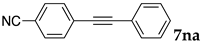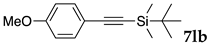Abstract
A few kinds of thermoresponsive diblock copolymers have been synthesized and utilized for palladium-catalyzed coupling reactions in water. Poly(N-isopropylacrylamide) (PNIPAAm) and poly(N,N-diethylacrylamide) (PDEAAm) are employed for thermoresponsive segments and poly(sodium 4-styrenesulfonate) (PSSNa) and poly(sodium 2-acrylamido-methylpropanesulfonate) (PAMPSNa) are employed for hydrophilic segments. Palladium-catalyzed Mizoroki–Heck reactions are performed in water and the efficiency of the extraction process is studied. More efficient extraction was observed for the PDEAAm copolymers when compared with the PNIPAAm copolymers and conventional surfactants. In the study of the Sonogashira coupling reactions in water, aggregative precipitation of the products was observed. Washing the precipitate with water gave the product with satisfactory purity with a good yield.
1. Introduction
The development of environmentally benign processes that enable organic syntheses to achieve the United Nations Sustainable Development Goals (SDGs) is an urgent subject. Transition metal-catalyzed chemical transformations are broadly utilized for producing fine chemicals; however, most catalytic reactions require organic solvents, which consequently results in an increase in the E-factor [1]. Conducting catalytic reactions in water is attractive to chemists who want to develop environmentally benign processes. In fact, many examples of palladium-catalyzed reactions conducted in water (or “on water”) have been reported over the past few decades [2,3,4,5,6,7,8,9,10,11,12,13]. The addition of surfactants to aqueous reaction mixtures causes the formation of oil/water (o/w) emulsions such that the organic reactions proceed in the micelle core. It is known that some chemical reactions can be accelerated in micellular systems [14,15,16,17]; however, organic reactions in o/w emulsions often require extraction processes using organic solvents to separate the products. Reducing the amount of the extraction solvent is an important subject for decreasing the E-factor of a process. Extraction processes might be more efficient if micelle formation can be “turned off” upon completion of the reaction. We envision that a thermoresponsive polymer micelle could be utilized for this purpose (Figure 1).
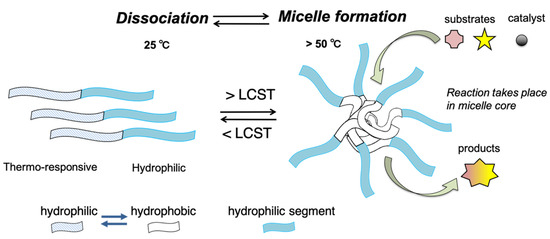
Figure 1.
Thermoresponsive micelle formed by diblock copolymers.
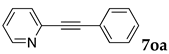
Poly(N-isopropylacrylamide) (PNIPAAm), which shows a lower critical solution temperature (LCST) at 32 °C in water, is known to be a thermoresponsive polymer and its applications in fields such as drug delivery systems and smart therapeutic materials have been studied extensively [18,19,20,21,22,23,24,25]. Thermoresponsive micelles that consist of PNIPAAm blocks have been vastly investigated, as has their utilization for therapeutic purposes [26,27,28,29,30]. For organic synthetic methods, there have also been many reports in which PNIPAAm was applied for organic reactions, as well as transition metal-catalyzed reactions. Many of these studies involve the use of a cross-linked PNIPAAm gel [31,32,33,34,35,36,37,38,39,40,41,42,43]; however, examples of thermoresponsive micelles applied for organic synthesis are still rare [44,45,46,47,48,49,50,51]. We previously reported the utilization of PNIPAAm block copolymers that form thermoresponsive micelles in water for organic synthesis. These micelles form at a temperature above 40 °C and dissociate at room temperature. We have tethered organocatalysts such as L-proline on the PNIPAAm block copolymers and demonstrated asymmetric cross-aldol reactions in water [44,46,47]. O’Reilly and coworkers also reported the use of PNIPAAm-based copolymer micelles bearing L-proline for asymmetric reactions in water [48]. We recently reported palladium-catalyzed Mizoroki–Heck reactions in water using these thermoresponsive polymer micelles [45] and showed that these reactions gave the products in high yields and with a good extraction efficiency. In the previous study, we reported that more efficient extraction was observed for aqueous solutions of the diblock copolymer poly(N-isopropylacrylamide)-b-poly(sodium 4-styrenesulfonate) (PNIPAAm-b-PSSNa, NS) compared to PNIPAAm-b-PEG, although the E-factor was still no less than 20. The extraction of more products from the aqueous reaction mixture with less organic solvent usage is important for elucidating an improved E-factor. Furthermore, the turnover number (TON) of palladium catalysts (2 mol %) was no more than 50, and reuse of the aqueous catalyst solutions was not achieved. Herein, we wish to report that Mizoroki–Heck reactions proceed in water using thermoresponsive micelles with a palladium catalyst 1 bearing 2,9-diphenyl-1,10-phenanthroline as a ligand. The TON reached 7800 due to high catalytic activity of 1. In this study, we employ a new system of three diblock copolymers (NA, DS and DA), and examine the reactions with these copolymers as well as the extraction efficiency from the aqueous solutions (Figure 2). We also report palladium-catalyzed Sonogashira coupling reactions using these copolymers in water.
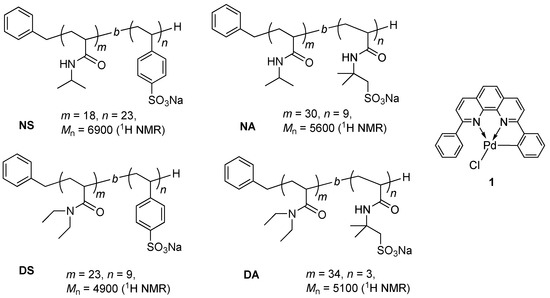
Figure 2.
Thermoresponsive diblock copolymers (with typical m and n) and palladium complex 1.
2. Materials and Methods
2.1. General
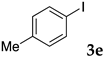
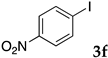
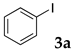
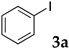
The preparation of copolymers was conducted under an argon atmosphere using standard Schlenk techniques unless otherwise mentioned. N-Isopropyl acrylamide (NIPAAm) was purchased from Kanto Chemical Co., Inc. and recrystallized from hexane/toluene prior to use. 2,2′-Azobis(isobutyronitrile) (AIBN), and dimethylacetamide (DMA) were purchased from Kanto Chemical Co., Inc. and used without further purification. Sodium dodecyl sulfate (SDS) and 4,4′-azobis(4-cyanovaleric acid) (V-501) were purchased from FUJIFILM Wako Pure Chemical Corporation and were used as received. N,N-Diethylacrylamide was purchased from Tokyo Chemical Industry Co., Ltd. and was distilled prior to use. Styrene was purchased from Tokyo Chemical Industry Co., Ltd., distilled, and kept under argon. Sodium 4-styrenesulfonate, 2-acrylamido-2-methylpropanesulfonic acid, dichlorobis(triphenylphosphine)palladium, 2,9-diphenyl-1,10-phenanthroline, iodobenzene, n-butyl acrylate, diisopropylethylamine, and α-methylstyrene were purchased from Tokyo Chemical Industry Co., Ltd. and were used as received. Other aryl halides, alkenes, ethynylarenes, and palladium catalysts were purchased and used as received. XPhos and Triton X-100 were purchased from Sigma-Aldrich Co. LLC. and used without further purification.
Palladium complex 1 was prepared from 2,9-diphenyl-1,10-phenanthroline and dichlorobis(acetonitrile)palladium according to the literature [52]. RAFT agent 2a and 2b were prepared according to the reported method in the literature [53,54]. Triethylammonium hypophosphite was prepared from triethylamine and hypophosphinic acid in toluene. The diblock copolymer NS was prepared as previously reported [45,55,56,57,58,59,60,61]. Dialysis was performed using Spectra/Por® RC tubing (MWCO: 3.5kD). Deionized water was obtained on WE-200 (Yamato Scientific Co., Ltd., Tokyo, Japan). NMR spectra were recorded on JEOL ECA 500 and Bruker Avance III HD400 spectrometers. Gel permeation chromatography (GPC) was measured on PU-4580 and RI-4030 system (JASCO Corporation, Tokyo, Japan) equipped with Shodex GPC KD-802.5 and KD-804 columns (Showa Denko K.K., Tokyo, Japan) using N,N-dimethylformamide (DMF) (0.1 wt % LiBr) as an eluent. The molecular weight of the polymers was determined based on monodispersed poly(ethylene oxide) as standard. Dynamic light scattering (DLS) measurements were made with the DLS-8000 and ELSZ-2000ZS (Otsuka Electronics Co., Ltd., Osaka, Japan) instruments. Scanning transmission electron microscopy (STEM) was recorded with a S-8000 (Hitachi High-Tech Corporation, Tokyo, Japan) instrument. Transmittance was recorded on a Shimadzu UV-2550 instrument.
2.2. Preparation of the Homopolymer PNIPAAm
A thoroughly dried Schlenk tube (100 mL) was filled with argon. In this tube, RAFT agent 2b (51 mg, 0.23 mmol), NIPAAm (0.54 g, 4.68 mmol), and AIBN (12 mg, 0.08 mmol) were dissolved in DMA (6 mL) and degassed in three freeze-pump-thaw cycles. The mixture was stirred at 60 °C for 24 h and the reaction mixture was poured into hexane/diethyl ether (75/75 mL) to precipitate a yellow solid. After the solvent was decanted, the yellow solid was dissolved in chloroform, the solution was collected, and the solvent was removed in a vacuum to leave the PNIPAAm homopolymer as a yellow solid (363 mg, 62%). The molecular weight was determined by 1H NMR spectroscopy. The DP (degree of polymerization) = 30, Mn = 3600 by 1H NMR. 1H NMR (D2O, Me3Si(CH2)3SO3Na, 500 MHz): δ 1.15 (CH3), 1.6 (CH2), 2.0–2.2 (CH), 3.89 (CH), 7.24–7.34 (br, Ph).
2.3. Preparation of the Copolymer PNIPAAm-b-PAMPSNa NA-T
The obtained PNIPAAm (227 mg, 0.063 mmol) was added to a dried Schlenk tube, and sodium 2-acrylamido-2-methylpropanesulfonic acid (0.19 g, 0.83 mmol) and AIBN (6 mg, 0.04 mmol) were dissolved in dimethylsulfoxide (DMSO) (5 mL) in the tube. The mixture was degassed in three freeze-pump-thaw cycles. The tube was stirred at 65 °C for 17 h and the yellow mixture was purified by dialysis for 3 days. The dialyzed yellow solution was dried in a vacuum to produce the product polymer NA-T as a white solid (380 mg, 89%). The molecular weight was determined by 1H NMR spectroscopy. The degree of polymerization (DP) of the AMPSNa units = 9 and Mn = 5700 by 1H NMR. Our attempts to record GPC has been unsuccessful so far due to highly ionic property of the polymer. 1H NMR (D2O, Me3Si(CH2)3SO3Na, 500 MHz): δ 1.15 (CH3), 1.56 (CH2), 2.0–2.2 (CH), 3.4–3.6 (br, SCH2), 3.89 (CH), 7.24–7.34 (br, Ph).
2.4. Removal of the Ethyl Xanthogenate Terminus in the PNIPAAm-b-PAMPSNa NA-T: Preparation of NA
The PNIPAAm-b-PAMPSNa NA-T (308 mg, 0.067 mmol), triethylammonium hypophosphite (82 mg, 0.4 mmol), and V-501 (11 mg, 0.04 mmol) were dissolved in DMSO (5 mL) and the solution was degassed by the freeze-pump-thaw method. [62] The mixture was stirred at 80 °C for 3 h and additional V-501 (11 mg, 0.04 mmol) was added to the solution. After the mixture was stirred at 60 °C for 17 h, the yellow solution was dialyzed. The resultant colorless solution with white precipitate was dried in vacuo to obtain the product NA as a white solid (281 mg, 76%). The molecular weight was determined by 1H NMR spectroscopy. DP of the PNIPAAm segment was 30, while PAMPSNa segment was 9, Mn = 5600 by 1H NMR. 1H NMR (D2O, Me3Si(CH2)3SO3Na, 500 MHz): δ 1.15 (CH3), 1.56 (CH2), 2.0–2.2 (CH), 3.4–3.6 (br, SCH2), 3.89 (CH), 7.24–7.34 (br, Ph).
2.5. Preparation of the Homopolymer PDEAAm
In a dried Schlenk tube (25 mL), RAFT agent 2a (92 mg, 0.36 mmol), N,N-diethylacrylamide (916 mg, 7.2 mmol), and AIBN (16 mg, 0.10 mmol) were dissolved in DMA (7 mL). The mixture was degassed in three freeze-pump-thaw cycles and was stirred at 60 °C for 24 h. The solution was poured into hexane (400 mL) and the yellow precipitate was dissolved in chloroform. The volatiles were removed in vacuo to produce PDEAAm as a yellow solid (529 mg, 53%). The average molecular weight of the polymer was determined as Mn = 2300, Mw/Mn = 1.20 by gel permeation chromatography (GPC) analysis using poly(ethylene oxide) as a standard and Mn = 3200 (DP = 23) as per 1H NMR. 1H NMR (D2O, Me3Si(CH2)3SO3Na, 500 MHz): δ 1.10–1.25 (CH-CH3), 1.5–1.8 (CH2), 2.5–2.8 (CH), 3.2–3.5 (NCH2), 7.24–7.34 (br, Ph).
2.6. Preparation of the Copolymer PDEAAm-b-PSSNa DS-T
In a thoroughly dried Schlenk tube, the obtained PDEAAm (255 mg, 0.08 mmol) was dissolved in DMSO (5 mL) and sodium 4-styrene sulfonate (167 mg, 0.8 mmol) and AIBN (5.2 mg, 0.032 mmol) were added. The mixture was degassed in 3 freeze-pump-thaw cycles and stirred at 65 °C for 24 h. The yellow mixture was dialyzed for 2 days. The volatiles were removed from the dialyzed mixture in vacuo to afford the title compound as a yellow solid (415 mg, 97%). 1H NMR (D2O, Me3Si(CH2)3SO3Na, 500 MHz): δ 1.10–1.25 (CH-CH3), 1.5–1.8 (CH2), 2.5–2.8 (CH), 3.2–3.5 (NCH2), 7.24–7.34 (br, Ph), 7.6–7.8 (C6H4).
2.7. Removal of Trithiocarbonate Terminus from DS-T; Synthesis of DS
In a thoroughly dried Schlenk tube (100 mL), the prepared DS-T (769 mg, 0.138 mmol), triethylammonium hypophosphite (172 mg, 0.833 mmol), and V-501 (28 mg, 0.1 mmol) were dissolved in DMSO (8 mL). The mixture was degassed by 3 freeze-pump-thaw cycles and heated at 80 °C for 24 h. The yellow solution was dialyzed for 25 h. The volatiles were removed in vacuo to give the title compound as a white solid (662 mg, 89%). DP was determined by 1H NMR (m = 23, n = 9, Mn = 4900). 1H NMR (D2O, Me3Si(CH2)3SO3Na, 500 MHz): δ 1.10–1.25 (CH-CH3), 1.5–1.8 (CH2), 2.5–2.8 (CH), 3.2–3.5 (NCH2), 7.24–7.34 (br, Ph), 7.6–7.8 (C6H4).
2.8. Preparation of the Diblock Copolymer Poly(DEAAm-b-AMPSNa) DA-T
The diethylacrylamide homopolymer was prepared as described above (DP = 34). The obtained PDEAAm homopolymer (428 mg, 0.12 mmol), sodium 2-acrylamide-2-methylpropane sulfonate (444 mg, 0.6 mmol), and AIBN (9 mg, 0.055 mmol) were dissolved in DMSO (7 mL) and degassed in three freeze-pump-thaw cycles. The mixture was stirred at 65 °C for 17 h. The yellow mixture was dialyzed, and the volatiles were removed in vacuo. The title compound was obtained as a white solid (380 mg, 78%). 1H NMR (D2O, Me3Si(CH2)3SO3Na, 500 MHz): δ 1.10–1.25 (CH-CH3), 1.5 (CH3), 1.6–1.8 (CH2), 2.5–2.8 (CH), 3.2–3.5 (NCH2 + SCH2), 7.24–7.34 (br, Ph).
2.9. Removal of Trithiocarbonate Terminus from DA-T; Synthesis of DA
The obtained polymer DA-T (444 mg, 0.12 mmol) was dissolved in DMSO (6 mL), and triethylammonium hypophosphite (112 mg, 0.54 mmol) and V-501 (15 mg, 0.054 mmol) were added to this solution. The mixture was degassed by the freeze-pump-thaw method and stirred at 80 °C for 3 h. Additional V-501 (15 mg, 0.054 mmol) was added to the solution. After the mixture was stirred at 60 °C for 17 h, the yellow solution was dialyzed. The resultant colorless solution with a white precipitate was dried in vacuo to afford the product as a white solid (281 mg, 76%). DP was determined by 1H NMR (m = 34, n = 3, Mn = 5100). 1H NMR (D2O, Me3Si(CH2)3SO3Na, 500 MHz): δ 1.10–1.25 (CH-CH3), 1.5 (CH3), 1.6–1.8 (CH2), 2.5–2.8 (CH), 3.2–3.5 (NCH2 + SCH2), 7.24–7.34 (br, Ph).
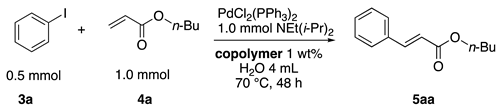
2.10. Mizoroki–Heck Reactions in Water Using the Copolymers, Initial Study
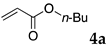
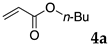
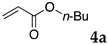
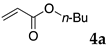
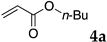
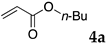
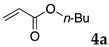
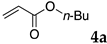
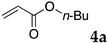
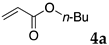
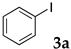
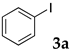
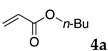
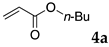
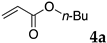
The typical procedure for Mizoroki–Heck reactions in water using the thermoresponsive micelles is as follows. In a test tube with a screw cap, the copolymer (40 mg) was dissolved in deionized water (4 mL) and the solution was stirred. With this solution, iodobenzene (102 mg, 0.5 mmol), n-butyl acrylate (128 mg, 1.0 mmol), PdCl2(PPh3)2 (7.0 mg, 0.01 mmol), and diisopropylethylamine (129 mg, 1.0 mmol) were added and the mixture was stirred at 70 °C for 48 h. The grayish turbid suspension was cooled in an ice bath and then diethyl ether (3 mL) was added and stirred for 1 h. The organic layer was taken up and extracted again with diethyl ether (3 mL) until the product was not detected by thin layer chromatography. The extract was analyzed by gas chromatography using tetradecane as an internal standard to determine the GC yield.
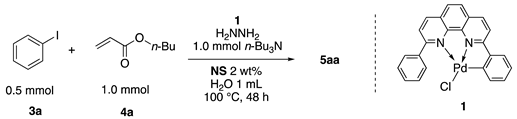
2.11. Mizoroki–Heck Reactions in Water Using the Copolymers Catalyzed by 1
Typically, in a test tube with a screw cap, the copolymer (20 mg) was dissolved in deionized water (1 mL) and the solution was stirred. With this solution, iodobenzene (102 mg, 0.5 mmol), n-butyl acrylate (128 mg, 1.0 mmol), and tri-n-butylamine (185 mg, 1.0 mmol) were added and the mixture was stirred. Meanwhile, palladium complex 1 (2.4 mg, 0.005 mmol) was dissolved in N-methyl-2-pyrrolidone (400 μL) in a vial. With this yellow solution, hydrazine monohydrate was added (1.6 mg, 0.032 mmol) and stirred for 10 s. This solution (40 μL) was added to the test tube and the mixture was stirred at 70 °C for 48 h. The turbid suspension was cooled in an ice bath and then ethyl acetate (0.4 mL) was added and vigorously stirred, then centrifuged at 1000 rpm for 10 min (180× g). The organic layer was taken up and extracted again with ethyl acetate (0.4 mL) until the product was not detected by thin layer chromatography. The extract was analyzed by gas chromatography using tetradecane as an internal standard to determine the GC yield (97%).
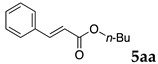
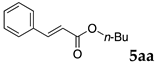
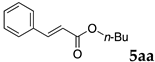
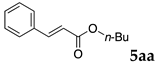
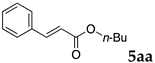
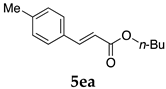
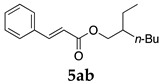
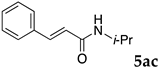
2.12. Evaluation of Extraction Efficiencies of Mizoroki–Heck Product 5aa
Typically, in a test tube with screw cap, the block copolymer DS (40 mg) was dissolved in deionized water (4 mL) and the solution was stirred for 0.5 h at room temperature. With this solution, n-butyl cinnamate (5aa, 102 mg, 0.5 mmol) was added and the mixture was stirred at 70 °C for 1 h. Ethyl acetate (1 mL) was added to this mixture, and this mixture was shaken at 120 rpm in a shaking apparatus at 0 °C for 0.5 h. The mixture was allowed to stand still for 0.5 h at room temperature, and then the organic layer was taken up and analyzed by gas chromatograph using tetradecane as an internal standard.
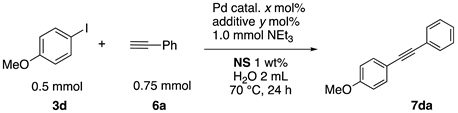
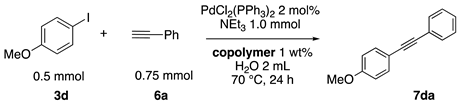
2.13. Sonogashira Coupling Reactions in Water Using the Copolymers
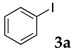
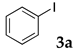
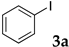
The typical procedure for Sonogashira coupling in water is as follows. In a dried test tube with a screw cap, copolymer NS (20 mg) was dissolved in deionized water (2 mL). Then, 4-iodoanisole (117 mg, 0.5 mmol), ethynylbenzene (77 mg, 0.75 mmol), PdCl2(PPh3)2 (7.0 mg, 0.01 mmol), and triethylamine (110 mg, 1.0 mmol) were added, and the mixture was then stirred at 70 °C for 24 h. The brown turbid solution was cooled in an ice bath while stirring until the supernatant became clear. The precipitated brown solid was collected by filtration when possible. Otherwise, the mixture was extracted with ethyl acetate and purified by column chromatograph on silica gel (hexane/ethyl acetate = 4/1). The solid was characterized by 1H NMR in CDCl3.
3. Results and Discussion
3.1. Preparation and Temperature-Dependent Properties of the Diblock Copolymers
We previously reported the use of the thermoresponsive diblock copolymer poly(N-isopropylacrylamide-b-sodium 4-styrenesulfonate) (PNIPAAm-b-PSSNa, NS) in palladium-catalyzed reactions in water. [45] In this study, we also employed poly(N,N-diethylacrylamide) (PDEAAm) as a thermoresponsive block that shows LCST at 35–40 °C in water [63]. We envisioned that the enhanced hydrophobic properties of the N,N-diethylamide moieties compared with PNIPAAm may facilitate intake of organic substrates into the micelle core. In addition, poly(sodium 2-acrylamide-2-methylpropane sulfonate) (PAMPSNa) was used as a hydrophilic segment as the amide moiety can be expected to be more hydrophilic than PSSNa that bears aromatic rings.
Thus, in addition to the previously reported NS, three diblock copolymers were prepared, i.e., PNIPAAm-b-PAMPSNa NA [64,65,66], PDEAAm-b-PSSNa DS [67], and PDEAAm-b-PAMPSNa DA. All polymers were synthesized by a reversible addition-fragmentation chain-transfer (RAFT) polymerization technique. For example, DEAAm was polymerized in the presence of RAFT agent 2a to give a PDEAAm homopolymer, followed by polymerization of AMPSNa on PDEAAm to give a PDEAAm-b-PAMPSNa diblock copolymer DA-T which has a trithiocarbonate terminus. Removal of the sulfur-containing moieties at the termini afforded DA (Scheme 1). Typical examples of the polymerization degree as determined by 1H NMR are shown in Scheme 1. To the best of our knowledge, diblock copolymer DA has not been reported to date, although the random copolymer was reported [68].
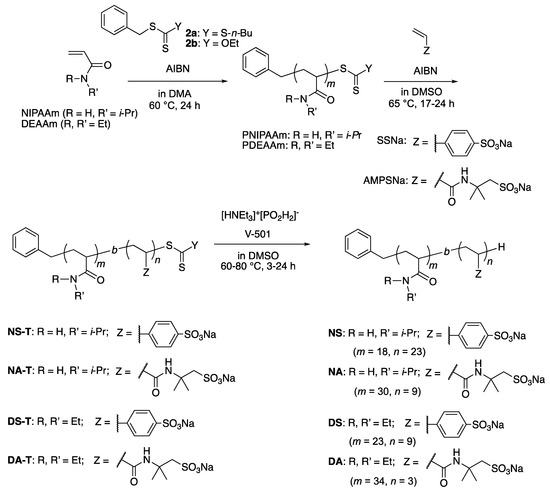
Scheme 1.
Synthesis of thermoresponsive diblock copolymers.
The temperature-dependent transmittance of the aqueous solution of the copolymers indicated their thermoresponsive behavior (Figure 3). Copolymer NA, which has a PNIPAAm block as the thermoresponsive segment, showed LCST at 30–35 °C, while copolymers DS and DA, which have a thermoresponsive PDEAAm block, showed LCST at 35–40 °C.
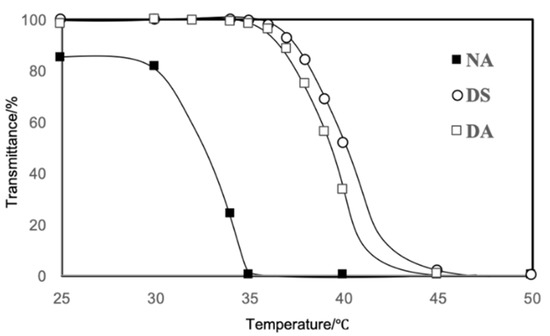
Figure 3.
Transmittance (λ = 600 nm) of the aqueous solutions of the copolymers.
Dynamic light scattering (DLS) analysis of aqueous solutions of the copolymers indicated that the particle sizes at 50 °C were significantly larger than those at 30 °C, showing the thermoresponsive formation of copolymer micelles in water (Figure 4). It is noteworthy that the copolymers NA and DA showed smaller particle sizes as ca. 10 nm at 30 °C, compared with NS and DS (40 nm and 150 nm, respectively). It is possibly because NA and DA are well dispersed at 30 °C, owing to more hydrophilic PAMPSNa segments. On the other hand, DS and DA formed larger particles at 50 °C (270–380 nm) than those formed by NA. These results might suggest that more hydrophobic PDEAAm segments tend to form more aggregated micelles above LCST.
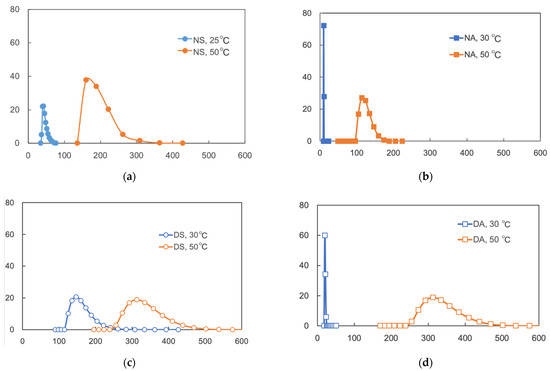
Figure 4.
DLS of the copolymers in aqueous solutions at 30 °C and 50 °C. (a) NS [45], (b) NA, (c) DS, and (d) DA.
3.2. Palladium-Catalyzed Mizoroki–Heck Reaction Using the Copolymers
We studied the Mizoroki–Heck reaction in water using the thermoresponsive polymer micelles. We previously reported that copolymer NS promoted the Mizoroki–Heck reaction in water with PdCl2(PPh3)2 as a catalyst precursor [45]. In this study, the three other copolymers were also investigated. Initially, we compared these polymers under our previous reaction conditions (Table 1). The copolymer was dissolved in water at room temperature and to this aqueous solution was added the substrates, base, and the palladium catalyst. As the mixture was heated at 70 °C and stirred, the mixture became opaque. After stirring, the reaction mixture was cooled to room temperature whereupon the solution became clear. The product was extracted and the yield was determined by gas chromatography or 1H NMR.

Table 1.
Mizoroki–Heck reaction in water using the copolymers a.
The reaction using copolymer NA delivered a moderate yield (entry 3), whereas those with DS and DA resulted in satisfactory results (entries 4 and 6). These results were superior to those obtained by using conventional surfactants such as sodium dodecyl sulfate (SDS) and Triton X-100 (entries 7 and 8), as well as those obtained without any surfactants (only water, entry 9) under the same reaction conditions. The aqueous solution of NA was much more viscous than other polymer solutions, and the lower yield obtained with NA might be due to slow diffusion of the substrates. In these reactions, 2 mol % of palladium complex PdCl2(PPh3)2 was required to complete the reactions. A decrease of the Pd load to 0.5 mol % resulted in lower yields (entries 2 and 5).
In our previous report, we examined a range of Pd complexes and concluded that PdCl2(PPh3)2 was optimal. In this study, we employed the Pd complex of NNC-pincer ligand 1 as a catalyst precursor [69,70,71,72]. Uozumi and coworkers reported excellent catalytic activity of ligand 1 for various reactions [52,73,74,75]. We adopted 1 for the Mizoroki–Heck reactions using NS (Table 2). When only 1 was added to the system, the reaction resulted in a moderate yield (entry 1). We considered that the Pd(II) species was not effectively reduced in the reaction system. Hydrazine hydrate was then mixed with 1 prior to use in a small amount of N-methyl-2-pyrrolidone (NMP) and the NMP solution was added to the reaction mixture. Addition of hydrazine improved the catalytic activity remarkably, and the reactions gave the product in quantitative yields with less Pd load (0.1 mol%), in shorter reaction time (entry 3). Even the use of 0.01 mol % Pd was sufficient to achieve a good yield, and the TON reached more than 6000, although the reaction was somewhat irreproducible. Thus, we decided to conduct further studies with 0.1 mol % of the Pd catalyst.

Table 2.
Mizoroki–Heck reaction in water with 1 a.
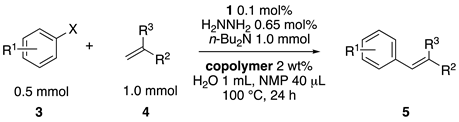
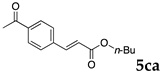
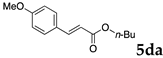
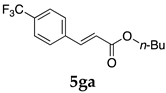
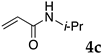
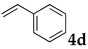
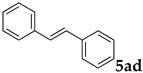
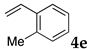
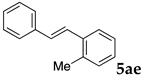
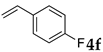
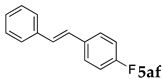
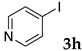
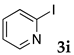
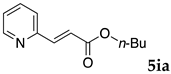
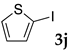
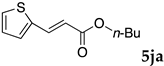
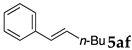
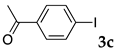
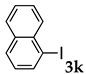
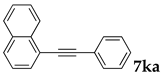
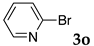
These results led us to investigate the reaction with various substrates with other copolymers using 1 in water (Table 3). Copolymers DS and DA delivered similar results to NS, whereas the use of NA resulted in lower yield (entries 1–4). Again, it is presumed that the low yield resulted from the high viscosity of the NA solution. Most iodoarenes reacted with n-butyl acrylate to give the products in good to excellent yields in the presence of 0.1 mol % ligand 1 and hydrazine regardless of the presence of electron-donating or electron-withdrawing groups on the aromatic ring (entries 6–10), whereas bromobenzene resulted in a low yield (entry 5). Other acrylic ester and amide gave the corresponding coupling products in good yield (entries 11–12). On the other hand, the use of styrene derivatives as a coupling partner gave lower yields (entries 13–15). In the attempts on heteroarenes such as iodopyridines and 2-iodothiophene, most of the starting materials remained intact (entries 16–18). Hex-1-ene gave a trace amount of the products as isomeric mixtures (entry 19).

Table 3.
Mizoroki–Heck reactions with various aryl halides and alkenes in water.
3.3. Reuse of the Aqueous Solution and Formation of Palladium Nanoparticles (PdNPs)
The aqueous solution after the reaction between iodobenzene and n-butyl acrylate was reused for further reactions. The substrates and bases were added and the mixture was stirred at 100 °C for 24 h. To our delight, the second reaction gave the product in 95% yield, and the third run delivered a 62% yield. Observation of the aqueous solution after the reactions by scanning transmission electron microscopy (STEM) indicated the formation of nanoparticles of palladium with diameters of 30–80 nm (Figure S1). Uozumi and coworkers proposed that the palladium atoms in 1 form nanoparticles (NPs) in the reaction solution and that single atoms liberated from the particles catalyze the reactions [52,73]. In this reaction, it is likely that PdNPs generated by the reduction with hydrazine are encapsuled and protected by the polymers in water, at room temperature. Gradual growth of the particle might cause the decrease of the yield.
3.4. Extraction Efficiency
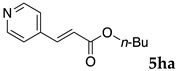
One of the problems in organic reactions in aqueous media when using micelles is the separation of the products from the reaction mixture. The products are commonly extracted with considerable amounts of organic solvents. Thus, reducing the amount of extraction solvents is important to achieve an environmentally benign system. In our previous study, we examined the efficiency in extracting the Mizoroki–Heck product 5aa from the aqueous solution of NS [45]. We herein also studied the other polymers.
Model aqueous mixtures were prepared that consisted of surfactants (1 wt %) and 5aa in water and these were stirred at 70 °C for 1 h. The mixtures were then cooled and extracted once at 0 °C with 1 mL of an extraction solvent such as diethyl ether or ethyl acetate. The results are summarized in Table 4. The extraction efficiency was estimated on the basis of the amount of recovered 5aa within a given period of extraction time. Overall, the extraction with ethyl acetate recovered more 5aa than with diethyl ether.

Table 4.
Extraction study from model aqueous solution a.
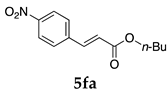
In the absence of any surfactants, 50–60% of 5aa was recovered within 30 min (entry 1). To our delight, more 5aa was recovered from most of the copolymer solutions (NA, DS and DA). This is probably due to salting out effect by sulfonate ions. Note that the DEAAm copolymer solutions of DS and DA showed better results compared with those of the NIPAAm copolymer NS. This is presumably because of the more hydrophobic nature of N,N-diethylamide moieties. The extraction efficiency from the suspension of SDS was comparable to that from water (entry 6), although the palladium-catalyzed reaction gave the product in lower yield in SDS suspension under the present reaction conditions, as we previously reported [45]. We conducted the same study using the reaction mixture with DA (entries 7–8). Single extraction with 1 mL of ethyl acetate gave 83% recovered product 5aa (entry 7), and 0.5 mL of ethyl acetate gave 68% recovered product (entry 8). The calculated E-factors in these entries were 12.6 and 8.9, respectively.
3.5. Sonogashira Coupling Reaction
Sonogashira coupling reaction is a powerful tool for the construction of alkynylarene scaffolds. The reaction was originally promoted by palladium catalysts and copper salts as co-catalysts. Recently, it was found that the reactions can proceed under copper-free conditions [76,77], especially in aqueous media [78,79]. We herein examined copper-free Sonogashira coupling reactions in the thermoresponsive micelle system.
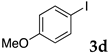
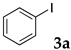
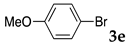
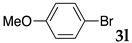
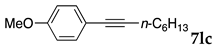
First, palladium catalysts were examined in the absence of copper salts for the reaction between 4-iodoanisole (3d) and phenylacetylene (6a) using NS as a copolymer surfactant (Table 5). Among a selection of palladium(II) catalyst precursors (entries 1–5), PdCl2(PPh3)2 gave the product 7da in 86% yield, even in the absence of Cu salt (entry 2), whereas the yield reached 99% when CuI was added (entry 11). Combination of Pd(II) species and phosphine ligands were also studied (entries 6–9). Although triphenylphosphine and tricyclohexylphosphine gave low to moderate yields (entries 6 and 7), the addition of XPhos, which is known to be effective for copper-free Sonogashira coupling [80,81,82], increased the yield (entries 8 and 9). Palladium complex 1, which was highly active for Mizoroki–Heck reactions, was not so active in this reaction (entry 10). Nickel complexes showed low activity (entries 12 and 13). Thus, we selected the conditions described in entry 2 for further studies on the effects of surfactant copolymers. The results are summarized in Table 6.

Table 5.
Copper-free Sonogashira coupling in water with Pd catalysts a.

Table 6.
Copper-free Sonogashira coupling in water with surfactants a.
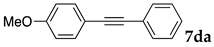
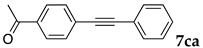
The reaction between 4-iodoanisole and phenylacetylene in water using various polymers gave the Sonogashira product 7da with moderate to good yields. Reactions with the copolymers NS, NA, DS, and DA gave slightly higher yields than with conventional surfactants. Interestingly, when polymer NS was applied (Table 6, entry 2), the product 7da precipitated at the bottom of the reaction vessel as aggregated chunks (Figure 5). Before the reaction, added substrates were biphasic and separated. The mixture was finely suspended with the formation of micelles during the reaction at 70 °C. The mixture was cooled after the reaction with stirring, then the aggregated precipitates could be easily taken up by filtration and washed with water. Analysis of the washed solid by 1H NMR spectroscopy indicated that it was an almost pure product (84% yield, see the supporting information for the NMR spectrum). The polymer NS gave better precipitates, whereas other copolymers and surfactants afforded sticky solids that made separation more difficult. Although the procedure was somewhat irreproducible with respect to the formation of the aggregated chunks, the calculated E-factor in this method was 1.8 and the process might provide an advantageous method for product separation from the reaction mixture.
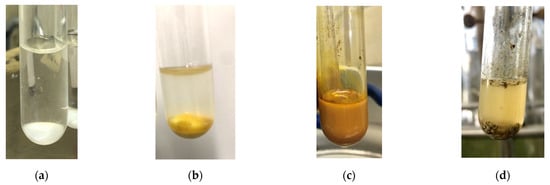
Figure 5.
The appearance of the reaction mixture of Sonogashira coupling with NS. (a) The aqueous polymer solution. (b) Before the reaction with substrates. (c) During the reaction. (d) Cooled after the reaction with precipitated chunks.
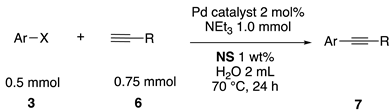
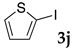
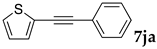
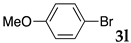
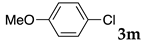
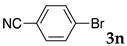
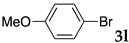
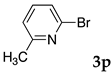
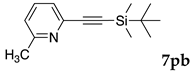
We then investigated the scope and limitations of the substrates in Sonogashira coupling reactions using NS as thermoresponsive micelles (Table 7). Aryl iodides bearing either electron-donating or electron-withdrawing groups gave the coupled products with good yields (entries 1 and 3), although aryl bromide gave the product in a low yield with PdCl2(PPh3)2 (entry 2). Pd(OAc)2 with XPhos, on the other hand, successfully promoted the reaction of aryl bromides (entries 6, 8–10, 12), except for the reaction between 2-bromo-6-methylpyridine and (tert-butyldimethylsilyl)acetylene (entry 11). The reaction of aryl chloride was disappointing (entry 7). It was demonstrated that Pd(OAc)2/XPhos was effective as a catalyst in water for various aryl bromides, including alkenyl bromide (entry 13), and terminal alkynes. [82] On the contrary to the Mizoroki–Heck reactions, heteroarenes such as 2-iodothiophene and 2-bromopyridine gave the coupling products in moderate to good yields (entries 5, 9 and 11).

Table 7.
Copper-free Sonogashira coupling in water using thermoresponsive copolymers a.
4. Conclusions
We have prepared thermoresponsive diblock copolymers that consist of poly(N-isopropylacrylamide)/poly(N,N-diethylacrylamide) as thermoresponsive segments and poly(sodium 4-styrenesulfonate)/poly(sodium 2-acrylamido-methylpropanesulfonate) as a hydrophilic segment. These copolymers formed micelles at 50 °C in water and they dissolved at room temperature. Palladium-catalyzed Mizoroki–Heck reactions proceeded in water using these thermoresponsive micelles. In particular, the palladium complex of NNC-pincer ligand 1 achieved a TON result as high as 7800. Copper-free Sonogashira coupling reactions were promoted in water using the copolymers. We achieved to decrease the use of organic extract by using the thermoresponsive polymer micelles.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/polym13162717/s1, Figure S1: STEM images of the aqueous solutions after the Mizoroki–Heck reactions.
Author Contributions
Investigation, S.K., R.K., N.E., R.A.; validation, S.K., R.K., N.E., R.A.; conceptualization, N.S. and F.-Y.T.; methodology, N.S., Y.T., M.R.; writing—original draft preparation, N.S.; writing—review and editing, Y.T., F.-Y.T.; project administration, N.S., F.-Y.T.; funding acquisition, N.S., Y.T., M.R. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by JSPS KAKENHI 21K05074.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
The authors thank Ken Watanabe and Daijiro Takayama for their assistance in the experiments.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Sheldon, R.A. The E Factor: Fifteen Years on. Green Chem. 2007, 9, 1273–1283. [Google Scholar] [CrossRef]
- Cortes-Clerget, M.; Yu, J.; Kincaid, J.R.A.; Walde, P.; Gallou, F.; Lipshutz, B.H. Water As the Reaction Medium in Organic Chemistry: From Our Worst Enemy to Our Best Friend. Chem. Sci. 2021, 12, 4237–4266. [Google Scholar] [CrossRef]
- Lipshutz, B.H.; Ghorai, S.; Cortes-Clerget, M. The Hydrophobic Effect Applied to Organic Synthesis: Recent Synthetic Chemistry “In Water”. Chem. A Eur. J. 2018, 24, 6672–6695. [Google Scholar] [CrossRef] [PubMed]
- Christoffel, F.; Ward, T.R. Palladium-Catalyzed Heck Cross-Coupling Reactions in Water: A Comprehensive Review. Catal. Lett. 2017, 148, 489–511. [Google Scholar] [CrossRef]
- Lipshutz, B.H.; Gallou, F.; Handa, S. Evolution of Solvents in Organic Chemistry. ACS Sustain. Chem. Eng. 2016, 4, 5838–5849. [Google Scholar] [CrossRef]
- Lipshutz, B.H.; Ghorai, S. Transitioning Organic Synthesis from Organic Solvents to Water. What’s E Factor? Green Chem. 2014, 16, 3660–3679. [Google Scholar] [CrossRef] [PubMed]
- Lipshutz, B.H.; Abela, A.R.; Bošković, Ž.V.; Nishikata, T.; Duplais, C.; Krasovskiy, A. “Greening Up” Cross-Coupling Chemistry. Top. Catal. 2010, 53, 985–990. [Google Scholar] [CrossRef] [Green Version]
- Kitanosono, T.; Kobayashi, S. Reactions in Water Involving the “On-Water” Mechanism. Chem. A Eur. J. 2020, 26, 9408–9429. [Google Scholar] [CrossRef]
- Kitanosono, T.; Masuda, K.; Xu, P.; Kobayashi, S. Catalytic Organic Reactions in Water toward Sustainable Society. Chem. Rev. 2018, 118, 679–746. [Google Scholar] [CrossRef]
- Guo, W.; Liu, X.; Liu, Y.; Li, C. Chiral Catalysis at the Water/Oil Interface. ACS Catal. 2017, 8, 328–341. [Google Scholar] [CrossRef]
- Uozumi, Y. Heterogeneous Asymmetric Catalysis in Water with Amphiphilic Polymer-Supported Homochiral Palladium Complexes. Bull. Chem. Soc. Jpn. 2008, 81, 1183–1195. [Google Scholar] [CrossRef] [Green Version]
- Pang, H.; Hu, Y.; Yu, J.; Gallou, F.; Lipshutz, B.H. Water-Sculpting of a Heterogeneous Nanoparticle Precatalyst for Mizoroki-Heck Couplings under Aqueous Micellar Catalysis Conditions. J. Am. Chem. Soc. 2021, 143, 3373–3382. [Google Scholar] [CrossRef] [PubMed]
- Lamblin, M.; Nassar-Hardy, L.; Hierso, J.-C.; Fouquet, E.; Felpin, F.-X. Recyclable Heterogeneous Palladium Catalysts in Pure Water: Sustainable Developments in Suzuki, Heck, Sonogashira and Tsuji-Trost Reactions. Adv. Synth. Catal. 2010, 352, 33–79. [Google Scholar] [CrossRef]
- Cordes, E.H.; Dunlap, R.B. Kinetics of Organic Reactions in Micellar Systems. Acc. Chem. Res. 2002, 2, 329–337. [Google Scholar] [CrossRef]
- Samiey, B.; Cheng, C.H.; Wu, J. Effects of Surfactants on the Rate of Chemical Reactions. J. Chem. 2014, 2014, 1–14. [Google Scholar] [CrossRef]
- Rideout, D.C.; Breslow, R. Hydrophobic Acceleration of Diels-Alder Reactions. J. Am. Chem. Soc. 1980, 102, 7816–7817. [Google Scholar] [CrossRef]
- Breslow, R. Hydrophobic Effects on Simple Organic Reactions in Water. Acc. Chem. Res. 1991, 24, 159–164. [Google Scholar] [CrossRef]
- Ward, M.A.; Georgiou, T.K. Thermoresponsive Polymers for Biomedical Applications. Polymers 2011, 3, 1215–1242. [Google Scholar] [CrossRef] [Green Version]
- Kobayashi, J.; Okano, T. Design of Temperature-Responsive Polymer-Grafted Surfaces for Cell Sheet Preparation and Manipulation. Bull. Chem. Soc. Jpn. 2019, 92, 817–824. [Google Scholar] [CrossRef] [Green Version]
- He, W.; Ma, Y.; Gao, X.; Wang, X.; Dai, X.; Song, J. Application of Poly(N-isopropylacrylamide) as Thermosensitive Smart Materials. J. Phys. Conf. Ser. 2020, 1676, 012063. [Google Scholar] [CrossRef]
- Harun-Ur-Rashid, M.; Seki, T.; Takeoka, Y. Structural Colored Gels for Tunable Soft Photonic Crystals. Chem. Rec. 2009, 9, 87–105. [Google Scholar] [CrossRef]
- Hellweg, T. Responsive Core-Shell Microgels: Synthesis, Characterization, and Possible Applications. J. Polym. Sci. Part B Polym. Phys. 2013, 51, 1073–1083. [Google Scholar] [CrossRef]
- Hertle, Y.; Hellweg, T. Thermoresponsive Copolymer Microgels. J. Mater. Chem. B 2013, 1, 5874–5885. [Google Scholar] [CrossRef] [PubMed]
- Klouda, L. Thermoresponsive Hydrogels in Biomedical Applications: A Seven-Year Update. Eur. J. Pharm. Biopharm. 2015, 97, 338–349. [Google Scholar] [CrossRef] [PubMed]
- Ichijo, H. Thermo-Responsive Polymer Gels. In Macromolecular Science and Engineering; Tanabe, Y., Ed.; Springer: Berlin/Heidelberg, Germany, 1999; pp. 71–83. [Google Scholar]
- Luo, G.-F.; Chen, W.-H.; Zhang, X.-Z. 100th Anniversary of Macromolecular Science Viewpoint: Poly(N-isopropylacrylamide)-Based Thermally Responsive Micelles. ACS Macro Lett. 2020, 9, 872–881. [Google Scholar] [CrossRef]
- Umekar, M.J.; Rarokar, N.R.; Tatode, A.A.; Agrawal, R.D. Polymeric Micelle as a Nanocarrier for Delivery of Therapeutic Agents: A Comprehensive Review. J. Drug Deliv. Ther. 2020, 10, 191–195. [Google Scholar] [CrossRef] [Green Version]
- Nakayama, M.; Okano, T. Intelligent Thermoresponsive Polymeric Micelles for Targeted Drug Delivery. J. Drug Deliv. Sci. Technol. 2006, 16, 35–44. [Google Scholar] [CrossRef]
- Chung, J.E.; Yokoyama, M.; Suzuki, K.; Aoyagi, T.; Sakurai, Y.; Okano, T. Reversibly Thermo-Responsive Alkyl-Terminated Poly(N-Isopropylacrylamide) Core-Shell Micellar Structures. Colloids Surf. B Biointerfaces 1997, 9, 37–48. [Google Scholar] [CrossRef]
- Winnik, F.M.; Adronov, A.; Kitano, H. Pyrene-Labeled Amphiphilic Poly-(N-Isopropylacrylamides) Prepared by Using a Lipophilic Radical Initiator: Synthesis, Solution Properties in Water, and Interactions with Liposomes. Can. J. Chem. 1995, 73, 2030–2040. [Google Scholar] [CrossRef]
- Seto, H.; Matsumoto, H.; Miura, Y. Preparation of Palladium-Loaded Polymer Hydrogel Catalysts with High Durability and Recyclability. Polym. J. 2020, 52, 671–679. [Google Scholar] [CrossRef]
- Yamada, Y.M.A.; Takeda, K.; Takahashi, H.; Ikegami, S. An Assembled Complex of Palladium and Non-Cross-linked Amphiphilic Polymer: A Highly Active and Recyclable Catalyst for the Suzuki-Miyaura Reaction. Org. Lett. 2002, 4, 3371–3374. [Google Scholar] [CrossRef] [PubMed]
- Hamamoto, H.; Suzuki, Y.; Yamada, Y.M.; Tabata, H.; Takahashi, H.; Ikegami, S. A Recyclable Catalytic System Based on a Temperature-Responsive Catalyst. Angew. Chem. Intl. Ed. Engl. 2005, 44, 4536–4538. [Google Scholar] [CrossRef] [PubMed]
- Hamamoto, H.; Suzuki, Y.; Takahashi, H.; Ikegami, S. Direct Transformation of Benzilic Amines to Carbonyls Using Polyacrylamide-Bound Tungstate under Phase-Transfer Catalysis Conditions. Tetrahedron Lett. 2007, 48, 4239–4242. [Google Scholar] [CrossRef]
- Ikegami, S.; Hamamoto, H. Novel Recycling System for Organic Synthesis Via Designer Polymer-Gel Catalysts. Chem. Rev. 2009, 109, 583–593. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, J.; Zhang, W.; Zhang, M. Pd-Catalyzed C-C Cross-Coupling Reactions within a Thermoresponsive and Ph-Responsive and Chelating Polymeric Hydrogel. J. Org. Chem. 2009, 74, 1923–1931. [Google Scholar] [CrossRef]
- Sato, T.; Ohno, A.; Sarkar, S.M.; Uozumi, Y.; Yamada, Y.M.A. A Convoluted Polymeric Imidazole Palladium Catalyst: Structural Elucidation and Investigation of the Driving Force for The Efficient Mizoroki-Heck Reaction. ChemCatChem 2015, 7, 2141–2148. [Google Scholar] [CrossRef]
- Yamada, Y.M.; Sarkar, S.M.; Uozumi, Y. Amphiphilic Self-Assembled Polymeric Copper Catalyst to Parts Per Million Levels: Click Chemistry. J. Am. Chem. Soc. 2012, 134, 9285–9290. [Google Scholar] [CrossRef]
- Yamada, Y.M.; Sarkar, S.M.; Uozumi, Y. Self-Assembled Poly(Imidazole-Palladium): Highly Active, Reusable Catalyst at Parts Per Million to Parts Per Billion Levels. J. Am. Chem. Soc. 2012, 134, 3190–3198. [Google Scholar] [CrossRef]
- Sarkar, S.M.; Uozumi, Y.; Yamada, Y.M. A Highly Active and Reusable Self-Assembled Poly(Imidazole/Palladium) Catalyst: Allylic Arylation/Alkenylation. Angew. Chem. Int. Ed. Engl. 2011, 50, 9437–9441. [Google Scholar] [CrossRef]
- Chen, T.; Fang, Q.; Zhou, L.; Xu, Z.; Qiu, J.; Wang, M.; Wang, J. Comparative Study of Cross-Linked and Linear Thermo-Responsive Carriers Supported Palladium Nanoparticles in the Reduction of 4-Nitrophenol: Structure, Catalytic Activity and Responsive Catalysis Property. React. Funct. Polym. 2019, 142, 104–111. [Google Scholar] [CrossRef]
- Yamada, Y.M.A.; Takeda, K.; Takahashi, H.; Ikegami, S. Assembled Catalyst of Palladium and Non-Cross-Linked Amphiphilic Polymer Ligand for the Efficient Heterogeneous Heck Reaction. Tetrahedron 2004, 60, 4097–4105. [Google Scholar] [CrossRef]
- Yamada, Y.M.A.; Takeda, K.; Takahashi, H.; Ikegami, S. An Efficient Heterogeneous Heck Reaction Promoted by a New Assembled Catalyst of Palladium and Non-Cross-Linked Amphiphilic Polymer. Tetrahedron Lett. 2003, 44, 2379–2382. [Google Scholar] [CrossRef]
- Suzuki, N.; Mizuno, D.; Guidote, A.M.; Koyama, S.; Masuyama, Y.; Rikukawa, M. Asymmetric Reactions in Water Catalyzed by L-Proline Tethered on Thermoresponsive Ionic Copolymers. Lett. Org. Chem. 2020, 17, 717–725. [Google Scholar] [CrossRef]
- Suzuki, N.; Takabe, T.; Yamauchi, Y.; Koyama, S.; Koike, R.; Rikukawa, M.; Liao, W.-T.; Peng, W.-S.; Tsai, F.-Y. Palladium-Catalyzed Mizoroki-Heck Reactions in Water Using Thermoresponsive Polymer Micelles. Tetrahedron 2019, 75, 1351–1358. [Google Scholar] [CrossRef]
- Suzuki, N.; Akebi, R.; Inoue, T.; Rikukawa, M.; Masuyama, Y. Asymmetric Aldol and Michael Reactions in Water Using Organocatalysts Immobilized on a Thermoresponsive “Linear” Block Copolymer. Curr. Organocatal. 2016, 3, 306–314. [Google Scholar] [CrossRef]
- Suzuki, N.; Inoue, T.; Asada, T.; Akebi, R.; Kobayashi, G.; Rikukawa, M.; Masuyama, Y.; Ogasawara, M.; Takahashi, T.; Thang, S.H. Asymmetric Aldol Reaction on Water Using an Organocatalyst Tethered on a Thermoresponsive Block Copolymer. Chem. Lett. 2013, 42, 1493–1495. [Google Scholar] [CrossRef]
- Zayas, H.A.; Lu, A.; Valade, D.; Amir, F.; Jia, Z.; O’Reilly, R.K.; Monteiro, M.J. Thermoresponsive Polymer-Supportedl-Proline Micelle Catalysts for the Direct Asymmetric Aldol Reaction in Water. ACS Macro Lett. 2013, 2, 327–331. [Google Scholar] [CrossRef]
- Wang, Z.-M.; Li, M.-H.; Feng, L.; Zhou, H.-Y.; Wang, J.-X. Construction Of Reversible Nano Reactor by Thermo-Responsive Polymeric Surfactant: Its Application in Chloromethylation of Naphthalene. J. Environ. Chem. Eng. 2019, 7, 103034. [Google Scholar] [CrossRef]
- Chen, Z.; Liang, Y.; Jia, D.-S.; Cui, Z.-M.; Song, W.-G. Simple Synthesis of Sub-Nanometer Pd Clusters: High Catalytic Activity of Pd/PEG-PNIPAM in Suzuki Reaction. Chin. J. Catal. 2017, 38, 651–657. [Google Scholar] [CrossRef]
- Dolya, N.A.; Kudaibergenov, S.E. Catalysis by Thermiresponsive Polymers. In Temperature-Responsive Polymers: Chemistry, Properties, and Applications; Khutoryanskiy, V.V., Georgiou, T.K., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2018; pp. 357–377. [Google Scholar]
- Hamasaka, G.; Ichii, S.; Uozumi, Y. A Palladium NNC-Pincer Complex as an Efficient Catalyst Precursor for the Mizoroki-Heck Reaction. Adv. Synth. Catal. 2018, 360, 1833–1840. [Google Scholar] [CrossRef]
- Zehm, D.; Laschewsky, A.; Gradzielski, M.; Prevost, S.; Liang, H.; Rabe, J.P.; Schweins, R.; Gummel, J. Amphiphilic Dual Brush Block Copolymers as “Giant Surfactants” and Their Aqueous Self-Assembly. Langmuir 2010, 26, 3145–3155. [Google Scholar] [CrossRef]
- Flynn, S.; Dale, S.D.; Dwyer, A.B.; Chambon, P.; Rannard, S.P. In Situ Xanthate Deprotection to Generate Thiol Chain Transfer Agents for Conventional Free Radical Linear and Branched Vinyl Polymerization. J. Polym. Sci. A Polym. Chem. 2017, 55, 3963–3967. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Cong, H.; Li, L.; Zheng, S. Thermoresponse Improvement of Poly(N-Isopropylacrylamide) Hydrogels Via Formation of Poly(Sodium P-Styrenesulfonate) Nanophases. ACS Appl. Mater. Interfaces 2014, 6, 13677–13687. [Google Scholar] [CrossRef] [PubMed]
- Kjøniksen, A.L.; Zhu, K.; Pamies, R.; Nyström, B. Temperature-Induced Formation and Contraction of Micelle-Like Aggregates in Aqueous Solutions of Thermoresponsive Short-Chain Copolymers. J. Phys. Chem. B 2008, 112, 3294–3299. [Google Scholar] [CrossRef] [PubMed]
- Kjøniksen, A.-L.; Zhu, K.; Karlsson, G.; Nyström, B. Novel Transition Behavior in Aqueous Solutions of A Charged Thermoresponsive Triblock Copolymer. Colloids Surf. A Physicochem. Eng. Asp. 2009, 333, 32–45. [Google Scholar] [CrossRef]
- McFaul, C.A.; Alb, A.M.; Drenski, M.F.; Reed, W.F. Simultaneous Multiple Sample Light Scattering Detection of LCST during Copolymer Synthesis. Polymer 2011, 52, 4825–4833. [Google Scholar] [CrossRef]
- Behrens, M.A.; Kjøniksen, A.-L.; Zhu, K.; Nyström, B.; Pedersen, J.S. Small-Angle X-Ray Scattering Study of Charged Triblock Copolymers as a Function of Polymer Concentration, Temperature, and Charge Screening. Macromolecules 2011, 45, 246–255. [Google Scholar] [CrossRef]
- Takeoka, H.; Wada, S.; Yusa, S.-I.; Sakurai, S.; Nakamura, Y.; Fujii, S. Thermo-Responsive Polypyrrole-Palladium NanocompositeParticles Synthesized by Aqueous Chemical Oxidative Dispersion Polymerization. J. Adhes. Soc. Jpn. 2015, 51, 255–263. [Google Scholar] [CrossRef]
- Mizusaki, M.; Endo, T.; Nakahata, R.; Morishima, Y.; Yusa, S.-I. pH-Induced Association and Dissociation of Intermolecular Complexes Formed by Hydrogen Bonding between Diblock Copolymers. Polymers 2017, 9, 367. [Google Scholar] [CrossRef] [Green Version]
- Farnham, W.B.; Moad, G.; Thang, S.H.; Rizzardo, E.; Fryd, M. (CSIRO), Method for Removing Sulfur-Containing End Groups. WO2005113612A1, 12 May 2004. [Google Scholar]
- Uğuzdoğan, E.; Denkbaş, E.B.; Kabasakal, O.S. Investigation of Temperature Sensitivity Behaviors of Water Soluble Polyacrylamides. J. Appl. Polym. Sci. 2013, 127, 4374–4384. [Google Scholar] [CrossRef]
- Masci, G.; Giacomelli, L.; Crescenzi, V. Atom Transfer Radical Polymerization of Sodium2-Acrylamido-2-Methylpropanesulfonate. J. Polym. Sci. A Polym. Chem. 2005, 43, 4446–4454. [Google Scholar] [CrossRef]
- Yusa, S.-i.; Shimada, Y.; Mitsukami, Y.; Yamamoto, T.; Morishima, Y. Heat-Induced Association and Dissociation Behavior of Amphiphilic Diblock Copolymers Synthesized via Reversible Addition-Fragmentation Chain Transfer Radical Polymerization. Macromolecules 2004, 37, 7507–7513. [Google Scholar] [CrossRef]
- Masci, G.; Diociaiuti, M.; Crescenzi, V. ATRP Synthesis and Association Properties of Thermoresponsive Anionic Block Copolymers. J. Polym. Sci. A Polym. Chem. 2008, 46, 4830–4842. [Google Scholar] [CrossRef]
- Yamazaki, M.; Akiyama, E.; Ozoe, S. Synthesis of Poly(styrenesulfonate)-poly(dialkylacrylamide) Block Copolymers and Preparation of Conductive PEDOT Composites. Kobunshi Ronbunshu 2017, 74, 524–533. [Google Scholar] [CrossRef]
- Liu, X.; Rao, P.; Xiao, W.; Xiao, Q.; Zhang, W. Synthesis And Performance of Fluid Loss Agents Based On Different Acrylamide Monomers. J. Petro. Explor. Prod. Technol. 2015, 5, 409–415. [Google Scholar] [CrossRef] [Green Version]
- Takeuchi, D.; Yasuda, A.; Osakada, K. Pd Complex-Catalyzed Copolymerization of A Bicyclic Methylenecyclopropane With Carbon Monoxide to Afford A New Polyketone. Dalton Trans. 2003, 2029–2035. [Google Scholar] [CrossRef]
- Takeuchi, D.; Okada, T.; Kuwabara, J.; Osakada, K. Living Alternating Copolymerization of a Methylenecyclopropane Derivative with CO to Afford Polyketone with Dihydrophenanthrene-1,10-diyl Groups. Macromol. Chem. Phys. 2006, 207, 1546–1555. [Google Scholar] [CrossRef]
- Sjoegren, M.; Hansson, S.; Norrby, P.O.; Aakermark, B.; Cucciolito, M.E.; Vitagliano, A. Selective Stabilization of The Anti Isomer of (η3-Allyl)Palladium And -Platinum Complexes. Organometallics 1992, 11, 3954–3964. [Google Scholar] [CrossRef]
- Burger, P.; Baumeister, J. Transition Metal Complexes With Sterically Demanding Ligands. I. Synthesis And X-Ray Crystal Structure of 1,5-Cyclooctadiene Palladium Methyl Triflate, (COD)Pd(Me)(Otf) And Its Cationic Penta-Coordinate Adducts with Sterically Demanding 2,9-Diaryl-Substituted 1,10-Phenanthroline Ligands. J. Organomet. Chem. 1999, 575, 214–222. [Google Scholar] [CrossRef]
- Uozumi, Y.; Purta, A.E.; Ichii, S.; Tazawa, A. C-H Arylation of Thiophenes with Aryl Bromides by a Parts-per-Million Loading of a Palladium NNC-Pincer Complex. Synlett 2020, 31, 1634–1638. [Google Scholar] [CrossRef]
- Ichii, S.; Hamasaka, G.; Uozumi, Y. The Hiyama Cross-Coupling Reaction at Parts Per Million Levels of Pd: In Situ Formation of Highly Active Spirosilicates in Glycol Solvents. Chem. Asian J. 2019, 14, 3850–3854. [Google Scholar] [CrossRef]
- Hamasaka, G.; Sakurai, F.; Uozumi, Y. A Palladium NNC-Pincer Complex: An Efficient Catalyst for Allylic Arylation at Parts Per Billion Levels. Chem. Commun. 2015, 51, 3886–3888. [Google Scholar] [CrossRef]
- Nguefack, J.-F.; Bolitt, V.; Sinou, D. An Efficient Palladium-Catalysed Coupling of Terminal Alkynes with Aryl Halides under Jeffery’s Conditions. Tetrahedron Lett. 1996, 37, 5527–5530. [Google Scholar] [CrossRef]
- Alami, M.; Ferri, F.; Linstrumelle, G. An Efficient Palladium-Catalysed Reaction of Vinyl And Aryl Halides or Triflates with Terminal Alkynes. Tetrahedron Lett. 1993, 34, 6403–6406. [Google Scholar] [CrossRef]
- Uozumi, Y.; Kobayashi, Y. The Sonogashira Reaction in Water via an Amphiphilic Resin-supported Palladium-Phosphine Complex under Copper-free Conditions. Heterocycles 2003, 59, 71–74. [Google Scholar] [CrossRef]
- Liang, B.; Dai, M.; Chen, J.; Yang, Z. Copper-Free Sonogashira Coupling Reaction with PdCl2 in Water under Aerobic Conditions. J. Org. Chem. 2005, 70, 391–393. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Anderson, K.W.; Zim, D.; Jiang, L.; Klapars, A.; Buchwald, S.L. Expanding Pd-Catalyzed C-N Bond-Forming Processes: The First Amidation of Aryl Sulfonates, Aqueous Amination, and Complementarity with Cu-Catalyzed Reactions. J. Am. Chem. Soc. 2003, 125, 6653–6655. [Google Scholar] [CrossRef]
- Handa, S.; Jin, B.; Bora, P.P.; Wang, Y.; Zhang, X.; Gallou, F.; Reilly, J.; Lipshutz, B.H. Sonogashira Couplings Catalyzed by Fe Nanoparticles Containing ppm Levels of Reusable Pd, under Mild Aqueous Micellar Conditions. ACS Catal. 2019, 9, 2423–2431. [Google Scholar] [CrossRef]
- Komaromi, A.; Novak, Z. Efficient Copper-Free Sonogashira Coupling of Aryl Chlorides with Palladium on Charcoal. Chem. Commun. 2008, 4968–4970. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).