Abstract
Stretchable, adhesive, and conductive hydrogels have been regarded as ideal interfacial materials for seamless and biocompatible integration with the human body. However, existing hydrogels can rarely achieve good mechanical, electrical, and adhesive properties simultaneously, as well as limited patterning/manufacturing techniques posing severe challenges to bioelectronic research and their practical applications. Herein, we develop a stretchable, adhesive, and conductive Ti3C2Tx-polyacrylic acid hydrogel by a simple pre-crosslinking method followed by successive direct ink writing 3D printing. Pre-polymerization of acrylic acid can be initiated by mechanical mixing with Ti3C2Tx nanosheet suspension, leading to the formation of viscous 3D printable ink. Secondary free radical polymerization of the ink patterns via 3D printing can achieve a stretchable, adhesive, and conductive Ti3C2Tx-polyacrylic acid hydrogel. The as-formed hydrogel exhibits remarkable stretchability (~622%), high electrical conductivity (5.13 S m−1), and good adhesion strength on varying substrates. We further demonstrate the capability of facilely printing such hydrogels into complex geometries like mesh and rhombus patterns with high resolution and robust integration.
1. Introduction
Bioelectronic interfacing with the human body has become a bridge to record/stimulate physiological information in our daily lives. Currently, most bioelectronic devices are fabricated from inorganic materials with proper electrical conductivity, such as metals and silicon [1,2,3]. However, these bioelectronic devices based on inorganic materials are limited by mechanical mismatches with biological tissues, leading to unstable and uncomfortable signal collection/stimulation [4,5,6]. Compared to traditional inorganic materials, tissue-like soft materials can replace traditional rigid electronics to improve compliance and improve performance to suffice for practical applications [3,6,7,8,9].
Hydrogels have been extensively explored in bioelectronics because of their tissue-like mechanical properties and tunable functionalities. Most recently, an increasing number of functional hydrogels have been employed for the fabrication/integration of bioelectronic devices [6,7,10]. Among them, stretchable, adhesive, and conductive hydrogels have been regarded as ideal candidates in human-machine interfaces [11]. However, the development of such hydrogels is still a considerable challenge due to the trade-offs among varying properties.
One of the effective strategies to realize stretchable, adhesive, and conductive hydrogels is rational compositing of highly conductive nanofillers within the hydrogel matrix. MXene is an emerging family of 2D materials with a general formula of Mn+1XnTx, which are obtained by selective etching A-group (generally group IIIA and IVA elements) layers from the MAX phases [12,13,14]. Typically, the “M” is a transition metal, “X” is carbon and/or nitrogen, and “T” represents the surface functional groups (=O, -OH, and -F). Particularly, Ti3C2Tx, as a representative MXene material, possesses high electrical conductivity (10,000 S cm−1), superior hydrophilicity [15,16], as well as good mechanical performance due to the presence of M-N or M-C bonds. Functional groups (=O, -OH, and -F) on the MXene surface [17] enables MXenes with excellent dispersibility, which can be processed by multiple manufacturing techniques [18,19,20], involving screen-printing [21,22], stamping [23], and spraying [24]. However, these processing techniques are usually limited by multiple factors, such as low-resolution, two-dimensional, and low aspect ratio [25]. Direct ink writing 3D printing is an advanced additive manufacturing technology that offers the capability to fabricate geometrically freeform 3D structures [26,27]. Although some interesting efforts have been devoted to develop Mxene-based printable inks [28,29,30], 3D printing of Mxene-based hydrogels simultaneously with good mechanical, electrical, and adhesive properties have been rarely investigated.
In this work, we prepare a stretchable, conductive, and adhesive Ti3C2Tx-polyacrylic acid (PAA) hydrogel by using a simple pre-crosslinking method followed by direct ink writing 3D printing. Our strategy is to employ Ti3C2Tx nanosheet aqueous suspension to initiate the pre-polymerization of acrylic acid monomers. Ti3C2Tx-PAA hydrogels can be further achieved by secondary radical polymerization of 3D printed ink patterns. The resultant Ti3C2Tx-PAA hydrogel exhibits high stretchability (~622%), high electrical conductivity (5.13 S m−1), and strong adhesion on varying substrates. The flexibility of 3D printing technology enables facile patterning of complex geometries like mesh and rhombus patterns with high resolution and robust integration. Based on these merits, Ti3C2Tx-PAA hydrogels are potential material candidates for biomedical applications [30,31,32].
2. Materials and Methods
2.1. Materials
Acrylic acid (AA, 99%; Shanghai Vita, Shanghai, China), ammonium persulfate (APS, ≥98%; Aladdin, Shanghai, China), lithium fluoride (LiF, 97%; J&K Scientific, Beijing, China), hydrochloric acid (HCl, 35–38%; Shanghai Vita, Shanghai, China), phosphate buffer saline (PBS, pH = 7.4; Howei Pharm, Guangzhou, China)), and Ti3AlC2 were purchased from 11 Technolog Co, Jilin, China.
Preparation of Ti3C2Tx MXene nanosheets. MXene was prepared by a selective etching method with LiF/HCl as the etching solution. Notably, the etching solution was composed of LiF (1 g) and HCl (20 mL, 9 M). After the etching solution was stirred for 5 min at room temperature, Ti3AlC2 powder (1 g) was slowly added to the etching solution and stirred at 35 °C for 24 h. When the reaction was completed, the acid suspension was repeatedly washed with deionized water 6 times and centrifuged at 6000 rpm for 2 min. Deionized water was added to the collected sediment, followed by the suspension being sonicated for 1 h and centrifuged at 2000 rpm for 30 min. Subsequently, a dark green MXene colloidal dispersion was collected [33]. Redispersible MXene powder can be obtained by drying MXene colloidal dispersion at 60 °C for 6 h. Such powder (0.1 g) was added to deionized water (1.33 g) and sonicated for 30 min to obtain aqueous MXene nanosheet suspension.
Preparation of 3D printable Ti3C2Tx-PAA inks. MXene suspension and AA monomers were mixed and filtered with a syringe filter (40 µm) to obtain viscous 3D printable Ti3C2Tx-PAA inks via pre-crosslinking at the optimal time (Figure S1). The detailed composition of different Ti3C2Tx-PAA inks is listed in Table S1.
3D printing of Ti3C2Tx-PAA inks. Direct ink writing 3D printing of Ti3C2Tx-PAA inks was conducted based on a 3D printer (DB 100, Shanghai Mifang Electronic Technology, Shanghai, China) with a 160-µm nozzle. The printing pressure and speed were 30 kPa and 40 mm s−1, respectively. Printing pattern paths were generated by AI drawings and converted into SVG. The detailed printing paths are shown in Figure S2. After printing, 3D-printed Ti3C2Tx-PAA patterns were put into APS solution for 10 min to yield Ti3C2Tx-PAA hydrogels via secondary radical polymerization of AA oligomer.
2.2. Characterization
Mechanical characterization. Mechanical properties of Ti3C2Tx-PAA hydrogels were performed by using a universal testing machine (Zhiqu-990L, ZHIQU Precision Instrument, Guangzhou, China) equipped with a U-stretch 5 N load cell at 100 mm min−1 rate.
Electrical conductivity measurement. Electrical conductivity of Ti3C2Tx-PAA hydrogels was measured by using a standard four-point probe (Keithley 2700 digital multimeter, Keithley, Beaverton, OG, USA). Ti3C2Tx-PAA hydrogels were cut into rectangle shapes (12 mm in length and 5 mm in width). Copper electrodes were adhered onto the surface of hydrogels by applying silver paste.
Electrochemical properties. Charge injection capacity (CIC) and electrochemical impedance spectroscopy (EIS) measurements of Ti3C2Tx-PAA hydrogels were carried out by using a Gamry instrument (Interface 1010, Gamry instruments, Warminster, PA, USA). The samples were attached onto platinum substrate. All measurements were obtained using a three-electrode configuration, e.g., Ti3C2Tx-PAA hydrogel as the working electrode, platinum wires as the counter electrode, and Ag/AgCl electrode as the reference electrode with PBS as the electrolyte.
Adhesive properties. The adhesion properties of Ti3C2Tx-PAA hydrogels were evaluated through lap shear tests based on the ASTM F2255-05 standard by using a universal testing machine (ZQ-990LB, ZHIQU Precision Instrument, Dongguan, China) at the testing speed of 10 mm min−1. The adhesion strength was determined by dividing the maximum separation force by the contact area.
3. Results and Discussion
3.1. Design and Preparation of Ti3C2Tx-PAA Hydrogels
To prepare a stretchable, adhesive, and conductive hydrogel, we present a pre-crosslinking strategy to pre-polymerize AA monomer within Ti3C2Tx nanosheets (Figure 1a), leading to the viscosity increase and the formation of viscous 3D printable inks (Figure 1b). With increasing concentration of Ti3C2Tx nanosheet suspension, AA can polymerize gradually, resulting in the transition of mixed solution into viscous 3D printable pastes (Figure S1). This is mainly due to the catalytic effect of Ti3C2Tx and also hydrogen bonding between hydroxyl groups in Ti3C2Tx and carboxylic groups of AA or PAA chains [34].
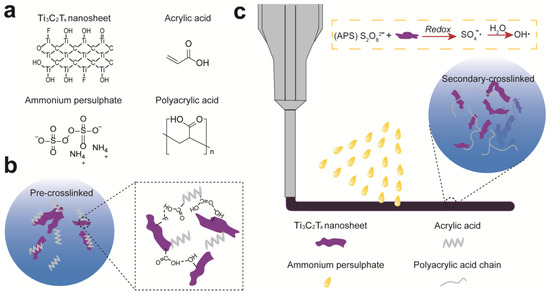
Figure 1.
Schematic illustration for preparing Ti3C2Tx-PAA hydrogels via 3D printing. (a) Chemical structures of Ti3C2Tx nanosheets, AA, APS, and PAA. (b) Pre-polymerization of AA with Ti3C2Tx nanosheets. (c) 3D printing and secondary-crosslinking of Ti3C2Tx-PAA hydrogels with APS.
After 3D printing, prepolymerized hydrogel patterns are further oxidized by putting them into APS solution for 10 min to obtain fully crosslinked Ti3C2Tx-PAA hydrogels (Figure 1c). During the soaking process, a redox reaction between the reductive (Ti3C2Tx nanosheets) and the initiator (APS) results in plenty of sulfate radical () generated from APS. Subsequently, hydrolyzes a large amount of hydroxyl radicals (), which accelerates the polymerization of AA monomers or oligomers to form PAA chains [10,35]. Meanwhile, the redox reaction releases enormous heat, also facilitating the generation of free radicals for faster cross-linking [35,36].
3.2. Mechanical Performance of Ti3C2Tx-PAA Hydrogels
Since we prepare the hydrogel by directly adding AA monomers into the Ti3C2Tx dispersion, the water content of the resultant Ti3C2Tx-PAA hydrogels is controlled by the Ti3C2Tx concentration, showing rising water content from 11.71% (1 wt.% Ti3C2Tx) to 66.54% (15 wt.% Ti3C2Tx) with increasing Ti3C2Tx concentration (Table S1). To quantify the mechanical properties of Ti3C2Tx-PAA hydrogels, we systematically characterize the stress-strain curves of varying Ti3C2Tx-PAA hydrogels by tensile tests. As shown in Figure 2, Ti3C2Tx-PAA hydrogels display excellent mechanical characteristics with various Ti3C2Tx contents. Apparently, the incorporation of Ti3C2Tx dispersion dramatically decreases the ultimate strain from 622% to 101% and reduces the tensile strength from 893 kPa to 111 kPa (Figure 2a,b). This phenomenon can be ascribed to the introduction of rigid Ti3C2Tx nanosheets as well as the water content changes in Ti3C2Tx-PAA hydrogels. The Young’s modulus of the Ti3C2Tx-PAA hydrogels also declines from 795.8 kPa to 78 kPa (Figure 2c) with increasing Ti3C2Tx concentration, owing to the rising water content.
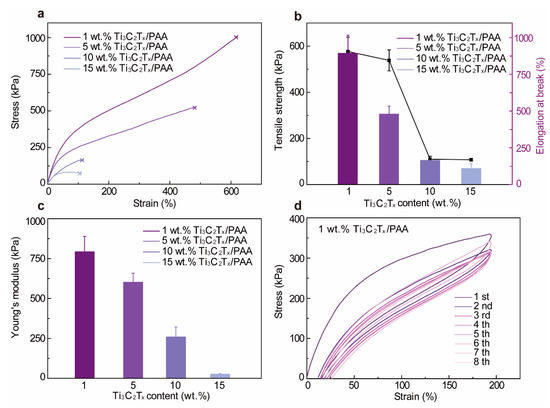
Figure 2.
Mechanical properties of Ti3C2Tx-PAA hydrogels. (a) Stress-strain curves, (b) tensile strength and elongation at the break, and (c) Young’s modulus with varying Ti3C2Tx contents. (d) Loading/unloading stress-strain curves at the strain of 200% for 1 wt.% Ti3C2Tx-PAA hydrogel.
Most hydrogels require robust mechanical performance to resist various mechanical deformations. Therefore, we further evaluate the energy dissipation capacity of 1 wt.% Ti3C2Tx-PAA hydrogel (Figure 2d). For consecutive tensile loading/unloading tests, the tensile stress decreases when increasing the cyclic time and maintains its elasticity (~88.55% of the original) after eight cycles. Evidently, the loading/unloading curve area gradually stabilizes from the second to the eighth lap, implying stable energy dissipation and further revealing the superior mechanical performance of Ti3C2Tx-PAA hydrogels. Taking advantage of these merits, the resultant Ti3C2Tx-PAA hydrogels exhibit favorable overall mechanical properties and can be easily tuned by varying the material composition.
3.3. Electrical and Electrochemical Properties of Ti3C2Tx-PAA Hydrogels
To assess the electrical conductivity of Ti3C2Tx-PAA hydrogels, we vary the Ti3C2Tx concentration in the Ti3C2Tx-PAA solution and keep the same soaking condition for 10 min in PBS solution. Evidently, when increasing the concentration of Ti3C2Tx, the electrical conductivity of Ti3C2Tx-PAA hydrogels is improved up to 5.13 S m−1 in PBS, displaying a positive linear relationship versus Ti3C2Tx concentration (Figure 3a) [37]. A significant increase in electrical conductivity is attributable to favorably connected Ti3C2Tx nanosheets for an extraordinary electron transport ability [37,38]. Notably, compared to other conductive hydrogels reported previously [39], our Ti3C2Tx-PAA hydrogel exhibits superior conductivity to most hydrogels so far (see detailed comparison in Table S2 [36,40,41,42,43]). Highly conductive Ti3C2Tx-PAA hydrogel may also be utilized for potential applications in flexible and wearable electronic devices [3,44].
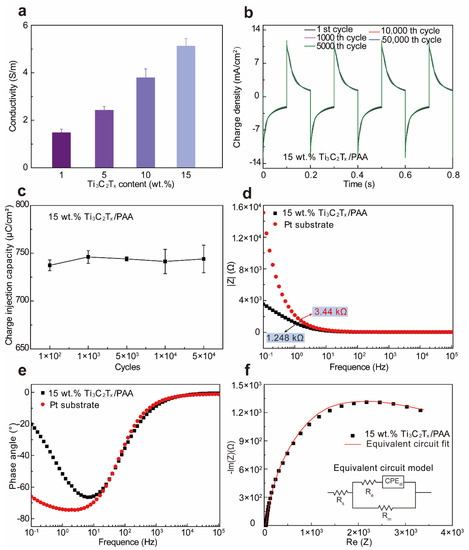
Figure 3.
Electrical conductivity and electrochemical performances of Ti3C2Tx-PAA hydrogels. (a) Electrical conductivity of Ti3C2Tx-PAA hydrogels. (b) Cyclic current pulse injection curves of the 15 wt.% Ti3C2Tx-PAA hydrogel on Pt electrode under between −1 V and 1 V (versus Ag/AgCl). (c) Charge injection capacity of the 15 wt.% Ti3C2Tx-PAA hydrogel. From the EIS characterization (versus frequency of 0.1~105 Hz), (d) plots of impedance, (e) phase angle, and (f) Nyquist plot of the 15 wt.% Ti3C2Tx-PAA hydrogel on Pt substrate are obtained. The corresponding equivalent circuit fitted values of the 15 wt.% Ti3C2Tx-PAA hydrogel are Rs = 32.47 Ω, Rm = 3678 Ω, Re = 0.6955 Ω, and CPEdl (Qp = 1.479 × 10−4 S·sn, np = 0.8315) [4,25].
To further investigate the electrochemical performance of Ti3C2Tx-PAA hydrogels, we performed the current density of the 15 wt.% Ti3C2Tx-PAA hydrogels. Under a biphasic voltage transient pulse test (±1 V voltage amplitude, 0.1 s duration), the 15 wt.% Ti3C2Tx-PAA hydrogel displays the highest charge density of ~11.82 mA cm−2 (Figure 3b). The charge injection capacity (CIC) of Ti3C2Tx-PAA hydrogel is measured to be about 742.6 ± 5 μC cm−2. Moreover, the CIC loss is less than 0.9% even after 50,000 cycles of bipolar voltage stimulation (Figure 3c), implying an excellent electrochemical stability of such hydrogels. The electrochemical impedance spectroscopy (EIS) analysis shows that the impedance of 15 wt.% Ti3C2Tx-PAA hydrogel is significantly lower than a bare Pt electrode in the frequency range of 1 Hz (Figure 3d), suggesting enhanced ion transportability in the 15 wt.% Ti3C2Tx-PAA physical hydrogel. Moreover, over a frequency range of 103~105 Hz (the high-frequency range), impedance plot of the hydrogel exhibits the phase angle (nearly 0°) as well as the value of solution resistance (Rs) that varies from 34.85 to 32.53 Ω. In light of the frequency range of 0.1~102 Hz (the low-frequency line), the 15 wt.% Ti3C2Tx-PAA hydrogel represents the electric double layer capacitance (CPEdl), membrane resistance (Rm), and charge transfer resistance (Re) (Figure 3d,e). A reasonable equivalent circuit model of the 15 wt.% Ti3C2Tx-PAA hydrogel in PBS solution (pH = 7.4) is also well fitted (Figure 3f).
3.4. Adhesion Performance of Ti3C2Tx-PAA Hydrogels
To demonstrate the extensive adhesion of hydrogels (Figure 4a), we adhere hydrogels on different substrates, including glass, metal, PTFE, rubber, plastic, wood, and pig skin. Interestingly, the Ti3C2Tx-PAA hydrogel can withstand 200 g loading cell when adhering on the substrate. To evaluate the adhesion performance of Ti3C2Tx-PAA hydrogels, we adopt standard mechanical tests to measure the adhesion strength on a range of typical substrates (Figure S3). It is found that Ti3C2Tx-PAA hydrogels generally exhibit good adhesion strengths on varying substrates (2.13 kPa for glass, 0.44 kPa for metal, 0.42 kPa for PTFE, 0.74 kPa for pigskin, 0.49 kPa for weight, 2.04 kPa for plastic, 0.38 kPa for rubber, and 0.52 kPa for wood).
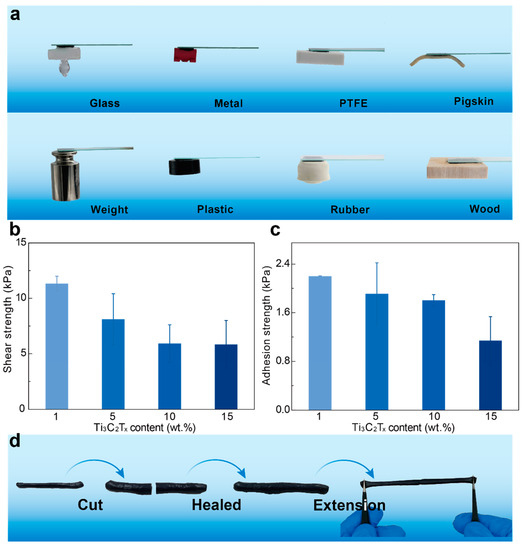
Figure 4.
Adhesion property and self-healing property of Ti3C2Tx-PAA hydrogels. (a) Photographs illustrate the adhesion ability of hydrogels on different substrates (glass, metal, PTFE, weight, rubber, plastic, wood and pigskin). (b) Shear adhesion strength of Ti3C2Tx-PAA hydrogels on PET substrates. (c) Adhesion strength on aluminum substrate. (d) Self-healing property of the hydrogel.
Additionally, we measure the adhesion strength on aluminum substrate via 90° peeling and adhesion of hydrogels on PET by the lap shear test. In general, the electrostatic force between carboxyl groups on PAA chains and various substrates is the main reason for the adhesion of hydrogels [36]. In our work, the shear strength (5.84 kPa to 11.32 kPa) and adhesion strength (1.14 kPa to 2.20 kPa) of hydrogels show an increasing trend (Figure 4b,c). Due to the increase of the concentration of AA monomers, the electrostatic force between hydrogels and substrates is obviously enhanced, leading to good adhesion of Ti3C2Tx-PAA hydrogels against varying substrates.
3.5. Self-Healing Properties of Ti3C2Tx-PAA Hydrogels
Ti3C2Tx-PAA hydrogels are able to self-heal immediately after cutting (Figure 4d). When we cut the hydrogels into two pieces and put them in touch with each other under external force, they heal automatically within several seconds. The self-healed hydrogel could be stretched without obvious decrease in ultimate strain.
3.6. Patterning Ti3C2Tx-PAA Hydrogels by 3D Printing
Complex patterning/manufacturing techniques have greatly hampered the development of stretchable, adhesive, and conductive hydrogels in various practical applications. Enlightened by the 3D printability of recent MXene materials and conducting polymer ink via controlling the viscosity [4], we developed the 3D printing techniques of Ti3C2Tx-PAA hydrogels. The viscosity of Ti3C2Tx-PAA inks increased rapidly with increasing Ti3C2Tx contents (Figure S2), rendering good 3D printability of such inks [45,46]. Notably, an excessively high Ti3C2Tx concentration will lead to significant aggregation of hydrogels and nozzle clogging.
To demonstrate the capability of 3D printing such hydrogels, we printed mesh (Figure 5a) and rhombus (Figure 5c) structures with 15 wt.% Ti3C2Tx-PAA ink through a 160-µm diameter nozzle onto PET film. The 3D-printed Ti3C2Tx-PAA hydrogel pattern displays superior flexibility, good stretchability, and excellent adhesion against the PET substrate (Figure 5e,f), offering a promising platform to fabricate multifunctional materials towards various applications like tissue engineering and neural science [47,48].
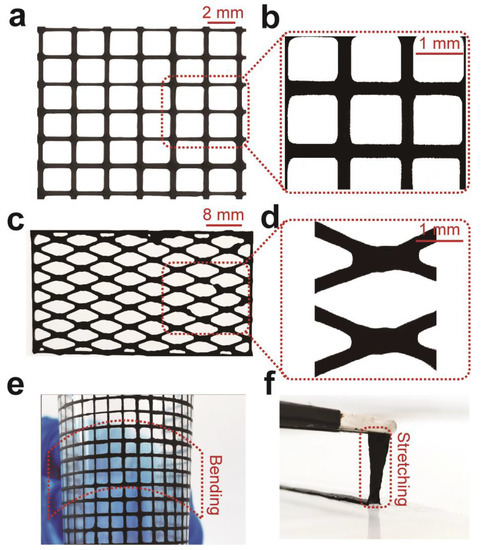
Figure 5.
3D printing of Ti3C2Tx-PAA hydrogels. (a) 3D-printing of a mesh structure and (b) its microstructure. (c) 3D-printing of a rhombus structure and (d) its microstructure. (e) Bending of the 3D-printed Ti3C2Tx-PAA mesh structure without defect. (f) Stretching of the 3D-printed Ti3C2Tx-PAA rectangle structure without failure.
4. Conclusions
We successfully developed a pre-crosslinking and secondary-crosslinking strategy for the 3D printing of stretchable, adhesive, and conductive Ti3C2Tx-PAA hydrogels. The pre-polymerization between Ti3C2Tx nanosheets and AA monomers yields viscous 3D printable inks. Secondary polymerization of 3D-printed patterns by 3D printing realizes a multifunctional Ti3C2Tx-PAA hydrogel. The resultant hydrogels are demonstrated to display high stretchability (~622%), high electrical conductivity (5.13 S m−1), excellent adhesion (11.32 kPa), and outstanding electrochemical activity and stability. Moreover, the 3D printable Ti3C2Tx-PAA inks can be readily printed into various complex patterns like mesh and rhombs with high resolution, which benefits robust integration of wearable and implantable devices. This work not only provides a simple strategy to achieve stretchable, conductive, and self-healable multifunctional hydrogels, but also sets up a 3D printing technique for facile fabrication and integration of diverse bioelectronic devices.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/polym14101992/s1, Figure S1: Digital images of varying Ti3C2Tx-PAA inks after 1 day (a), 2 days (b), and 3 days (c), showing the viscosity increase with time, Figure S2: Rhombus and square patterns for 3D printing of Ti3C2Tx-PAA hydrogels. Designing and printing paths for (a) rhombus pattern, (b) square pattern (size: 2 mm × 2 mm), and (c) square pattern (size: 3 mm × 3 mm), Figure S3: Adhesion strength of 1 wt.% Ti3C2Tx-PAA hydrogel with varying substrates.; Table S1: Compositions of Ti3C2Tx-PAA hydrogels, Table S2: Electrical conductivity comparison of our Ti3C2Tx-PAA hydrogels with previously reported conductive hydrogels. References [36,40,41,42,43] are cited in Supplementary Materials.
Author Contributions
W.Z. (Weijing Zhao): conceptualization, visualization, methodology, software, formal analysis, investigation, data curation, writing—original draft preparation, writing—review and editing; J.C.: conceptualization, visualization, methodology, software, formal analysis, investigation, writing—review and editing; F.W.: conceptualization, visualization, software, formal analysis, investigation, writing—review and editing; F.T.: software investigation, formal analysis, writing—review and editing; W.Z. (Wenqiang Zheng): software investigation, formal analysis, writing—review and editing; Y.B.: Supervision, resources, funding acquisition, writing—review and editing; K.Z.: visualization, formal analysis, review and editing; Z.Z.: visualization, formal analysis, review and editing; J.Y.: visualization, software, formal analysis, review and editing; J.X.: visualization, review and editing; X.L.: Supervision, resources, funding acquisition, formal analysis, investigation, data curation, writing—review and editing. B.L.: Supervision, resources, funding acquisition, formal analysis, investigation, data curation, writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the Natural Science Foundation of China (8210087, 51963011), and the Interdisciplinary Program of Shanghai Jiao Tong University (YG2021QN105), Technological Expertise and Academic Leaders Training Program of Jiangxi Province (20194BCJ22013), Training Program of Natural Science Foundation of China Youth Fund (20202ZDB01007), Jiangxi Provincial Double Thousand Talents Plan-Youth Program (JXSQ2019201108), Natural Science Foundation of Jiangxi Province (20202ACB214001), Jiangxi Key Laboratory of Flexible Electronics (20212BCD42004), the Science and Technology Project of the Education Department of Jiangxi Province (GJJ201101), Jiangxi Science & Technology Normal University (2020XJZD006). Z. Z. and J. Y. thank Jiangxi Science & Technology Normal University for the Provincial Postgraduate Innovation Program grants (YC2021-S754 and YC2021-S759).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The raw/processed data required to reproduce these findings cannot be shared at this time due to technical limitations. They are available on request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Song, Y.; Min, J.; Gao, W. Wearable and implantable electronics: Moving toward precision therapy. ACS Nano 2019, 13, 12280–12286. [Google Scholar] [CrossRef] [PubMed]
- Jia, M.; Rolandi, M. Soft and ion-conducting materials in bioelectronics: From conducting polymers to hydrogels. Adv. Healthc. Mater. 2020, 9, 1901372. [Google Scholar] [CrossRef] [PubMed]
- Hu, F.; Xue, Y.; Xu, J.; Lu, B. PEDOT-Based Conducting Polymer Actuators. Front. Robot. AI 2019, 6, 114. [Google Scholar]
- Lu, B.; Yuk, H.; Lin, S.; Jian, N.; Qu, K.; Xu, J.; Zhao, X. Pure PEDOT:PSS hydrogels. Nat. Commun. 2019, 10, 1043. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wood, C.S.; Thomas, M.R.; Budd, J.; Tivani, P.; Johnson, A.M.; Mckendry, R.A.; Stevens, M.M.; Pillay, D.; Rosanna, W. Taking connected mobile-health diagnostics of infectious diseases to the field. Nature 2019, 566, 467–474. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yuk, H.; Lu, B.; Zhao, X. Hydrogel bioelectronics. Chem. Soc. Rev. 2019, 48, 1642–1667. [Google Scholar]
- Lei, Z.; Wang, Q.; Sun, S.; Zhu, W.; Wu, P. A bioinspired mineral hydrogel as a self-healable, mechanically adaptable ionic skin for highly sensitive pressure sensing. Adv. Mater. 2017, 29, 1700321. [Google Scholar] [CrossRef] [PubMed]
- Fu, F.; Wang, J.; Zeng, H.; Yu, J. Functional conductive hydrogels for bioelectronics. ACS Mater. Lett. 2020, 2, 1287–1301. [Google Scholar] [CrossRef]
- Zhao, Q.; Liu, J.; Wu, Z.; Xu, X.; Ma, H.; Hou, J.; Xu, Q.; Yang, R.; Zhang, K.; Zhang, M.; et al. Robust PEDOT: PSS-based hydrogel for highly efficient interfacial solar water purification. Chem. Eng. J. 2022, 442, 136284. [Google Scholar]
- Hu, Y.; Cui, Y.; Que, X.; Zhang, Z.; Peng, J.; Li, J.; Zhai, M. Super adhesive MXene-based nanocomposite hydrogel with self-healable and conductivity properties via radiation synthesis. Adv. Eng. Mater. 2022, 24, 2101692. [Google Scholar] [CrossRef]
- Inoue, A.; Yuk, H.; Lu, B.; Zhao, X. Strong adhesion of wet conducting polymers on diverse substrates. Sci. Adv. 2020, 6, 5394. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.; Xie, X.; Anasori, B.; Sarycheva, A.; Makaryan, T.; Zhao, M.; Urbankowski, P.; Miao, L.; Jiang, J.; Gogotsi, Y. MoS2-on-MXene heterostructures as highly reversible anode materials for lithium-ion batteries. Angew. Chem. Int. Ed. 2022, 580, 152371. [Google Scholar]
- Wu, Z.; Shang, T.; Deng, Y.; Tao, Y.; Yang, Q.H. The assembly of MXenes from 2D to 3D. Adv. Sci. 2020, 7, 1903077. [Google Scholar] [CrossRef] [Green Version]
- Alhabeb, M.; Maleski, K.; Mathis, T.S.; Sarycheva, A.; Hatter, C.B.; Uzun, S.; Levitt, A.; Gogotsi, Y. Selective etching of silicon from Ti3SiC2(MAX) to obtain 2D titanium carbide (MXene). Angew. Chem. Int. Ed. 2018, 130, 5542–5546. [Google Scholar] [CrossRef]
- Li, K.; Liang, M.; Wang, H.; Wang, X.; Huang, Y.; Coelho, J.; Pinilla, S.; Zhang, Y.; Qi, F.; Nicolosi, V.; et al. 3D MXene architectures for efficient energy storage and conversion. Adv. Funct. Mater. 2020, 30, 2000842. [Google Scholar] [CrossRef]
- Gong, K.; Zhou, K.; Qian, X.; Shi, C.; Yu, B. MXene as emerging nanofillers for high-performance polymer composites: A review. Compos. Part B Eng. 2021, 217, 108867. [Google Scholar] [CrossRef]
- Gkountaras, A.; Kim, Y.; Coraux, J.; Bouchiat, V.; Lisi, S.; Barsoum, M.W.; Ouisse, T. Mechanical exfoliation of select MAX phases and Mo4Ce4Al7C3 single crystals to produce MAXenes. Small 2020, 16, 1905784. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.L.; Bian, S.W. Vacuum-filtration assisted layer-by-layer strategy to design MXene/Carbon Nanotube@MnO2 all-in-one supercapacitors. J. Mater. Chem. A 2021, 9, 21347–21356. [Google Scholar] [CrossRef]
- Wu, X.; Ding, M.; Xu, H.; Yang, W.; Zhang, K.; Tian, H.; Wang, H.; Xie, Z. Scalable Ti3C2TxMXene Interlayered forward osmosis membranes for enhanced water purification and organic solvent recovery. ACS Nano 2020, 14, 9125–9135. [Google Scholar] [CrossRef]
- Zhang, Z.; Yan, Q.; Liu, Z.; Zhao, X.; Wang, Z.; Sun, J.; Wang, Z.L.; Wang, R.; Li, L. Flexible MXene composed triboelectric nanogenerator via facile vacuum-assistant filtration method for self-powered biomechanical sensing. Nano Energy 2021, 88, 106257. [Google Scholar] [CrossRef]
- Abdolhosseinzadeh, S.; Schneider, R.; Verma, A.; Heier, J.; Nüesch, F.; Zhang, C. Turning trash into treasure: Additive free MXene sediment inks for screen-printed micro-supercapacitors. Adv. Mater. 2020, 32, 2000716. [Google Scholar] [CrossRef]
- Wu, H.; Xie, Y.; Ma, Y.; Zhang, B.; Xia, B.; Zhang, P.; Qian, W.; He, D.; Zhang, X.; Li, B.; et al. Aqueous MXene/Xanthan gum hybrid inks for screen-printing electromagnetic shielding, joule heater, and piezoresistive sensor. Small 2022, 2107087. [Google Scholar] [CrossRef]
- Zhang, C.J.; Kremer, M.P.; Seral-Ascaso, A.; Park, S.H.; McEvoy, N.; Anasori, B.; Gogotsi, Y.; Nicolosi, V. Stamping of flexible, coplanar micro-supercapacitors using MXene inks. Adv. Funct. Mater. 2018, 28, 1705506. [Google Scholar] [CrossRef]
- Dong, J.; Luo, S.; Ning, S.; Yang, G.; Pan, D.; Ji, Y.; Feng, Y.; Su, F.; Liu, C. MXene-coated wrinkled fabrics for stretchable and multifunctional electromagnetic interference shielding and electro/photo-thermal conversion applications. ACS Appl. Mater. Interfaces 2021, 13, 60478–60488. [Google Scholar] [CrossRef] [PubMed]
- Yuk, H.; Lu, B.; Lin, S.; Qu, K.; Xu, J.; Luo, J.; Zhao, X. 3D printing of conducting polymers. Nat. Commun. 2020, 11, 1604. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shahrubudin, N.; Lee, T.C.; Ramlan, R. An overview on 3D printing technology: Technological, materials, and applications. Procedia Manuf. 2019, 35, 1286–1296. [Google Scholar] [CrossRef]
- Yuk, H.; Zhao, X. A New 3D Printing strategy by harnessing deformation, instability, and fracture of viscoelastic inks. Adv. Mater. 2018, 30, 1704028. [Google Scholar] [CrossRef]
- Orangi, J.; Hamade, F.; Davis, V.A.; Beidaghi, M. 3D Printing of additive-free 2D Ti3C2Tx (MXene) ink for fabrication of micro-supercapacitors with ultra-high energy densities. ACS Nano 2020, 14, 640–650. [Google Scholar] [CrossRef]
- Yu, L.; Fan, Z.; Shao, Y.; Tian, Z.; Sun, J.; Liu, Z. Versatile N-doped MXene ink for printed electrochemical energy storage application. Adv. Energy Mater. 2019, 9, 1901839. [Google Scholar] [CrossRef]
- Nutan, B.; Chandel, A.K.S.; Jewrajka, S.K. Liquid prepolymer-based in situ formation of degradable poly(ethylene glycol)-linked-poly(caprolactone)-linked-poly(2-dimethylaminoethyl)methacrylate amphiphilic conetwork gels showing polarity driven gelation and bioadhesion. ACS Appl. Bio Mater. 2018, 1, 1606–1619. [Google Scholar] [CrossRef]
- Chandel, A.K.S.; Shimizu, A.; Hasegawa, K.; Ito, T. Advancement of biomaterial-based postoperative adhesion barriers. Macromol. Biosci. 2021, 21, 1–34. [Google Scholar] [CrossRef]
- Madhavikutty, A.S.; Ohta, S.; Chandel, A.K.S.; Qi, P.; Ito, T. Analysis of endoscopic injectability and post-ejection dripping of yield stress fluids: Laponite, Carbopol and Xanthan Gum. J. Chem. Eng. Japan 2021, 54, 500–511. [Google Scholar] [CrossRef]
- Qin, L.; Jiang, J.; Tao, Q.; Wang, C.; Persson, I.; Fahlman, M.; Persson, P.O.A.; Hou, L.; Rosen, J.; Zhang, F. A flexible semitransparent photovoltaic supercapacitor based on water-processed MXene electrodes. J. Mater. Chem. A 2020, 8, 5467–5475. [Google Scholar] [CrossRef] [Green Version]
- Guo, J.; Li, Q.; Zhang, R.; Li, B.; Zhang, J.; Yao, L.; Lin, Z.; Zhang, L.; Cao, X.; Duan, B. Loose pre-cross-linking mediating cellulose self-assembly for 3D printing strong and tough biomimetic scaffolds. Biomacromolecules 2022, 23, 877–888. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Pan, X.; Lin, C.; Gao, H.; Cao, S.; Ni, Y.; Ma, X. Modified Ti3C2Tx (MXene) nanosheet-catalyzed self-assembled, anti-aggregated, ultra-stretchable, conductive hydrogels for wearable bioelectronics. Chem. Eng. J. 2020, 401, 126129. [Google Scholar] [CrossRef]
- Afewerki, S.; Wang, X.; Ruiz-esparza, G.U.; Tai, C.; Kong, X.; Zhou, S.; Welch, K.; Huang, P.; Bengtsson, R.; Xu, C.; et al. Combined catalysis for engineering bioinspired lignin-based, long-lasting, adhesive, self-mending, antimicrobial hydrogels. ACS Nano 2020, 14, 17004–17017. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Wang, L.; Zheng, Y.; Zhao, S.; Wei, W.; Zhang, D.; Fu, X.; Jiang, K.; Shen, G.; Han, W. Highly-stable polymer-crosslinked 2D MXene-based flexible biocompatible electronic skins for in vivo biomonitoring. Nano Energy 2021, 84, 105921. [Google Scholar] [CrossRef]
- Chen, Y.; Xie, X.; Xin, X.; Tang, Z.R.; Xu, Y.J. Ti3C2Tx-based three-dimensional hydrogel by a graphene oxide-assisted self-convergence process for enhanced photoredox catalysis. ACS Nano 2019, 13, 295–304. [Google Scholar] [CrossRef]
- Li, Y.; Yan, J.; Liu, Y.; Xie, X.M. Super tough and intelligent multibond network physical hydrogels facilitated by Ti3C2TxMXene nanosheets. ACS Nano 2022, 16, 1567–1577. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, K.; Li, Y.; Lan, J.; Yan, B.; Shi, L.; Ran, R. High-strength, self-Healable, temperature-sensitive, MXene-containing composite hydrogel as a smart compression sensor. ACS Appl. Mater. Interfaces 2019, 11, 47350–47357. [Google Scholar]
- Feng, Y.; Liu, H.; Zhu, W.; Guan, L.; Yang, X.; Zvyagin, A.; Zhao, Y.; Shen, C.; Yang, B.; Lin, Q. Muscle-inspired MXene conductive hydrogels with anisotropy and low-temperature tolerance for wearable flexible sensors and arrays. Adv. Funct. Mater. 2021, 31, 2105264. [Google Scholar]
- Liu, J.; Mckeon, L.; Garcia, J.; Pinilla, S.; Barwich, S.; Möbius, M.; Stamenov, P.; Coleman, J.; Nicolosi, V. Additive manufacturing of Ti3C2-MXene-functionalized conductive polymer hydrogels for electromagnetic-interference shielding. Adv. Mater. 2022, 34, 2106253. [Google Scholar]
- Li, S.; Yu, Z.; Guo, B.; Guo, K.; Li, Y.; Gong, L.; Zhao, L.; Bae, J.; Tang, L. Environmentally stable, mechanically flexible, self-Adhesive, and electrically conductive Ti3C2TX MXene hydrogels for wide-temperature strain sensing. Nano Energy 2021, 90, 106502. [Google Scholar]
- Han, T.H.; Shin, H.; Eom, W.; Lee, K.H.; Jeong, W.; Kang, D.J. highly electroconductive and mechanically strong Ti3C2Tx Mxene fibers using a deformable Mxene gel. ACS Nano 2021, 15, 3320–3329. [Google Scholar]
- Nakagawa, Y.; Oki, Y.; Da, X.; Singh Chandel, A.K.; Ohta, S.; Ito, T. Injectable bottlebrush triblock copolymer hydrogel crosslinked with ferric ions. Polymer 2022, 240, 1–8. [Google Scholar] [CrossRef]
- Patrickios, C.S. Amphiphilic Polymer Co-Networks Synthesis, Properties, Modelling and Applications, 3rd ed.; Royal Society of Chemistry: London, UK, 2020; pp. 67–96. [Google Scholar]
- Lei, Q.; He, J.; Li, D. Electrohydrodynamic 3D printing of layer-specifically oriented, multiscale conductive scaffolds for cardiac tissue engineering. Nanoscale 2019, 11, 15195–15205. [Google Scholar] [CrossRef]
- Rinoldi, C.; Lanzi, M.; Fiorelli, R.; Nakielski, P.; Zembrzycki, K.; Kowalewski, T.; Urbanek, O.; Grippo, V.; Jezierska-Woźniak, K.; Maksymowicz, W.; et al. Three-dimensional printable conductive semi-interpenetrating polymer network hydrogel for neural tissue applications. Biomacromolecules 2021, 22, 3084–3098. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).