concentrations of sorbic acid. Food Packag. Shelf Life 2018, 18, 87–94. [Google Scholar] [CrossRef]
- da Rocha, M.; Prietto, L.; de Souza, M.M.; Furlong, E.B.; Prentice, C. Effect of Organic Acids on Physical-Mechanical and Antifungicidal Properties of Anchovy Protein Films. J. Aquat. Food Prod. Technol. 2018, 27, 316–326. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, J.; Ding, W.; Zhang, D.; Reed, K.; Zhang, B. Alternatives to carcinogenic preservatives in Chinese Sausage-Sorbic acid-loaded chitosan/tripolyphosphate nanoparticles. Int. J. Biol. Macromol. 2018, 120, 28–33. [Google Scholar] [CrossRef] [PubMed]
- Nemes, D.; Kovács, R.; Nagy, F.; Tóth, Z.; Herczegh, P.; Borbás, A.; Kelemen, V.; Pfliegler, W.P.; Rebenku, I.; Hajdu, P.B.; et al. Comparative biocompatibility and antimicrobial studies of sorbic acid derivates. Eur. J. Pharm. Sci. 2020, 143, 105162. [Google Scholar] [CrossRef]
- Wangprasertkul, J.; Siriwattanapong, R.; Harnkarnsujarit, N. Antifungal packaging of sorbate and benzoate incorporated biodegradable films for fresh noodles. Food Control 2021, 123, 107763. [Google Scholar] [CrossRef]
- Küçük, G.S.; Çelik, Ö.F.; Mazi, B.G.; Türe, H. Evaluation of alginate and zein films as a carrier of natamycin to increase the shelf life of kashar cheese. Packag. Technol. Sci. 2020, 33, 39–48. [Google Scholar] [CrossRef]
- Phothisarattana, D.; Harnkarnsujarit, N. Migration, aggregations and thermal degradation behaviors of TiO2 and ZnO incorporated PBAT/TPS nanocomposite blown films. Food Packag. Shelf Life 2022, 33, 100901. [Google Scholar] [CrossRef]
- Wadaugsorn, K.; Panrong, T.; Wongphan, P.; Harnkarnsujarit, N. Plasticized hydroxypropyl cassava starch blended PBAT for improved clarity blown films: Morphology and properties. Ind. Crop. Prod. 2022, 176, 114311. [Google Scholar] [CrossRef]
- Wongphan, P.; Khowthong, M.; Supatrawiporn, T.; Harnkarnsujarit, N. Novel edible starch films incorporating papain for meat tenderization. Food Packag. Shelf Life 2022, 31, 100787. [Google Scholar] [CrossRef]
- Ezati, P.; Rhim, J.-W.; Molaei, R.; Rezaei, Z. Carbon quantum dots-based antifungal coating film for active packaging application of avocado. Food Packag. Shelf Life 2022, 33, 100878. [Google Scholar] [CrossRef]
- Riahi, Z.; Priyadarshi, R.; Rhim, J.-W.; Lotfali, E.; Bagheri, R.; Pircheraghi, G. Alginate-based multifunctional films incorporated with sulfur quantum dots for active packaging applications. Colloids Surf. B Biointerfaces 2022, 215, 112519. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, F.G.; Grisi, C.V.B.; Costa Araújo, R.; Botrel, D.A.; Sousa, S. Active cellulose acetate-oregano essential oil films to conservation of hamburger buns: Antifungal, analysed sensorial and mechanical properties. Packag. Technol. Sci. 2021, 35, 175–182. [Google Scholar] [CrossRef]
- Suwanamornlert, P.; Kerddonfag, N.; Sane, A.; Chinsirikul, W.; Zhou, W.; Chonhenchob, V. Poly(lactic acid)/poly(butylene-succinate-co-adipate) (PLA/PBSA) blend films containing thymol as alternative to synthetic preservatives for active packaging of bread. Food Packag. Shelf Life 2020, 25, 100515. [Google Scholar] [CrossRef]
- Sharma, P.; Ahuja, A.; Dilsad Izrayeel, A.M.; Samyn, P.; Rastogi, V.K. Physicochemical and thermal characterization of poly (3-hydroxybutyrate-co-4-hydroxybutyrate) films incorporating thyme essential oil for active packaging of white bread. Food Control 2022, 133, 108688. [Google Scholar] [CrossRef]
- Heras-Mozos, R.; Muriel-Galet, V.; López-Carballo, G.; Catalá, R.; Hernandez-Munoz, P.; Gavara, R. Development and optimization of antifungal packaging for sliced pan loaf based on garlic as active agent and bread aroma as aroma corrector. Int. J. Food Microbiol. 2019, 290, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Mirón-Mérida, V.A.; Yáñez-Fernández, J.; Montañez-Barragán, B.; Barragán Huerta, B.E. Valorization of coffee parchment waste (Coffea arabica) as a source of caffeine and phenolic compounds in antifungal gellan gum films. LWT 2019, 101, 167–174. [Google Scholar] [CrossRef]
- Boonruang, K.; Kerddonfag, N.; Chinsirikul, W.; Mitcham, E.J.; Chonhenchob, V. Antifungal effect of poly(lactic acid) films containing thymol and R-(-)-carvone against anthracnose pathogens isolated from avocado and citrus. Food Control 2017, 78, 85–93. [Google Scholar] [CrossRef]
- Robledo, N.; Vera, P.; López, L.; Yazdani-Pedram, M.; Tapia, C.; Abugoch, L. Thymol nanoemulsions incorporated in quinoa protein/chitosan edible films; antifungal effect in cherry tomatoes. Food Chem. 2018, 246, 211–219. [Google Scholar] [CrossRef]
- Sapper, M.; Martin-Esparza, M.E.; Chiralt, A.; Gonzalez Martinez, C. Antifungal Polyvinyl Alcohol Coatings Incorporating Carvacrol for the Postharvest Preservation of Golden Delicious Apple. Coatings 2020, 10, 1027. [Google Scholar] [CrossRef]
- Cheng, M.; Wang, J.; Zhang, R.; Kong, R.; Lu, W.; Wang, X. Characterization and application of the microencapsulated carvacrol/sodium alginate films as food packaging materials. Int. J. Biol. Macromol. 2019, 141, 259–267. [Google Scholar] [CrossRef] [PubMed]
- Kong, R.; Wang, J.; Cheng, M.; Lu, W.; Chen, M.; Zhang, R.; Wang, X. Development and characterization of corn starch/PVA active films incorporated with carvacrol nanoemulsions. Int. J. Biol. Macromol. 2020, 164, 1631–1639. [Google Scholar] [CrossRef]
- Liu, H.; Chen, J.; Xia, Z.; An, M.; Wu, Y. Effects of ε-poly-l-lysine on vegetative growth, pathogenicity and gene expression of Alternaria alternata infecting Nicotiana tabacum. Pestic. Biochem. Physiol. 2020, 163, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Luz, C.; Calpe, J.; Saladino, F.; Luciano, F.B.; Fernandez-Franzón, M.; Mañes, J.; Meca, G. Antimicrobial packaging based on ε-polylysine bioactive film for the control of mycotoxigenic fungi in vitro and in bread. J. Food Process. Preserv. 2018, 42, e13370. [Google Scholar] [CrossRef] [PubMed]
- Mateo, E.M.; Gómez, J.V.; Domínguez, I.; Gimeno-Adelantado, J.V.; Mateo-Castro, R.; Gavara, R.; Jiménez, M. Impact of bioactive packaging systems based on EVOH films and essential oils in the control of aflatoxigenic fungi and aflatoxin production in maize. Int. J. Food Microbiol. 2017, 254, 36–46. [Google Scholar] [CrossRef]
- Promsorn, J.; Harnkarnsujarit, N. Pyrogallol loaded thermoplastic cassava starch based films as bio-based oxygen scavengers. Ind. Crop. Prod. 2022, 186, 115226. [Google Scholar] [CrossRef]
- Promsorn, J.; Harnkarnsujarit, N. Oxygen absorbing food packaging made by extrusion compounding of thermoplastic cassava starch with gallic acid. Food Control 2022, 142, 109273. [Google Scholar] [CrossRef]
- Li, Z.; Lin, S.; An, S.; Liu, L.; Hu, Y.; Wan, L. Preparation, characterization and anti-aflatoxigenic activity of chitosan packaging films incorporated with turmeric essential oil. Int. J. Biol. Macromol. 2019, 131, 420–434. [Google Scholar] [CrossRef] [PubMed]
- Chein, S.H.; Sadiq, M.B.; Anal, A.K. Antifungal effects of chitosan films incorporated with essential oils and control of fungal contamination in peanut kernels. J. Food Process. Preserv. 2019, 43, e14235. [Google Scholar] [CrossRef]
- Guo, X.; Chu, L.; Gu, T.; Purohit, S.; Kou, L.; Zhang, B. Long-term quality retention and decay inhibition of chestnut using thymol loaded chitosan nanoparticle. Food Chem. 2022, 374, 131781. [Google Scholar] [CrossRef]
- Muñoz-Tébar, N.; Carmona, M.; de Elguea-Culebras, G.O.; Molina, A.; Berruga, M.I. Chia Seed Mucilage Edible Films with Origanum vulgare and Satureja montana Essential Oils: Characterization and Antifungal Properties. Membranes 2022, 12, 213. [Google Scholar] [CrossRef] [PubMed]
- McDaniel, A.; Tonyali, B.; Yucel, U.; Trinetta, V. Formulation and development of lipid nanoparticle antifungal packaging films to control postharvest disease. J. Agric. Food Res. 2019, 1, 100013. [Google Scholar] [CrossRef]
- Trinetta, V.; McDaniel, A.; Batziakas, K.G.; Yucel, U.; Nwadike, L.; Pliakoni, E. Antifungal Packaging Film to Maintain Quality and Control Postharvest Diseases in Strawberries. Antibiotics 2020, 9, 618. [Google Scholar] [CrossRef] [PubMed]
- Grafia, A.L.; Vazquez, M.B.; Bianchinotti, M.V.; Barbosa, S.E. Development of an antifungal film by polyethylene surface modification with natamycin. Food Packag. Shelf Life 2018, 18, 191–200. [Google Scholar] [CrossRef]
- Karabulut, G.; Cagri-Mehmetoglu, A. Antifungal, Mechanical, and Physical Properties of Edible Film Containing Williopsis saturnus var. Saturnus Antagonistic Yeast. J. Food Sci. 2018, 83, 763–769. [Google Scholar] [CrossRef] [PubMed]
- Sahraee, S.; Milani, J.M.; Ghanbarzadeh, B.; Hamishehkar, H. Development of emulsion films based on bovine gelatin-nano chitin-nano ZnO for cake packaging. Food Sci. Nutr. 2020, 8, 1303–1312. [Google Scholar] [CrossRef]
- Jha, P. Effect of plasticizer and antimicrobial agents on functional properties of bionanocomposite films based on corn starch-chitosan for food packaging applications. Int. J. Biol. Macromol. 2020, 160, 571–582. [Google Scholar] [CrossRef]
- Erkmen, O.; Bozoglu, T.F. Types of Microorganisms in Foods. In Food Microbiology: Principles into Practice; John Wiley & Sons: Hoboken, NJ, USA, 2016; pp. 35–80. [Google Scholar]
- Russell, N.J.; Fukunaga, N. A comparison of thermal adaptation of membrane lipids in psychrophilic and thermophilic bacteria. FEMS Microbiol. Lett. 1990, 75, 171–182. [Google Scholar] [CrossRef]
- Marianski, S.; Mariański, A. The Art of Making Fermented Sausages; Bookmagic: Andhra Pradesh, India, 2009. [Google Scholar]
- Gould, G.W.; Russell, N.J. Major, new, and emerging food-poisoning and food-spoilage microorganisms. In Food Preservatives; Russell, N.J., Gould, G.W., Eds.; Springer: Boston, MA, USA, 2003; pp. 1–13. [Google Scholar]
- Pongsetkul, J.; Benjakul, S. Development of modified atmosphere packaging (MAP) on shelf-life extension of pla-duk-ra (dried fermented catfish) stored at room temperature. Food Control 2021, 124, 107882. [Google Scholar] [CrossRef]
- Jakobsen, A.N.; Shumilina, E.; Lied, H.; Hoel, S. Growth and spoilage metabolites production of a mesophilic Aeromonas salmonicida strain in Atlantic salmon (Salmo salar L.) during cold storage in modified atmosphere. J. Appl. Microbiol. 2020, 129, 935–946. [Google Scholar] [CrossRef]
- Bassey, A.P.; Chen, Y.; Zhu, Z.; Odeyemi, O.A.; Gao, T.; Olusola, O.O.; Ye, K.; Li, C.; Zhou, G. Evaluation of spoilage indexes and bacterial community dynamics of modified atmosphere packaged super-chilled pork loins. Food Control 2021, 130, 108383. [Google Scholar] [CrossRef]
- Chan, S.S.; Skare, M.; Rotabakk, B.T.; Sivertsvik, M.; Lerfall, J.; Løvdal, T.; Roth, B. Evaluation of physical and instrumentally determined sensory attributes of Atlantic salmon portions packaged in modified atmosphere and vacuum skin. LWT 2021, 146, 111404. [Google Scholar] [CrossRef]
- Hilgarth, M.; Lehner, E.M.; Behr, J.; Vogel, R.F. Diversity and anaerobic growth of Pseudomonas spp. isolated from modified atmosphere packaged minced beef. J. Appl. Microbiol. 2019, 127, 159–174. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, J.S.; Biduski, B.; dos Santos, L.R. Listeria monocytogenes: Health risk and a challenge for food processing establishments. Arch. Microbiol. 2021, 203, 5907–5919. [Google Scholar] [CrossRef] [PubMed]
- Aymerich, T.; Jofré, A.; Bover-Cid, S. Enterocin A-based antimicrobial film exerted strong antilisterial activity in sliced dry-cured ham immediately and after 6 months at 8 °C. Food Microbiol. 2022, 105, 104005. [Google Scholar] [CrossRef] [PubMed]
- Woraprayote, W.; Pumpuang, L.; Tosukhowong, A.; Zendo, T.; Sonomoto, K.; Benjakul, S.; Visessanguan, W. Antimicrobial biodegradable food packaging impregnated with Bacteriocin 7293 for control of pathogenic bacteria in pangasius fish fillets. LWT 2018, 89, 427–433. [Google Scholar] [CrossRef]
- Silveira, V.A.I.; Marim, B.M.; Hipólito, A.; Gonçalves, M.C.; Mali, S.; Kobayashi, R.K.T.; Celligoi, M.A.P.C. Characterization and antimicrobial properties of bioactive packaging films based on polylactic acid-sophorolipid for the control of foodborne pathogens. Food Packag. Shelf Life 2020, 26, 100591. [Google Scholar] [CrossRef]
- Glicerina, V.; Siroli, L.; Canali, G.; Chinnici, F.; Capelli, F.; Lanciotti, R.; Colombo, V.; Romani, S. Efficacy of biodegradable, antimicrobial packaging on safety and quality parameters maintenance of a pear juice and rice milk-based smoothie product. Food Control 2021, 128, 108170. [Google Scholar] [CrossRef]
- Ordoñez, R.; Atarés, L.; Chiralt, A. Effect of ferulic and cinnamic acids on the functional and antimicrobial properties in thermo-processed PLA films. Food Packag. Shelf Life 2022, 33, 100882. [Google Scholar] [CrossRef]
- Phothisarattana, D.; Wongphan, P.; Promhuad, K.; Promsorn, J.; Harnkarnsujarit, N. Biodegradable Poly(Butylene Adipate-Co-Terephthalate) and Thermoplastic Starch-Blended TiO2 Nanocomposite Blown Films as Functional Active Packaging of Fresh Fruit. Polymers 2021, 13, 4192. [Google Scholar] [CrossRef]
- Wongphan, P.; Panrong, T.; Harnkarnsujarit, N. Effect of different modified starches on physical, morphological, thermomechanical, barrier and biodegradation properties of cassava starch and polybutylene adipate terephthalate blend film. Food Packag. Shelf Life 2022, 32, 100844. [Google Scholar] [CrossRef]
- Contini, L.R.F.; Zerlotini, T.D.S.; Brazolin, I.F.; dos Santos, J.W.S.; Silva, M.F.; Lopes, P.S.; Sampaio, K.A.; Carvalho, R.A.; Venturini, A.C.; Yoshida, C.M.P. Antioxidant chitosan film containing lemongrass essential oil as active packaging for chicken patties. J. Food Process. Preserv. 2021, 46, e16136. [Google Scholar] [CrossRef]
- Xiao, L.; Kang, S.; Lapu, M.; Jiang, P.; Wang, X.; Liu, D.; Li, J.; Liu, M. Preparation and characterization of chitosan/pullulan film loading carvacrol for targeted antibacterial packaging of chilled meat. Int. J. Biol. Macromol. 2022, 211, 140–149. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.; Mohammadi Nafchi, A.; Baghaie, H. Development of an active packaging based on polyethylene containing linalool or thymol for mozzarella cheese. Food Sci. Nutr. 2021, 9, 3732–3739. [Google Scholar] [CrossRef] [PubMed]
- Jo, Y.; Garcia, C.V.; Ko, S.; Lee, W.; Shin, G.H.; Choi, J.C.; Park, S.-J.; Kim, J.T. Characterization and antibacterial properties of nanosilver-applied polyethylene and polypropylene composite films for food packaging applications. Food Biosci. 2018, 23, 83–90. [Google Scholar] [CrossRef]
- Kim, Y.H.; Kim, G.H.; Yoon, K.S.; Shankar, S.; Rhim, J.-W. Comparative antibacterial and antifungal activities of sulfur nanoparticles capped with chitosan. Microb. Pathog. 2020, 144, 104178. [Google Scholar] [CrossRef]
- Wang, L.; Xu, J.; Zhang, M.; Zheng, H.; Li, L. Preservation of soy protein-based meat analogues by using PLA/PBAT antimicrobial packaging film. Food Chem. 2022, 380, 132022. [Google Scholar] [CrossRef]
- Gan, L.; Jiang, G.; Yang, Y.; Zheng, B.; Zhang, S.; Li, X.; Tian, Y.; Peng, B. Development and characterization of levan/pullulan/chitosan edible films enriched with ε-polylysine for active food packaging. Food Chem. 2022, 388, 132989. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Zhai, X.; Cheng, Y.; Zhang, R.; Wang, W.; Hou, H. Starch/PBAT blown antimicrobial films based on the synergistic effects of two commercial antimicrobial peptides. Int. J. Biol. Macromol. 2022, 204, 457–465. [Google Scholar] [CrossRef]
- Mathew, S.; Snigdha, S.; Jyothis, M.; Radhakrishnan, E.K. Biodegradable and active nanocomposite pouches reinforced with silver nanoparticles for improved packaging of chicken sausages. Food Packag. Shelf Life 2019, 19, 155–166. [Google Scholar] [CrossRef]
- Pattanayaiying, R.; Sane, A.; Photjanataree, P.; Cutter, C.N. Thermoplastic starch/polybutylene adipate terephthalate film coated with gelatin containing nisin Z and lauric arginate for control of foodborne pathogens associated with chilled and frozen seafood. Int. J. Food Microbiol. 2019, 290, 59–67. [Google Scholar] [CrossRef]
- Haghighi, H.; Leugoue, S.K.; Pfeifer, F.; Siesler, H.W.; Licciardello, F.; Fava, P.; Pulvirenti, A. Development of antimicrobial films based on chitosan-polyvinyl alcohol blend enriched with ethyl lauroyl arginate (LAE) for food packaging applications. Food Hydrocoll. 2020, 100, 105419. [Google Scholar] [CrossRef]
- Pobiega, K.; Przybył, J.L.; Żubernik, J.; Gniewosz, M. Prolonging the Shelf Life of Cherry Tomatoes by Pullulan Coating with Ethanol Extract of Propolis During Refrigerated Storage. Food Bioprocess Technol. 2020, 13, 1447–1461. [Google Scholar] [CrossRef]
- Guo, Z.; Ge, X.; Gou, Q.; Yang, L.; Han, M.; Han, G.; Yu, Q.-L.; Han, L. Changes in chilled beef packaged in starch film containing sea buckthorn pomace extract and quality changes in the film during super-chilled storage. Meat Sci. 2021, 182, 108620. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-García, G.J.; Quintana-Romero, L.A.; Morales-Figueroa, G.G.; Esparza-Romero, J.; Pérez-Morales, R.; López-Mata, M.A.; Juárez, J.; Sánchez-Escalante, J.J.; Peralta, E.; Quihui-Cota, L.; et al. Effect of Yucca baccata butanolic extract on the shelf life of chicken and development of an antimicrobial packaging for beef. Food Control 2021, 127, 108142. [Google Scholar] [CrossRef]
- Phothisarattana, D.; Wongphan, P.; Promhuad, K.; Promsorn, J.; Harnkarnsujarit, N. Blown film extrusion of PBAT/TPS/ZnO nanocomposites for shelf-life extension of meat packaging. Colloids Surf. B Biointerfaces 2022, 214, 112472. [Google Scholar] [CrossRef]
- Cardoso, L.G.; Pereira Santos, J.C.; Camilloto, G.P.; Miranda, A.L.; Druzian, J.I.; Guimarães, A.G. Development of active films poly (butylene adipate co-terephthalate)–PBAT incorporated with oregano essential oil and application in fish fillet preservation. Ind. Crop. Prod. 2017, 108, 388–397. [Google Scholar] [CrossRef]
- Laorenza, Y.; Harnkarnsujarit, N. Carvacrol, citral and α-terpineol essential oil incorporated biodegradable films for functional active packaging of Pacific white shrimp. Food Chem. 2021, 363, 130252. [Google Scholar] [CrossRef] [PubMed]
- Shruthy, R.; Jancy, S.; Preetha, R.; Ramesh, S.; Stephen, J.; Radhakrishnan, P. Cellulose nanoparticles synthesised from potato peel for the development of active packaging film for enhancement of shelf life of raw prawns (Penaeus monodon) during frozen storage. Int. J. Food Sci. Technol. 2020, 56, 3991–3999. [Google Scholar] [CrossRef]
- Llana-Ruiz-Cabello, M.; Pichardo, S.; Bermudez, J.M.; Baños, A.; Ariza, J.J.; Guillamón, E.; Aucejo, S.; Cameán, A.M. Characterisation and antimicrobial activity of active polypropylene films containing oregano essential oil and Allium extract to be used in packaging for meat products. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess 2018, 35, 783–792. [Google Scholar] [CrossRef]
- Jaballah, S.; Fhoula, I.; Boumaiza, M.; Najjari, A.; Mhajbi, N.; Boudabous, A.; Klibi, N.; Ouzari, H. Prevalence and risk factors of potential pathogenic Yersinia enterocolitica in Tunisian frozen ground beef through a shelf-life monitoring protocol validation. J. Food Process. Preserv. 2022, 46, e16360. [Google Scholar] [CrossRef]
- Reichel, J.; Kehrenberg, C.; Krischek, C. Inactivation of Yersinia enterocolitica and Brochothrix thermosphacta on pork by UV-C irradiation. Meat Sci. 2019, 158, 107909. [Google Scholar] [CrossRef]
- Abel, N.; Rotabakk, B.T.; Lerfall, J. Effect of heat treatment and packaging technology on the microbial load of lightly processed seafood. LWT 2019, 101, 123–129. [Google Scholar] [CrossRef]
- Ukuku, D.O.; Bari, M.L. Yersinia enterocolitica. In Food Microbiology; American Society for Microbiology: Washington, DC, USA, 2019; pp. 437–450. [Google Scholar]
- Cai, L.; Wang, Y.; Cao, A. The physiochemical and preservation properties of fish sarcoplasmic protein/chitosan composite films containing ginger essential oil emulsions. J. Food Process Eng. 2020, 43, e13495. [Google Scholar] [CrossRef]
- Safaei, M.; Azad, R.R. Preparation and characterization of poly-lactic acid based films containing propolis ethanolic extract to be used in dry meat sausage packaging. J. Food Sci. Technol. 2020, 57, 1242–1250. [Google Scholar] [CrossRef] [PubMed]
- Sganzerla, W.G.; da Rosa, C.G.; da Silva, A.P.G.; Ferrareze, J.P.; Azevedo, M.S.; Forster-Carneiro, T.; Nunes, M.R.; de Lima Veeck, A.P. Application in situ of biodegradable films produced with starch, citric pectin and functionalized with feijoa (Acca sellowiana (Berg) Burret) extracts: An effective proposal for food conservation. Int. J. Biol. Macromol. 2021, 189, 544–553. [Google Scholar] [CrossRef] [PubMed]
- Shivangi, S.; Dorairaj, D.; Negi, P.S.; Shetty, N.P. Development and characterisation of a pectin-based edible film that contains mulberry leaf extract and its bio-active components. Food Hydrocoll. 2021, 121, 107046. [Google Scholar] [CrossRef]
- Bayani Bandpey, N.; Aroujalian, A.; Raisi, A.; Fazel, S. Surface coating of silver nanoparticles on polyethylene for fabrication of antimicrobial milk packaging films. Int. J. Dairy Technol. 2017, 70, 204–211. [Google Scholar] [CrossRef]
- Valipour Motlagh, N.; Aghazamani, J.; Gholami, R. Investigating the Effect of Nano-silver Contained Packaging on the Olivier Salad Shelf-life. Bio. Nano. Sci. 2021, 11, 838–847. [Google Scholar] [CrossRef]
- Gourama, H. Foodborne Pathogens. In Food Safety Engineering; Demirci, A., Feng, H., Krishnamurthy, K., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 25–49. [Google Scholar]
- Appleton, H. VIRUSES. In Encyclopedia of Food Sciences and Nutrition, 2nd ed.; Caballero, B., Ed.; Academic Press: Oxford, UK, 2003; pp. 6004–6011. [Google Scholar]
- Vasickova, P.; Pavlik, I.; Verani, M.; Carducci, A. Issues Concerning Survival of Viruses on Surfaces. Food Environ. Virol. 2010, 2, 24–34. [Google Scholar] [CrossRef]
- Yeargin, T.; Gibson, K.E. Key characteristics of foods with an elevated risk for viral enteropathogen contamination. J. Appl. Microbiol. 2019, 126, 996–1010. [Google Scholar] [CrossRef]
- Di Cola, G.; Fantilli, A.C.; Pisano, M.B.; Ré, V.E. Foodborne transmission of hepatitis A and hepatitis E viruses: A literature review. Int. J. Food Microbiol. 2021, 338, 108986. [Google Scholar] [CrossRef] [PubMed]
- Randazzo, W.; Sánchez, G. Hepatitis A infections from food. J. Appl. Microbiol. 2020, 129, 1120–1132. [Google Scholar] [CrossRef]
- Bozkurt, H.; Phan-Thien, K.-Y.; van Ogtrop, F.; Bell, T.; McConchie, R. Outbreaks, occurrence, and control of norovirus and hepatitis a virus contamination in berries: A review. Crit. Rev. Food Sci. Nutr. 2021, 61, 116–138. [Google Scholar] [CrossRef]
- Gyawali, P.; Fletcher, G.C.; McCoubrey, D.-J.; Hewitt, J. Norovirus in shellfish: An overview of post-harvest treatments and their challenges. Food Control 2019, 99, 171–179. [Google Scholar] [CrossRef]
- Thornton, A.C.; Jennings-Conklin, K.S.; McCormick, M.I. Noroviruses: Agents in outbreaks of acute gastroenteritis. Disaster Manag. Response 2004, 2, 4–9. [Google Scholar] [CrossRef]
- Lee, A.; Grove, S. Virus Inactivation During Food Processing. In Viruses in Foods; Goyal, S.M., Cannon, J.L., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 421–447. [Google Scholar]
- Hirneisen, K.A.; Black, E.P.; Cascarino, J.L.; Fino, V.R.; Hoover, D.G.; Kniel, K.E. Viral Inactivation in Foods: A Review of Traditional and Novel Food-Processing Technologies. Compr. Rev. Food Sci. Food Saf. 2010, 9, 3–20. [Google Scholar] [CrossRef] [PubMed]
- Mallakpour, S.; Azadi, E.; Hussain, C.M. Recent breakthroughs of antibacterial and antiviral protective polymeric materials during COVID-19 pandemic and after pandemic: Coating, packaging, and textile applications. Curr. Opin. Colloid Interface Sci. 2021, 55, 101480. [Google Scholar] [CrossRef] [PubMed]
- Olaimat, A.N.; Shahbaz, H.M.; Fatima, N.; Munir, S.; Holley, R.A. Food Safety during and after the Era of COVID-19 Pandemic. Front. Microbiol. 2020, 11, 1854. [Google Scholar] [CrossRef]
- Priyadarshi, R.; Purohit, S.D.; Roy, S.; Ghosh, T.; Rhim, J.-W.; Han, S.S. Antiviral Biodegradable Food Packaging and Edible Coating Materials in the COVID-19 Era: A Mini-Review. Coatings 2022, 12, 577. [Google Scholar] [CrossRef]
- Oliveira, W.Q.; Azeredo, H.M.C.; Neri-Numa, I.A.; Pastore, G.M. Food packaging wastes amid the COVID-19 pandemic: Trends and challenges. Trends Food Sci. Technol. 2021, 116, 1195–1199. [Google Scholar] [CrossRef]
- Xue, X.; Ball, J.K.; Alexander, C.; Alexander, M.R. All Surfaces are not Equal in Contact Transmission of SARS-CoV-2. Matter 2020, 3, 1433–1441. [Google Scholar] [CrossRef] [PubMed]
- Chitrakar, B.; Zhang, M.; Bhandari, B. Improvement strategies of food supply chain through novel food processing technologies during COVID-19 pandemic. Food Control 2021, 125, 108010. [Google Scholar] [CrossRef]
- Han, S.; Roy, P.K.; Hossain, I.; Byun, K.H.; Choi, C.; Ha, S.D. COVID-19 pandemic crisis and food safety: Implications and inactivation strategies. Trends Food Sci. Technol. 2021, 109, 25–36. [Google Scholar] [CrossRef]
- Baert, L.; Debevere, J.; Uyttendaele, M. The efficacy of preservation methods to inactivate foodborne viruses. Int. J. Food Microbiol. 2009, 131, 83–94. [Google Scholar] [CrossRef]
- Pogan, R.; Dülfer, J.; Uetrecht, C. Norovirus assembly and stability. Curr. Opin. Virol. 2018, 31, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Falcó, I.; Randazzo, W.; Sánchez, G.; López-Rubio, A.; Fabra, M.J. On the use of carrageenan matrices for the development of antiviral edible coatings of interest in berries. Food Hydrocoll. 2019, 92, 74–85. [Google Scholar] [CrossRef]
- Fabra, M.J.; Falcó, I.; Randazzo, W.; Sánchez, G.; López-Rubio, A. Antiviral and antioxidant properties of active alginate edible films containing phenolic extracts. Food Hydrocoll. 2018, 81, 96–103. [Google Scholar] [CrossRef]
- Falcó, I.; Flores-Meraz, P.L.; Randazzo, W.; Sánchez, G.; López-Rubio, A.; Fabra, M.J. Antiviral activity of alginate-oleic acid based coatings incorporating green tea extract on strawberries and raspberries. Food Hydrocoll. 2019, 87, 611–618. [Google Scholar] [CrossRef]
- Martínez-Abad, A.; Ocio, M.J.; Lagarón, J.M.; Sánchez, G. Evaluation of silver-infused polylactide films for inactivation of Salmonella and feline calicivirus in vitro and on fresh-cut vegetables. Int. J. Food Microbiol. 2013, 162, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Moreno, M.A.; Bojorges, H.; Falcó, I.; Sánchez, G.; López-Carballo, G.; López-Rubio, A.; Zampini, I.C.; Isla, M.I.; Fabra, M.J. Active properties of edible marine polysaccharide-based coatings containing Larrea nitida polyphenols enriched extract. Food Hydrocoll. 2020, 102, 105595. [Google Scholar] [CrossRef]
- Park, K.; Sadeghi, K.; Panda, P.K.; Seo, J.; Seo, J. Ethylene vinyl acetate/low-density polyethylene/oyster shell powder composite films: Preparation, characterization, and antimicrobial properties for biomedical applications. J. Taiwan Inst. Chem. Eng. 2022, 134, 104301. [Google Scholar] [CrossRef]
- Kim, H.; Panda, P.K.; Sadeghi, K.; Lee, S.; Chung, C.; Park, Y.; Park, J.; Seo, J. Facile thermal and hydrolytic conversion of tannic acid: Enhancement of antimicrobial activity and biocompatibility for biomedical applications. Mater. Chem. Phys. 2022, 285, 126141. [Google Scholar] [CrossRef]
- Sharif, N.; Falcó, I.; Martínez-Abad, A.; Sánchez, G.; López-Rubio, A.; Fabra, M.J. On the Use of Persian Gum for the Development of Antiviral Edible Coatings against Murine Norovirus of Interest in Blueberries. Polymers 2021, 13, 224. [Google Scholar] [CrossRef]
- Amankwaah, C.; Li, J.; Lee, J.; Pascall, M.A. Development of antiviral and bacteriostatic chitosan-based food packaging material with grape seed extract for murine norovirus, Escherichia coli and Listeria innocua control. Food Sci. Nutr. 2020, 8, 6174–6181. [Google Scholar] [CrossRef] [PubMed]
- Castro-Mayorga, J.L.; Randazzo, W.; Fabra, M.J.; Lagaron, J.M.; Aznar, R.; Sánchez, G. Antiviral properties of silver nanoparticles against norovirus surrogates and their efficacy in coated polyhydroxyalkanoates systems. LWT-Food Sci. Technol. 2017, 79, 503–510. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).