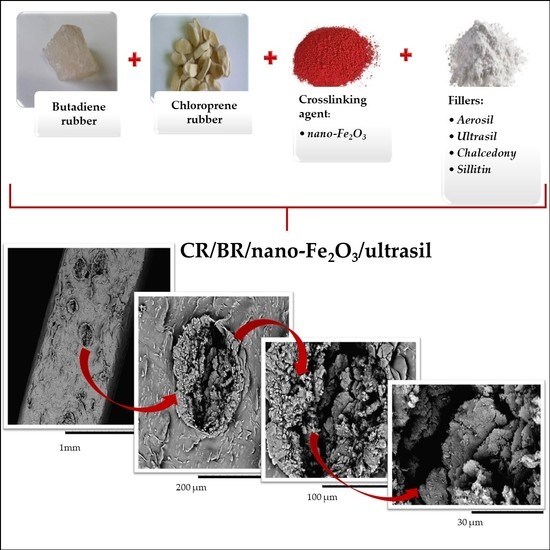
Curing Behaviors, Mechanical and Dynamic Properties of Composites Containing Chloroprene and Butadiene Rubbers Crosslinked with Nano-Iron(III) Oxide
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation and Vulcanization of the Rubber Blends
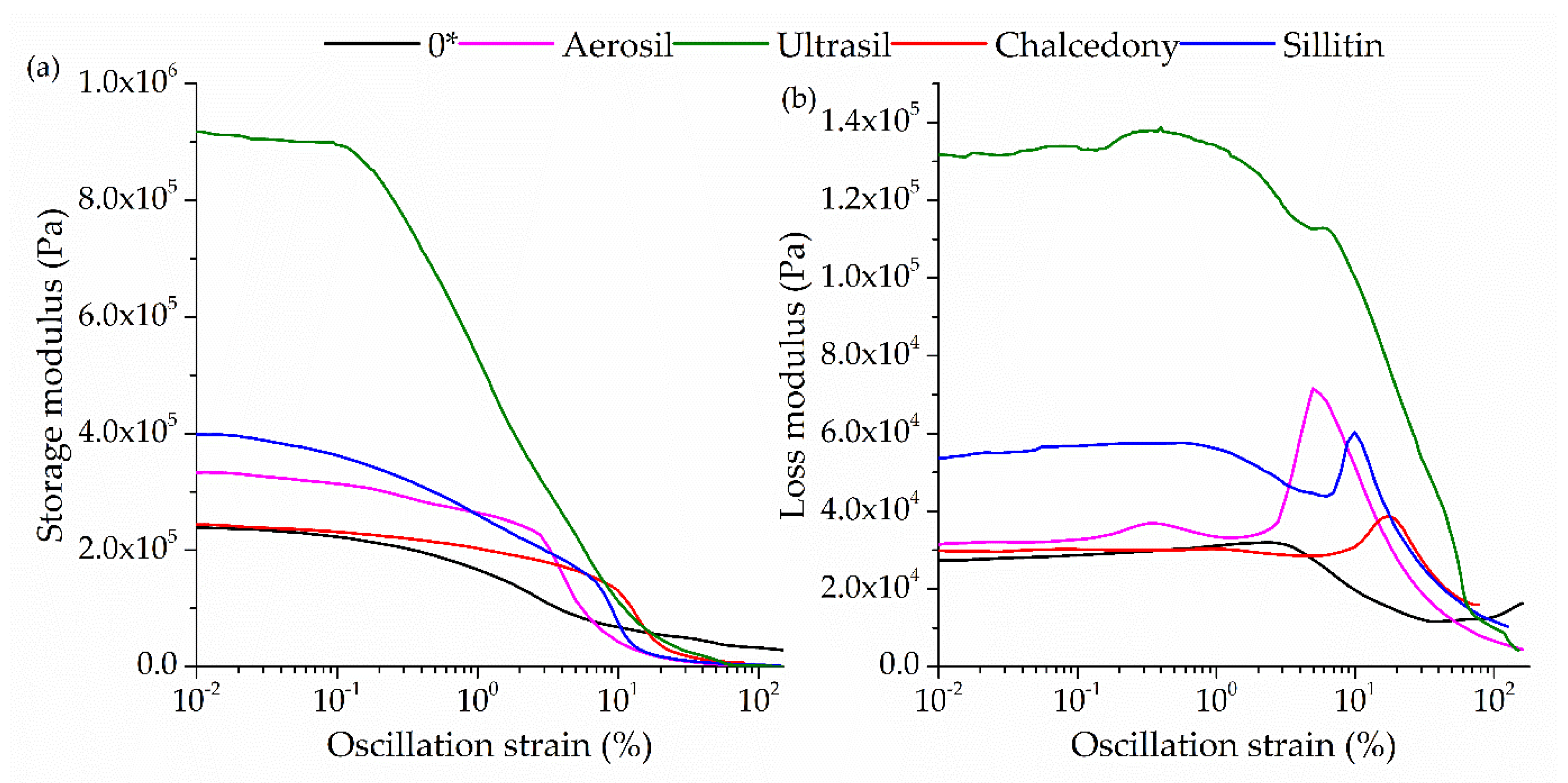
2.3. Dynamic Properties
2.4. Hysteresis Losses and Mullins Effect
2.5. Differential Scanning Calorimetry
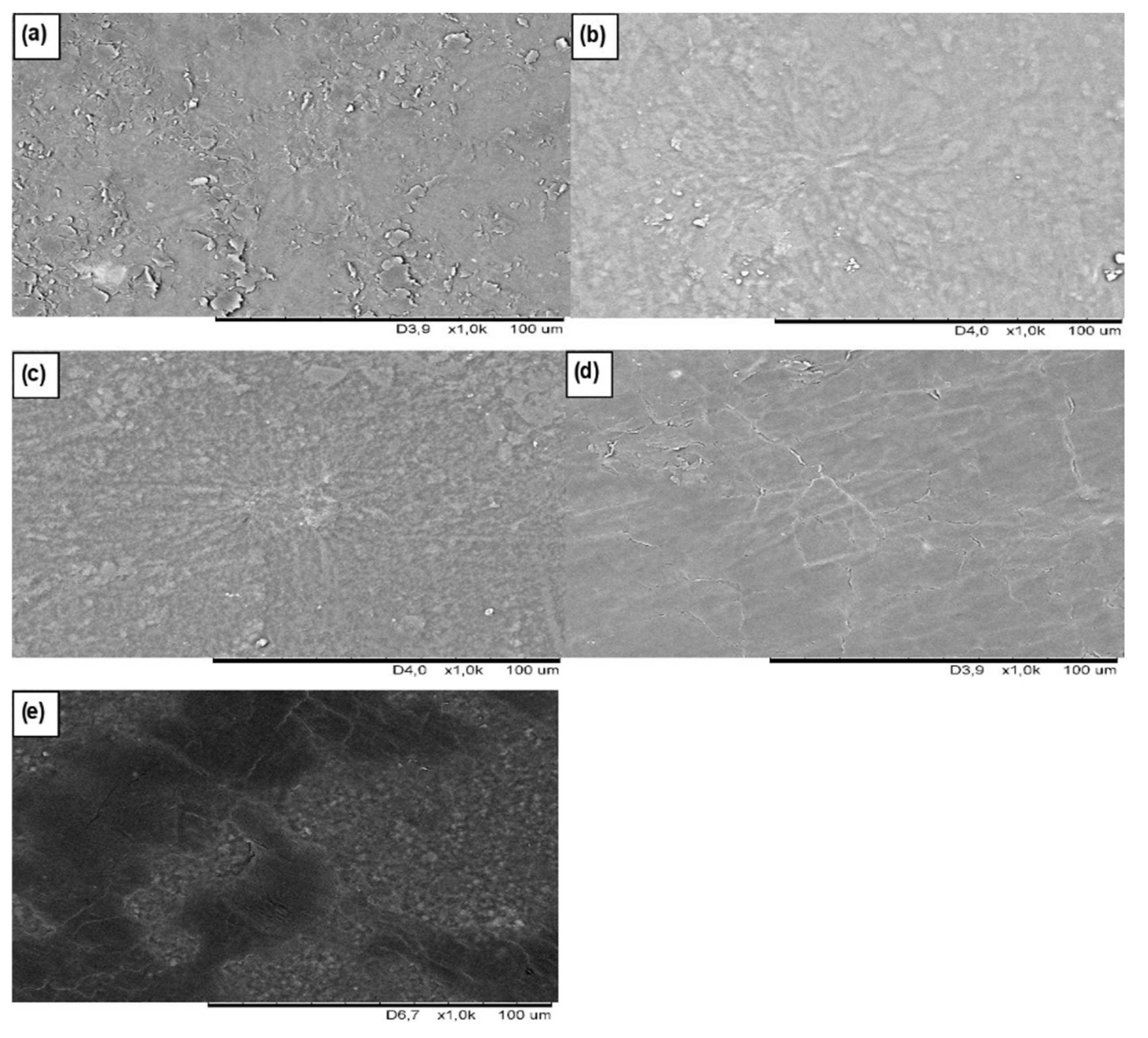
2.6. Morphology Studies
2.7. Cure Characteristic
2.8. Mechanical Test
2.9. Thermal Aging
2.10. Flammability
2.11. Thermogravimetry Analysis and First Derivative Thermogravimetry
3. Results and Discussion
3.1. Morphology Analysis of Unfilled and Filled CR/BR/Nano-Fe2O3 Vulcanizates
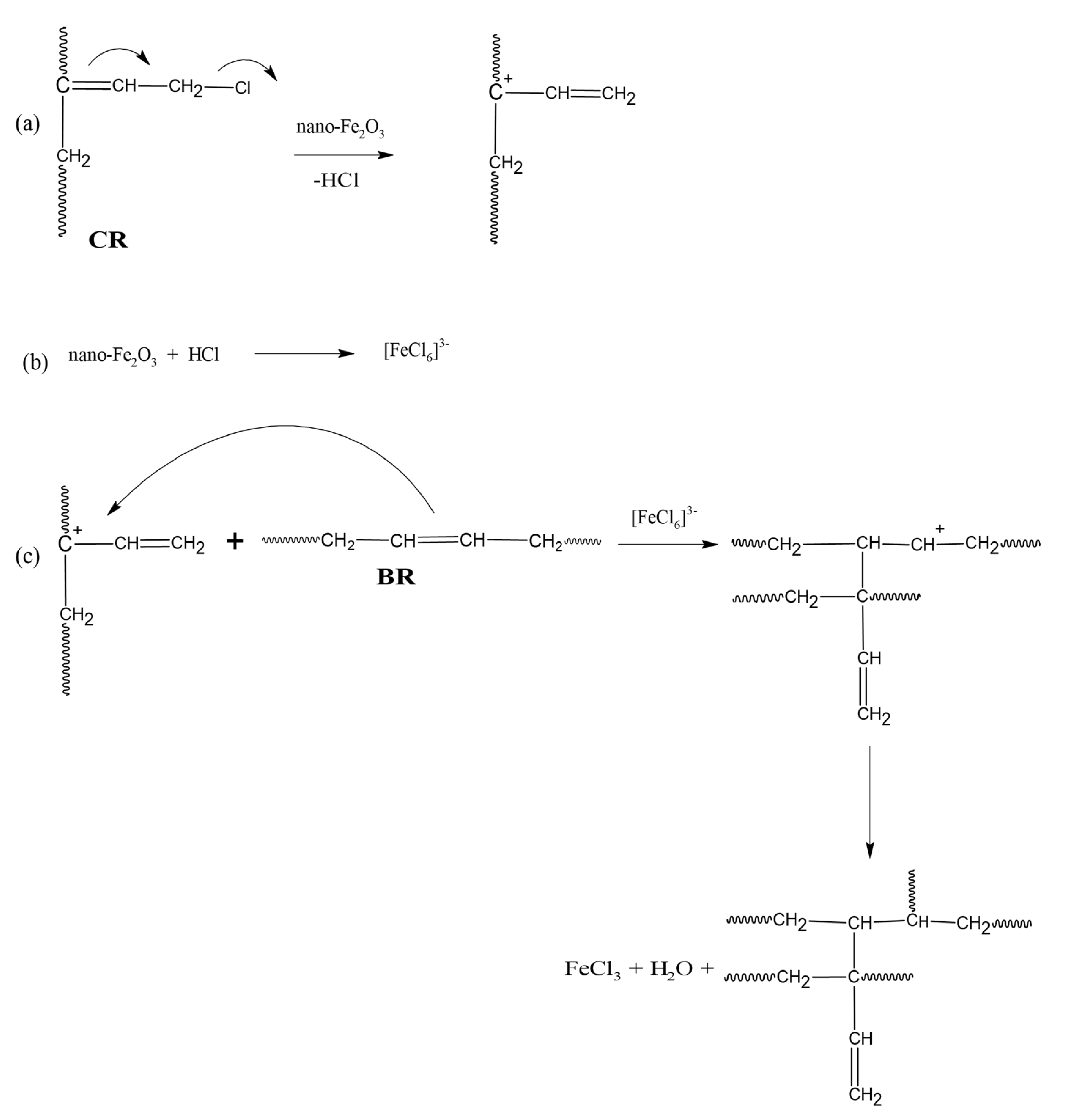
3.2. Cure Characteristic of Unfilled and Filled CR/BR/Nano-Fe2O3 Composites
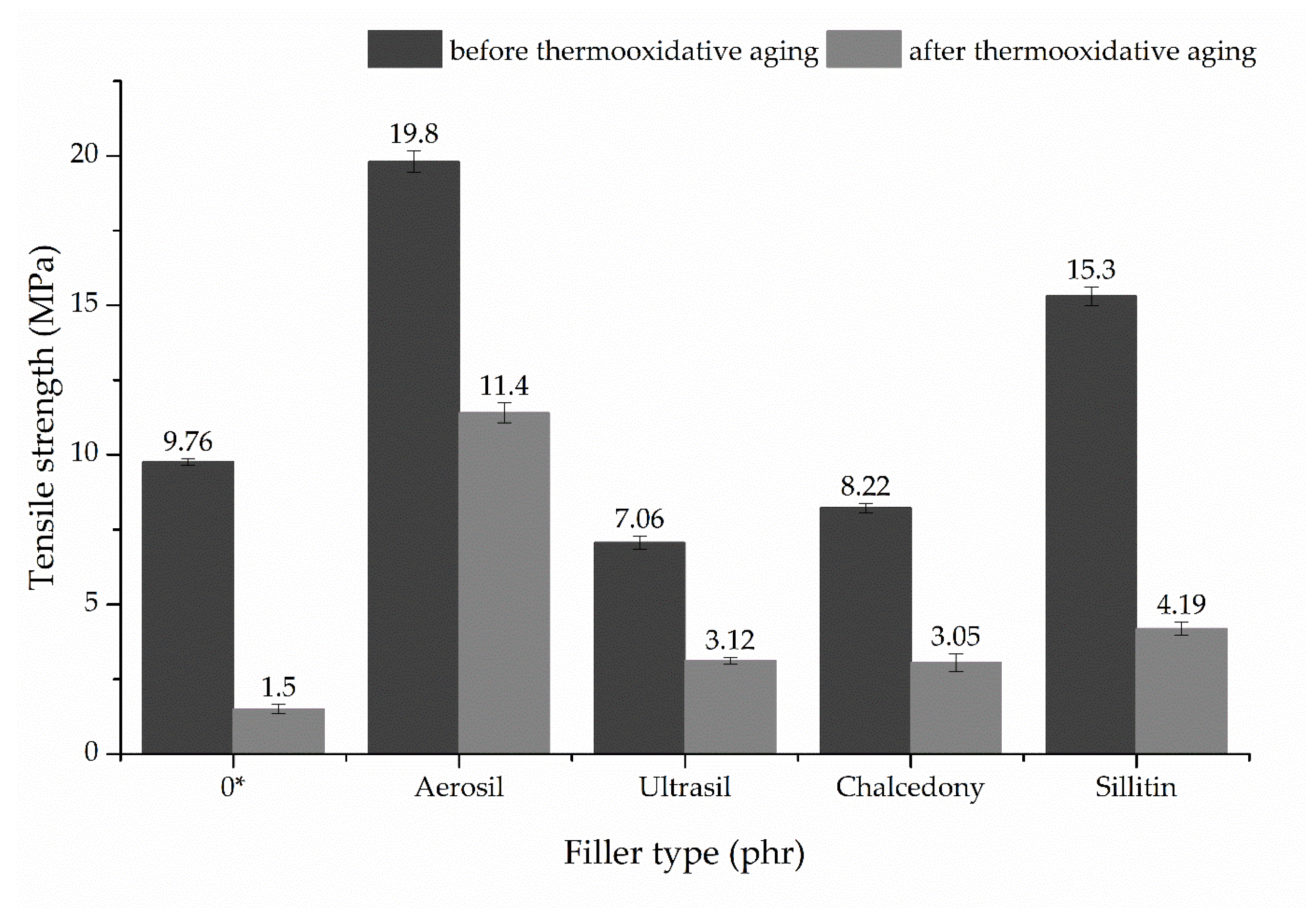
3.3. Mechanical Properties of Unfilled and Filled CR/BR/Nano-Fe2O3 Vulcanizates
3.4. Flammability of Unfilled and Filled CR/BR/Nano-Fe2O3 Vulcanizates
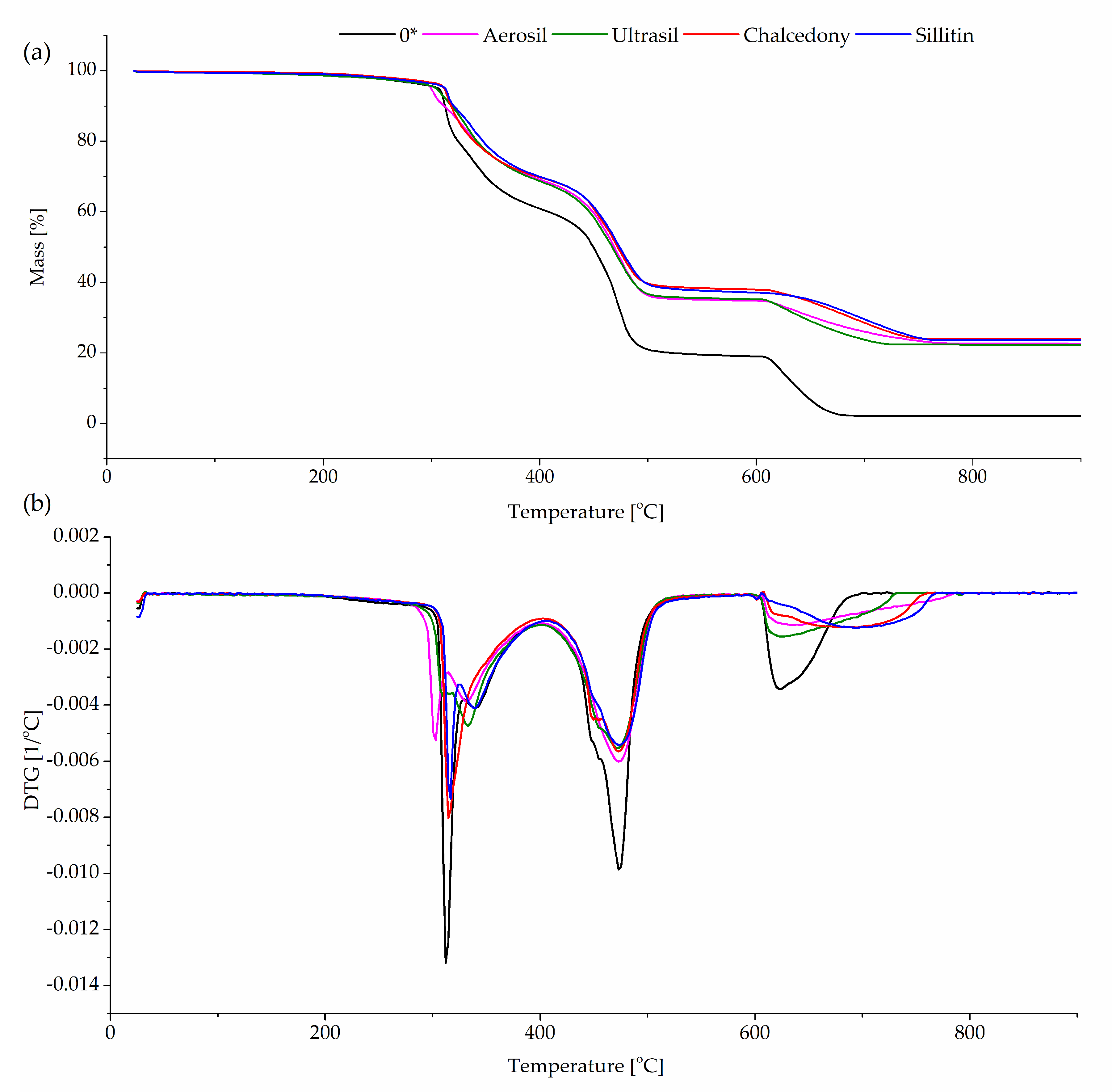
3.5. Thermal Stability of Unfilled and Filled CR/Br/Nano-Fe2O3 Vulcanizates
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Feldman, D. Elastomer Nanocomposite; Properties. J. Macromol. Sci. Part A 2012, 49, 784–793. [Google Scholar] [CrossRef]
- Song, K. Micro- and nano-fillers used in the rubber industry. In Progress in Rubber Nanocomposites; Elsevier BV: Amsterdam, The Netherlands, 2017; Volume 40, pp. 41–80. [Google Scholar]
- Zaborski, M.; Masłowski, M.; Pietrasik, J. Elastomers Containing Fillers with Magnetic Properties. Solid State Phenom. 2009, 154, 121–126. [Google Scholar] [CrossRef]
- Irez, A.; Bayraktar, E.; Miskioglu, I. Recycled and devulcanized rubber modified epoxy-based composites reinforced with nano-magnetic iron oxide, Fe3O4. Compos. Part B Eng. 2018, 148, 1–13. [Google Scholar] [CrossRef]
- Iacob, M.; Tugui, C.; Tiron, V.; Bele, A.; Vlad, S.; Vasiliu, T.; Cazacu, M.; Vasiliu, A.-L.; Racles, C. Iron oxide nanoparticles as dielectric and piezoelectric enhancers for silicone elastomers. Smart Mater. Struct. 2017, 26, 105046. [Google Scholar] [CrossRef]
- Khayam, S.U.; Usman, M.; Umer, M.A.; Rafique, A. Development and characterization of a novel hybrid magnetorheological elastomer incorporating micro and nano size iron fillers. Mater. Des. 2020, 192, 108748. [Google Scholar] [CrossRef]
- Harandi, M.H.; Alimoradi, F.; Rowshan, G.; Faghihi, M.; Keivani, M.; Abadyan, M. Morphological and mechanical properties of styrene butadiene rubber/nano copper nanocomposites. Results Phys. 2017, 7, 338–344. [Google Scholar] [CrossRef]
- Pazhooh, H.N.; Bagheri, R.; Adloo, A. Fabrication of semi-conductive natural rubber nanocomposites with low copper nanoparticle contents. Polymers 2017, 108, 135–145. [Google Scholar] [CrossRef]
- Rao, J.K.; Raizada, A.; Ganguly, D.; Mankad, M.M.; Satyanarayana, S.V.; Madhu, G.M. Enhanced mechanical properties of polyvinyl alcohol composite films containing copper oxide nanoparticles as filler. Polym. Bull. 2015, 72, 2033–2047. [Google Scholar] [CrossRef]
- Mammadov, S.M.; Khankishiyeva, R.F.; Mammadov, D.S.; Akhundzada, H.N.; Mahmudova, A.U. Influence nanopowders metal oxide on the rheological and structural properties of vulcanizates. Am. J. Polym. Sci. 2016, 6, 59–67. [Google Scholar] [CrossRef]
- Khankishiyeva, R.F.; Akberov, O.H.; Akberov, E.O.; Mammadov, S.M.; Aslanli, Z.A.; Akhundzada, H.N. Effect of nano-dimensional powders of metal oxides on the physicomechanical properties of vulcanized nitrile rubber. New Mater. Compd. Appl. 2018, 1, 90–102. [Google Scholar]
- Przybyszewska, M. The effect of zinc oxide nanoparticle morphology on activity in crosslinking of carboxylated nitrile elastomer. Express Polym. Lett. 2009, 3, 542–552. [Google Scholar] [CrossRef]
- Eslami, Z.; Mirzapour, M. Compatibilizing effect and reinforcing efficiency of nanosilica on ethylene-propylene diene monomer/chloroprene rubber blends. Polym. Compos. 2021. [Google Scholar] [CrossRef]
- Lim, G.E.; Park, K.; Cheon, J.M.; Jeong, B.Y.; Choi, M.J.; Lee, S.-K.; Lee, W.-K.; Jang, K.D.; Chun, J.H. Chloroprene rubber modified by nanosilica for performance adhesion strength. Mol. Cryst. Liq. Cryst. 2018, 660, 121–127. [Google Scholar] [CrossRef]
- Wu, C.; Gao, Y.; Liang, X.; Gubanski, S.M.; Wang, Q.; Bao, W.; Li, S. Manifestation of Interactions of Nano-Silica in Silicone Rubber Investigated by Low-Frequency Dielectric Spectroscopy and Mechanical Tests. Polymers 2019, 11, 717. [Google Scholar] [CrossRef] [PubMed]
- Smejda-Krzewicka, A.; Olejnik, A.; Strzelec, K. The role of iron(III) oxide in chloroprene and butadiene rubber blends’ cross-linking, structure, thermal and mechanical characteristics. Iran. Polym. J. 2019, 28, 313–323. [Google Scholar] [CrossRef]
- Smejda-Krzewicka, A.; Olejnik, A.; Strzelec, K. The effect of metal oxide on the cure, morphology, thermal and mechanical characteristics of chloroprene and butadiene rubber blends. Polym. Bull. 2019, 77, 4131–4146. [Google Scholar] [CrossRef]
- Olejnik, A.; Smejda-Krzewicka, A.; Strzelec, K.; Szynkowska, M.I. Curing and properties of chloroprene and butadiene rubber (CR/BR) blends cross-linked with copper(I) oxide or copper(II) oxide. Int. J. Polym. Anal. Charact. 2019, 24, 18–31. [Google Scholar] [CrossRef]
- Sae-Oui, P.; Sirisinha, C.; Thepsuwan, U.; Hatthapanit, K. Dependence of mechanical and aging properties of chloroprene rubber on silica and ethylene thiourea loadings. Eur. Polym. J. 2007, 43, 185–193. [Google Scholar] [CrossRef]
- Rothon, R. (Ed.) Polymers and Polymeric Composites: A Reference Series; Springer International Publishing: Berlin, Germany, 2017. [Google Scholar]
- Kashiwagi, T.; Gilman, J.W.; Butler, K.M.; Harris, R.H.; Shields, J.R.; Asano, A. Flame retardant mechanism of silica gel/silica. Fire. Mater. 2000, 24, 277–289. [Google Scholar] [CrossRef]
- Rybiński, P. Thermal Stability and Flammability of Elastomers and Elastomeric Materials; Lodz University of Technology: Lodz, Poland, 2014; p. 1182. [Google Scholar]



| Symbol | Trade Name | Company | Content of Bound Chlorine (%) | Content of cis Structure | Mooney Viscosity (100 °C) (ML) | Density (g/cm3) |
|---|---|---|---|---|---|---|
| CR | Baypren®216 | Lanxess GmbH, Dormagen, Germany | ~40 | 5–13 | 38–48 | 1.23 |
| BR | SYNTECA®44 | Synthos S.A., Oswiecim, Poland | - | ~97 | 39–49 | 0.91 |
| Filler | Trade Name | Company | Specific Surface Area (BET), (m2/g) |
|---|---|---|---|
| Sillica | Ultrasil®7000GR | Evonik Industries AG, Essen, Germany | 175 |
| Aerosil®380 | Evonik Industries AG, Essen, Germany | 380 | |
| Chalcedony | Crusil M12 | Crusil Co., Inowlodz, Poland | 10 |
| Neuburg siliceous earth | Sillitin Z86 | Hoffmann Mineral, Neuburg (Donau), Germany | 12 |
| Component | Component Content (phr) | |||
|---|---|---|---|---|
| CR | 80 | |||
| BR | 20 | |||
| Stearic acid | 1 | |||
| Aerosil | 30 | - | - | - |
| Ultrasil | - | 30 | - | - |
| Chalcedony | - | - | 30 | - |
| Sillitin | - | - | - | 30 |
| Nano-Fe2O3 | 2.5 | |||
| 0* | Aerosil | Ultrasil | Chalcedony | Sillitin | |
|---|---|---|---|---|---|
| ΔG [Pa] | 210,865 | 332,015 | 898,076 | 242,744 | 397,508 |
| G′max [Pa] | 238,314 | 333,081 | 899,439 | 247,517 | 399,321 |
| G″max [Pa] | 31,961 | 68,170 | 138,001 | 38,860 | 57,512 |
| 0* | Aerosil | Ultrasil | Chalcedony | Sillitin | |
|---|---|---|---|---|---|
| ΔWi (Nmm) | 38.1 | 550.8 | 179.0 | 86.3 | 90.3 |
| EM (%) | 18.5 | 77.1 | 70.0 | 27.5 | 45.3 |
| Filler Type (30 phr) | t02 (min) | t90 (min) | Mmin (dNm) | ∆M30 (dNm) | CRI (min−1) | QvT (mL/mL) | QvH (mL/mL) |
|---|---|---|---|---|---|---|---|
| 0* | 7.30 | 16.70 | 0.68 | 5.93 | 10.64 | 6.20 ± 0.11 | 0.86 ± 0.03 |
| Aerosil | 0.06 | 34.31 | 6.17 | 15.76 | 2.92 | 3.98 ± 0.15 | 0.73 ± 0.02 |
| Ultrasil | 1.10 | 14.00 | 3.23 | 21.49 | 7.75 | 6.21 ± 0.09 | 0.83 ± 0.04 |
| Chalcedony | 3.78 | 11.94 | 0.72 | 9.23 | 12.25 | 6.61 ± 0.13 | 0.91 ± 0.06 |
| Sillitin | 4.70 | 11.10 | 0.84 | 9.19 | 15.63 | 6.66 ± 0.22 | 1.00 ± 0.03 |
| Filler Type (30 phr) | Se100 (MPa) | Se200 (MPa) | Se300 (MPa) | TSb (MPa) | Eb (%) | TSb′ (MPa) | Eb′ (%) | K (-) | HA (°ShA) |
|---|---|---|---|---|---|---|---|---|---|
| 0* | 0.70 ± 0.02 | 0.99 ± 0.03 | 1.28 ± 0.04 | 9.76 ± 0.40 | 1120 ± 26 | 1.50 ± 0.11 | 192 ± 15 | 0.026 | 30.0 ± 0.5 |
| Aerosil | 4.93 ± 0.23 | 7.78 ± 0.31 | 10.60 ± 0.40 | 19.80 ± 0.21 | 650 ± 27 | 11.40 ± 0,35 | 140 ± 10 | 0.124 | 65.1 ± 0.1 |
| Ultrasil | 1.47 ± 0.06 | 2.04 ± 0.04 | 2.67 ± 0.03 | 7.06 ± 0.26 | 688 ± 35 | 3.12 ± 0.09 | 42 ± 5 | 0.029 | 55.2 ± 0.4 |
| Chalcedony | 0.98 ± 0.07 | 1.56 ± 0.06 | 2.16 ± 0.07 | 8.22 ± 0.36 | 845 ± 30 | 3.05 ± 0.20 | 138 ± 8 | 0.061 | 36.5 ± 0.3 |
| Sillitin | 1.08 ± 0.05 | 1.70 ± 0.06 | 2.31 ± 0.05 | 15.30 ± 0.82 | 1145 ± 35 | 4.19 ± 0.28 | 214 ± 16 | 0.051 | 41.9 ± 0.7 |
| Filler Type (30 phr) | Ts (N/mm) | Tτw (%) |
|---|---|---|
| 0* | 1.99 ± 0.06 | 10.00 |
| Aerosil | 12.60 ± 0.70 | 25.83 |
| Ultrasil | 8.16 ± 0.16 | 27.07 |
| Chalcedony | 3.93 ± 0.33 | 31.05 |
| Sillitin | 5.11 ± 0.23 | 28.71 |
| Sample | 0* | Aerosil | Ultrasil | Chalcedony | Sillitin |
|---|---|---|---|---|---|
| OI (%) | 31.0 | >37.5 | >37.5 | >37.5 | 37.5 |
| Filler Type (30 phr) | Temperature (°C) | Weight Loss (%) |
|---|---|---|
| 0* | 285–400 | 38.8 |
| 405–512 | 41.9 | |
| 600–700 | 16.9 | |
| Aerosil | 270–400 | 30.6 |
| 405–522 | 34.1 | |
| 600–790 | 12.3 | |
| Ultrasil | 270–400 | 31 |
| 405–520 | 33.3 | |
| 600–740 | 12.9 | |
| Chalcedony | 290–400 | 29.8 |
| 405–540 | 32 | |
| 590–760 | 14 | |
| Sillitin | 290–400 | 29.9 |
| 405–520 | 32.4 | |
| 585–770 | 13.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Słubik, A.; Smejda-Krzewicka, A.; Strzelec, K. Curing Behaviors, Mechanical and Dynamic Properties of Composites Containing Chloroprene and Butadiene Rubbers Crosslinked with Nano-Iron(III) Oxide. Polymers 2021, 13, 853. https://doi.org/10.3390/polym13060853
Słubik A, Smejda-Krzewicka A, Strzelec K. Curing Behaviors, Mechanical and Dynamic Properties of Composites Containing Chloroprene and Butadiene Rubbers Crosslinked with Nano-Iron(III) Oxide. Polymers. 2021; 13(6):853. https://doi.org/10.3390/polym13060853
Chicago/Turabian StyleSłubik, Anna, Aleksandra Smejda-Krzewicka, and Krzysztof Strzelec. 2021. "Curing Behaviors, Mechanical and Dynamic Properties of Composites Containing Chloroprene and Butadiene Rubbers Crosslinked with Nano-Iron(III) Oxide" Polymers 13, no. 6: 853. https://doi.org/10.3390/polym13060853
APA StyleSłubik, A., Smejda-Krzewicka, A., & Strzelec, K. (2021). Curing Behaviors, Mechanical and Dynamic Properties of Composites Containing Chloroprene and Butadiene Rubbers Crosslinked with Nano-Iron(III) Oxide. Polymers, 13(6), 853. https://doi.org/10.3390/polym13060853