Citrus Pomace Biomass as a Source of Pectin and Lignocellulose Fibers: From Waste to Upgraded Biocomposites for Mulching Applications
Abstract
1. Introduction
2. Materials and Methods
2.1. Material and Chemicals
2.2. Extraction and Purification of Pectin from Citrus Waste
2.3. Pectin Film and Biocomposites
2.4. Characterizations Methods
2.4.1. Gel Permeation Chromatography (GPC)
2.4.2. Degree of Methoxylation (DM) Measurement
2.4.3. Attenuated Total Reflection Fourier Transform Infrared Spectroscopy (FTIR-ATR)
2.4.4. Total Phenolic Content (TPC)
2.4.5. Total Flavonoids Content (TFC)
2.4.6. Thermogravimetric Analysis (TGA)
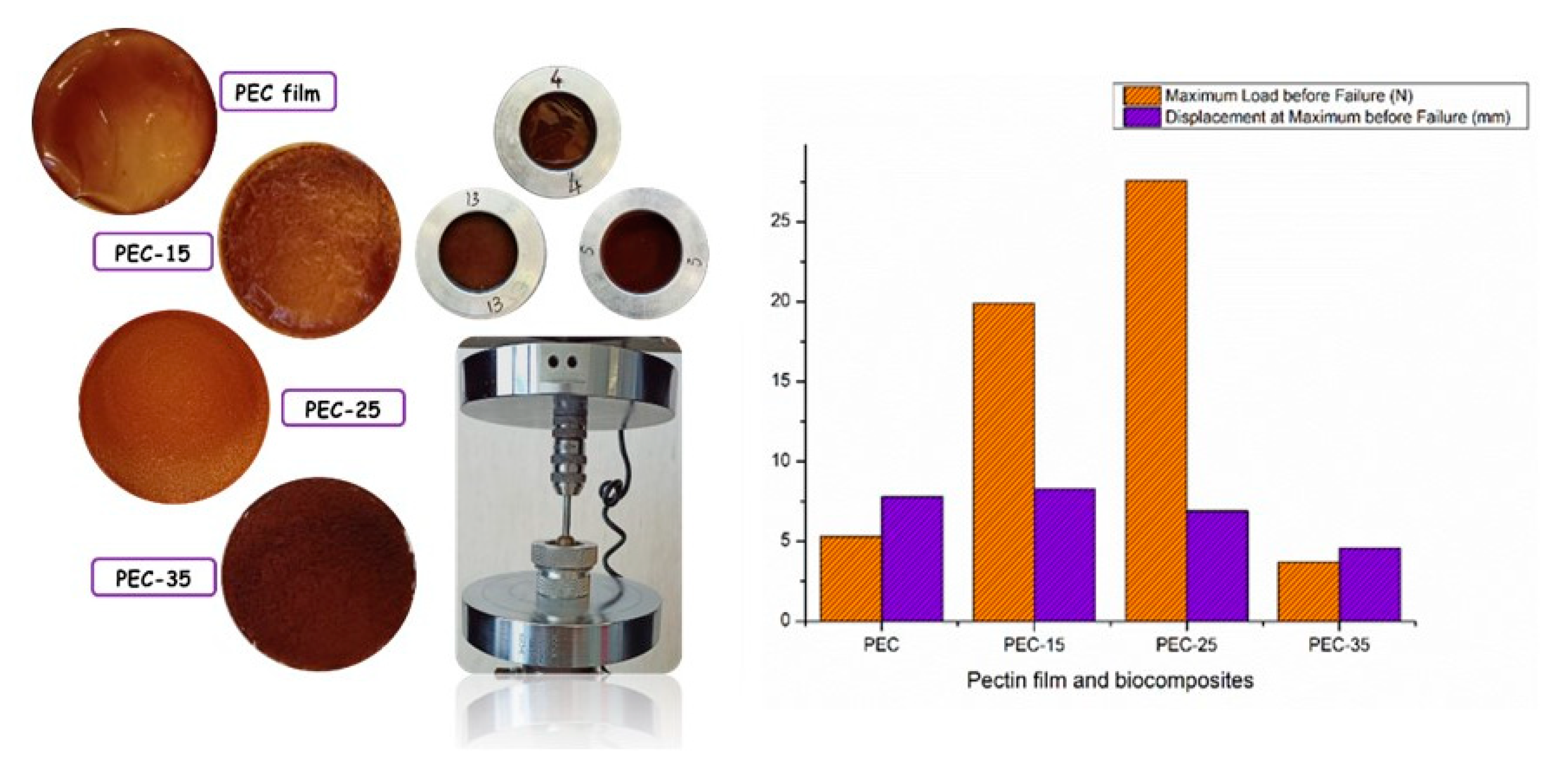
2.4.7. Mechanical Tests: Tensile Test and Puncture Test
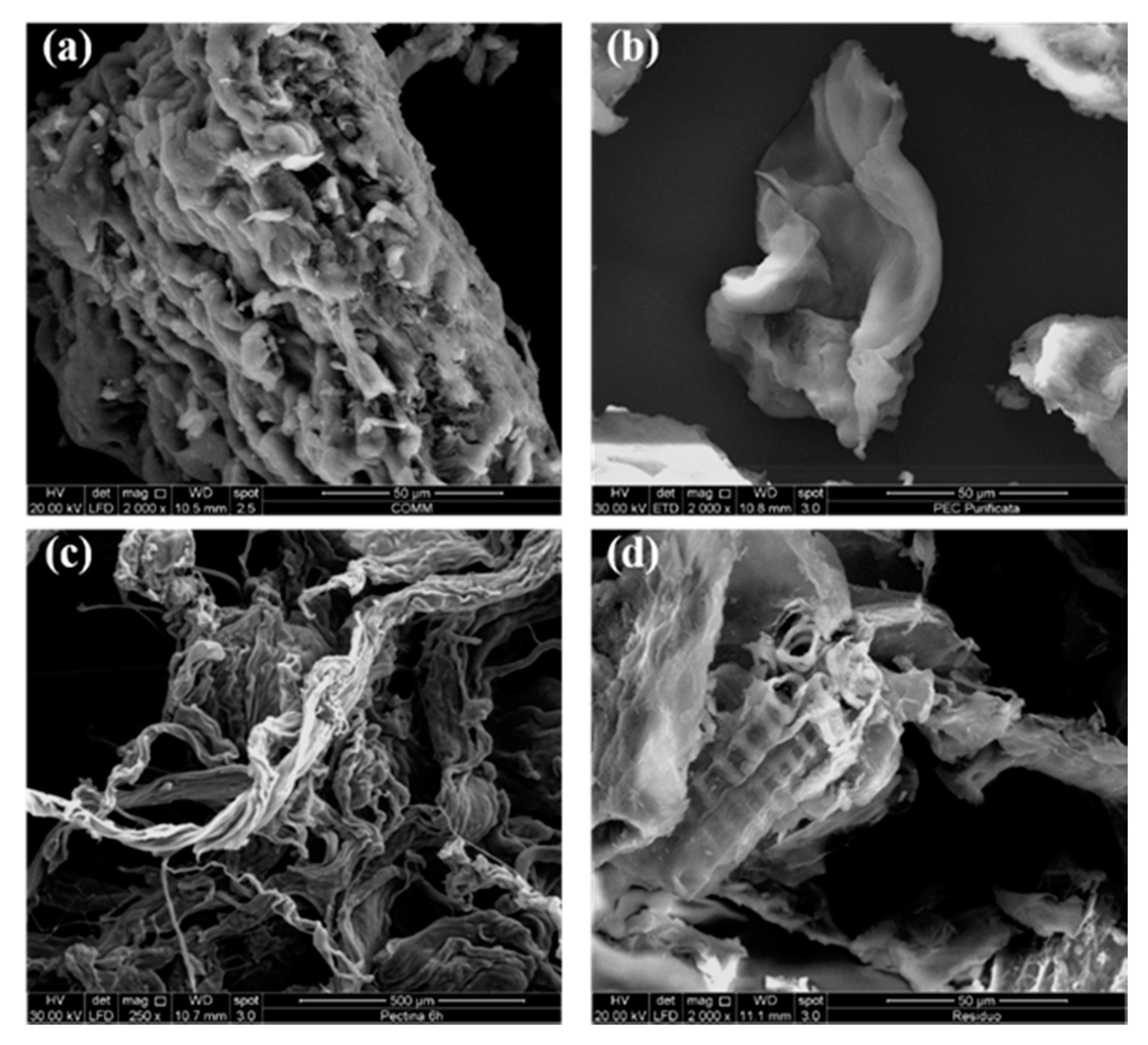
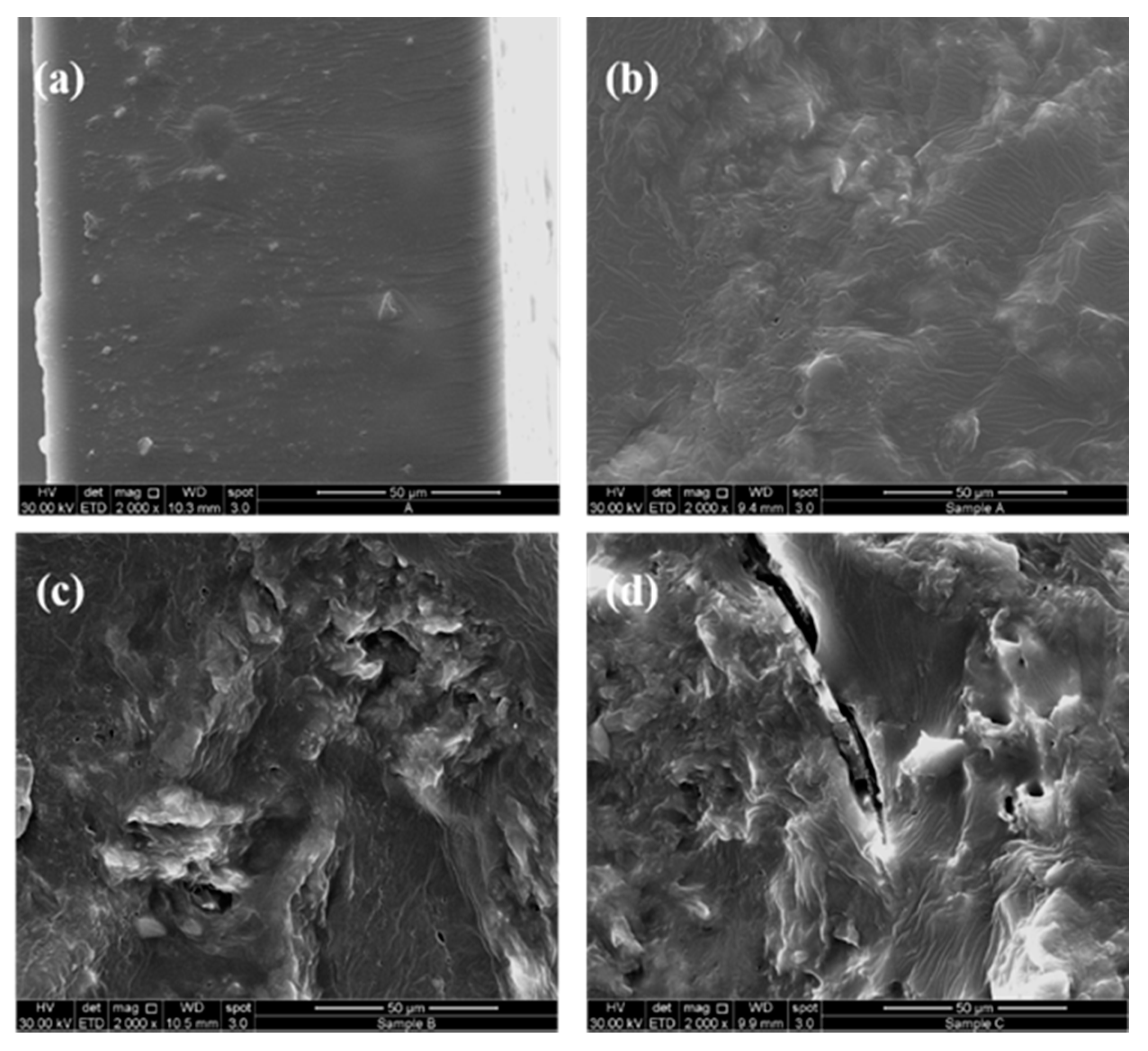
2.4.8. Scanning Electron Microscope (SEM)
3. Results
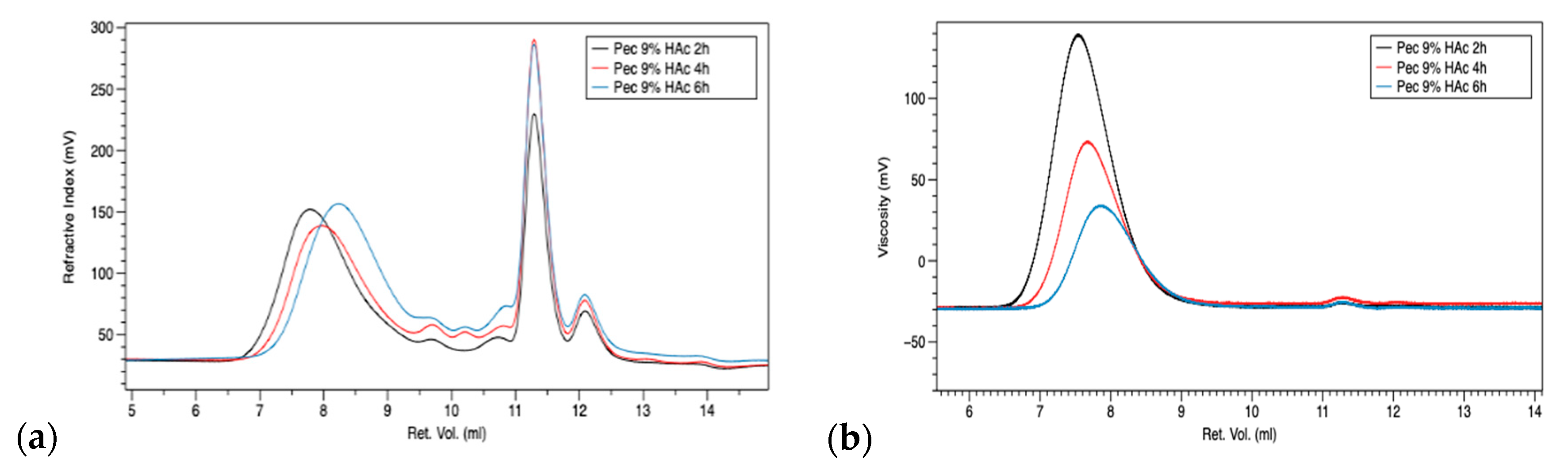
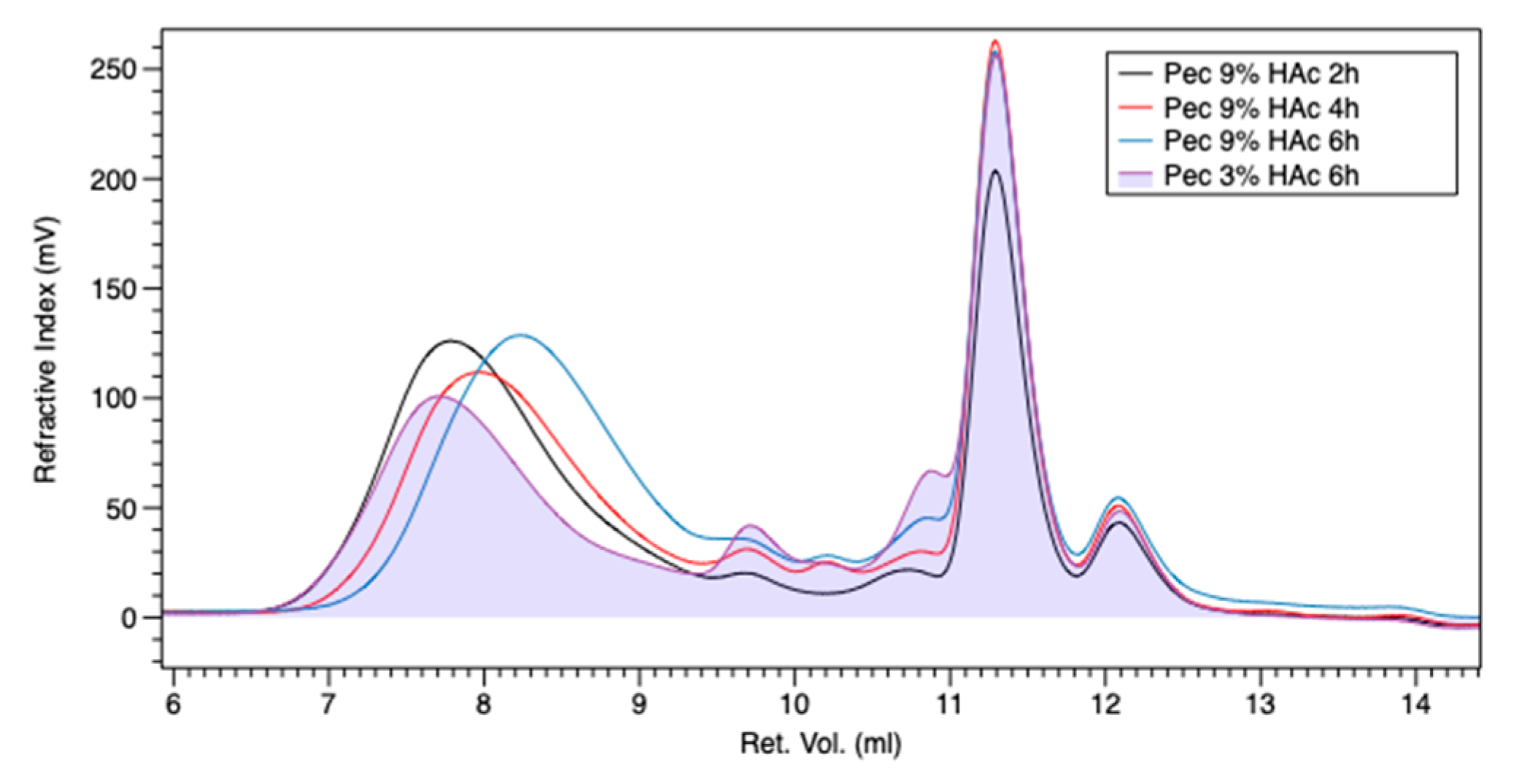
3.1. Gel Permeation Chromatography (GPC)
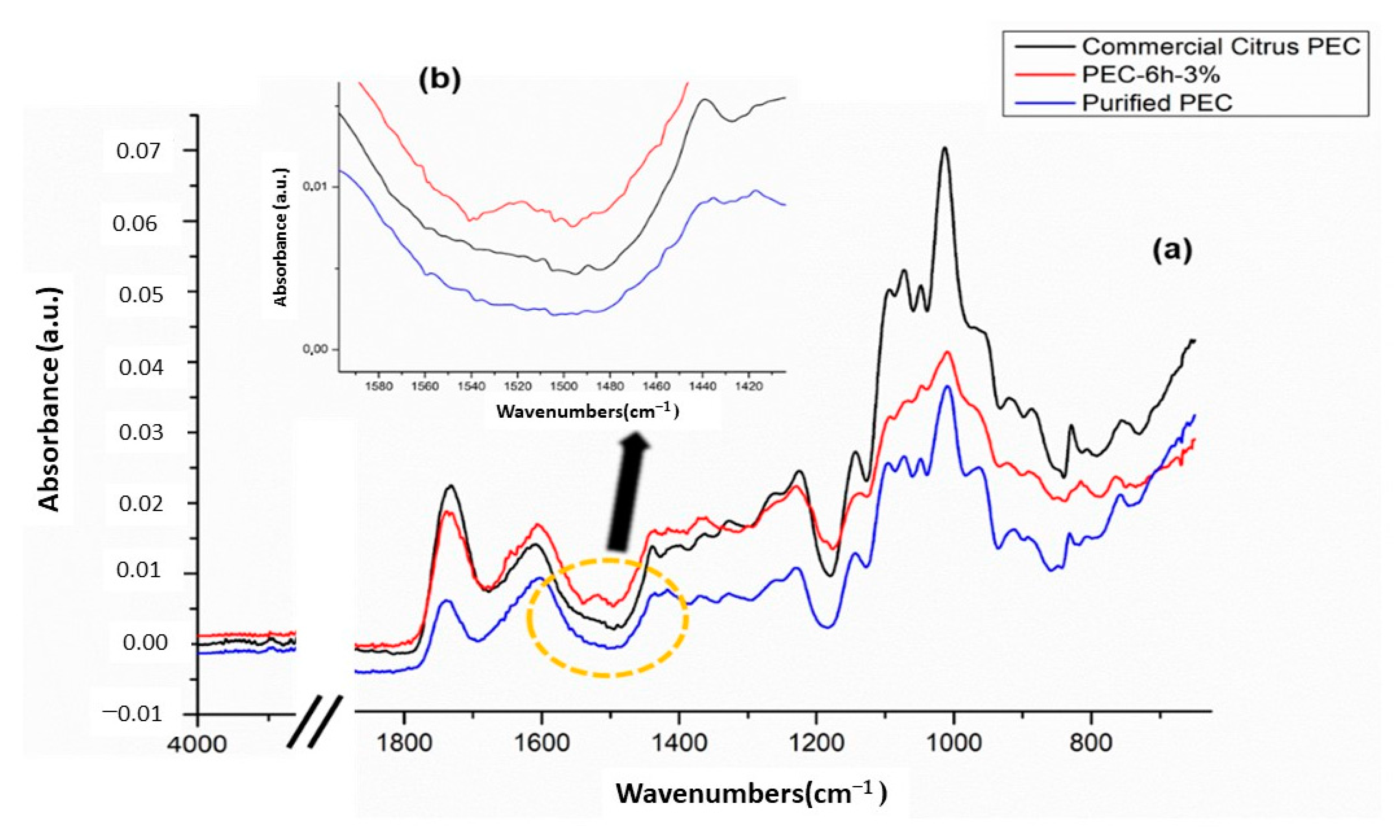
3.2. Attenuated Total Reflectance Fourier Transform Infrared (FTIR-ATR) Spectroscopy
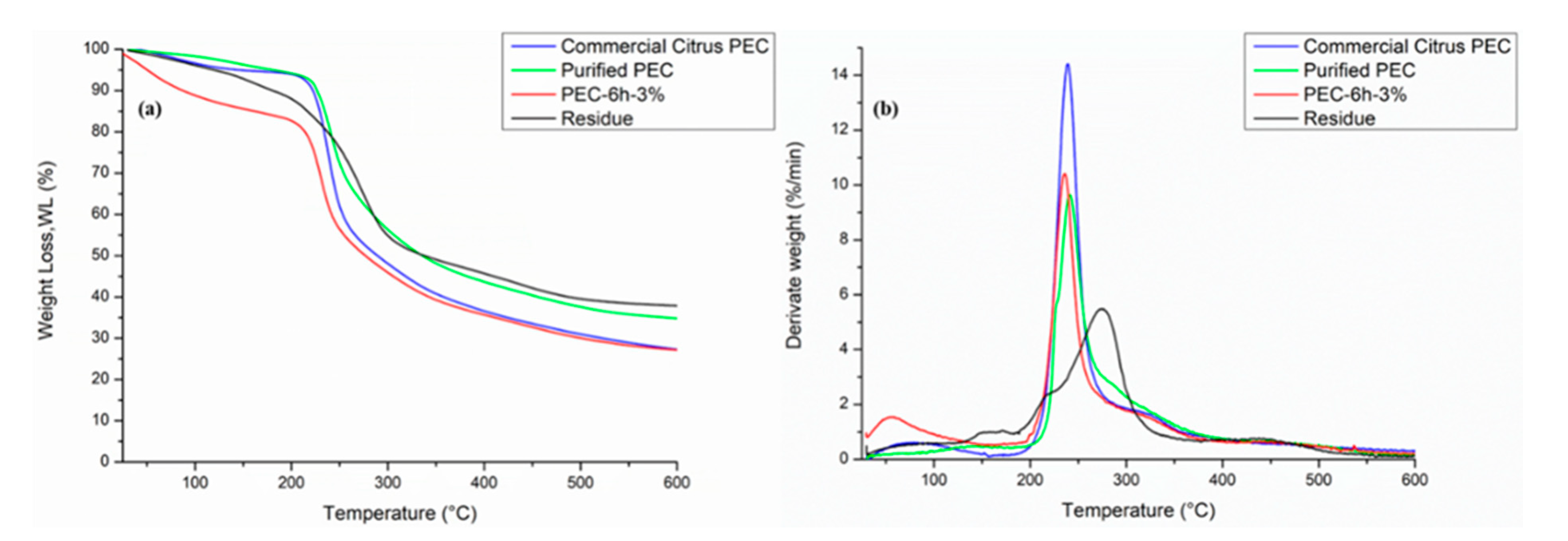
3.3. Thermogravimetric Analysis (TGA)
3.4. Mechanical Tests: Tensile Test and Puncture Test
3.5. Scanning Electron Microscope (SEM)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Castro, M.D.L.D. Towards a Comprehensive Exploitation of Agrofood Residues: Olive Tree—Olive Oil as Example. Comptes Rendus Chim. 2014, 17, 252–260. [Google Scholar] [CrossRef]
- Desai, B.H.; Gavouneli, M.; Koivurova, T. From the Editors-in-Chief. Yearb. Int. Environ. Law 2017, 28, xi–xii. [Google Scholar] [CrossRef]
- Dao, T.A.T.; Webb, H.K.; Malherbe, F. Optimization of Pectin Extraction from Fruit Peels by Response Surface Method: Conventional versus Microwave-Assisted Heating. Food Hydrocoll. 2021, 113, 106475. [Google Scholar] [CrossRef]
- De Albuquerque, M.A.C.; Levit, R.; Beres, C.; Bedani, R.; de Moreno, M.A.; Isay Saad, S.M.; Leblanc, J.G.J. Tropical Fruit By-Products Water Extracts of Tropical Fruit by-Products as Sources of Soluble Fibres and Phenolic Compounds with Potential Antioxidant, Anti-Inflammatory, and Functional Properties. J. Funct. Foods 2019, 52, 724–733. [Google Scholar] [CrossRef]
- Jiang, B.; Wang, X.; Wang, L.; Wu, S.; Li, D.; Liu, C.; Feng, Z. Fabrication and Characterization of a Microemulsion Stabilized by Integrated Phosvitin and Gallic Acid. J. Agric. Food Chem. 2020, 68, 5437–5447. [Google Scholar] [CrossRef] [PubMed]
- Jiang, B.; Wang, M.; Wang, X.; Wu, S.; Li, D.; Liu, C.; Feng, Z.; Li, J. Effective Separation of Prolyl Endopeptidase from Aspergillus Niger by Aqueous Two Phase System and Its Characterization and Application. Int. J. Biol. Macromol. 2021, 169, 384–395. [Google Scholar] [CrossRef] [PubMed]
- Jiang, B.; Na, J.; Wang, L.; Li, D.; Liu, C.; Feng, Z. Reutilization of Food Waste: One-Step Extration, Purification and Characterization of Ovalbumin from Salted Egg White by Aqueous Two-Phase Flotation. Foods 2019, 8, 286. [Google Scholar] [CrossRef] [PubMed]
- Sharma, K.; Mahato, N.; Lee, Y.R. Extraction, Characterization and Biological Activity of Citrus Flavonoids. Rev. Chem. Eng. 2019, 35, 265–284. [Google Scholar] [CrossRef]
- Ke, Z.; Xu, X.; Nie, C.; Zhou, Z. Citrus Flavonoids and Human Cancers. J. Food Nutr. Res. 2015, 3, 341–351. [Google Scholar] [CrossRef]
- Benavente-García, O.; Castillo, J.; Marin, F.R.; Ortuño, A.; Del Río, J.A. Uses and Properties of Citrus Flavonoids. Am. Chem. Soc. 1997, 45, 4505–4515. [Google Scholar]
- Jandrić, Z.; Islam, M.; Singh, D.K.; Cannavan, A. Authentication of Indian Citrus Fruit/Fruit Juices by Untargeted and Targeted Metabolomics. Food Control 2017, 72, 181–188. [Google Scholar] [CrossRef]
- Miller, E.V.; Winston, J.R.; Schomer, H.A. Physiological Studies of Plastid Pigments in Rinds of Maturing Oranges. J. Agric. Res. 1940, 60, 259–267. [Google Scholar]
- Chen, X.M.; Tait, A.R.; Kitts, D.D. Flavonoid Composition of Orange Peel and Its Association with Antioxidant and Anti-Inflammatory Activities. Food Chem. 2017, 218, 15–21. [Google Scholar] [CrossRef]
- Chavan, P.; Singh, A.K.; Kaur, G. Recent Progress in the Utilization of Industrial Waste and By-products of Citrus Fruits: A Review. J. Food Process Eng. 2018, 41, e12895. [Google Scholar] [CrossRef]
- Schmidt, U.S.; Pietsch, V.L.; Rentschler, C.; Kurz, T.; Endreß, H.U.; Schuchmann, H.P. Influence of the Degree of Esterification on the Emulsifying Performance of Conjugates Formed between Whey Protein Isolate and Citrus Pectin. Food Hydrocoll. 2016, 56, 1–8. [Google Scholar] [CrossRef]
- Willats, W.G.T.; Knox, J.P.; Mikkelsen, J.D. Pectin: New Insights into an Old Polymer Are Starting to Gel. Trends Food Sci. Technol. 2006, 17, 97–104. [Google Scholar] [CrossRef]
- Caffall, K.H.; Mohnen, D. The Structure, Function, and Biosynthesis of Plant Cell Wall Pectic Polysaccharides. Carbohydr. Res. 2009, 344, 1879–1900. [Google Scholar] [CrossRef]
- Yapo, B.M. Rhamnogalacturonan-I: A Structurally Puzzling and Functionally Versatile Polysaccharide from Plant Cell Walls and Mucilages; Taylor & Francis Group: Abingdon, UK, 2011; Volume 51. [Google Scholar]
- Marić, M.; Grassino, A.N.; Zhu, Z.; Barba, F.J.; Brnčić, M.; Rimac Brnčić, S. An Overview of the Traditional and Innovative Approaches for Pectin Extraction from Plant Food Wastes and By-Products: Ultrasound-, Microwaves-, and Enzyme-Assisted Extraction. Trends Food Sci. Technol. 2018, 76, 28–37. [Google Scholar] [CrossRef]
- O’Neill, M.A.; Ishii, T.; Albersheim, P.; Darvill, A.G. RHAMNOGALACTURONAN II: Structure and Function of a Borate Cross-Linked Cell Wall Pectic Polysaccharide. Annu. Rev. Plant Biol. 2004, 55, 109–139. [Google Scholar] [CrossRef]
- Oosterveld, A.; Beldman, G.; Schols, H.A.; Voragen, A.G.J. Arabinose and Ferulic Acid Rich Pectic Polysaccharides Extracted from Sugar Beet Pulp. Carbohydr. Res. 1996, 288, 143–153. [Google Scholar] [CrossRef]
- Ridley, B.L.; O’Neill, M.A.; Mohnen, D. Pectins: Structure, Biosynthesis, and Oligogalacturonide-Related Signaling. Phytochemistry 2001, 57, 929–967. [Google Scholar] [CrossRef]
- Pellerin, P.; O’Neill, M.A. The Interaction of the Pectic Polysaccharide Rhamnogalacturonan II with Heavy Metals and Lanthanides in Wines and Fruit Juices. Analusis 1998, 26, 32–36. [Google Scholar] [CrossRef]
- Mohnen, D. Pectin Structure and Biosynthesis. Curr. Opin. Plant Biol. 2008, 11, 266–277. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.S.; Mu, T.H.; Ma, M.M. Extraction, Structure, and Emulsifying Properties of Pectin from Potato Pulp. Food Chem. 2018, 244, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Šešlija, S.; Nešić, A.; Ružić, J.; Kalagasidis Krušić, M.; Veličković, S.; Avolio, R.; Santagata, G.; Malinconico, M. Edible Blend Films of Pectin and Poly (Ethylene Glycol): Preparation and Physico-Chemical Evaluation. Food Hydrocoll. 2018, 77, 494–501. [Google Scholar] [CrossRef]
- Nešić, A.; Onjia, A.; Davidović, S.; Dimitrijević, S.; Errico, M.E.; Santagata, G.; Malinconico, M. Design of Pectin-Sodium Alginate Based Films for Potential Healthcare Application: Study of Chemico-Physical Interactions between the Components of Films and Assessment of Their Antimicrobial Activity. Carbohydr. Polym. 2017, 157, 981–990. [Google Scholar] [CrossRef]
- Chua, B.L.; Tang, S.F.; Ali, A.; Chow, Y.H. Optimisation of Pectin Production from Dragon Fruit Peels Waste: Drying, Extraction and Characterisation Studies. SN Appl. Sci. 2020, 2. [Google Scholar] [CrossRef]
- Seixas, F.L.; Fukuda, D.L.; Turbiani, F.R.B.; Garcia, P.S.; de, O. Petkowicz, C.L.; Jagadevan, S.; Gimenes, M.L. Extraction of Pectin from Passion Fruit Peel (Passiflora Edulis f.Flavicarpa) by Microwave-Induced Heating. Food Hydrocoll. 2014, 38, 186–192. [Google Scholar] [CrossRef]
- Sayah, M.Y.; Chabir, R.; Benyahia, H.; Rodi, K.Y.; Ouazzani, C.F.; Touzani, H.; Errachidi, F. Yield, Esterification Degree and Molecular Weight Evaluation of Pectins Isolated from Orange and Grapefruit Peels under Different Conditions. PLoS ONE 2016, 11, e0161751. [Google Scholar] [CrossRef]
- Immirzi, B.; Santagata, G.; Vox, G.; Schettini, E. Preparation, Characterisation and Field-Testing of a Biodegradable Sodium Alginate-Based Spray Mulch. Biosyst. Eng. 2009, 102, 461–472. [Google Scholar] [CrossRef]
- Kang, S.-M.; Bilal, S.; Shahzad, R.; Kim, Y.-N.; Park, C.-W.; Lee, K.-E.; Lee, J.-R.; Lee, I.-J. Effect of Ammonia and Indole-3-Acetic Acid Producing Endophytic Klebsiella Pneumoniae YNA12 as a Bio-Herbicide for Weed Inhibition: Special Reference with Evening Primroses. Plants 2020, 9, 761. [Google Scholar] [CrossRef]
- Orzali, L. Chitosan in Agriculture: A New Challenge for Managing Plant Disease. In Biological Activities and Application of Marine Polysaccharides; Corsi, B., Ed.; IntechOpen: Rijeka, Croatia, 2017; pp. 17–36. ISBN 978-953-51-2860-1. [Google Scholar]
- Giaccone, M.; Cirillo, C.; Scognamiglio, P.; Teobaldelli, M.; Mataffo, A.; Stinca, A.; Pannico, A.; Immirzi, B.; Santagata, G.; Malinconico, M.; et al. Biodegradable Mulching Spray for Weed Control in the Cultivation of Containerized Ornamental Shrubs. Chem. Biol. Technol. Agric. 2018, 5, 21. [Google Scholar] [CrossRef]
- Vox, G.; Santagata, G.; Malinconico, M.; Immirzi, B.; Scarascia Mugnozza, G.; Schettini, E. Biodegradable Films and Spray Coatings as Eco-Friendly Alternative to Petro-Chemical Derived Mulching Films. J. Agric. Eng. 2013, 44. [Google Scholar] [CrossRef]
- Winans, K.; Kendall, A.; Deng, H. The History and Current Applications of the Circular Economy Concept. Renew. Sustain. Energy Rev. 2017, 68, 825–833. [Google Scholar] [CrossRef]
- Twinomuhwezi, H.; Godswill, A.C.; Kahunde, D. Extraction and Characterization of Pectin from Orange (Citrus Sinensis), Lemon (Citrus Limon) and Tangerine (Citrus Tangerina). Am. J. Phys. Sci. 2020, 1, 17–30. [Google Scholar]
- Fakayode, O.A.; Abobi, K.E. Optimization of Oil and Pectin Extraction from Orange (Citrus Sinensis) Peels: A Response Surface Approach. J. Anal. Sci. Technol. 2018, 9, 20. [Google Scholar] [CrossRef]
- Asgari, K.; Labbafi, M.; Khodaiyan, F.; Kazemi, M.; Hosseini, S.S. High-Methylated Pectin from Walnut Processing Wastes as a Potential Resource: Ultrasound Assisted Extraction and Physicochemical, Structural and Functional Analysis. Int. J. Biol. Macromol. 2020, 152, 1274–1282. [Google Scholar] [CrossRef] [PubMed]
- Dewanto, V.; Wu, X.; Adom, K.K.; Hai Liu, R. Thermal Processing Enhances the Nutritional Value of Tomatoes by Increasing Total Antioxidant Activity. J. Agric. Food Chem. 2002, 50, 3010–3014. [Google Scholar] [CrossRef] [PubMed]
- Papoutsis, K.; Pristijono, P.; Golding, J.B.; Stathopoulos, C.E.; Bowyer, M.C.; Scarlett, C.J.; Vuong, Q.V. Optimizing a Sustainable Ultrasound-Assisted Extraction Method for the Recovery of Polyphenols from Lemon by-Products: Comparison with Hot Water and Organic Solvent Extractions. Eur. Food Res. Technol. 2018, 244, 1353–1365. [Google Scholar] [CrossRef]
- Malinconico, M.; Immirzi, B.; Schettini, E.; Vox, G.; Santagata, G.; Scarascia Mugnozza, G. An Overview on Innovative Biodegradable Materials for Agricultural Applications. In Progress in Polymer Degradation and Stability Research; Nova Science Publishers: New York, NY, USA, 2008; ISBN 978-1-60021-828-6. [Google Scholar]
- Kuuva, T.; Lantto, R.; Reinikainen, T.; Buchert, J.; Autio, K. Rheological Properties of Laccase-Induced Sugar Beet Pectin Gels. Food Hydrocoll. 2003, 17, 679–684. [Google Scholar] [CrossRef]
- Diaz, J.V.; Anthon, G.E.; Barrett, D.M. Nonenzymatic Degradation of Citrus Pectin and Pectate during Prolonged Heating: Effects of PH, Temperature, and Degree of Methyl Esterification. J. Agric. Food Chem. 2007, 55, 5131–5136. [Google Scholar] [CrossRef] [PubMed]
- Fraeye, I.; De Roeck, A.; Duvetter, T.; Verlent, I.; Hendrickx, M.; Van Loey, A. Influence of Pectin Properties and Processing Conditions on Thermal Pectin Degradation. Food Chem. 2007, 105, 555–563. [Google Scholar] [CrossRef]
- Jiang, Y.; Xu, Y.; Li, F.; Li, D.; Huang, Q. Pectin Extracted from Persimmon Peel: A Physicochemical Characterization and Emulsifying Properties Evaluation. Food Hydrocoll. 2020, 101, 105561. [Google Scholar] [CrossRef]
- Nisar, T.; Wang, Z.C.; Yang, X.; Tian, Y.; Iqbal, M.; Guo, Y. Characterization of Citrus Pectin Films Integrated with Clove Bud Essential Oil: Physical, Thermal, Barrier, Antioxidant and Antibacterial Properties. Int. J. Biol. Macromol. 2018, 106, 670–680. [Google Scholar] [CrossRef] [PubMed]
- Singthong, J.; Cui, S.W.; Ningsanond, S.; Douglas Goff, H. Structural Characterization, Degree of Esterification and Some Gelling Properties of Krueo Ma Noy (Cissampelos Pareira) Pectin. Carbohydr. Polym. 2004, 58, 391–400. [Google Scholar] [CrossRef]
- Gnanasambandam, R.; Proctor, A. Determination of Pectin Degree of Esterification by Diffuse Reflectance Fourier Transform Infrared Spectroscopy. Food Chem. 2000, 68, 327–332. [Google Scholar] [CrossRef]
- Baron, R.D.; Pérez, L.L.; Salcedo, J.M.; Córdoba, L.P.; do Amaral, S.P.J. Production and Characterization of Films Based on Blends of Chitosan from Blue Crab (Callinectes Sapidus) Waste and Pectin from Orange (Citrus Sinensis Osbeck) Peel. Int. J. Biol. Macromol. 2017, 98, 676–683. [Google Scholar] [CrossRef] [PubMed]
- Nisar, T.; Wang, Z.-C.; Alim, A.; Iqbal, M.; Yang, X.; Sun, L.; Guo, Y. Citrus Pectin Films Enriched with Thinned Young Apple Polyphenols for Potential Use as Bio-Based Active Packaging. CyTA 2019, 17, 695–705. [Google Scholar] [CrossRef]
- Ghaffari, A.; Navaee, K.; Oskoui, M.; Bayati, K.; Rafiee-Tehrani, M. Preparation and Characterization of Free Mixed-Film of Pectin/Chitosan/Eudragit® RS Intended for Sigmoidal Drug Delivery. Eur. J. Pharm. Biopharm. 2007, 67, 175–186. [Google Scholar] [CrossRef]
- Einhorn-Stoll, U.; Kunzek, H.; Dongowski, G. Thermal Analysis of Chemically and Mechanically Modified Pectins. Food Hydrocoll. 2007, 21, 1101–1112. [Google Scholar] [CrossRef]
- Wang, W.; Ma, X.; Jiang, P.; Hu, L.; Zhi, Z.; Chen, J.; Ding, T.; Ye, X.; Liu, D. Characterization of Pectin from Grapefruit Peel: A Comparison of Ultrasound-Assisted and Conventional Heating Extractions. Food Hydrocoll. 2016, 61, 730–739. [Google Scholar] [CrossRef]
- Zhang, Y.; Qi, J.; Zeng, W.; Huang, Y.; Yang, X. Properties of Dietary Fiber from Citrus Obtained through Alkaline Hydrogen Peroxide Treatment and Homogenization Treatment. Food Chem. 2020, 311, 125873. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.; Ye, X.; Chantapakul, T.; Chen, S.; Zheng, J. Manosonication Extraction of RG-I Pectic Polysaccharides from Citrus Waste: Optimization and Kinetics Analysis. Carbohydr. Polym. 2020, 235, 115982. [Google Scholar] [CrossRef]
- Qin, Z.; Liu, H.M.; Cheng, X.C.; Wang, X.D. Effect of Drying Pretreatment Methods on Structure and Properties of Pectins Extracted from Chinese Quince Fruit. Int. J. Biol. Macromol. 2019, 137, 801–808. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Takamizu, A.; Hoshino, M.; Sasaki, M.; Goto, M. Extraction of Dietary Fiber from Citrus Junos Peel with Subcritical Water. Food Bioprod. Process. 2012, 90, 180–186. [Google Scholar] [CrossRef]
- Cabello, P.S.D.; Takara, E.A.; Marchese, J.; Ochoa, N.A. Influence of Plasticizers in Pectin Films: Microstructural Changes. Mater. Chem. Phys. 2015, 162, 491–497. [Google Scholar] [CrossRef]
- Schettini, E.; Santagata, G.; Malinconico, M.; Immirzi, B.; Mugnozza, G.S.; Vox, G. Recycled Wastes of Tomato and Hemp Fibres for Biodegradable Pots: Physico-Chemical Characterization and Field Performance. Resour. Conserv. Recycl. 2013, 70, 9–19. [Google Scholar] [CrossRef]
- Nešić, A.; Ružić, J.; Gordić, M.; Ostojić, S.; Micić, D.; Onjia, A. Pectin-Polyvinylpyrrolidone Films: A Sustainable Approach to the Development of Biobased Packaging Materials. Compos. Part B 2017, 110, 56–61. [Google Scholar] [CrossRef]
- Dalpasquale, M.; Mariani, F.Q.; Müller, M.; Anaissi, F.J. Citrus Pectin as a Template for Synthesis of Colorful Aluminates. Dye. Pigment. 2016, 125, 124–131. [Google Scholar] [CrossRef]
- Tovar, A.K.; Godínez, L.A.; Espejel, F.; Ramírez-Zamora, R.M.; Robles, I. Optimization of the Integral Valorization Process for Orange Peel Waste Using a Design of Experiments Approach: Production of High-Quality Pectin and Activated Carbon. Waste Manag. 2019, 85, 202–213. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Abomohra, A.E.F.; Elsayed, M.; Zhang, X.; Fan, Q.; Ai, P. Compositional Changes of Rice Straw Fibers after Pretreatment with Diluted Acetic Acid: Towards Enhanced Biomethane Production. J. Clean. Prod. 2019, 230, 775–782. [Google Scholar] [CrossRef]
- Naz, S.; Ahmad, N.; Akhtar, J.; Ahmad, N.M.; Ali, A.; Zia, M. Management of Citrus Waste by Switching in the Production of Nanocellulose. IET Nanobiotech. 2016, 10, 395–399. [Google Scholar] [CrossRef] [PubMed]
- Belouadah, Z.; Ati, A.; Rokbi, M. Characterization of New Natural Cellulosic Fiber from Lygeum Spartum L. Carbohydr. Polym. 2015, 134, 429–437. [Google Scholar] [CrossRef] [PubMed]
- Zafeiropoulos, N.E.; Williams, D.R.; Baillie, C.A.; Matthews, F.L. Engineering and Characterisation of the Interface in Flax Fibre/Polypropylene Composite Materials. Part I. Development and Investigation of Surface Treatments. Compos. Part A 2002, 33, 1083–1093. [Google Scholar] [CrossRef]
- Lopes, I.A.; Santos, J., Jr.; Da Silva, D.C.; Da Silva, L.J.S.; Barros, A.K.; Villa-Vélez, H.A.; Santana, A.A. Characterization of Pectin Biofilms with the Addition of Babassu Mesocarp and Whey Protein Concentrate. Am. J. Mater. Sci. 2017, 7, 64–70. [Google Scholar] [CrossRef]
- Bátori, V.; Jabbari, M.; Åkesson, D.; Lennartsson, P.R.; Taherzadeh, M.J.; Zamani, A. Production of Pectin-Cellulose Biofilms: A New Approach for Citrus Waste Recycling. Int. J. Polym. Sci. 2017, 2017. [Google Scholar] [CrossRef]
| Identification Code | Pectin (PEC) (g) | Residue (g) |
|---|---|---|
| Purified PEC | 1 | - |
| PEC | 1 | - |
| PEC-15 | 1 | 0.15 |
| PEC-25 | 1 | 0.25 |
| PEC-35 | 1 | 0.35 |
| Data/Samples | PEC-2h-9% | PEC-4h-9% | PEC-6h-9% | PEC-6h-3% |
|---|---|---|---|---|
| Yield (%) | 16.4 ± 3.4 | 20.3 ± 2.2 | 23.7 ± 1.8 | 24.1 ± 2.6 |
| DM (%) | 70.6 ± 3.2 | 58.3 ± 3.4 | 57.8 ± 3.0 | 70.3 ± 3.3 |
| Samples | Mn (Da) | Mw (Da) | IV |
|---|---|---|---|
| PEC-2h-9% | 55,503 | 140,719 | 1.53 |
| PEC-4h-9% | 39,320 | 116,889 | 0.96 |
| PEC-6h-9% | 25,443 | 91,293 | 0.57 |
| PEC-6h-3% | 12,6486 | 308,847 | 1.69 |
| Data/Samples | Young Modulus (E) (MPa) | Tensile Strength (σ) (MPa) | Elongation at Break (ε) (%) |
|---|---|---|---|
| Purified PEC film | 4045.0 ± 1051 | 22.0 ± 22 | 0.7 ± 0.4 |
| PEC film | 46.0 ± 33 | 3.2 ± 2 | 31.2 ± 9.3 |
| Data/Samples | Force to Break (N) | Displacement (mm) |
|---|---|---|
| PEC | 5.3 ± 1.8 | 7.8 ± 1.2 |
| PEC-15 | 19.9 ± 7.4 | 8.3 ± 0.7 |
| PEC-25 | 27.6 ± 7.9 | 6.9 ± 3.3 |
| PEC-35 | 3.7 ± 1.2 | 4.6 ± 0.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zannini, D.; Dal Poggetto, G.; Malinconico, M.; Santagata, G.; Immirzi, B. Citrus Pomace Biomass as a Source of Pectin and Lignocellulose Fibers: From Waste to Upgraded Biocomposites for Mulching Applications. Polymers 2021, 13, 1280. https://doi.org/10.3390/polym13081280
Zannini D, Dal Poggetto G, Malinconico M, Santagata G, Immirzi B. Citrus Pomace Biomass as a Source of Pectin and Lignocellulose Fibers: From Waste to Upgraded Biocomposites for Mulching Applications. Polymers. 2021; 13(8):1280. https://doi.org/10.3390/polym13081280
Chicago/Turabian StyleZannini, Domenico, Giovanni Dal Poggetto, Mario Malinconico, Gabriella Santagata, and Barbara Immirzi. 2021. "Citrus Pomace Biomass as a Source of Pectin and Lignocellulose Fibers: From Waste to Upgraded Biocomposites for Mulching Applications" Polymers 13, no. 8: 1280. https://doi.org/10.3390/polym13081280
APA StyleZannini, D., Dal Poggetto, G., Malinconico, M., Santagata, G., & Immirzi, B. (2021). Citrus Pomace Biomass as a Source of Pectin and Lignocellulose Fibers: From Waste to Upgraded Biocomposites for Mulching Applications. Polymers, 13(8), 1280. https://doi.org/10.3390/polym13081280








