Fabrication and Testing of Multi-Hierarchical Porous Scaffolds Designed for Bone Regeneration via Additive Manufacturing Processes
Abstract
1. Introduction
2. Materials, Equipment, and Methods
2.1. Materials
2.2. Equipment
2.3. Methods
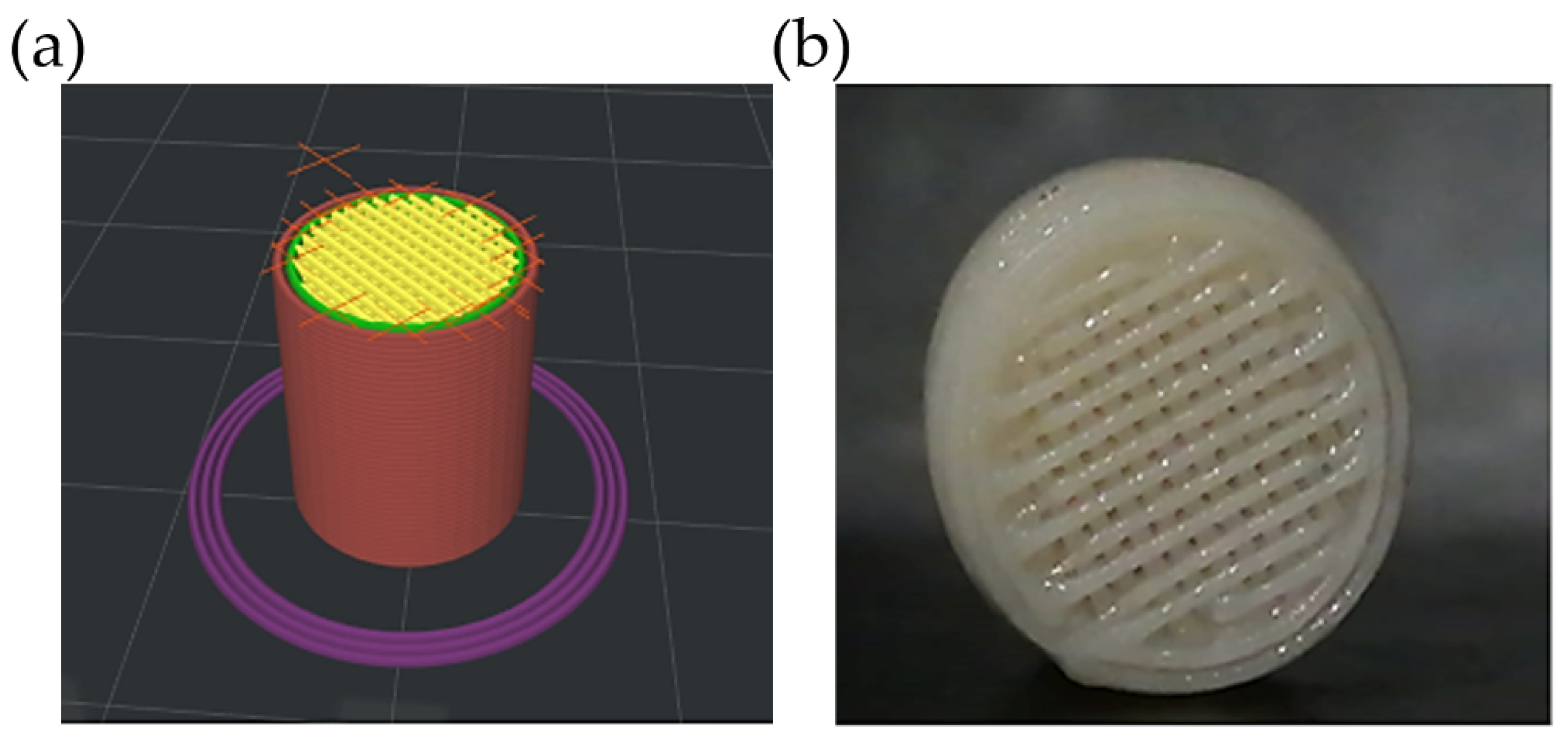
2.3.1. Design and Printing of Scaffolds via FDM
2.3.2. Copolymer Synthesis
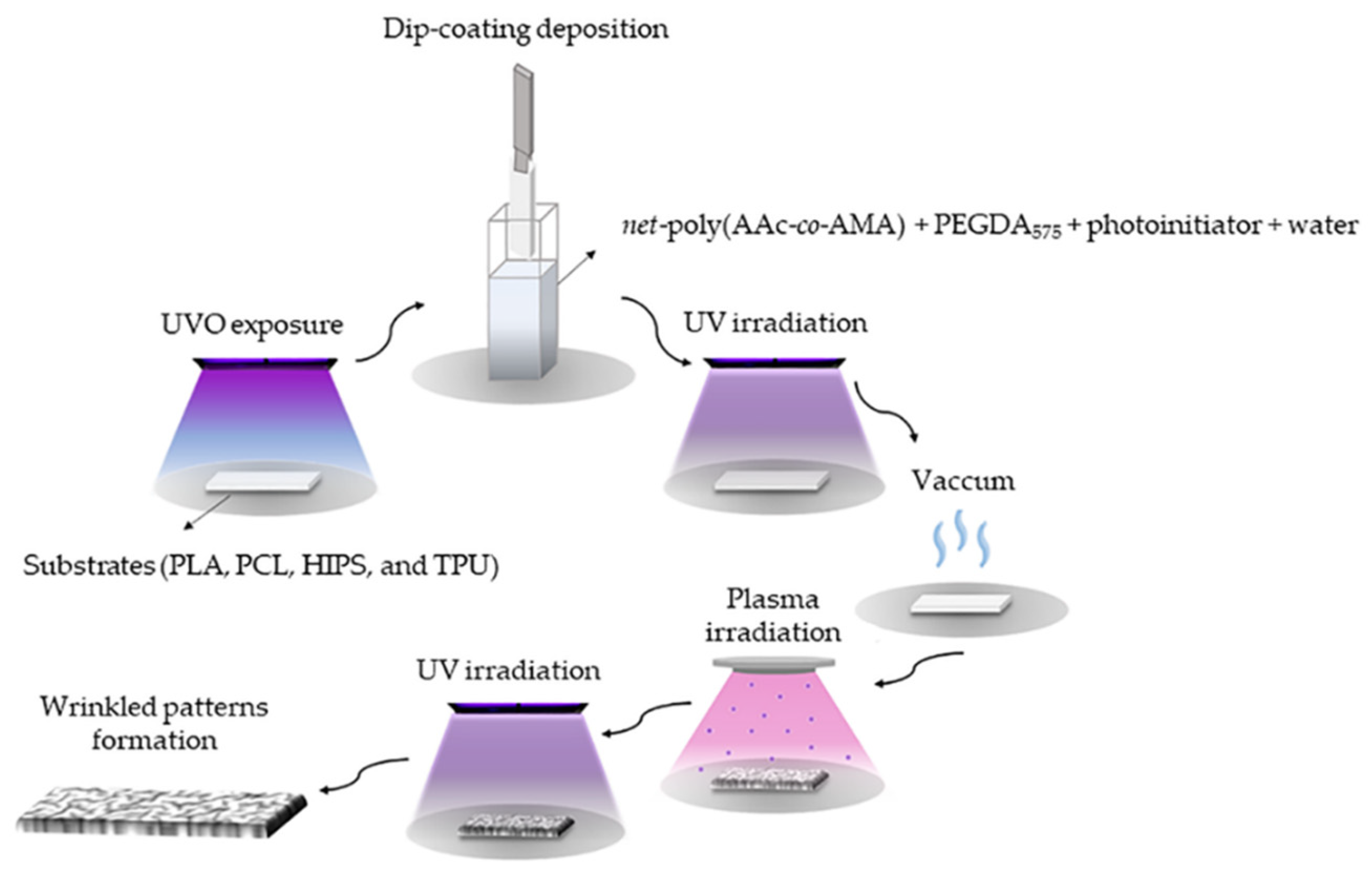
2.3.3. Wrinkles on Different Scaffolds
Hydrogel Synthesis and Deposition over the Scaffolds
2.3.4. PCL Filament Modification and Printing of the Porous Structures
Incorporation of Porogen and Bioactive Agents in PCL
Optimization of Printing Parameters and Salt Leaching Process
2.3.5. Mechanical Tests
2.3.6. Biological Evaluation (Proliferation Studies)
3. Results and Discussion
3.1. Characterization of the Copolymers
3.2. Substrates Characterization
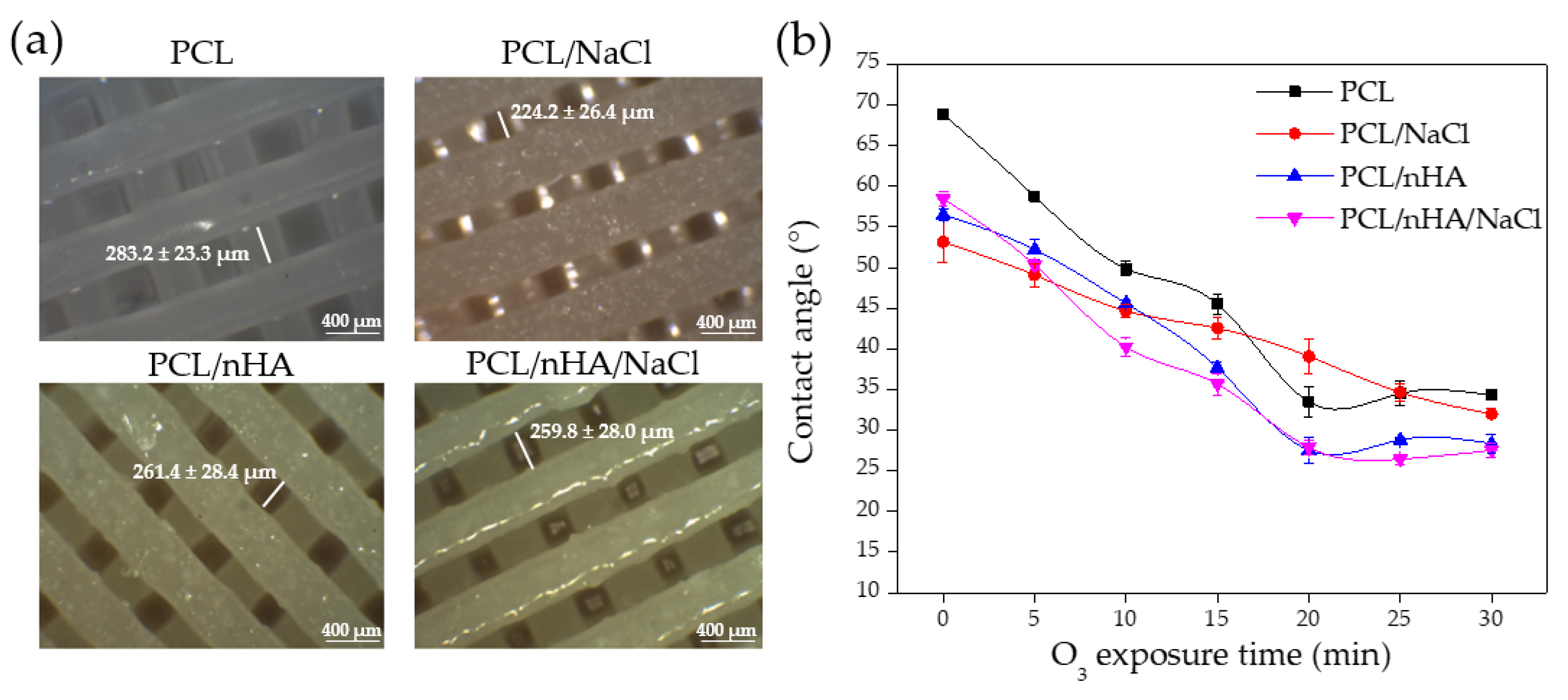
Contact Angle Analysis of the Substrates
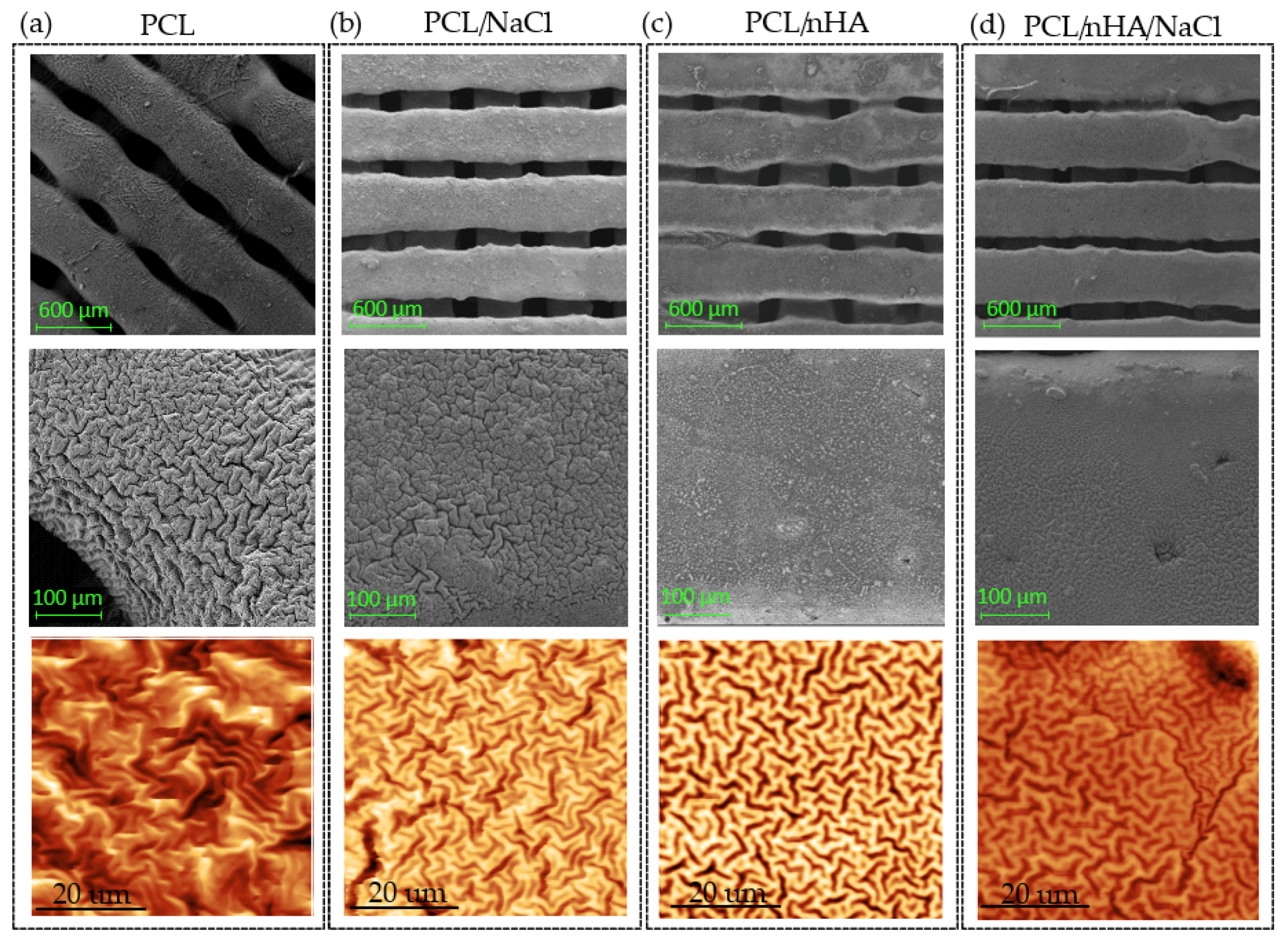
3.3. Morphological Study of the Wrinkle Patterns in the Different Substrates
3.4. Bioactive Agent Synthesis
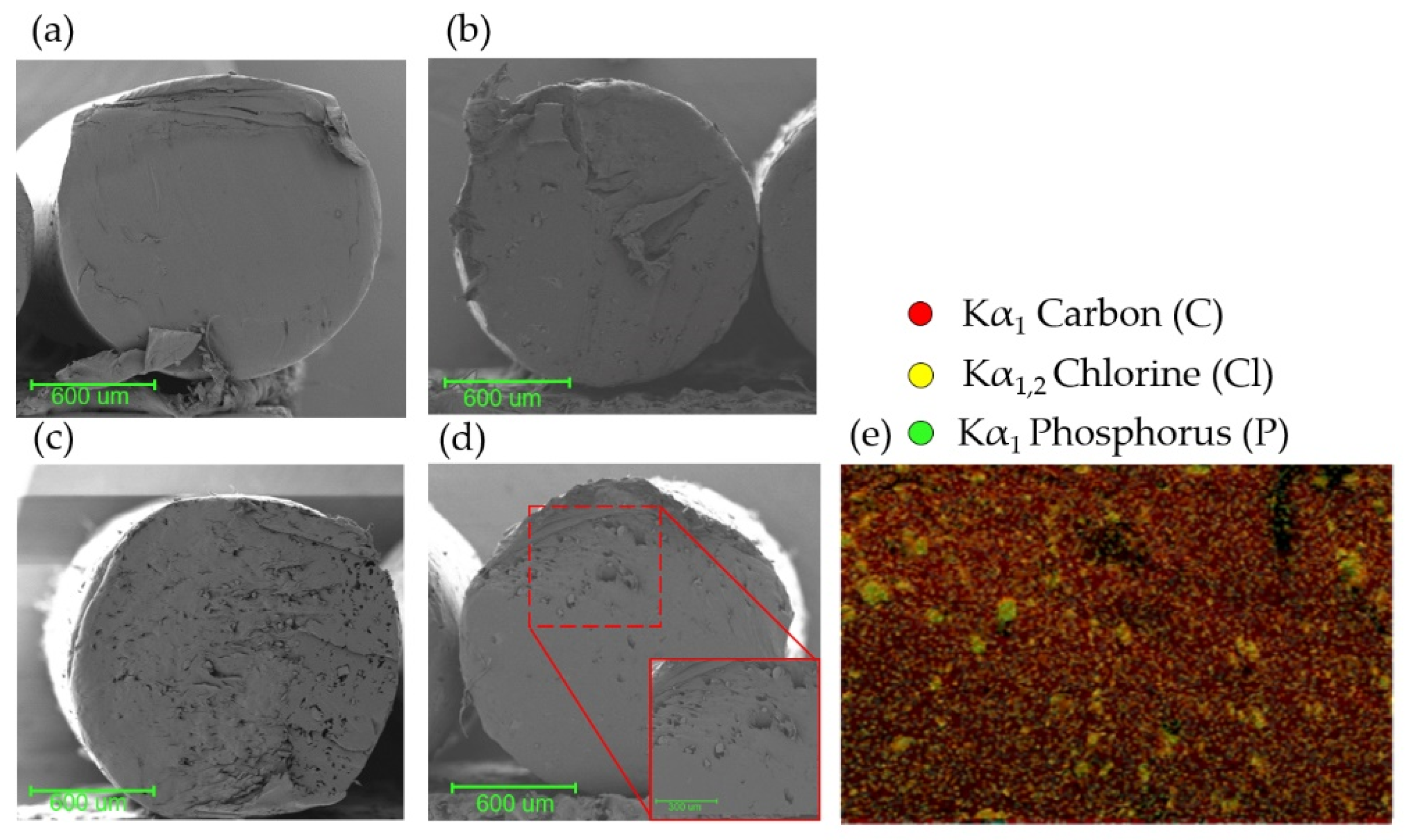
3.5. Modified Filament Characterization and Printing of 3D Pieces
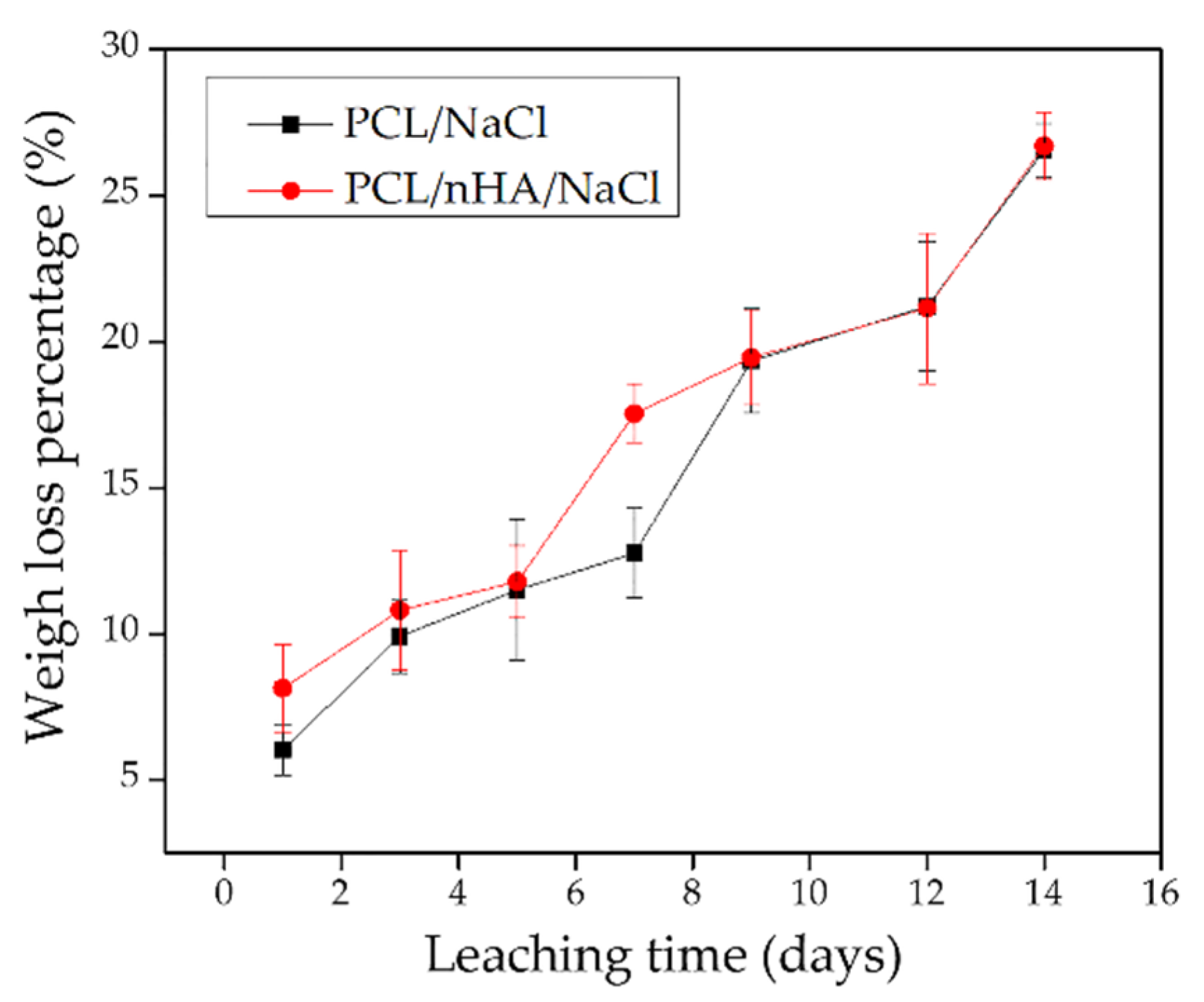
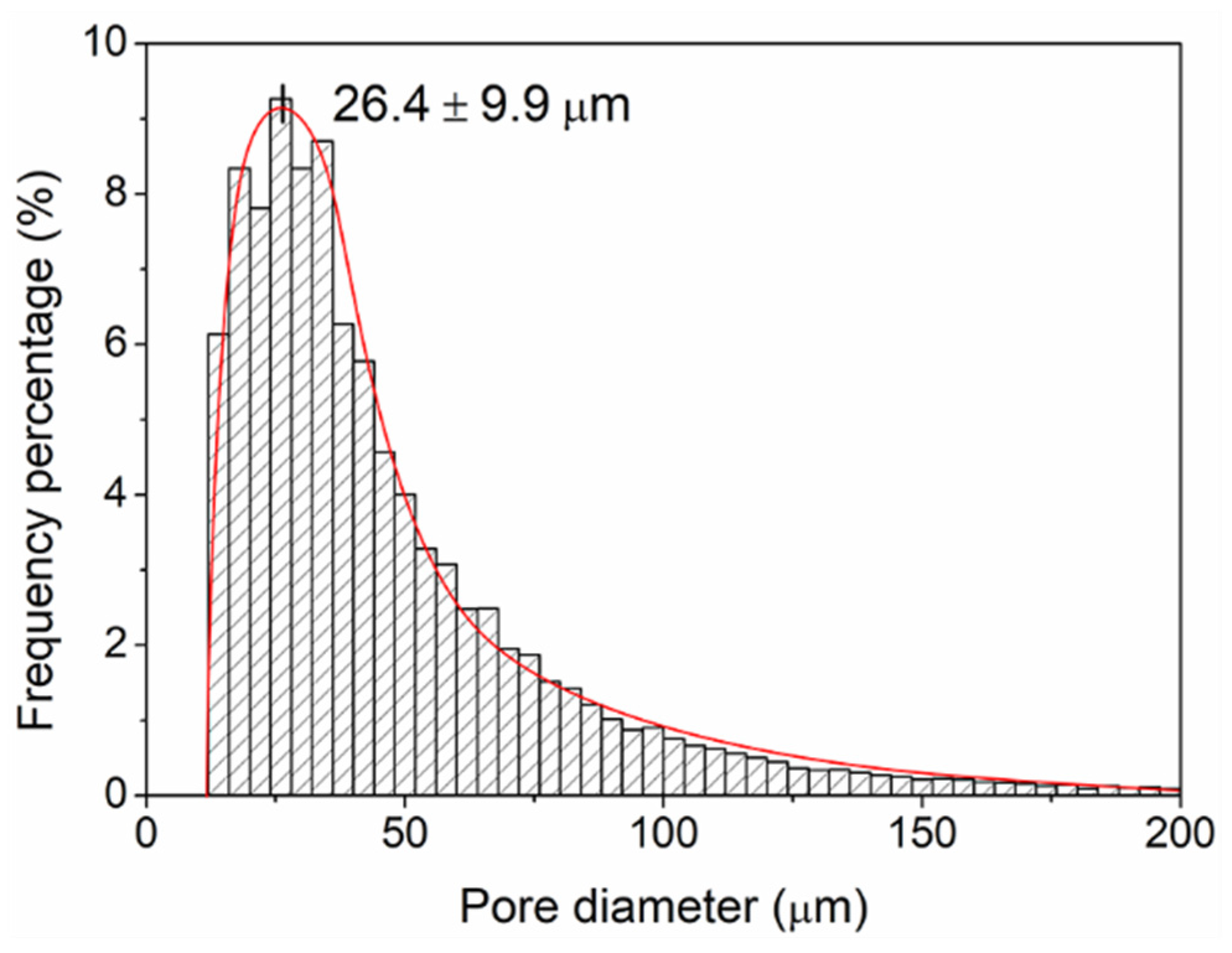
3.6. Leached 3D Pieces
3.7. Wrinkled Micropattern Analysis
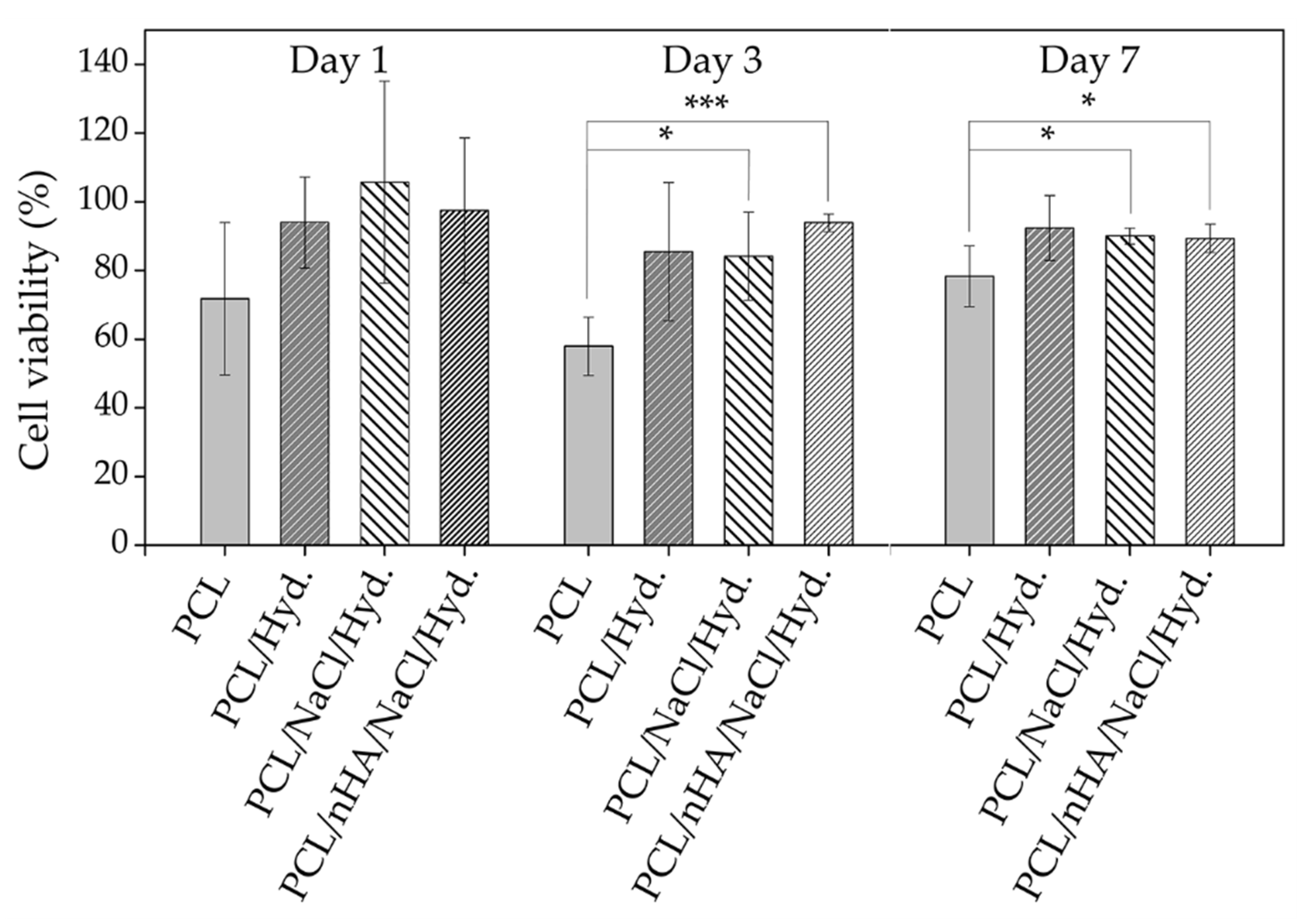
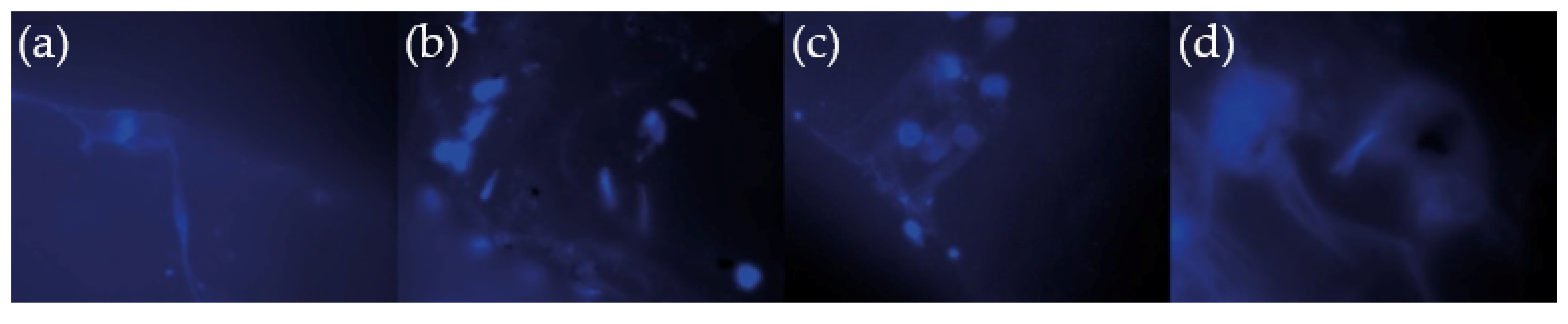
3.8. Cell Viability Studies
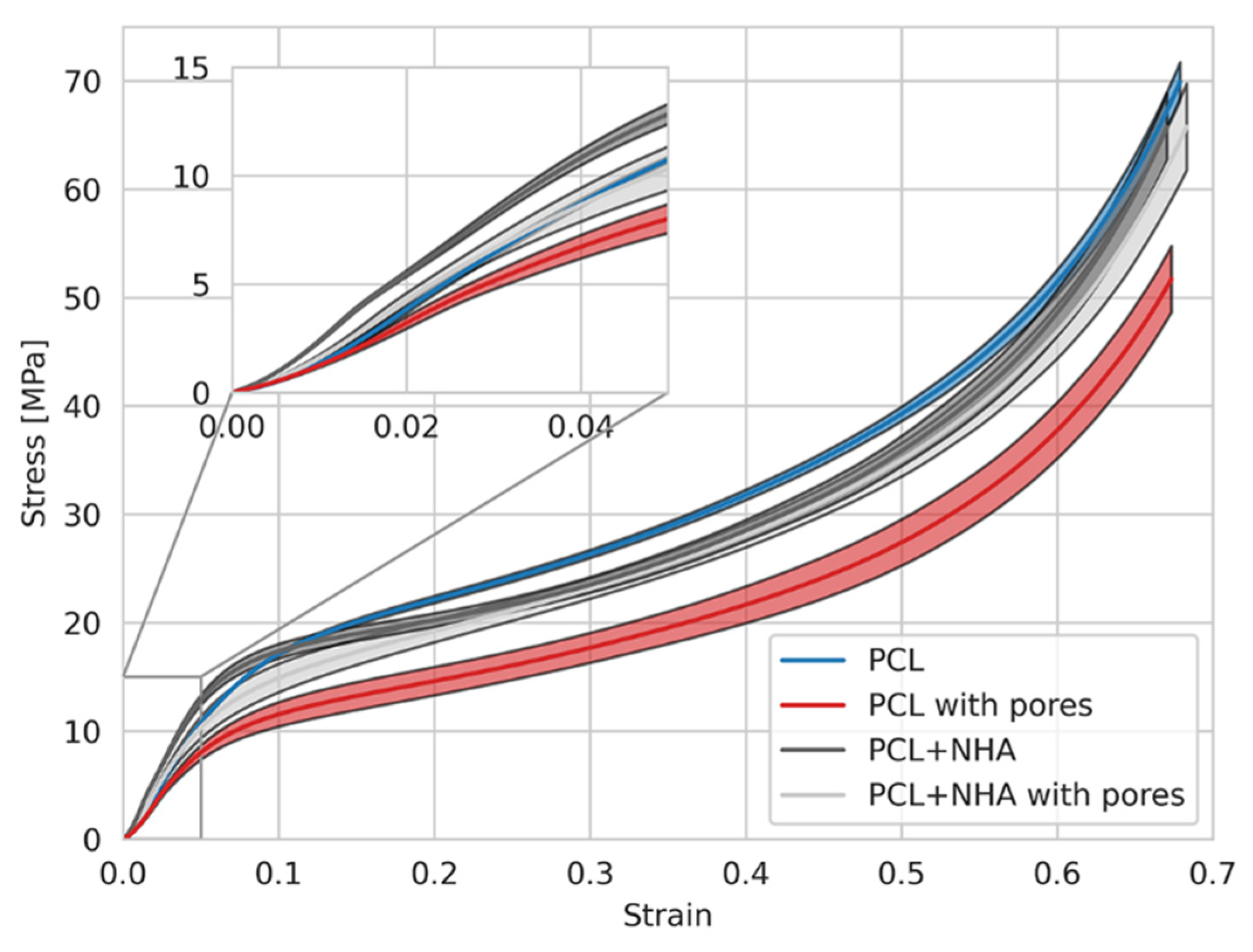
3.9. Mechanical Tests
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Ronca, D.; Langella, F.; Chierchia, M.; D’Amora, U.; Russo, T.; Domingos, M.; Gloria, A.; Bartolo, P.; Ambrosio, L. Bone Tissue Engineering: 3D PCL-based Nanocomposite Scaffolds with Tailored Properties. Procedia CIRP 2016, 49, 51–54. [Google Scholar] [CrossRef]
- Buckwalter, J.A. Articular cartilage: Injuries and potential for healing. J. Orthop. Sports Phys. Ther. 1998, 28, 192–202. [Google Scholar] [CrossRef]
- Jiao, C.; Xie, D.; He, Z.; Liang, H.; Shen, L.; Yang, Y.; Tian, Z.; Wu, G.; Wang, C. Additive manufacturing of Bio-inspired ceramic bone Scaffolds: Structural Design, mechanical properties and biocompatibility. Mater. Des. 2022, 217, 110610. [Google Scholar] [CrossRef]
- Hasirci, V.; Hasirci, N. Tissue Engineering and Regenerative Medicine. In Fundamentals of Biomaterials; Springer: New York, NY, USA, 2018; pp. 281–302. ISBN 978-1-4939-8854-9. [Google Scholar]
- Pereira, H.; Frias, A.M.; Oliveira, J.M.; Espregueira-Mendes, J.; Reis, R.L. Tissue engineering and regenerative medicine strategies in meniscus lesions. Arthrosc. J. Arthrosc. Relat. Surg. 2011, 27, 1706–1719. [Google Scholar] [CrossRef] [PubMed]
- Jammalamadaka, U.; Tappa, K. Recent advances in biomaterials for 3D printing and tissue engineering. J. Funct. Biomater. 2018, 9, 22. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Zhang, B.; Li, M.; Li, J.; Zhang, C.; Han, Y.; Wang, L.; Wang, K.; Zhou, C.; Liu, L.; et al. 3D printing of PLA/n-HA composite scaffolds with customized mechanical properties and biological functions for bone tissue engineering. Compos. Part B Eng. 2021, 224, 109192. [Google Scholar] [CrossRef]
- Gómez-Lizárraga, K.K.; Flores-Morales, C.; Del Prado-Audelo, M.L.; Álvarez-Pérez, M.A.; Piña-Barba, M.C.; Escobedo, C. Polycaprolactone- and polycaprolactone/ceramic-based 3D-bioplotted porous scaffolds for bone regeneration: A comparative study. Mater. Sci. Eng. C 2017, 79, 326–335. [Google Scholar] [CrossRef]
- Kim, C.G.; Han, K.S.; Lee, S.; Kim, M.C.; Kim, S.Y.; Nah, J. Fabrication of biocompatible polycaprolactone–hydroxyapatite composite filaments for the FDM 3D printing of bone scaffolds. Appl. Sci. 2021, 11, 6351. [Google Scholar] [CrossRef]
- Totaro, A.; Salerno, A.; Imparato, G.; Domingo, C.; Urciuolo, F.; Netti, P.A. PCL–HA microscaffolds for in vitro modular bone tissue engineering. J. Tissue Eng. Regen. Med. 2017, 11, 1865–1875. [Google Scholar] [CrossRef]
- Rezaei, A.; Mohammadi, M.R. In vitro study of hydroxyapatite/polycaprolactone (HA/PCL) nanocomposite synthesized by an in situ sol-gel process. Mater. Sci. Eng. C 2013, 33, 390–396. [Google Scholar] [CrossRef]
- Zimmerling, A.; Yazdanpanah, Z.; Cooper, D.M.L.; Johnston, J.D.; Chen, X. 3D printing PCL/nHA bone scaffolds: Exploring the influence of material synthesis techniques. Biomater. Res. 2021, 25, 3. [Google Scholar] [CrossRef] [PubMed]
- Gibson, I.; Rosen, D.; Stucker, B. Additive Manufacturing Technologies; Springer: New York, NY, USA, 2015; Volume 9, ISBN 978-1-4939-2112-6. [Google Scholar]
- Cho, Y.S.; Yang, S.; Choi, E.; Kim, K.H.; Gwak, S.J. Fabrication of a porous hydroxyapatite scaffold with enhanced human osteoblast-like cell response via digital light processing system and biomimetic mineralization. Ceram. Int. 2021, 47, 35134–35143. [Google Scholar] [CrossRef]
- González-Henríquez, C.M.; Sarabia-Vallejos, M.A.; Rodriguez-Hernandez, J. Polymers for additive manufacturing and 4D-printing: Materials, methodologies, and biomedical applications. Prog. Polym. Sci. 2019, 94, 57–116. [Google Scholar] [CrossRef]
- Sohrabian, M.; Vaseghi, M.; Khaleghi, H.; Dehrooyeh, S.; Kohan, M.S.A. Structural Investigation of Delicate-Geometry Fused Deposition Modeling Additive Manufacturing Scaffolds: Experiment and Analytics. J. Mater. Eng. Perform. 2021, 30, 6529–6541. [Google Scholar] [CrossRef]
- González-Henríquez, C.M.; Rodríguez-Umanzor, F.E.; Alegría-Gómez, M.N.; Terraza-Inostroza, C.A.; Martínez-Campos, E.; Cue-López, R.; Sarabia-Vallejos, M.A.; García-Herrera, C.; Rodríguez-Hernández, J. Wrinkling on Stimuli-Responsive Functional Polymer Surfaces as a Promising Strategy for the Preparation of Effective Antibacterial/Antibiofouling Surfaces. Polymers 2021, 13, 4262. [Google Scholar] [CrossRef]
- Nemani, S.K.; Annavarapu, R.K.; Mohammadian, B.; Raiyan, A.; Heil, J.; Haque, M.A.; Abdelaal, A.; Sojoudi, H. Surface Modification of Polymers: Methods and Applications. Adv. Mater. Interfaces 2018, 5, 1801247. [Google Scholar] [CrossRef]
- Toosi, S.F.; Moradi, S.; Hatzikiriakos, S. Fabrication of Micro/Nano Patterns on Polymeric Substrates Using Laser Ablation Methods to Control Wettability Behaviour: A Critical Review. Rev. Adhes. Adhes. 2017, 5, 55–78. [Google Scholar] [CrossRef]
- González-Henríquez, C.M.; Rodriguez-Umanzor, F.E.; Almagro-Correa, J.; Sarabia-Vallejos, M.A.; Martínez-Campos, E.; Esteban-Lucía, M.; del Campo-García, A.; Rodríguez-Hernández, J. Biocompatible fluorinated wrinkled hydrogel films with antimicrobial activity. Mater. Sci. Eng. C 2020, 114, 111031. [Google Scholar] [CrossRef]
- Zou, J.; Wu, S.; Chen, J.; Lei, X.; Li, Q.; Yu, H.; Tang, S.; Ye, D. Highly Efficient and Environmentally Friendly Fabrication of Robust, Programmable, and Biocompatible Anisotropic, All-Cellulose, Wrinkle-Patterned Hydrogels for Cell Alignment. Adv. Mater. 2019, 31, 1904762. [Google Scholar] [CrossRef]
- Izawa, H.; Okuda, N.; Yonemura, T.; Kuroda, K.; Ochi, K.; Ifuku, S.; Morimoto, M.; Saimoto, H.; Noda, M.; Azuma, K.; et al. Application of Bio-Based Wrinkled Surfaces as Cell Culture Scaffolds. Colloids Interfaces 2018, 2, 15. [Google Scholar] [CrossRef]
- González-Henríquez, C.M.; Rodríguez-Hernández, J. Wrinkled Polymer Surfaces; González-Henríquez, C.M., Rodríguez-Hernández, J., Eds.; Springer International Publishing: Cham, Switzerland, 2019; ISBN 978-3-030-05122-8. [Google Scholar]
- Rodríguez-Hernández, J. Wrinkled interfaces: Taking advantage of surface instabilities to pattern polymer surfaces. Prog. Polym. Sci. 2015, 42, 1–41. [Google Scholar] [CrossRef]
- González-Henríquez, C.M.; Rodriguez-Umanzor, F.E.; Sarabia-Vallejos, M.A.; Terraza, C.A.; Martínez-Campos, E.; Rodriguez-Hernandez, J. Innovative procedure for precise deposition of wrinkled hydrogel films using direct inkjet printing. Mater. Des. 2020, 194, 108959. [Google Scholar] [CrossRef]
- Klapetek, P.; Valtr, M.; Nečas, D.; Salyk, O.; Dzik, P. Atomic force microscopy analysis of nanoparticles in non-ideal conditions. Nanoscale Res. Lett. 2011, 6, 514. [Google Scholar] [CrossRef] [PubMed]
- Lejnieks, J.; Mourran, A.; Tillmann, W.; Keul, H.; Möller, M. Thin film of poly(acrylic acid-co-allyl acrylate) as a sacrificial protective layer for hydrophilic self cleaning glass. Materials 2010, 3, 3369–3384. [Google Scholar] [CrossRef]
- Karampour, H.; Parsa, M.A.; Moghadam, A.H.; Pourhasan, B.; Ashiri, R. Facile solution-based synthesis of impurity-free hydroxyapatite nanocrystals at ambient conditions. J. Mater. Res. Technol. 2022, 16, 656–674. [Google Scholar] [CrossRef]
- Griffin, D.R.; Weaver, W.M.; Scumpia, P.O.; Di Carlo, D.; Segura, T. Accelerated wound healing by injectable microporous gel scaffolds assembled from annealed building blocks. Nat. Mater. 2015, 14, 737–744. [Google Scholar] [CrossRef]
- de Rutte, J.M.; Koh, J.; Di Carlo, D. Scalable High-Throughput Production of Modular Microgels for In Situ Assembly of Microporous Tissue Scaffolds. Adv. Funct. Mater. 2019, 29, 1900071. [Google Scholar] [CrossRef]
- Xie, Y.; Moreno, N.; Calo, V.M.; Cheng, H.; Hong, P.-Y.; Sougrat, R.; Behzad, A.R.; Tayouo, R.; Nunes, S.P. Synthesis of highly porous poly(tert-butyl acrylate)-b-polysulfone-b-poly(tert-butyl acrylate) asymmetric membranes. Polym. Chem. 2016, 7, 3076–3089. [Google Scholar] [CrossRef]
- Davis, K.A.; Matyjaszewski, K. Atom Transfer Radical Polymerization of tert -Butyl Acrylate and Preparation of Block Copolymers. Macromolecules 2000, 33, 4039–4047. [Google Scholar] [CrossRef]
- Greving, N.; Keul, H.; Millaruelo, M.; Weberskirch, R.; Moeller, M. Synthesis of α,ω-isocyanate-telechelic poly(methyl methacrylate-co-allyl methacrylate) soft segments. Eur. Polym. J. 2013, 49, 235–246. [Google Scholar] [CrossRef]
- Song, B.; Walczyk, W.; Schönherr, H. Contact Angles of Surface Nanobubbles on Mixed Self-Assembled Monolayers with Systematically Varied Macroscopic Wettability by Atomic Force Microscopy. Langmuir 2011, 27, 8223–8232. [Google Scholar] [CrossRef] [PubMed]
- Howlader, M.M.R.; Kibria, M.G.; Zhang, F.; Kim, M.J. Hybrid plasma bonding for void-free strong bonded interface of silicon/glass at 200 °C. Talanta 2010, 82, 508–515. [Google Scholar] [CrossRef] [PubMed]
- Francis, M. Mirabella Internal Reflection Spectroscopy; CRC Press: Boca Raton, FL, USA, 1993; ISBN 0824787307. [Google Scholar]
- Murakami, T.N.; Fukushima, Y.; Hirano, Y.; Tokuoka, Y.; Takahashi, M.; Kawashima, N. Surface modification of polystyrene and poly(methyl methacrylate) by active oxygen treatment. Colloids Surfaces B Biointerfaces 2003, 29, 171–179. [Google Scholar] [CrossRef]
- Youssef, W.; Wickett, R.R.; Hoath, S.B. Surface free energy characterization of vernix caseosa. Potential role in waterproofing the newborn infant. Ski. Res. Technol. 2001, 7, 10–17. [Google Scholar] [CrossRef]
- Ensikat, H.J.; Ditsche-Kuru, P.; Neinhuis, C.; Barthlott, W. Superhydrophobicity in perfection: The outstanding properties of the lotus leaf. Beilstein J. Nanotechnol. 2011, 2, 152–161. [Google Scholar] [CrossRef] [PubMed]
- Higham, T.E.; Russell, A.P.; Niewiarowski, P.H.; Wright, A.; Speck, T. The Ecomechanics of Gecko Adhesion: Natural Surface Topography, Evolution, and Biomimetics. Integr. Comp. Biol. 2019, 59, 148–167. [Google Scholar] [CrossRef]
- Dean, B.; Bhushan, B. Shark-skin surfaces for fluid-drag reduction in turbulent flow: A review. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2010, 368, 4775–4806. [Google Scholar] [CrossRef]
- Bi, Y.; Huang, M. Characterization and Analysis of Nano-hydroxy Apatite Material. In Advanced Material Engineering; WORLD SCIENTIFIC: Singapore, 2015; pp. 582–589. [Google Scholar]
- Kalita, S.J.; Verma, S. Nanocrystalline hydroxyapatite bioceramic using microwave radiation: Synthesis and characterization. Mater. Sci. Eng. C 2010, 30, 295–303. [Google Scholar] [CrossRef]
- Yelten, A.; Yilmaz, S. Various Parameters Affecting the Synthesis of the Hydroxyapatite Powders by the Wet Chemical Precipitation Technique. Mater. Today Proc. 2016, 3, 2869–2876. [Google Scholar] [CrossRef]
- Schantz, J.-T.; Brandwood, A.; Hutmacher, D.W.; Khor, H.L.; Bittner, K. Osteogenic differentiation of mesenchymal progenitor cells in computer designed fibrin-polymer-ceramic scaffolds manufactured by fused deposition modeling. J. Mater. Sci. Mater. Med. 2005, 16, 807–819. [Google Scholar] [CrossRef]
- Shor, L.; Güçeri, S.; Wen, X.; Gandhi, M.; Sun, W. Fabrication of three-dimensional polycaprolactone/hydroxyapatite tissue scaffolds and osteoblast-scaffold interactions in vitro. Biomaterials 2007, 28, 5291–5297. [Google Scholar] [CrossRef] [PubMed]
- Draganov, I.R.; Mironov, R.P. 3D SUSAN Filtering of CT Images with Adaptive Selection of the Brightness Threshold. In Proceedings of the 2021 56th International Scientific Conference on Information, Communication and Energy Systems and Technologies (ICEST), Sozopol, Bulgaria, 16–18 June 2021; pp. 89–92. [Google Scholar] [CrossRef]
- Sarabia-Vallejos, M.A.; Ayala-Jeria, P.; Hurtado, D.E. Three-Dimensional Whole-Organ Characterization of the Regional Alveolar Morphology in Normal Murine Lungs. Front. Physiol. 2021, 12, 755468. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Wang, L.; Song, P.; Pei, X.; Sun, H.; Wu, L.; Zhou, C.; Wang, K.; Fan, Y.; Zhang, X. 3D printed bone tissue regenerative PLA/HA scaffolds with comprehensive performance optimizations. Mater. Des. 2021, 201, 109490. [Google Scholar] [CrossRef]
- Chen, Y.; Li, Z.; Wen, S.; Yang, Q.; Zhang, L.; Zhong, C.; Liu, L. Molecular simulation study of role of polymer–particle interactions in the strain-dependent viscoelasticity of elastomers (Payne effect). J. Chem. Phys. 2014, 141, 104901. [Google Scholar] [CrossRef]
- Fabbri, P.; Bondioli, F.; Messori, M.; Bartoli, C.; Dinucci, D.; Chiellini, F. Porous scaffolds of polycaprolactone reinforced with in situ generated hydroxyapatite for bone tissue engineering. J. Mater. Sci. Mater. Med. 2010, 21, 343–351. [Google Scholar] [CrossRef]
- Cui, Z.; Nelson, B.; Peng, Y.; Li, K.; Pilla, S.; Li, W.-J.; Turng, L.-S.; Shen, C. Fabrication and characterization of injection molded poly (ε-caprolactone) and poly (ε-caprolactone)/hydroxyapatite scaffolds for tissue engineering. Mater. Sci. Eng. C 2012, 32, 1674–1681. [Google Scholar] [CrossRef] [PubMed]





| Filament | PCL (g) | nHA (g) 10% | NaCl (g) 30% |
|---|---|---|---|
| PCL | 40.0 | 0 | 0 |
| PCL/nHA | 40.0 | 4.5 | 0 |
| PCL/NaCl | 40.0 | 0 | 16.0 |
| PCL/nHA/NaCl | 40.0 | 4.5 | 18.0 |
| Substrate | Contact Angle (°) | Surface Tension Components (dyne/cm) | Total, SFE (dyne/cm) | |||
|---|---|---|---|---|---|---|
| Water | Glycerol Anhydrous | Diiodomethane | γsD | γsP | ||
| PLA | 29.53 ± 0.80 | 50.91 ± 0.64 | 23.53 ± 0.80 | 38.70 | 26.95 | 65.65 |
| PCL | 33.50 ± 1.00 | 55.81 ± 1.77 | 23.11 ± 0.72 | 37.32 | 21.54 | 58.86 |
| HIPS | 22.15 ± 2.41 | 47.89 ± 2.39 | 11.70 ± 0.80 | 40.98 | 28.40 | 69.38 |
| TPU | 27.11 ± 0.68 | 71.15 ± 0.23 | 22.80 ± 0.43 | 22.65 | 33.81 | 56.46 |
| Young Modulus (Mpa) | |
|---|---|
| PCL | 257.15 ± 7.70 |
| PCL/nHA | 283.68 ± 16.84 |
| PCL/NaCl | 202.94 ± 22.40 |
| PCL/nHA/NaCl | 253.84 ± 25.27 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
González-Henríquez, C.M.; Rodríguez-Umanzor, F.E.; Acuña-Ruiz, N.F.; Vera-Rojas, G.E.; Terraza-Inostroza, C.; Cohn-Inostroza, N.A.; Utrera, A.; Sarabia-Vallejos, M.A.; Rodríguez-Hernández, J. Fabrication and Testing of Multi-Hierarchical Porous Scaffolds Designed for Bone Regeneration via Additive Manufacturing Processes. Polymers 2022, 14, 4041. https://doi.org/10.3390/polym14194041
González-Henríquez CM, Rodríguez-Umanzor FE, Acuña-Ruiz NF, Vera-Rojas GE, Terraza-Inostroza C, Cohn-Inostroza NA, Utrera A, Sarabia-Vallejos MA, Rodríguez-Hernández J. Fabrication and Testing of Multi-Hierarchical Porous Scaffolds Designed for Bone Regeneration via Additive Manufacturing Processes. Polymers. 2022; 14(19):4041. https://doi.org/10.3390/polym14194041
Chicago/Turabian StyleGonzález-Henríquez, Carmen M., Fernando E. Rodríguez-Umanzor, Nicolas F. Acuña-Ruiz, Gloria E. Vera-Rojas, Claudio Terraza-Inostroza, Nicolas A. Cohn-Inostroza, Andrés Utrera, Mauricio A. Sarabia-Vallejos, and Juan Rodríguez-Hernández. 2022. "Fabrication and Testing of Multi-Hierarchical Porous Scaffolds Designed for Bone Regeneration via Additive Manufacturing Processes" Polymers 14, no. 19: 4041. https://doi.org/10.3390/polym14194041
APA StyleGonzález-Henríquez, C. M., Rodríguez-Umanzor, F. E., Acuña-Ruiz, N. F., Vera-Rojas, G. E., Terraza-Inostroza, C., Cohn-Inostroza, N. A., Utrera, A., Sarabia-Vallejos, M. A., & Rodríguez-Hernández, J. (2022). Fabrication and Testing of Multi-Hierarchical Porous Scaffolds Designed for Bone Regeneration via Additive Manufacturing Processes. Polymers, 14(19), 4041. https://doi.org/10.3390/polym14194041








