In Situ Modification of Polyisoprene by Organo-Nanoclay during Emulsion Polymerization for Reinforcing Natural Rubber Thin Films
Abstract
1. Introduction
2. Experimental
2.1. Materials
2.2. Synthesis of the Nanoclay-Modified Polyisoprene Latexes
2.2.1. Starve-Fed Emulsion Polymerization
2.2.2. Batch Emulsion Polymerization
2.3. Latex Compounding
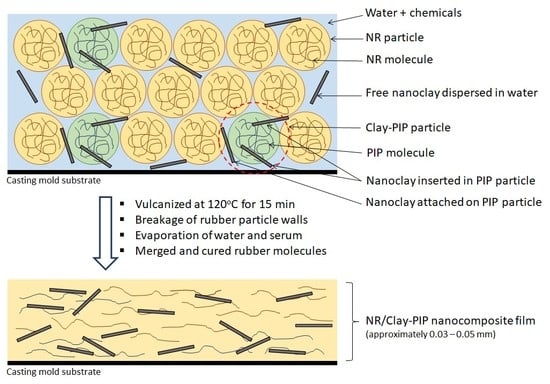
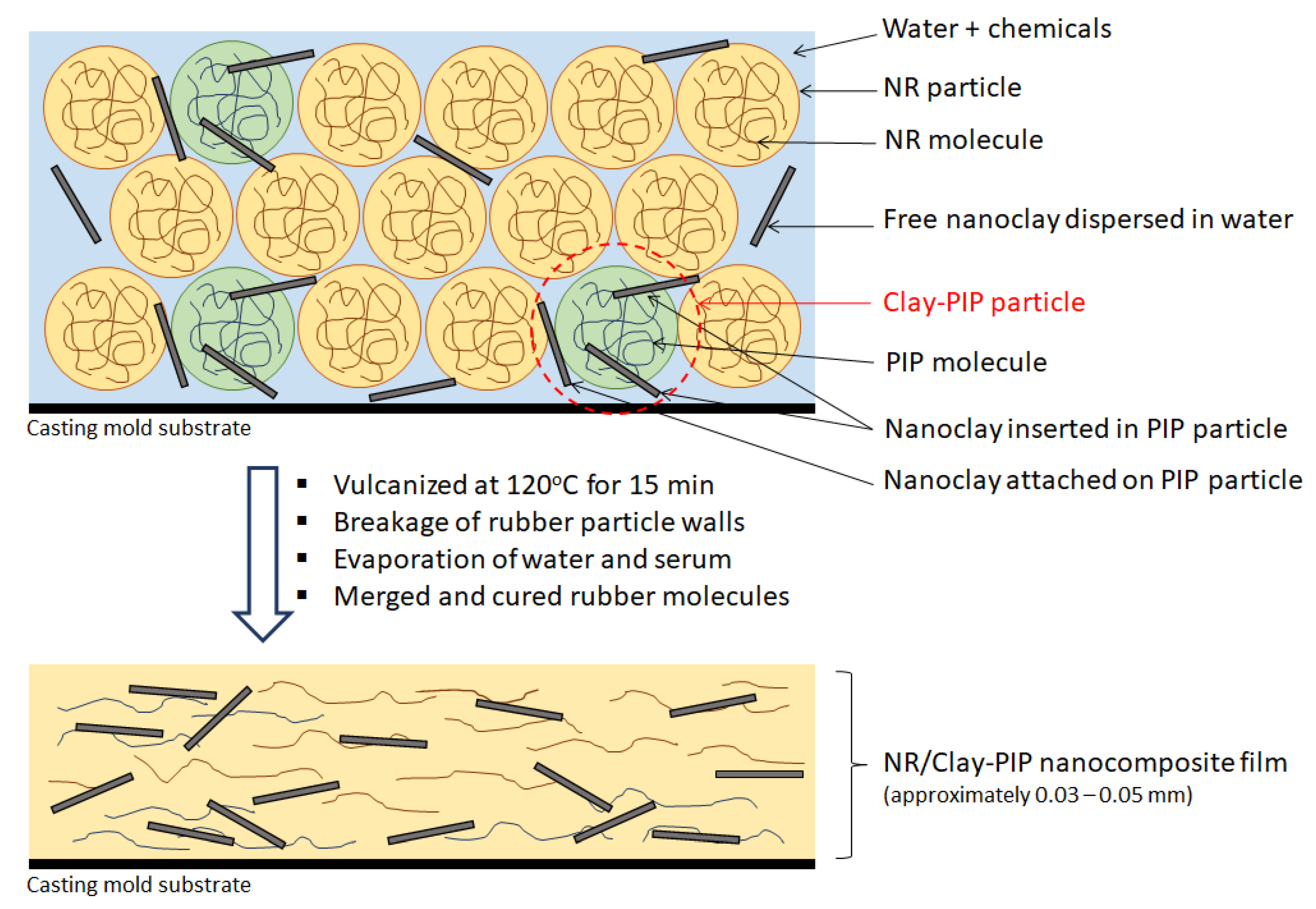
2.4. Preparation of the Natural Rubber Thin Films
2.5. Characterizations of the Clay-PIP Latexes
2.5.1. Conversion, Coagulation, and Gel Content
2.5.2. Morphology
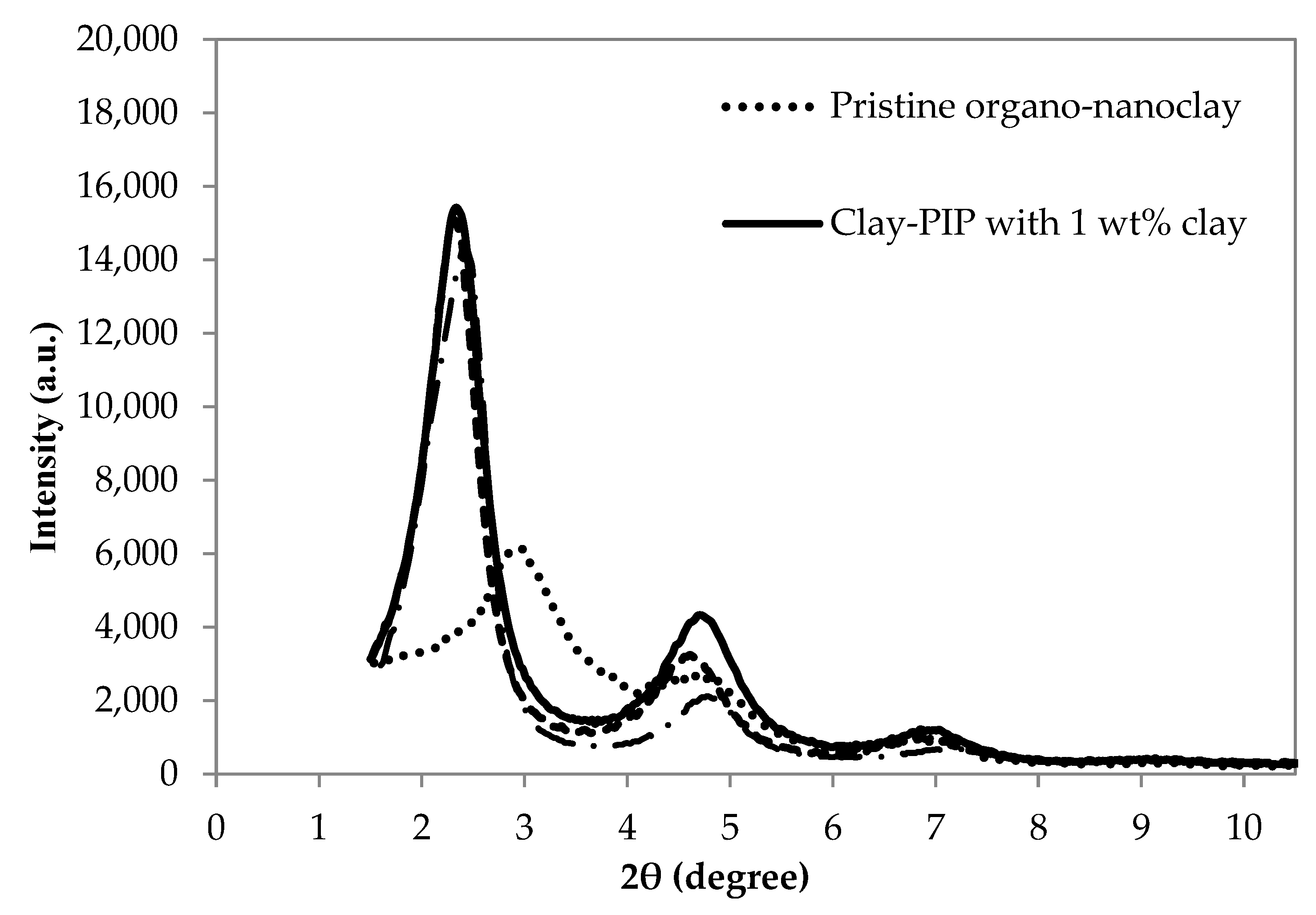
2.5.3. Clay Nanostructure Analysis
2.5.4. Particle Size and Particle Size Distribution
2.5.5. Mechanical Properties
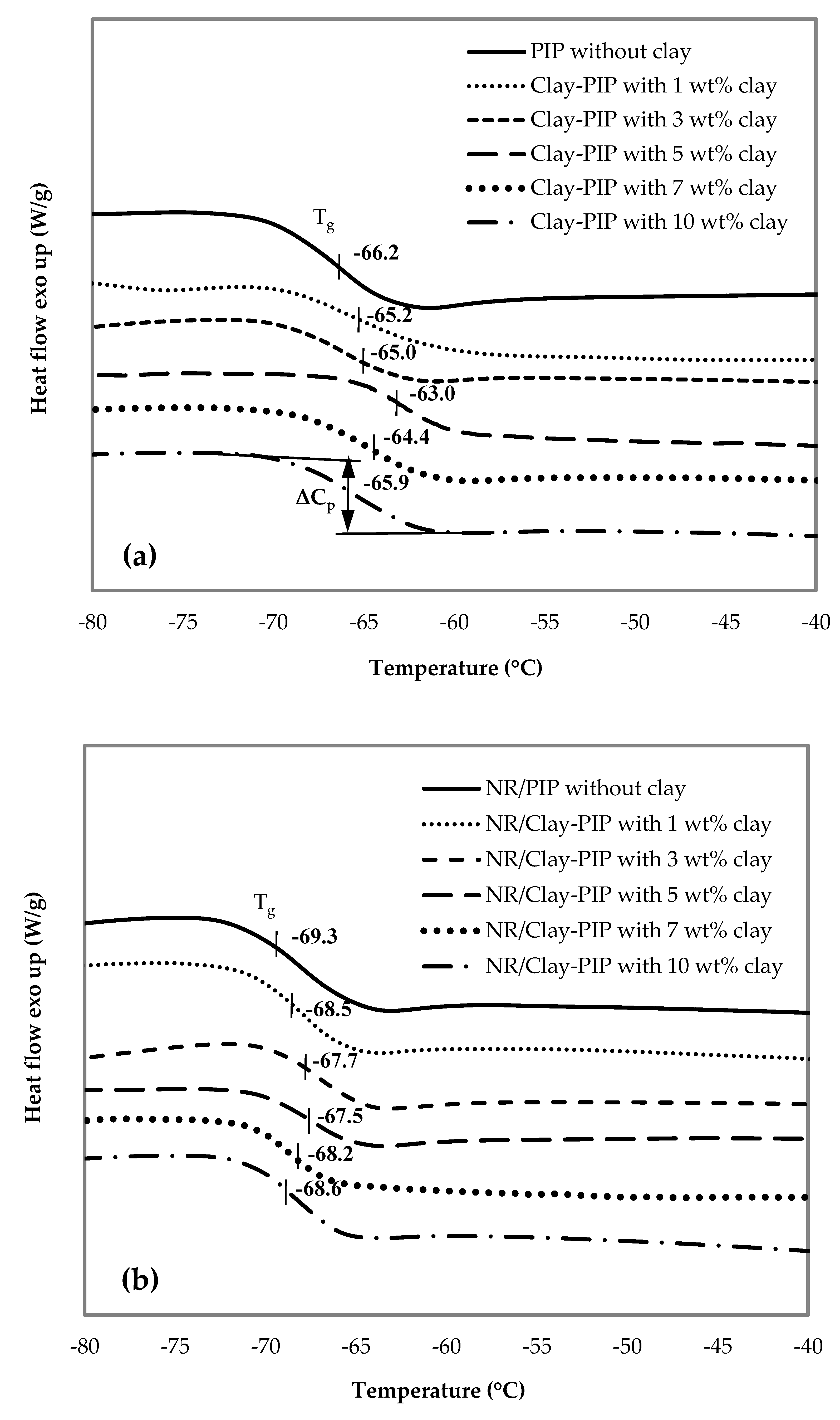
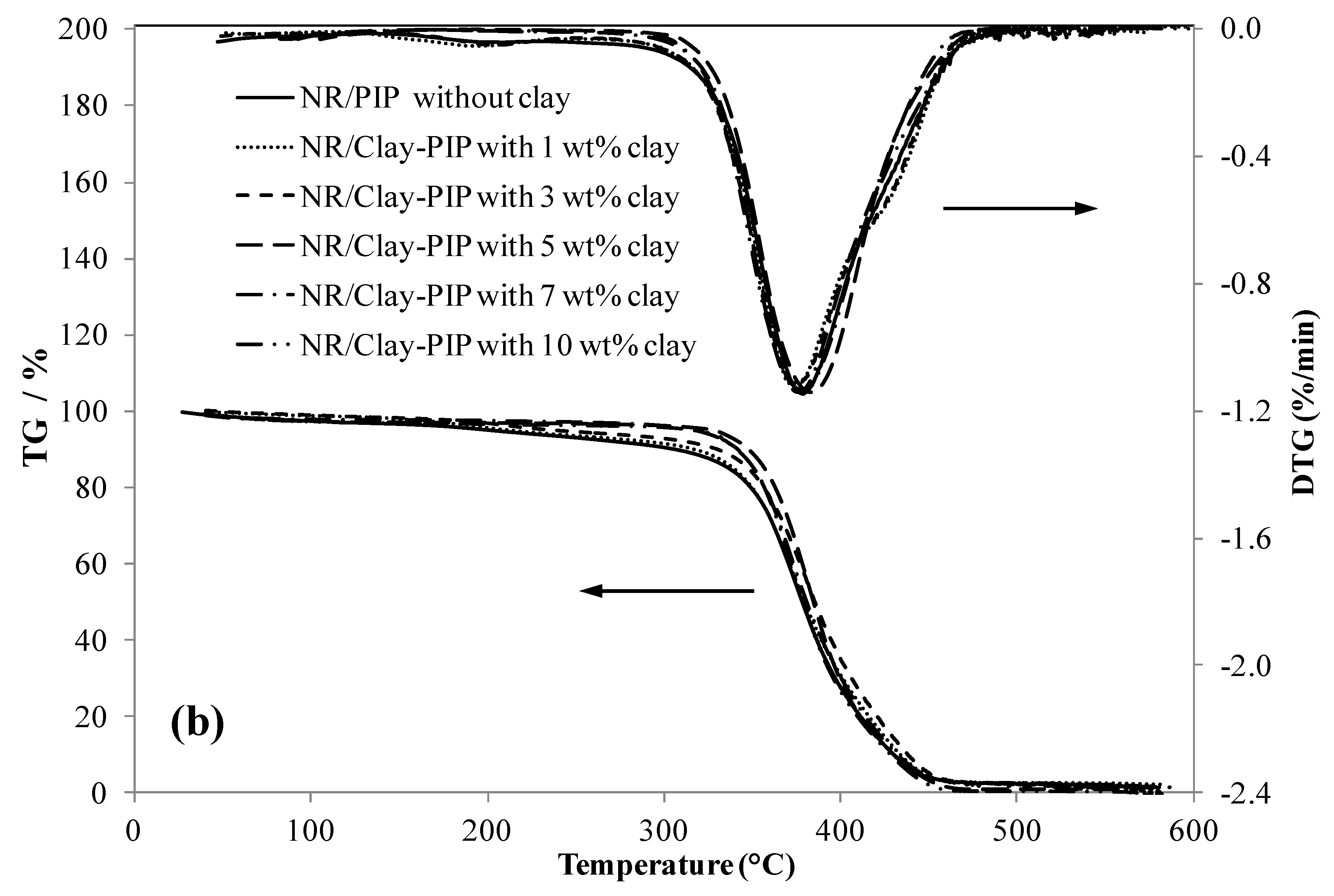
2.5.6. Thermal Analyses
3. Results and Discussion
3.1. Synthesis of the Nanoclay-Modified Polyisoprene Latexes
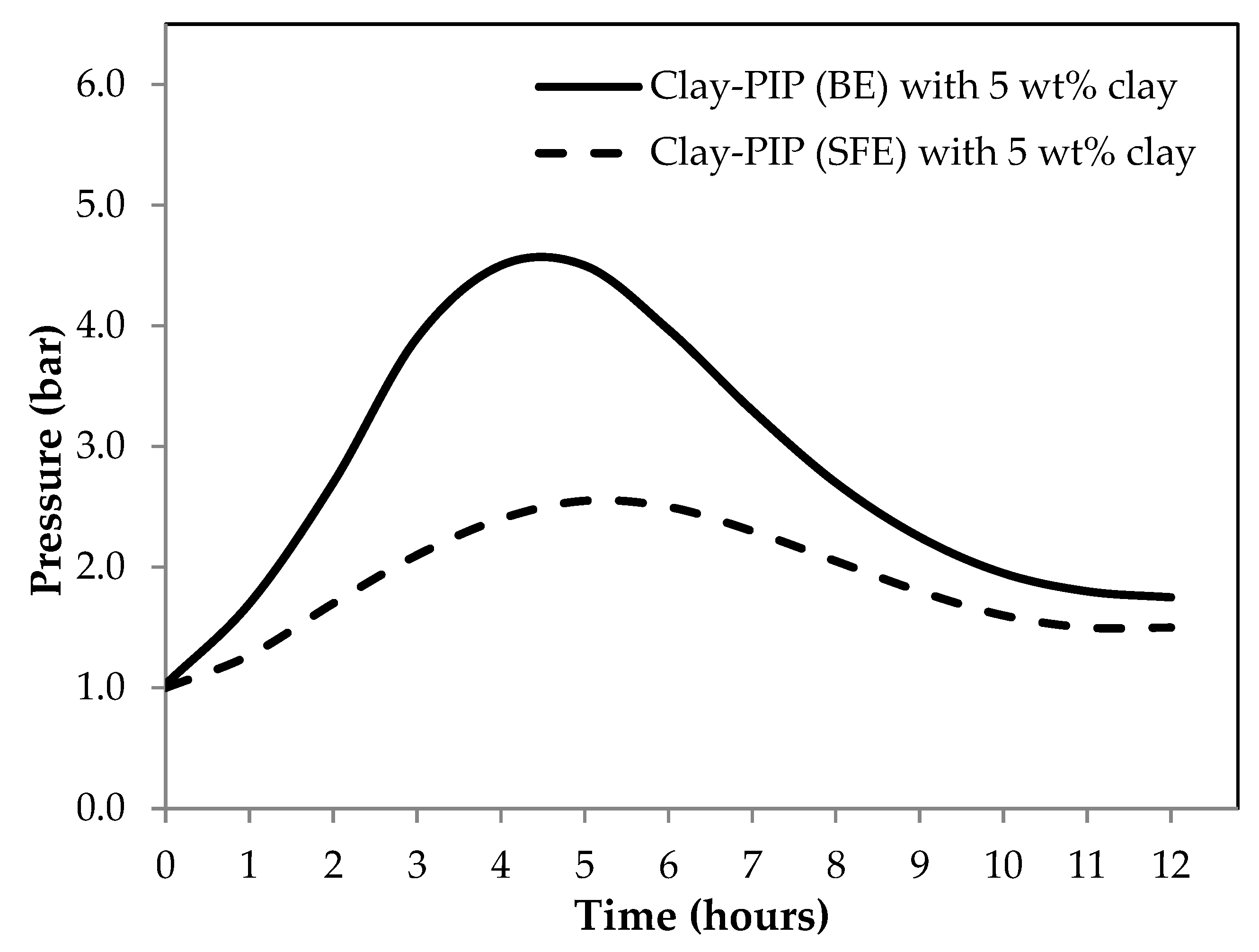
3.1.1. Effect of Different Emulsion Polymerization Techniques
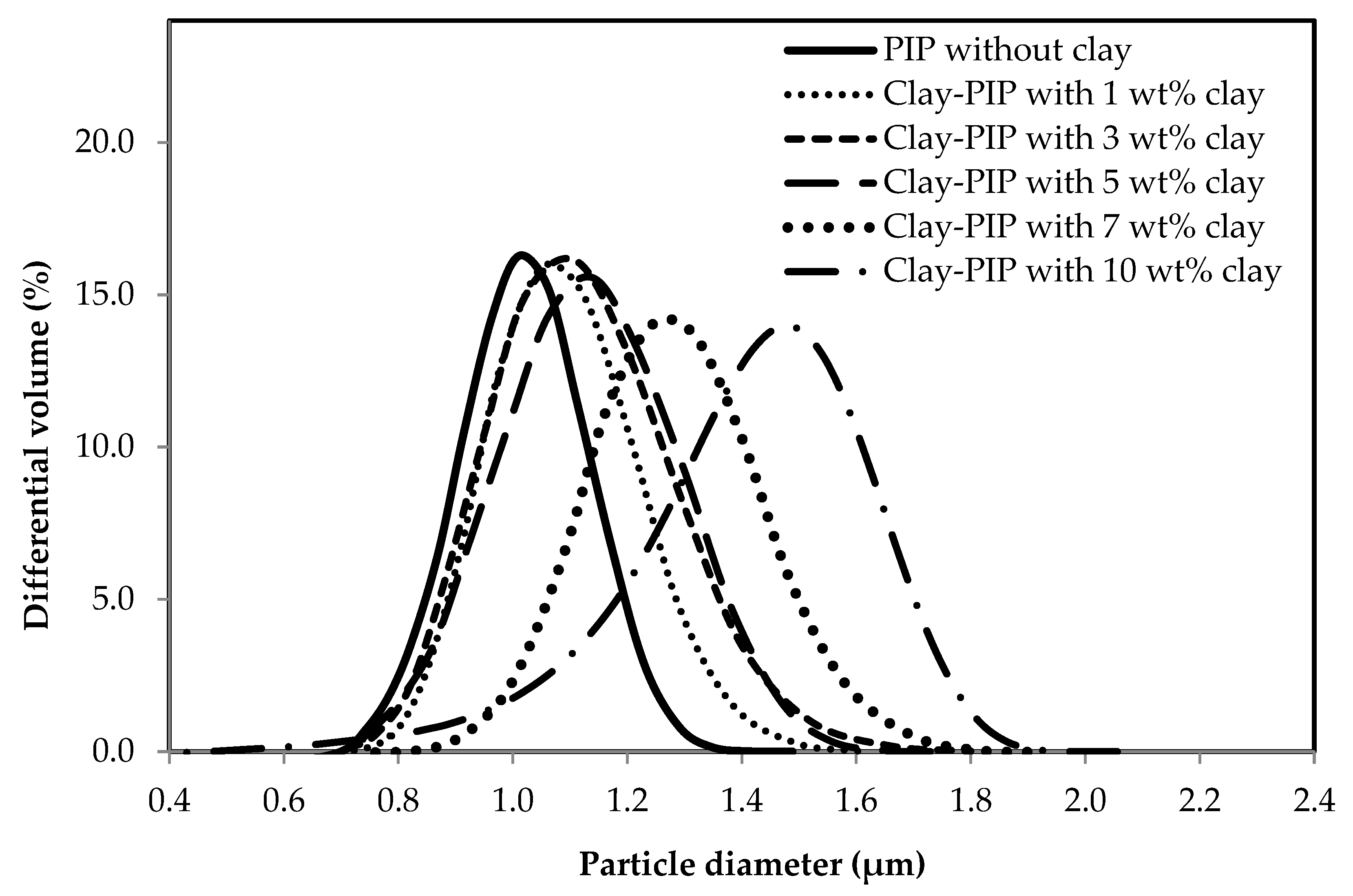
3.1.2. Variation of Nanoclay Contents in the Clay-PIP Latexes
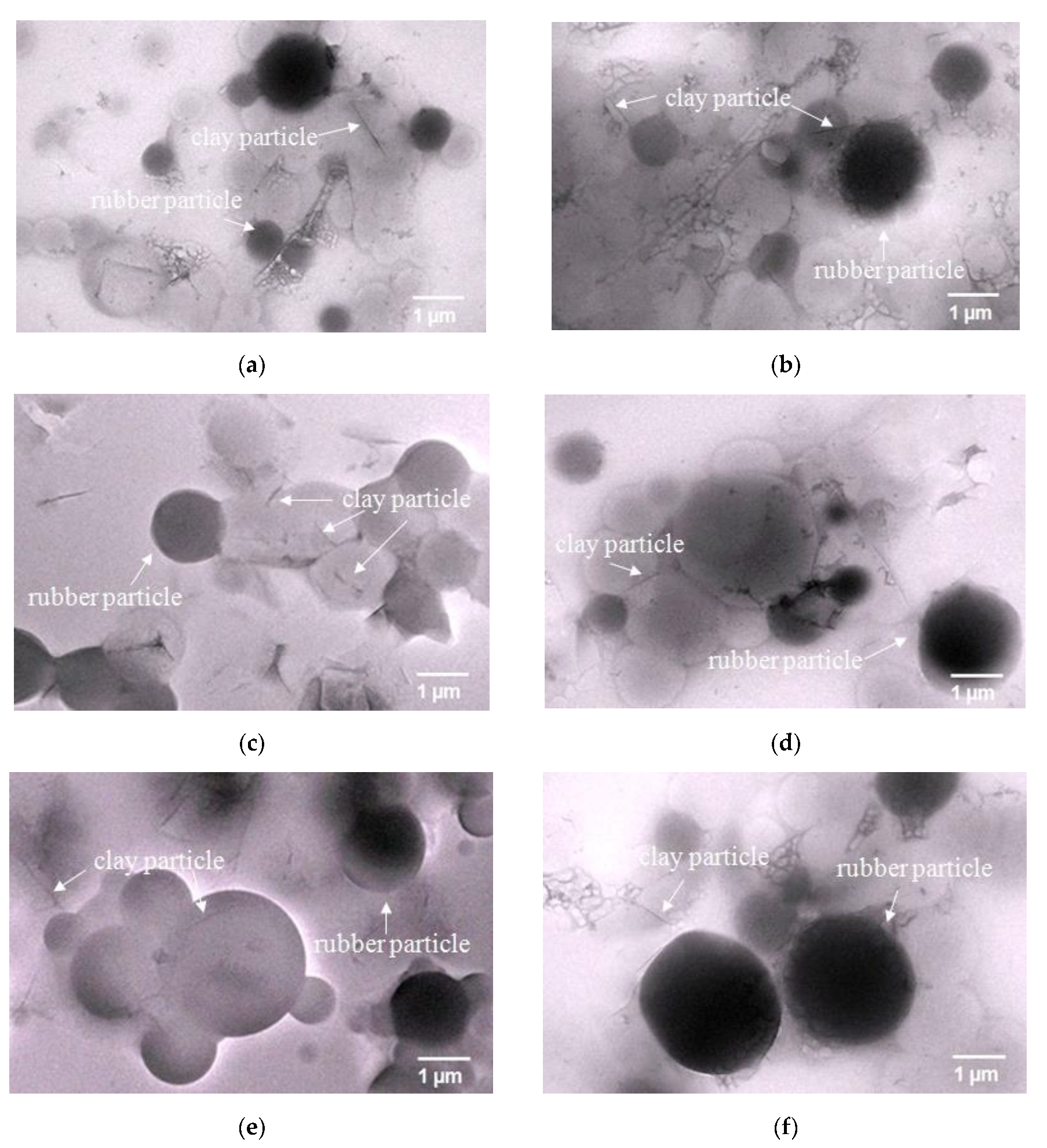
3.1.3. Morphology of the Obtained Clay-PIP Latexes
3.2. Use of the Clay-PIP Latexes as a Reinforcing Agent for NR Latex Compounds
3.2.1. Mechanical Properties of the NR Thin Films Reinforced with Clay-PIP
3.2.2. Indication of Immobilized Polymer in the Composites
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Utama, M.; Suhartini, M.; Herwinarni, H.; Siswanto, S.; Yoharmus, S.; Sundaru, H.; Halik, H.M.; Prayitno, P.; Muklis, H.M.; Ruslim, S. Pilot scale production of irradiated natural rubber latex and its dipping products. At. Indones. 2005, 31, 61–78. [Google Scholar] [CrossRef][Green Version]
- Ng, K.P.; Yip, E.; Mok, K.L. Production of natural rubber latex gloves with low extractable protein content: Some practical recommendations. J. Nat. Rubb. Res. 1994, 9, 87–95. [Google Scholar]
- Blackley, D.C. Polymer Latices: Science and Technology; Springer Science & Business Media: London, UK, 1997. [Google Scholar]
- Groves, R.; Routh, A.F. Film deposition and consolidation during thin glove coagulant dipping. J. Polym. Sci. B 2017, 55, 1633–1648. [Google Scholar] [CrossRef]
- Nakade, S.; Kuga, A.; Hayashi, M.; Tanaka, Y. Highl purified natural rubber IV. Preparation and characteristics of gloves and condoms. J. Nat. Rubb. Res. 1997, 12, 33–42. [Google Scholar]
- Kaewsakul, W.; Sahakaro, K.; Dierkes, W.K.; Noordermeer, J.W.M. Mechanistic aspects of silane coupling agents with different functionalities on reinforcement of silica-filled natural rubber compounds. Polym. Eng. Sci. 2015, 55, 836–842. [Google Scholar] [CrossRef]
- Goyl, M.; Goyal, N.; Kaur, H.; Gera, A.; Minocha, K.; Jinda, P. Fabrication and characterisation of low density polyethylene (LDPE)/multi walled carbon nanotubes (MWCNTs) nano-composites. Perspect. Sci. 2006, 8, 403–405. [Google Scholar] [CrossRef]
- Kocsis, J.K.; Fakirov, S. Nano-and Micro-Mechanics of Polymer Blends and Composites; Carl Hanser Verlag: Munich, Germany, 2009. [Google Scholar]
- Bokobza, L. The reinforcement of elastomeric networks by fillers. Macromol. Mater. Eng. 2004, 289, 607–621. [Google Scholar] [CrossRef]
- Kaewsakul, W.; Sahakaro, K.; Dierkes, W.K.; Noordermeer, J.W.M. Optimization of mixing conditions for silica-reinforced natural rubber tire tread compounds. Rubber Chem. Technol. 2012, 85, 277–294. [Google Scholar] [CrossRef]
- Moser, A.; Feuchter, M. Properties of polymer composites used in high-voltage applications. Polymers 2016, 8, 260. [Google Scholar] [CrossRef]
- Dennis, H.R.; Hunter, D.; Chang, D.; Kim, S.; Paul, D.R. Effect of melt processing condition on the extent of exfoliation in organoclay-based nanocomposites. Polymer 2001, 42, 9513–9522. [Google Scholar] [CrossRef]
- Rehab, A.; Salahuddin, N. Nanocomposites materials based on polyurethane intercalated into montmorillonite. Mater. Sci. Eng. A 2005, 399, 368–376. [Google Scholar] [CrossRef]
- Vaia, R.A.; Jandt, K.D.; Kramer, E.K.; Giannelis, E.P. Microstructural evaluation of melt intercalated polymer-organically modified layered silicate nanocomposites. Chem. Mater. 1996, 8, 2628–2635. [Google Scholar] [CrossRef]
- Tatou, M.; Genix, A.C.; Imaz, A.; Forcada, J.; Banc, A.; Schweins, R.; Grillo, I.; Oberdisse, J. Reinforcement and polymer mobility in silica-latex nanocomposites with controlled aggregation. Macromolecules 2011, 44, 9029–9039. [Google Scholar] [CrossRef]
- Xia, L.; Song, J.; Wang, H.; Kan, Z. Silica nanoparticles reinforced natural rubber latex composites: The effects of silica dimension and polydispersity on performance. J. Appl. Polym. Sci. 2019, 136, 47449. [Google Scholar] [CrossRef]
- Prasertsria, S.; Rattanasom, N. Fumed and precipitated silica reinforced natural rubber composites prepared from latex system: Mechanical and dynamic properties. Polym. Test. 2012, 31, 593–605. [Google Scholar] [CrossRef]
- Yah, C.S.; Simate, G.S.; Hlangothi, P.; Somai, B.M. Nanotechnology and the future of condoms in the prevention of sexually transmitted infections. Ann. Afr. Med. 2018, 17, 49–57. [Google Scholar] [CrossRef]
- Sun, Q.; Schork, F.J.; Deng, Y. Water-based polymer/clay nanocomposite suspension for improving water and moisture barrier in coating. Compos. Sci. Technol. 2007, 67, 1823–1829. [Google Scholar] [CrossRef]
- Reyes, Y.; Peruzzo, P.J.; Fernandez, M.; Paulis, M.; Leiza, J.R. Encapsulation of clay within polymer particles in a high-solids content aqueous dispersion. Langmuir 2013, 29, 9849–9856. [Google Scholar] [CrossRef]
- Xu, T.; Jia, Z.; Luo, Y.; Jia, D.; Peng, Z. Interfacial interaction between the epoxidized natural rubber and silica in natural rubber/silica composites. Appl. Surf. Sci. 2015, 328, 306–313. [Google Scholar] [CrossRef]
- Stockelhuber, K.W.; Svistkov, A.S.; Pelevin, A.G.; Heinrich, G. Impact of filler surface modification on large scale mechanics of styrene butadiene/silica rubber composites. Macromolecules 2011, 44, 4366–4381. [Google Scholar] [CrossRef]
- Jana, S.C.; Jain, S. Dispersion of nanofillers in high performance polymers using reactive solvents as processing aids. Polymer 2001, 42, 6897–6905. [Google Scholar] [CrossRef]
- Liao, Y.H.; Tondin, O.M.; Liang, Z.; Zhang, C.; Wang, B. Investigation of the dispersion process of SWNTs/SC-15 epoxy resin nanocomposites. Mater. Sci. Eng. A 2004, 385, 175–181. [Google Scholar] [CrossRef]
- Almasi, A.; Porumb, A.; Podariu, A.C.; Todor, L.; Tofan, S.A.; Popovici, R.A. The effects of nanofillers on composite materials mechanical properties. Rev. Chim. 2017, 68, 192–199. [Google Scholar]
- Manjunath, M.; Renukappa, N.; Suresha, B. Influence of micro and nanofillers on mechanical properties of pultruded unidirectional glass fiber reinforced epoxy composite systems. J. Compos. Mater. 2015, 50, 1–13. [Google Scholar] [CrossRef]
- Panda, A.; Dyadyura, K.; Valicek, J.; Harnicarova, M.; Zajac, J.; Modrak, V.; Pandova, I.; Vrabel, P.; Marcincinova, E.N.; Pavelek, Z. Manufacturing technology of composite materials-principles of modification of polymer composite materials technology based on polytetrafluoroethylene. Materials 2017, 10, 377. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Pang, X.J.; Yu, Z.L. Study on polycarbonate/multi-walled carbon nanotubes composite produced by melt processing. Mater. Sci. Eng. A 2007, 457, 287–291. [Google Scholar] [CrossRef]
- Nor, N.A.; Othman, N. Effect of filler loading on curing characteristic and tensile properties of palygorskite natural rubber nanocomposites. Procedia Chem. 2016, 19, 351–358. [Google Scholar] [CrossRef]
- Thaithae, W.; Antonio, C.; Wattanachai, P. Properties characterisation of polycarbonate and multi-walled carbon nanotubes composites prepared by solution technique. Asia Pac. J. Chem. Eng. 2015, 11, 34–50. [Google Scholar] [CrossRef]
- Rakmae, S.; Ruksakulpiwat, Y.; Sutapun, W.; Suppakarn, N. Effects of mixing technique and filler content on physical properties of bovine bone-based CHA/PLA composites. J. Appl. Polym. Sci. 2011, 122, 2433–2441. [Google Scholar] [CrossRef]
- Duy, N.Q.; Rashid, A.A.; Ismail, H. Effects of filler loading and different preparation methods on properties of cassava starch/natural rubber composites. Polym. Plast. Technol. Eng. 2012, 51, 940–944. [Google Scholar] [CrossRef]
- Grishin, B.S. Innovative technologies for the production of reinforced elastomer composites. Int. J. Polym. Sci. 2016, 43, 58–63. [Google Scholar] [CrossRef]
- Motaung, T.E.; Mochane, M.J.; Linganiso, Z.L.; Mashigo, P.M. In-situ polymerization of nylon-cellulose nanocomposite. Polym. Sci. 2017, 3, 1–8. [Google Scholar] [CrossRef]
- Wang, Q.; Luo, Y.; Feng, C.; Yi, Z.; Qiu, Q.; Kong, L.; Peng, Z. Reinforcement of natural rubber with core-shell structure silica-poly(methyl methacrylate) nanoparticles. J. Nanomater. 2012, 6, 1–9. [Google Scholar] [CrossRef]
- Bensebaa, F. Nanoparticle Technologies: From Lab to Market; Elsevier: Oxford, UK, 2013. [Google Scholar]
- Tong, Z.; Deng, Y. Synthesis of polystyrene encapsulated nanosaponite composite latex via miniemulsion polymerization. Polymers 2007, 48, 4337–4343. [Google Scholar] [CrossRef]
- Ianchis, R.; Donescu, D.; Cinteza, L.O. Polymer-clay nanocomposites obtained by solution polymerization of vinyl benzyl triammonium chloride in the presence of advanced functionalized clay. J. Chem. Sci. 2014, 126, 609–616. [Google Scholar] [CrossRef]
- Tumnantong, D.; Rempel, G.L.; Prasassarakich, P. Polyisoprene-silica nanoparticles synthesized via RAFT emulsifier-free emulsion polymerization using water-soluble initiators. Polymers 2017, 9, 637. [Google Scholar] [CrossRef]
- Boonchoo, P.; Rempel, G.L.; Prasassarakich, P. Synthesis of polyisoprene-montmorillonite nanocomposites via differential microemulsion polymerization and application of PIP–Mt in natural rubber. Appl. Clay Sci. 2014, 88, 186–193. [Google Scholar] [CrossRef]
- Shahriar, S. Extending the limits of emulsifier-free emulsion polymerization to achieve small uniform particles. RSC Adv. 2015, 5, 58549–58560. [Google Scholar]
- Chen, Y.; Jahanzad, F.; Sajjadi, S. Semicontinuous monomer-starved emulsion polymerization as a means to produce nanolatexes: Analysis of nucleation stage. Langmuir 2013, 29, 5650–5658. [Google Scholar] [CrossRef]
- Chouytan, J.; Beraheng, S.; Kalkornsurapranee, E.; Fellows, C.M.; Kaewsakul, W. Synthesis of polyisoprene via miniemulsion polymerisation: Effect on thermal behaviour, colloidal properties and stereochemistry. J. Rubb. Res. 2018, 21, 236–255. [Google Scholar] [CrossRef]
- Lami, E.B.; Othman, N.S.; Dos Santos, A.M. Polymer-clay nanocomposite particles and soap-free latexes stabilized by clay platelets: State of the art and recent advances. RSC Nanosci. Nanotechnol. 2010, 16, 269–311. [Google Scholar]
- Pardhy, N.P.; Budhlall, B.M. Pickering emulsion as a template to synthesize Janus colloids with anisotropy in the surface potential. Langmuir 2010, 26, 13130–13141. [Google Scholar] [CrossRef]
- Kim, D.H.; Ryu, Y.S.; Kim, S.H. Improvements in the oxygen barrier property of polypropylene nanocomposites. Polym. Adv. Technol. 2018, 29, 2655–2664. [Google Scholar] [CrossRef]
- Ghari, H.S.; Arani, A.J. Nanocomposites based on natural rubber, organoclay and nano-calcium carbonate: Study on the structure, cure behavior, static and dynamic-mechanical properties. Appl. Clay Sci. 2016, 119, 348–357. [Google Scholar] [CrossRef]
- Melo, T.J.; Araujo, E.M.; Brito, G.F.; Agrawal, P. Development of nanocomposites from polymer blends: Effect of organoclay on the morphology and mechanical properties. J. Alloy. Compd. 2014, 615, 389–391. [Google Scholar] [CrossRef]
- Alexander, M.; Dubois, P. Polymer-layered silicate nanocomposites: Preparation, properties and uses of a new class of materials. Mater. Sci. Eng. 2001, 28, 1–63. [Google Scholar] [CrossRef]
- Pal, K.; Rajasekar, R.; Kang, D.J.; Zhang, Z.X.; Kim, J.K.; Das, C.K. Effect of epoxidized natural rubber–organoclay nanocomposites on NR/high styrene rubber blends with fillers. Mater. Des. 2009, 30, 4035–4042. [Google Scholar] [CrossRef]
- Choi, S.S. Difference in bound rubber formation of silica and carbon black with styrene-butadiene rubber. Polym. Adv. Technol. 2002, 13, 466–474. [Google Scholar] [CrossRef]
- Fragiadakis, D.; Pissis, P. Glass transition and segmental dynamics in poly(dimethylsiloxane)/silica nanocomposites studied by various techniques. J. Non Cryst. Solids 2007, 353, 4344. [Google Scholar] [CrossRef]
- Corcione, C.E.; Frigione, M. Characterization of Nanocomposites by thermal analysis. Materials 2012, 5, 2960–2980. [Google Scholar] [CrossRef]
- Moisture Diffusion through Organomodified Montmorillonite/Polymer Nanocomposites and Resulting Property Degradations. Available online: https://shodhganga.inflibnet.ac.in/bitstream/10603/73026/3/appendices.pdf (accessed on 15 May 2019).



| Latexes | System | Nanoclay (wt%) |
|---|---|---|
| Clay-PIP (BE) with 5 wt% clay | BE | 0.5 |
| PIP without clay | SFE | - |
| Clay-PIP with 1 wt% clay | SFE | 0.1 |
| Clay-PIP with 3 wt% clay | SFE | 0.3 |
| Clay-PIP with 5 wt% clay | SFE | 0.5 |
| Clay-PIP with 7 wt% clay | SFE | 0.7 |
| Clay-PIP with 10 wt% clay | SFE | 1.0 |
| Ingredients | Dry Weight (phr) |
|---|---|
| Rubber latex a | 100 |
| KOH | 0.5 |
| K-Laurate | 0.5 |
| Sulfur | 1.4 |
| ZDEC | 0.9 |
| ZMBT | 0.5 |
| Antioxidant | 0.9 |
| ZnO | 2.7 |
| Latexes | Conversion (wt%) | Gelation (wt%) | Coagulation (wt%) |
|---|---|---|---|
| Clay-PIP (BE) with 5 wt% clay | 65.4 ± 5.9 | 21.7 ± 4.3 | 0.8 ± 0.2 |
| PIP without clay | 91.7 ± 8.2 | 22.5 ± 5.2 | 0.3 ± 0.0 |
| Clay-PIP with 1 wt% clay | 78.3 ± 7.1 | 21.8 ± 4.8 | 0.3 ± 0.1 |
| Clay-PIP with 3 wt% clay | 77.8 ± 6.3 | 21.1 ± 4.0 | 0.4 ± 0.1 |
| Clay-PIP with 5 wt% clay | 69.9 ± 5.1 | 18.9 ± 3.5 | 0.5 ± 0.2 |
| Clay-PIP with 7 wt% clay | 67.3 ± 5.8 | 18.1 ± 3.1 | 0.7 ± 0.2 |
| Clay-PIP with 10 wt% clay | 63.4 ± 6.3 | 19.2 ± 3.9 | 1.5 ± 0.4 |
| Latexes | Particle Size (µm) | Particle Dispersity | Particle Number (L−1) |
|---|---|---|---|
| Clay-PIP (BE) with 5 wt% clay | 1.40 | 0.43 | 5.2 × 1013 |
| Clay-PIP without clay | 1.01 | 0.10 | 15.5 × 1013 |
| Clay-PIP with 1 wt% clay | 1.05 | 0.19 | 14.0 × 1013 |
| Clay-PIP with 3 wt% clay | 1.11 | 0.36 | 11.7 × 1013 |
| Clay-PIP with 5 wt% clay | 1.14 | 0.35 | 9.8 × 1013 |
| Clay-PIP with 7 wt% clay | 1.24 | 0.40 | 7.3 × 1013 |
| Clay-PIP with 10 wt% clay | 1.46 | 0.62 | 4.2 × 1013 |
| Composites | Tg | ΔCp | Td | Actual Nanoclay |
|---|---|---|---|---|
| (°C) | (W/g) | (°C) | Content (wt%) a | |
| PIP without clay | −66.2 | 0.31 | 374 | 0.00 |
| Clay-PIP with 1 wt% clay | −65.2 | 0.25 | 377 | 0.60 |
| Clay-PIP with 3 wt% clay | −65.0 | 0.27 | 378 | 1.70 |
| Clay-PIP with 5 wt% clay | −63.0 | 0.20 | 382 | 4.20 |
| Clay-PIP with 7 wt% clay | −64.4 | 0.22 | 380 | 5.90 |
| Clay-PIP with 10 wt% clay | −65.9 | 0.31 | 375 | 7.60 |
| NR/PIP without clay | −69.3 | 0.38 | 378 | 0.00 |
| NR/Clay-PIP with 1 wt% clay | −68.5 | 0.36 | 371 | 0.01 |
| NR/Clay-PIP with 3 wt% clay | −67.7 | 0.31 | 376 | 0.04 |
| NR/Clay-PIP with 5 wt% clay | −67.5 | 0.31 | 383 | 0.08 |
| NR/Clay-PIP with 7 wt% clay | −68.2 | 0.34 | 369 | 0.12 |
| NR/Clay-PIP with 10 wt% clay | −68.6 | 0.36 | 371 | 0.15 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chouytan, J.; Kalkornsurapranee, E.; Fellows, C.M.; Kaewsakul, W. In Situ Modification of Polyisoprene by Organo-Nanoclay during Emulsion Polymerization for Reinforcing Natural Rubber Thin Films. Polymers 2019, 11, 1338. https://doi.org/10.3390/polym11081338
Chouytan J, Kalkornsurapranee E, Fellows CM, Kaewsakul W. In Situ Modification of Polyisoprene by Organo-Nanoclay during Emulsion Polymerization for Reinforcing Natural Rubber Thin Films. Polymers. 2019; 11(8):1338. https://doi.org/10.3390/polym11081338
Chicago/Turabian StyleChouytan, Jadsadaporn, Ekwipoo Kalkornsurapranee, Christopher M. Fellows, and Wisut Kaewsakul. 2019. "In Situ Modification of Polyisoprene by Organo-Nanoclay during Emulsion Polymerization for Reinforcing Natural Rubber Thin Films" Polymers 11, no. 8: 1338. https://doi.org/10.3390/polym11081338
APA StyleChouytan, J., Kalkornsurapranee, E., Fellows, C. M., & Kaewsakul, W. (2019). In Situ Modification of Polyisoprene by Organo-Nanoclay during Emulsion Polymerization for Reinforcing Natural Rubber Thin Films. Polymers, 11(8), 1338. https://doi.org/10.3390/polym11081338