Anionic Polymerization of β-Butyrolactone Initiated with Sodium Phenoxides. The Effect of the Initiator Basicity/Nucleophilicity on the ROP Mechanism
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.3. General Polymerization Procedure
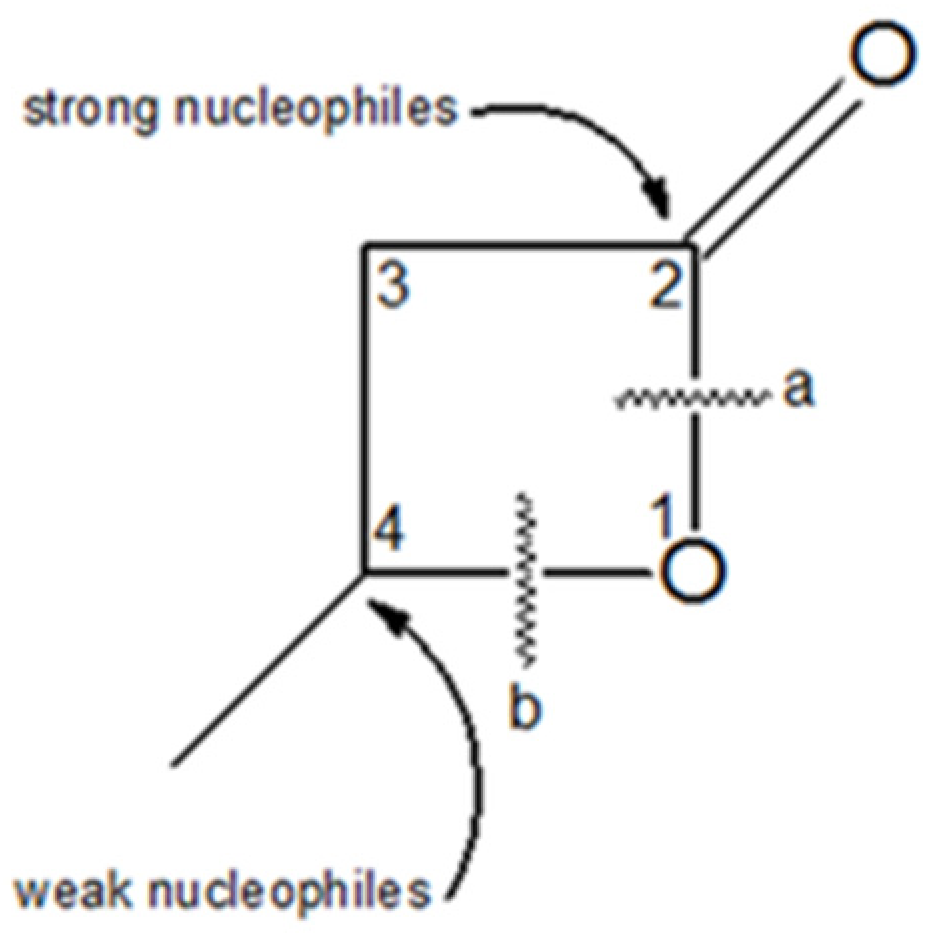
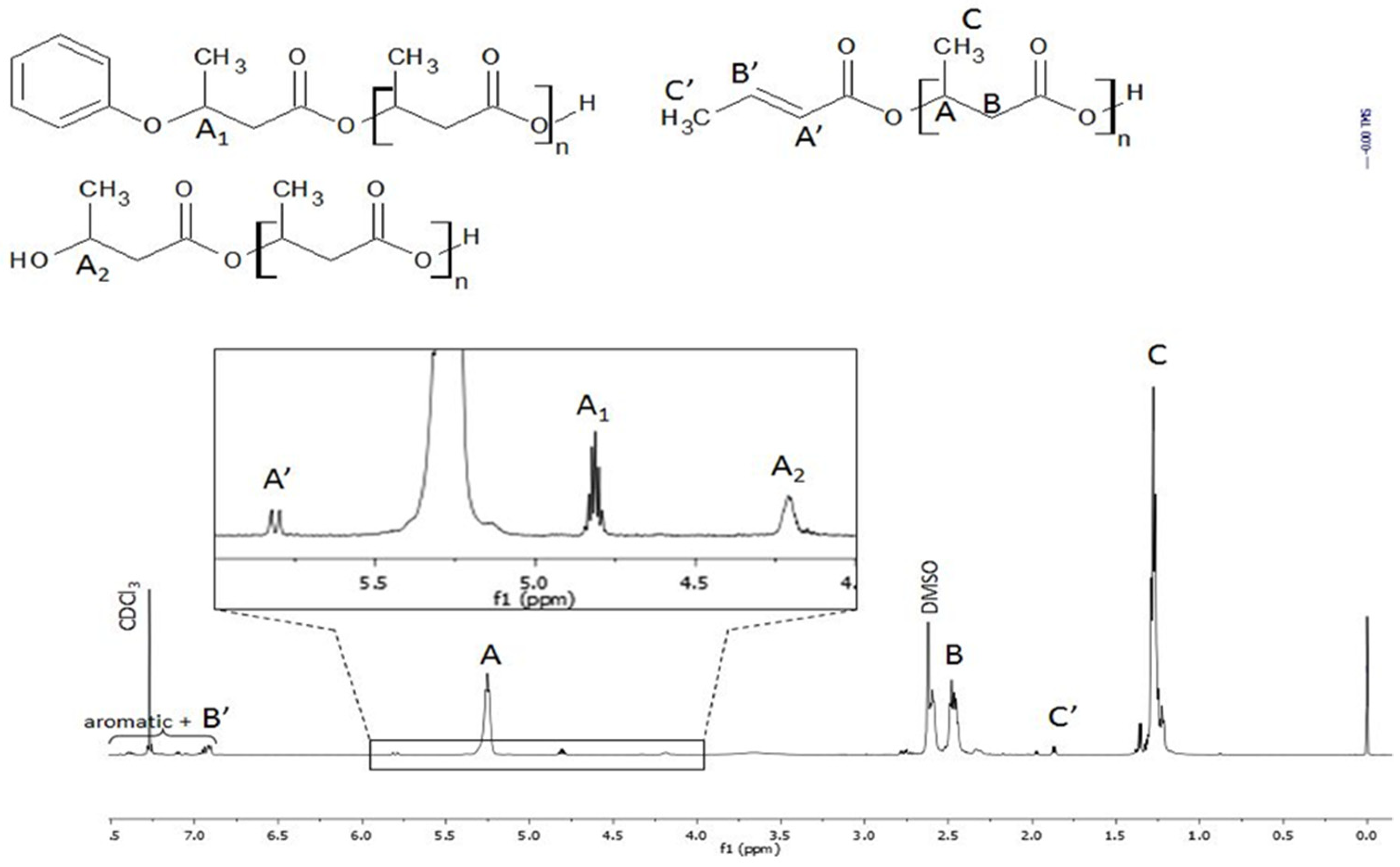
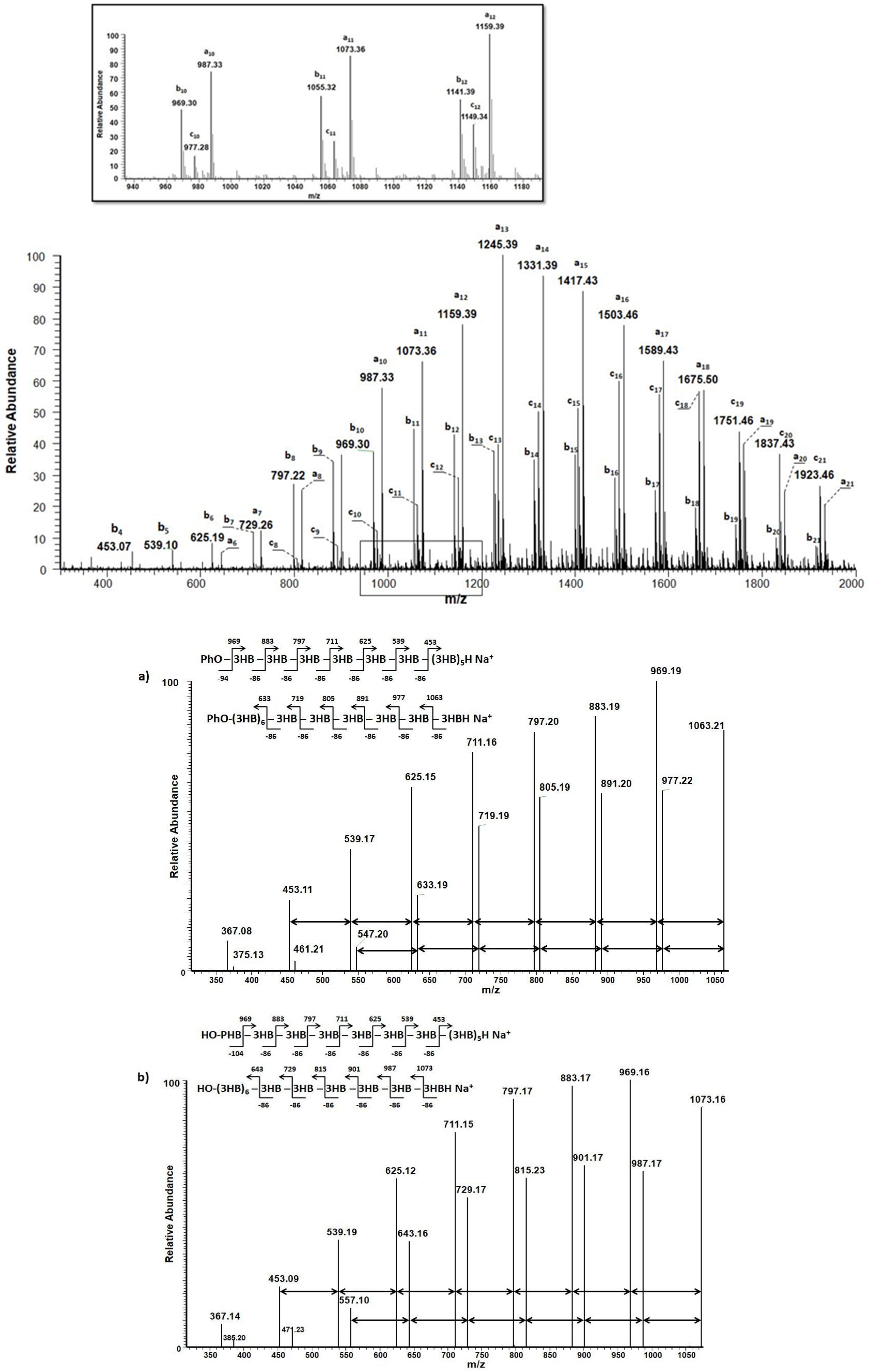
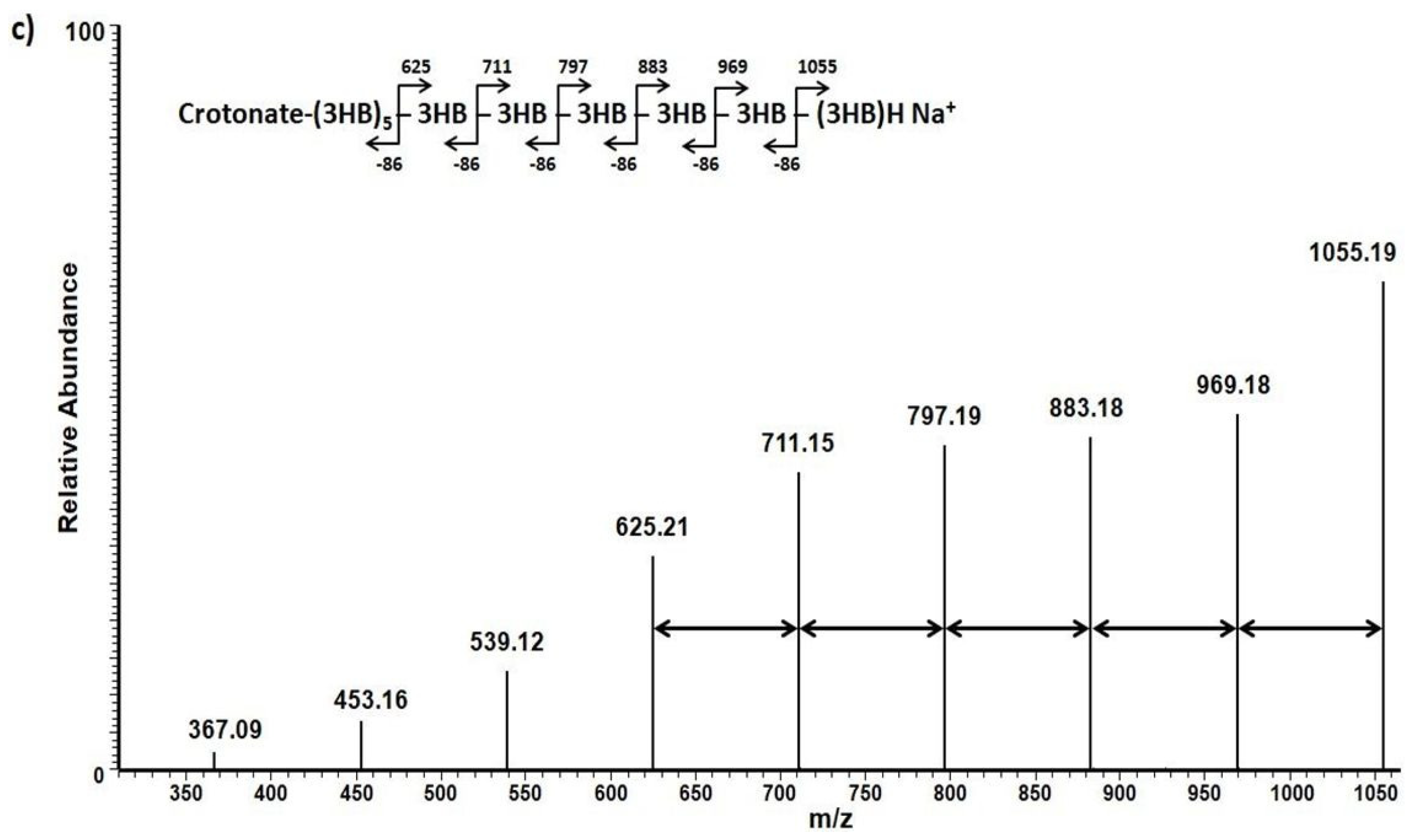
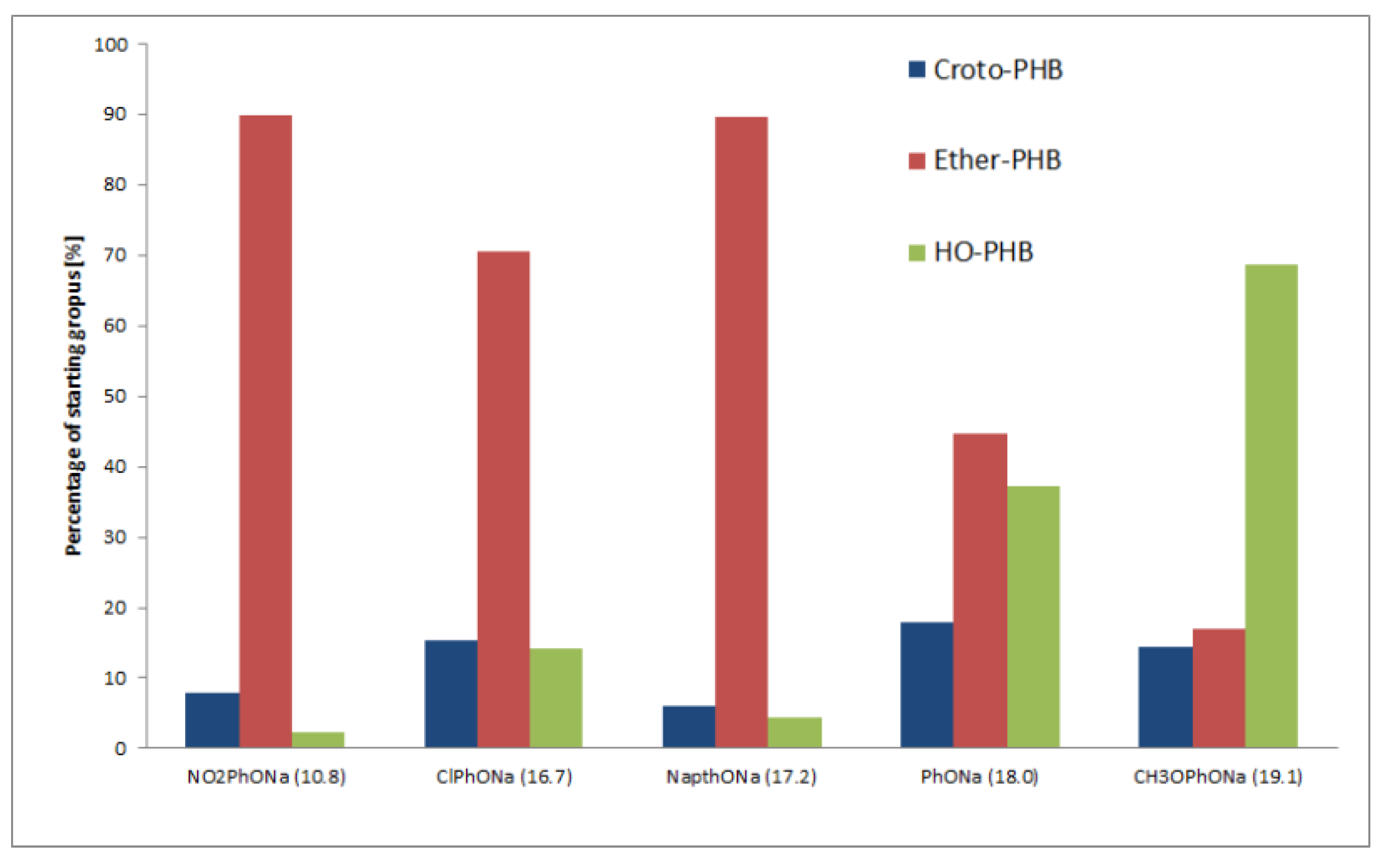
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Steinbüchel, A. Perspectives for biotechnological production and utilization of biopolymers: Metabolic engineering of polyhydroxyalkanoate biosynthesis pathways as a successful example. Macromol. Biosci. 2001, 1, 1–24. [Google Scholar] [CrossRef]
- Philip, S.; Keshavarz, T.; Roy, I. Polyhydroxyalkanoates: biodegradable polymers with a range of applications. J. Chem Technol Biotechnol. 2007, 82, 233–247. [Google Scholar] [CrossRef]
- Raza, Z.A.; Abid, S.; Banat, I.M. Polyhydroxyalkanoates: Characteristics, production, recent developments and applications. Int. Biodeter. Biodegr. 2018, 126, 45–56. [Google Scholar] [CrossRef]
- Shabina, M.; Afzal, M.; Hameed, S. Bacterial polyhydroxyalkanoates-eco-friendly next generation plastic: Production, biocompatibility, biodegradation, physical properties and applications. Green Chem. Lett. Rev. 2015, 8, 56–77. [Google Scholar]
- Anjum, A.; Zuber, M.; Zia, K.M.; Noreen, A.; Anjum, M.N.; Tabasum, S. Microbial production of polyhydroxyalkanoates (PHAs) and its copolymers: A review of recent advancements. Int. J. Biol. Macromol. 2016, 89, 161–174. [Google Scholar] [CrossRef] [PubMed]
- Jiang, G.; Hill, D.J.; Kowalczuk, M.; Johnston, B.; Adamus, G.; Iorere, V.; Radecka, I. Carbon Sources for Polyhydroxyalkanoates and an Integrated Biorefinery. Int. J. Mol. Sci. 2016, 17, 1157. [Google Scholar] [CrossRef] [PubMed]
- Piddubnyak, V.; Kurcok, P.; Matuszowicz, A.; Głowala, M.; Fiszer-Kierzkowska, A.; Jedliński, Z.; Juzwa, M.; Krawczyk, Z. Oligo-3-hydroxybutyrates as potential carriers for drug delivery. Biomaterials 2004, 25, 5271–5279. [Google Scholar] [CrossRef]
- Suriyamongkol, P.; Weselake, R.; Narine, S.; Moloney, M.; Shah, S. Biotechnological approaches for the production of polyhydroxyalkanoates in microorganisms and plants—A review. Biotechnol. Adv. 2007, 25, 148–175. [Google Scholar] [CrossRef]
- Tian, H.; Tang, Z.; Zhuang, X.; Chen, X.; Jing, X. Biodegradable synthetic polymers: Preparation, functionalization and biomedical application. Progr. Polym. Sci. 2012, 37, 237–280. [Google Scholar] [CrossRef]
- Tang, X.; Chen, E.Y.-X. Chemical synthesis of perfectly isotactic and high melting bacterial poly(3-hydroxybutyrate) from bio-sourced racemic cyclic diolide. Nat. Commun. 2018, 9, 2345. [Google Scholar] [CrossRef]
- Fang, J.; Tschan, M.J.L.; Roisnel, T.; Trivelli, X.; Gauvin, R.M.; Thomas, C.M.; Maron, L. Yttrium catalysts for syndioselective β-butyrolactone polymerization: on the origin of ligand-induced stereoselectivity. Polym. Chem. 2013, 4, 360–367. [Google Scholar] [CrossRef]
- Rieth, L.R.; Moore, D.R.; Lobkovsky, E.B.; Coates, G.W. Single-Site β-Diiminate Zinc Catalysts for the Ring-Opening Polymerization of β-Butyrolactone and β-Valerolactone to Poly(3-hydroxyalkanoates). J. Am. Chem. Soc. 2002, 124, 15239–15248. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-C.; Lin, C.-H.; Ko, B.-T.; Ho, R.-M. Ring-Opening Polymerization of β-Butyrolactone Catalyzed by Efficient Magnesium and Zinc Complexes Derived from Tridentate Anilido-Aldimine Ligand. J. Polym. Sci. Polym. Chem. 2010, 48, 5339–5347. [Google Scholar] [CrossRef]
- Okamoto, Y. Cationic Ring-Opening Polymerization of Lactones in the presence of alcohol. Makromol. Chem. Macromol. Symp. 1991, 42/43, 117–133. [Google Scholar] [CrossRef]
- Jaipuri, F.A.; Bower, B.D.; Pohl, N.L. Protic acid-catalyzed polymerization of β-lactones for the synthesis of chiral polyesters. Tetrahedron: Asymmetr. 2003, 14, 3249–3252. [Google Scholar] [CrossRef]
- Jedliński, Z.; Kurcok, P.; Lenz, R.W. First Facile Synthesis of Biomimetic Poly-(R)-3-hydroxybutyrate via Regioselective Anionic Polymerization of (S)-β-Butyrolactone. Macromolecules 1998, 31, 6718–6720. [Google Scholar] [CrossRef]
- Jedliński, Z. Regioselective Ring-Opening Anionic Polymerization of β-lactones. Macromol. Symp. 1998, 132, 377–383. [Google Scholar] [CrossRef]
- Jedliński, Z.; Kowalczuk, M.; Kurcok, P.; Adamus, G.; Matuszowicz, A.; Sikorska, W.; Gross, R.A.; Xu, J.; Lenz, R.W. Stereochemical Control in the Anionic Polymerization of β-Butyrolactone Initiated with Alkali-Metal Alkoxides. Macromolecules 1996, 29, 3773–3777. [Google Scholar] [CrossRef]
- Kowalczuk, M.; Kurcok, P.; Główkowski, W.; Jedliński, Z. New Reactions of Potassium Naphthalenide with β-, γ- and β-Lactones: An Efficient Route to α-Alkyl γ- and β-Lactones and α,β-Unsaturated Carboxylic Acid Esters. J. Org. Chem. 1992, 57, 391–393. [Google Scholar] [CrossRef]
- Khalil, A.; Cammas-Marion, S.; Coulembier, O. Organocatalysis Applied to the Ring-Opening Polymerization of β-Lactones: A Brief Overview. J. Polym. Sci. Part A 2019, 57, 657–672. [Google Scholar] [CrossRef]
- Jedliński, Z.; Kowalczuk, M. Novel Degradable Engineering Polyesters-Synthesis and Applications. Intern. J. Polymeric Mater. 1994, 24, 253–261. [Google Scholar] [CrossRef]
- Kurcok, P.; Matuszowicz, A.; Jedliński, Z. Anionic polymerization of β-lactones initiated with potassium hydride. A convenient route to polyester macromonomers. Macromol. Rapid Commun. 1995, 16, 201–206. [Google Scholar] [CrossRef]
- Lenz, R.W.; Jedliński, Z. Anionic and Coordination Polymerization of 3-butyrolactone. Macromol. Symp. 1996, 107, 149–161. [Google Scholar] [CrossRef]
- Jaffredo, C.G.; Carpentier, J.-F.; Guillaume, S.M. Controlled ROP of β-Butyrolactone Simply Mediated by Amidine, Guanidine, and Phosphazene Organocatalysts. Macromol. Rapid Commun. 2012, 22, 1938–1944. [Google Scholar] [CrossRef] [PubMed]
- Jaffredo, C.G.; Carpentier, J.-F.; Guillaume, S.M. Organocatalyzed controlled ROP of β-lactones towards poly(hydroxyalkanoate)s: from β-butyrolactone to benzyl β-malolactone polymers. Polym. Chem. 2013, 4, 3837–3850. [Google Scholar] [CrossRef]
- Moins, S.; Henoumont, C.; De Winter, J.; Khalil, A.; Laurent, S.; Cammas-Marion, S.; Coulembier, O. Reinvestigation of the Mechanism of Polymerization of β-Butyrolactone from 1,5,7-Triazabicyclo [4.4.0]dec-5-ene. Polym. Chem. 2018, 9, 1840–1847. [Google Scholar] [CrossRef]
- Kawalec, M.; Śmiga-Matuszowicz, M.; Kurcok, P. Counterion and solvent effects on the anionic polymerization of β-butyrolactone initiated with acetic acid salts. Eur. Polym. J. 2008, 44, 3556–3563. [Google Scholar] [CrossRef]
- Kurcok, P.; Jedliński, Z.; Kowalczuk, M. Reactions of β-Lactones with Potassium Alkoxides and Their Complexes with 18-Crown-6 in Aprotic Solvents. J. Org. Chem. 1993, 58, 4219–4220. [Google Scholar] [CrossRef]
- Juzwa, M.; Jedliński, Z. Novel Synthesis of Poly(3-hydroxybutyrate). Macromolecules 2006, 39, 4627–4630. [Google Scholar] [CrossRef]
- Kurcok, P.; Śmiga, M.; Jedliński, Z. β-Butyrolactone Polymerization Initiated with Tetrabutylammonium Carboxylates: A Novel Approach to Biomimetic Polyester Synthesis. J. Polym. Sci. Polym. Chem. 2002, 40, 2184–2189. [Google Scholar] [CrossRef]
- Adamus, G.; Kowalczuk, M. Anionic Ring-Opening Polymerization of β-Alkoxymethyl-Substituted β-Lactones. Biomacromolecules 2008, 9, 696–703. [Google Scholar] [CrossRef] [PubMed]
- Jedliński, Z.; Kurcok, P.; Kowalczuk, M.; Matuszowicz, A.; Dubois, P.; Jerome, R.; Kricheldor, H.R. Anionic Polymerization of Pivalolactone Initiated by Alkali Metal Alkoxides. Macromolecules 1996, 28, 7276–7280. [Google Scholar] [CrossRef]
- Kurcok, P.; Kowalczuk, M.; Hennek, K.; Jedliński, Z. Anionic Polymerization of β-Lactones Initiated with Alkali-Metal Alkoxides: Reinvestigation of the Polymerization Mechanism. Macromolecules 1992, 25, 2017–2020. [Google Scholar] [CrossRef]
- Kurcok, P.; Kowalczuk, M.; Jedliński, Z. Response to “On the Ambident Reactivity of β-Lactones in Their Reactions with Alcoholates Initiating Polymerization”. Macromolecules 1994, 27, 4833–4835. [Google Scholar] [CrossRef]
- Grobelny, Z.; Matlengiewicz, M.; Skrzeczyna, K.; Swinarew, A.; Golba, S.; Jurek-Suliga, J.; Michalak, M.; Swinarew, B. Ring-Opening Polymerization of Lactones Initiated with Metal Hydroxide-Activated Macrocyclic Ligands: Determination of Mechanism and Structure of Polymers. Int. J. Polym. Anal. Charact. 2015, 20, 457–468. [Google Scholar] [CrossRef]
- Grobelny, Z.; Golba, S.; Jurek-Suliga, J. Ring opening polymerization of β-butyrolactone in the presence of alkali metal salts: investigation of initiation course and determination of polymers structure by MALDI-TOF mass spectrometry. Polym. Bull. 2018. [Google Scholar] [CrossRef]
- Kawalec, M.; Coulembier, O.; Gerbaux, P.; Sobota, M.; De Winter, J.; Dubois, P.; Kowalczuk, M.; Kurcok, P. Traces do matter—Purity of 4-methyl-2-oxetanone and its effect on anionic ring-opening polymerization as evidenced by phosphazene superbase catalysis. React. Funct. Polym. 2012, 72, 509–520. [Google Scholar] [CrossRef]
- Bordwell pKa Table (Acidity in DMSO). Available online: https://www.chem.wisc.edu/areas/reich/pkatable/ (accessed on 12 June 2019).
- Kawalec, M.; Adamus, G.; Kurcok, P.; Kowalczuk, P.; Foltran, I.; Focarete, L.; Scandola, M. Carboxylate induced degradation of poly(3-hydroxybutyrate)s. Biomacromolecules 2007, 8, 1053–1058. [Google Scholar] [CrossRef] [PubMed]
- Kawalec, M.; Sobota, M.; Scandola, M.; Kowalczuk, M.; Kurcok, P. A convenient route to PHB macromonomers via anionically controlled moderate-temperature degradation of PHB. J. Polym. Sci., Part A 2010, 48, 5490–5497. [Google Scholar] [CrossRef]
- Grobelny, Z.; Stolarzewicz, A.; Morejko, B.; Pisarski, W.; Maercker, A.; Skibinski, A.; Krompiec, S.; Rzepa, J. C–O and Not C–C Bond Cleavage Starts the Polymerization of b-Butyrolactone with Potassium Anions of Alkalide. Macromolecules 2006, 39, 6832–6837. [Google Scholar] [CrossRef]
- Borgi, H.B.; Dunitz, J.D.; Lehn, J.M.; Wipff, G. Stereochemistry of reactions paths at carbonyl centres. Tetrahedron 1974, 30, 1563–1572. [Google Scholar] [CrossRef]

| Entry b | Initiator | Mn,th d [g·mol−1] | Mn,NMR e [g·mol−1] | Mn,SEC [g·mol−1] | Đ |
|---|---|---|---|---|---|
| 1 | sodium p-nitrophenoxide | 1100 | 1350 | 1300 | 1.19 |
| 2 | sodium p-chlorophenoxide | 1100 | 1150 | 1100 | 1.25 |
| 3 | sodium 1-naphtoxide | 1100 | 800 | 800 | 1.22 |
| 4 | sodium phenoxide | 1100 | 900 | 800 | 1.21 |
| 5 | sodium p-methoxyphenoxide | 1100 | 850 | 950 | 1.13 |
| 6 | sodium p-nitrophenoxide | 10000 | 3400 | 3800 | 1.62 |
| 7 | sodium p-chlorophenoxide | 10000 | 3050 | 4400 | 1.62 |
| 8 | sodium 1-naphtoxide | 10000 | 3000 | 4100 | 1.76 |
| 9 | sodium phenoxide | 10000 | 2400 | 4600 | 1.90 |
| 10 | sodium p-methoxyphenoxide | 10000 | 3100 | 4100 | 1.78 |
| 11 c | sodium phenoxide | 10000 | 7200 | 10200 | 1.27 |
| 12 c | sodium p-nitrophenoxide | 10000 | 7000 | 9100 | 1.21 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Domiński, A.; Konieczny, T.; Zięba, M.; Klim, M.; Kurcok, P. Anionic Polymerization of β-Butyrolactone Initiated with Sodium Phenoxides. The Effect of the Initiator Basicity/Nucleophilicity on the ROP Mechanism. Polymers 2019, 11, 1221. https://doi.org/10.3390/polym11071221
Domiński A, Konieczny T, Zięba M, Klim M, Kurcok P. Anionic Polymerization of β-Butyrolactone Initiated with Sodium Phenoxides. The Effect of the Initiator Basicity/Nucleophilicity on the ROP Mechanism. Polymers. 2019; 11(7):1221. https://doi.org/10.3390/polym11071221
Chicago/Turabian StyleDomiński, Adrian, Tomasz Konieczny, Magdalena Zięba, Magdalena Klim, and Piotr Kurcok. 2019. "Anionic Polymerization of β-Butyrolactone Initiated with Sodium Phenoxides. The Effect of the Initiator Basicity/Nucleophilicity on the ROP Mechanism" Polymers 11, no. 7: 1221. https://doi.org/10.3390/polym11071221
APA StyleDomiński, A., Konieczny, T., Zięba, M., Klim, M., & Kurcok, P. (2019). Anionic Polymerization of β-Butyrolactone Initiated with Sodium Phenoxides. The Effect of the Initiator Basicity/Nucleophilicity on the ROP Mechanism. Polymers, 11(7), 1221. https://doi.org/10.3390/polym11071221






