Abstract
Six examples of aluminum 5,6-dihydro-7,7-dimethylquinolin-8-olates, [{2-R1-7,7-Me2-8-R2C9H6N-8-O}AlR32]2 (R1 = R2 = H, R3 = Me C1; R1 = R2 = H, R3 = Et C2; R1 = R2 = H, R3 = i-Bu C3; R1 = Cl, R2 = H, R3 = Me C4; R1 = H, R2 = R3 = Me C5; R1 = Cl, R2 = R3 = Me C6), have been prepared by treating the corresponding pro-ligand (L1–L4) with either AlMe3, AlEt3 or Al(i-Bu)3. All complexes have been characterized by 1H and 13C NMR spectroscopy and in the case of C1 and C4 by single crystal X-ray diffraction; dimeric species are a feature of their molecular structures. In the presence of PhCH2OH (BnOH), C1–C6 displayed good control and efficiency for the ROP of ε-CL with almost 100% conversion achievable in 10 min at 90 °C; the chloro-substituted C4 and C6 notably exhibited the lowest activity of the series. However, in the absence of BnOH, C1 showed only low activity with 15% conversion achieved in 30 min forming a linear polymer capped with either a methyl or a L1 group. By contrast, when one or more equivalents of BnOH was employed in combination with C1, the resulting catalyst was not only more active but gave linear polymers capped with BnO end-groups. By using 1H and 27Al NMR spectroscopy to monitor solutions of C1, C1/BnOH and C1/BnOH/10 ε-CL over a range of temperatures, some support for a monomeric species being the active initiator at the operational temperature is presented.
1. Introduction
The past 10 years or so have seen some rapid progress in the synthesis of biodegradable polymers and in particular, aliphatic polyesters such as polylactides (PLA) and poly(ε-caprolactone) (PCL). These developments can be attributed, in a large measure, to the good biodegradability and biocompatibility properties of these materials as well as to their ease of preparation [1,2,3,4]. Typically, such polyesters can be prepared by the ring-opening polymerization (ROP) of cyclic esters catalyzed by metal complexes such as those based on Al, Ca, Sn and rare earth metals. In addition, immobilized catalysts have been considered for the improvement of the mechanical properties of PCLs [5]. Several review articles have documented advances in catalyst design for the ROP of cyclic esters [6,7]. Among the numerous reports, aluminum complexes bearing multidentate ligands such as Salen-Al and Salan-Al have attracted much attention due to their relatively high Lewis acidity, good controllability, as well as their decent selectivity towards the ROP of rac-lactide (rac-LA) and ε-caprolactone (ε-CL) [8,9,10,11]. By way of contrast, there are still relatively few examples of bidentate N^O-type aluminum complexes that have been used effectively for the ROP of cyclic esters.
With regard to N^O-aluminum complexes containing six-membered chelate rings (A–G, Chart 1), the 2-iminophenolates A constitute the most studied class of pro-initiator and indeed are highly effective in the presence of benzyl alcohol for the ROP of ε-CL and LA [12,13,14,15]. They can also efficiently promote the copolymerization of rac-lactide and glycolide [16], rac-β-butyrolactone and L-lactide [17] and the random copolymerization of rac-LA and ε-CL with various degrees of control [18]. Related bimetallic aluminum complexes incorporating two linked iminophenolate units have also been evaluated in the ROP of cyclic esters [19]. The dialkylaluminum aminophenolate complexes B have been shown as effective (pro-)initiators for the ROP of ε-CL and epoxides [20,21,22,23], while their dinuclear analogues showed higher activity [22,23]; good selectivity for the ROP of rac-LA have also been noted for some B-type systems [24]. Other aluminum phenolates such as dialkylaluminum 2-imidazolylphenolates (C) and 2-benzoxazole phenolates (D) have also been reported as efficient (pro-)initiators for the ROP of rac-lactide and ε-CL [25,26]. The β-ketiminato-aluminum complex E showed good efficiency for the random copolymerization of LA and ε-CL [18,27], while the fluorinated alkoxy-imino aluminum species F are active for the ROP of ε-CL [28]. Conversely, the amido-phosphinoxide aluminum complex G showed virtually no activity for the ROP of ε-CL [29].
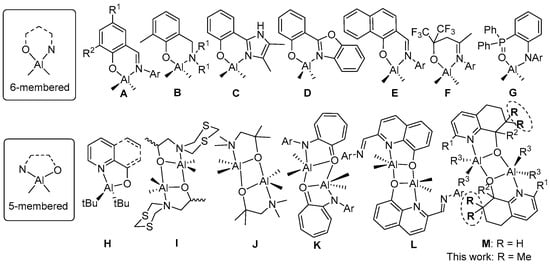
Chart 1.
Reported N^O-bidentate aluminum dialkyl complexes.
In comparison with the six-membered ring examples highlighted above (A–G, Chart 1), those based on five-membered N^O-chelate rings have been considerably less studied as ROP (pro-)initiators, though a tendency to form dimeric species is a feature of their structural chemistry (H–M, Chart 1). Nevertheless, a recent report has shown that five-membered ring aluminum complexes can display significantly higher polymerization rates when compared to their six-membered ring counterparts (e.g., two to three-fold increase for ε-CL polymerization) [30]. Elsewhere, N^O-bidentate aluminum complexes of type H [31,32] and J have been reported but have not been the subject of ROP studies though H has been discussed in terms of the relative stability of its mono- and dimeric forms [33]. On the other hand, dimeric J was shown to catalyze the cycloaddition of CO2 with epoxides [34]. Moreover, the anilinotropone-based dimeric aluminum complexes K exhibited, in the presence of BnOH, high activity in the ROP of rac-lactide [35]. Nonetheless, common to J and K it remains uncertain whether the dimeric structural forms are maintained during the polymerization or undergo dissociation to their monomeric forms. Interestingly, we have found the di- and monomeric complexes of dialkylaluminum 2-(arylimino)-quinolin-8-olates (L) display very different catalytic performance for the ROP of ε-CL. For example, the binuclear systems showed very low activity, while the mononuclear examples gave much higher efficiency and in a more controlled manner [36]. Furthermore, our group has recently synthesized a series of bimetallic dialkylaluminum hydroquinolin-8-olates (M: R1 = H, Chart 1) that showed good activity only at high temperature for the ROP of ε-CL. By using variable temperature 1H and 27Al NMR spectroscopy, we proposed that the di-aluminum complexes partly dissociated into mononuclear species at high temperature [37]. However, these complexes displayed only poor solubility at room temperature which precluded full assignments of their peaks in their 1H NMR spectra.
With a view to improve the solubility of M (Chart 1) and to re-investigate the nuclearity of the active initiator, we report herein the introduction of two methyl groups at the 7-position of the ligand framework. In particular, we report a series of bimetallic aluminum 5,6-dihydro-7,7-dimethylquinolin-8-olates, [{2-R1-7,7-Me2-8-R2C9H6N-8-O}AlR32]2 (R1 = R2 = H, R3 = Me; R1 = R2 = H, R3 = Et; R1 = R2 = H, R3 = i-Bu; R1 = Cl, R2 = H, R3 = Me; R1 = H, R2 = R3 = Me; R1 = Cl, R2 = R3 = Me), that differ in the substitution pattern at the R1, R2 and R3 positions. A full evaluation of these complexes as either initiators or pro-initiators for the ROP of ε-CL is conducted and these results compared with those observed using parent M. In addition, the pathway by which pro-initiator is transformed in to the initiator is probed using both 1H and 27Al NMR spectroscopy.
2. Materials and Methods
2.1. General Considerations and Materials
All manipulations of air or moisture-sensitive compounds were performed using standard Schlenk techniques under an atmosphere of high-purity nitrogen or using glove box techniques. Toluene was dried by refluxing it over sodium/benzophenone and distilled under nitrogen and stored over activated molecular sieves (4 Å) for 24 h in a glove box prior to use. n-Hexane and CDCl3 were dried over CaH2 for 48 h, distilled under nitrogen and stored over activated molecular sieves (4 Å) in a glove box prior to use. Solutions of Me3Al (1.0 M in toluene), Et3Al (1.0 M in toluene), i-Bu3Al (1.0 M in toluene) and methyllithium (1.0 M in toluene) were purchased from Aldrich and used as received. Elemental analyses were performed using a PE2400II Series instrument (Perkin-Elmer Co., Shanghai, China). The 1H and 13C NMR spectra were recorded on a Bruker DMX-400/300 MHz (Karlsruhe, Germany) spectrometer using TMS as an internal standard; δ values are given in ppm and J values in Hz. The 27Al NMR spectra were recorded on Bruker 500 MHZ (Beijing, China) spectrometer. The NMR spectra of the complexes and ligands were recorded in CDCl3 at room temperature. IR spectra were recorded on a Perkin-Elmer System 2000 (Shanghai, China) FT-IR spectrometer. The GPC measurements were performed using a set-up based on a Waters-1515 HPLC pump, a Waters 2414 (Beijng, China) refractive index detector and a combination of Styragel HT-2, HT-3 and HT-4 columns, the effective molar mass ranges of which are 100–10,000, 500–30,000 and 5000–600,000, respectively. THF was used as the eluent (flow rate: 1 mL/min, at 35 °C). Molecular weights and molecular weight distributions were calculated using polystyrene as a standard.
2.2. Syntheses of 2-R1-7,7-Me2,8-R2C9H6N-8-OH (L)
R1 = R2 = H L1. A mixture of 5,6-dihydro-7,7-dimethylquinolin-8-one (0.88 g, 5.0 mmol) and sodium borohydride (0.19 g, 5.0 mmol) was dissolved in methanol (30 mL) in a 100 mL round flask and stirred for 5 h at room temperature. The reaction was then quenched with water, extracted with dichloromethane and the organic phase dried over anhydrous magnesium sulfate and filtered. The filtrate was collected and the solvent evaporated under reduced pressure to give L1 as a light yellow solid. Yield: 0.86 g (96%). 1H NMR (400 MHz, CDCl3, ppm): δ 8.40 (d, J = 6.0 Hz, 1H, Py–H), 7.41 (d, J = 8.0 Hz, 1H, Py–H), 7.14–7.11 (m, 1H, Py–H), 4.38 (s, 1H, CH–OH), 4.31 (s, 1H, OH), 2.92–2.79 (m, 2H, CH2), 1.75–1.65 (m, 2H, CH2), 1.19 (s, 3H, CH3), 0.85 (s, 3H, CH3). 13C NMR (100 MHz, CDCl3, ppm): δ 157.3, 146.3, 136.5, 130.4, 122.0, 76.2, 34.0, 33.3, 27.5, 24.8, 18.9. IR (cm−1): 3117 (m), 2953 (m), 2922 (m), 2859 (w), 2714 (w), 1583 (m), 1446 (m), 1377 (m), 1359 (m), 1307 (w), 1260 (m), 1160 (m), 1107 (m), 1042 (s), 981 (m), 936 (w), 904 (w), 857 (w), 788 (s), 751 (m), 713 (m). Anal. Calcd for C11H15NO: C, 74.54; H, 8.53; N, 7.90%. Found: C, 74.40; H, 8.53; N, 7.81%.
R1 = Cl, R2 = H L2. Using a similar procedure to that described for L1, but with 2-chloro-5,6-dihydro-7,7-dimethylquinolin-8-one as the ketone, gave L2 as a white powder. Yield: 1.03 g (97%). 1H NMR (400 MHz, CDCl3, ppm): δ 7.38 (d, J = 8.0 Hz, 1H, Py–H), 7.14 (d, J = 8.0 Hz, 1H, Py–H), 4.27 (s, 1H, CH–OH), 3.76 (s, 1H, OH), 2.85–2.69 (m, 2H, CH2), 1.71–1.67 (m, 2H, CH2), 1.16 (s, 3H, CH3), 0.87 (s, 3H, CH3). 13C NMR (100 MHz, CDCl3, ppm): δ 158.3, 148.6, 139.6, 129.0, 123.0, 76.0, 34.0, 33.3, 27.5, 24.3, 19.0. IR (cm−1): 3281 (m), 2943 (m), 2905 (m), 2865 (w), 1570 (m), 1469 (m), 1440 (m), 1416 (w), 1377 (m), 1363 (m), 1256 (m), 1230 (w), 1197 (w), 1164 (m), 1131 (w), 1090 (m), 1033 (s), 946 (m), 904 (w), 870 (w), 842 (m), 789 (s), 756 (m), 727 (m). Anal. Calcd for C11H14ClNO: C, 62.41; H, 6.67; N, 6.62%. Found: C, 62.58; H, 6.59; N, 6.43%.
R1 = H, R2 = Me L3. Methyllithium (5.0 mL, 5.0 mmol, 1.0 M solution in toluene) was added dropwise to a stirred solution of 5,6-dihydro-7,7-dimethylquinolin-8-one (0.88 g, 5.0 mmol) in toluene (10.0 mL) at −30 °C. The reaction mixture was allowed to warm slowly to room temperature and stirred overnight. Following quenching with water and extraction with dichloromethane, the organic phase was dried over anhydrous magnesium sulfate and filtered. The filtrate was collected and the solvent removed under reduced pressure to give L3 as a white solid. Yield: 0.93 g (97%). 1H NMR (400 MHz, CDCl3, ppm): δ 8.39 (d, J = 4.0 Hz, 1H, Py–H), 7.38 (d, J = 8.0 Hz, 1H, Py–H), 7.11–7.08 (m, 1H, Py–H), 4.25 (s, 1H, OH), 2.90–2.74 (m, 2H, CH2), 1.94–1.86 (m, 1H, CH2), 1.63–1.58 (m, 1H, CH2), 1.37 (s, 1H, CH3), 1.15 (s, 1H, CH3), 0.93 (s, 1H, CH3). 13C NMR (100 MHz, CDCl3, ppm): δ 162.3, 148.3, 139.6, 128.2, 122.4, 74.7, 36.1, 33.0, 26.7, 24.5, 23.9, 21.9. IR (cm−1): 3413 (m), 2959 (m), 2872 (w), 1702 (w), 1580 (m), 1447 (m), 1424 (m), 1386 (m), 1359 (m), 1336 (w), 1283 (m), 1260 (m), 1169 (m), 1131 (m), 1077 (s), 1018 (m), 978 (m), 934 (w), 907 (w), 789 (s). Anal. Calcd for C12H17NO: C, 75.35; H, 8.96; N, 7.32%. Found: C, 75.26; H, 8.82; N, 7.46%.
R1 = Cl, R2 = Me L4. Using a similar procedure to that described for L3, but with 2-chloro-5,6-dihydro-7,7-dimethylquinolin-8-one as the ketone, gave L4 as a white powder. Yield: 1.09 g (97%). 1H NMR (400 MHz, CDCl3, ppm): 7.34 (d, J = 8.0 Hz, 1H, Py–H), 7.11 (d, J = 8.0 Hz, 1H, Py–H), 3.65 (s, 1H, OH), 2.87–2.70 (m, 2H, CH2), 1.89–1.81 (m, 1H, CH2), 1.65–1.59 (m, 1H, CH2), 1.38 (s, 3H, CH3), 1.12 (s, 3H, CH3), 0.94 (s, 3H, CH3). 13C NMR (100 MHz, CDCl3, ppm): δ 161.4, 146.5, 136.5, 129.2, 121.8, 74.9, 36.2, 33.0, 27.1, 24.5, 24.4, 22.0. IR (cm−1): 3477 (m), 2963 (m), 2924 (m), 1697 (m), 1572 (m), 1444 (m), 1427 (m), 1385 (m), 1360 (m), 1328 (m), 1316 (w), 1261 (m), 1191 (w), 1166 (m), 1127 (m), 1069 (s), 1016 (m), 932 (w), 854 (w), 815 (s), 748 (m). Anal. Calcd for C12H16ClNO: C, 63.86; H, 7.15; N, 6.21%. Found: C, 63.45; H, 7.28; N, 6.46%.
2.3. Syntheses of [{2-R1-7,7-Me2-8-R2C9H6N-8-O}AlR32]2 (C)
R1 = R2 = H, R3 = Me C1. Me3Al (5.0 mL, 5.0 mmol, 1.0 M solution in toluene) was added dropwise to a stirred solution of L1 (0.89 g, 5.0 mmol) in toluene (10 mL) at −78 °C. The resulting solution was allowed to warm slowly to room temperature and stirred for 3 h. Following concentration of the reaction mixture to ca. 1 mL, hexane (10 mL) was added to induce precipitation. The precipitate was filtered affording C1 as a white powder. Yield: 0.75 g, 65%. 1H NMR (400 MHz, toluene-d8, ppm): δ 7.44 (d, J = 8.0 Hz, 1H, Py–H), 6.63 (d, J = 8.0 Hz, 1H, Py–H), 6.47–6.41 (m, 1H, Py–H), 4.58 (s, 1H, CH–O), 2.07–1.98 (m, 2H, CH2), 1.52 (s, 3H, CH3), 1.21–1.13 (m, 2H, CH2), 0.68 (s, 3H, CH3), −0.12 (s, 3H, Al–CH3), −0.26 (s, 3H, Al–CH3). 13C NMR (100 MHz, toluene-d8, ppm): δ 157.0, 141.7, 140.1, 134.2, 124.0, 82.1, 36.2, 35.8, 30.1, 24.2, 17.7, −3.26. Anal. Calcd for C13H20AlNO: C, 66.93; H, 8.64; N, 6.00%. Found: C, 67.13; H, 8.77; N, 5.88%.
R1 = R2 = H, R3 = Et C2. Using a similar procedure to that described for C1, but with Et3Al (1.0 M solution in toluene) as the alkyl aluminum reagent, gave C2 as a white powder. Yield: 0.55 g, 42%. 1H NMR (400 MHz, toluene-d8, ppm): δ 8.11 (d, J = 4.0 Hz, 1H, Py–H), 6.74 (d, J = 5.6 Hz, 1H, Py–H), 6.57 (t, J = 6.0 Hz, 1H, Py–H), 5.01 (s, 1H, CH–O), 2.34–2.22 (m, 2H, CH2), 1.54 (s, 3H, CH3), 1.45–1.37 (m, 1H, Al–CH2CH3), 1.30–1.25 (m, 2H, CH2), 1.21–1.15 (m, 3H, Al–CH2CH3), 0.94 (s, 3H, CH3 and Al–CH2CH3), 0.51–0.44 (m, 2H, Al–CH2CH3), 0.35–0.26 (m, 2H, Al–CH2CH3). 13C NMR (100 MHz, toluene-d8, ppm): δ 164.4, 158.5, 142.5, 132.1, 122.7, 80.9, 36.7, 36.5, 30.2, 24.9, 18.6, 11.4, 11.3, 1.9. Anal. Calcd for C15H24AlNO: C, 68.94; H, 9.26; N, 5.36%. Found: C, 69.17; H, 9.44; N, 5.23%.
R1 = R2 = H, R3 = i-Bu C3. Using a similar procedure to that described for C1, but with i-Bu3Al (1.0 M solution in toluene) as the alkyl aluminum reagent, gave C3 as a white powder. Yield: 0.33 g, 21%. 1H NMR (400 MHz, CDCl3, ppm): δ 8.23 (d, J = 8.0 Hz, 1H, Py–H), 7.67 (d, J = 4.0 Hz, 1H, Py–H), 7.38–7.34 (m, 1H, Py–H), 4.61 (s, 1H, CH–O), 2.94–2.80 (m, 2H, CH2), 1.86–1.67 (m, 3H, CH2 and Al–CH2CH(CH3)2), 1.23 (s, 3H, CH3), 0.90–0.75 (m, 12H, Al–CH2CH(CH3)2), 0.73 (s, 3H, CH3), −0.07–−0.09 (m, 4H, Al–CH2CH(CH3)2). 13C NMR (100 MHz, CDCl3): δ 164.0, 142.2, 139.6, 133.6, 122.8, 80.4, 35.3, 34.3, 28.2, 28.1, 28.0, 27.9, 26.2, 26.0, 24.5, 22.6, 17.4. Anal. Calcd for C19H32AlNO: C, 71.89; H, 10.16; N, 4.41%. Found: C, 71.56; H, 10.52; N, 4.67%.
R1 = Cl, R2 = H, R3 = Me C4. Using a similar procedure to that described for C1, but with L2 as the pro-ligand, gave C4 as a white powder. Yield: 1.14 g, 85%. 1H NMR (400 MHz, CDCl3, ppm): 7.74 (d, J = 8.0 Hz, 1H, Py–H), 7.44 (d, J = 8.0 Hz, 1H, Py–H), 4.92 (s, 1H, CH–O), 2.85–2.81 (m, 2H, CH2), 1.92–1.84 (m, 1H, CH2), 1.71–1.65 (m, 1H, CH2), 1.51 (s, 3H, CH3), 0.84 (s, 3H, CH3), -0.58 (s, 6H, Al–CH3). 13C NMR (100 MHz, CDCl3, ppm): δ 158.4, 145.5, 142.4, 132.8, 126.7, 81.5, 36.1, 35.8, 31.6, 29.5, 23.9, 18.1, 14.3, −4.5. Anal. Calcd for C13H19AlClNO: C, 58.32; H, 7.15; N, 5.23%. Found: C, 58.11; H, 7.24; N, 5.38%.
R1 = H, R2 = R3 = Me C5. Using a similar procedure to that described for C1, but with L3 as the pro-ligand, gave C5 as a white powder. Yield: 0.36 g, 29%. 1H NMR (400 MHz, Toluene-d8, ppm): δ 7.49 (d, J = 4.0 Hz, 1H, Py–H), 6.74 (d, J = 8.0 Hz, 1H, Py–H), 6.51–6.48 (m, 1H, Py–H), 2.21–2.18 (m, 2H, CH2), 1.56–1.51 (m, 1H, CH2), 1.49 (s, 3H, CH3), 1.43 (s, 3H, CH3), 1.22–1.15 (m, 1H, CH2), 0.83 (s, 3H, CH3), −0.10 (s, 3H, Al–(CH3)2), −0.30 (s, 3H, Al–(CH3)2). 13C NMR (100 MHz, toluene-d8, ppm): δ 163.2, 143.4, 142.5, 134.3, 126.0, 87.7, 40.3, 36.72, 28.9, 27.8, 25.2, 23.3, −2.09, −2.92. Anal. Calcd. for C14H22AlNO: C, 67.99; H, 8.97; N, 5.66%. Found: C, 68.20; H, 8.83; N, 5.79%.
R1 = Cl, R2 = R3 = Me C6. Using a similar procedure to that described for C1, but with L4 as the pro-ligand, gave C6 as a white powder. Yield: 0.48 g, 34%. 1H NMR (300 MHz, CDCl3, ppm): δ 7.52 (d, J = 9.0 Hz, 1H, Py–H), 7.28 (t, J = 9.0 Hz, 1H, Py–H), 2.88–2.83 (m, 2H, CH2), 2.02–1.91 (m, 1H, CH2), 1.70–1.62 (m, 1H, CH2), 1.44 (s, 3H, CH3), 1.29 (s, 3H, CH3), 0.91 (s, 3H, CH3), −0.69 (s, 6H, Al–(CH3)2). 13C NMR (100 MHz, CDCl3, ppm): δ 161.1, 146.7, 141.1, 128.2, 125.0, 81.0, 37.4, 33.8, 27.3, 25.1, 23.2, 21.0, 14.4, −8.3. Anal. Calcd for C14H21AlClNO: C, 59.68; H, 7.51; N, 4.97%. Found: C, 59.55; H, 7.32; N, 4.85%.
2.4. X-ray Crystallographic Studies
Single crystal X-ray diffraction data for C1 and C4 were collected on a Rigaku Sealed Tube CCD (Saturn 724+) diffractometer (Tokyo, Japan) with graphite monochromated Mo-Kα radiation (λ = 0.71073 Å) at 293(2) K. Cell parameters were obtained by global refinement of the positions of all collected reflections. Intensities were corrected for Lorentz and polarization effects and empirical absorption. The structures were solved by direct methods and refined by full-matrix least squares on F2. All hydrogen atoms were placed in calculated positions. Structure solution and refinement were performed using the SHELXTL-97 package [38,39]. Details of the X-ray structure determinations and refinements are provided in Table 1. The Cambridge Crystallographic Data Centre CCDC 1851289 and 1851290 for C1 and C4, respectively, contain the supplementary crystallographic data for the paper. These data can be obtained free of charge from the Cambridge Crystallographic Data Centre (Cambridge, UK).

Table 1.
Crystal data and structure refinement for C1 and C4.
2.5. General Procedure for ε-Caprolactone Polymerization
A typical polymerization procedure in the presence of one equivalent of benzyl alcohol is outlined as follows. Precatalyst C1 (0.0047 g, 0.020 mmol) was dissolved in toluene (2 mL) in a Schlenk flask at room temperature and a solution of benzyl alcohol (0.020 mmol) in toluene added and the mixture stirred at room temperature for 5 min. The flask was then placed in a temperature-controlled oil bath preheated to 90 °C and ε-CL (0.571 g, 5.0 mmol) was injected. After the solution was stirred for the pre-determined time, the polymerization was terminated by the addition of glacial acetic acid (ca. 0.2 mL). The resulting viscous solution was diluted with dichloromethane and then transferred to a beaker containing cold methanol (100 mL) with stirring. The resultant polymer was collected on filter paper and dried under reduced pressure to give a white solid.
3. Results and Discussion
3.1. Synthesis and Characterization of C1–C6
Based on previous synthetic approaches, reduction of the 5,6-dihydro-7,7-dimethylquinolin-8-ones, 2-R1-7,7-Me2C9H6N-8-O (R1 = H; R1 = Cl), with NaBH4 or LiMe gave the dihydroquinolin-8-ols, 2-R1-7,7-Me2,8-R2C9H6N-8-OH (R1 = R2 = H L1; R1 = Cl, R2 = H L2; R1 = H, R2 = Me L3; R1 = Cl, R2 = Me L4), in high yield (Scheme 1). All four compounds have been fully characterized by 1H/13C NMR, FT-IR spectroscopy as well as by elemental analysis. Treatment of L1 with one equivalent of (R3)3Al (R3 = Me, Et, i-Bu) in toluene at −78 °C afforded [{2-R1-7,7-Me2-8-R2C9H6N-8-O}AlR32]2 (R1 = R2 = H, R3 = Me C1; R1 = R2 = H, R3 = Et C2; R1 = R2 = H, R3 = i-Bu C3), while reaction of L2, L3 and L4 with solely Me3Al gave [{2-R1-7,7-Me2-8-R2C9H6N-8-O}AlMe2]2 (R1 = Cl, R2 = H C4; R1 = H, R2 = Me C5; R1 = Cl, R2 = Me C6), in moderate to good yields. All six complexes have been characterized by 1H and 13C NMR spectroscopy as well as by elemental analysis. In addition, C1 and C4 have been the subject of single crystal X-ray diffraction studies.
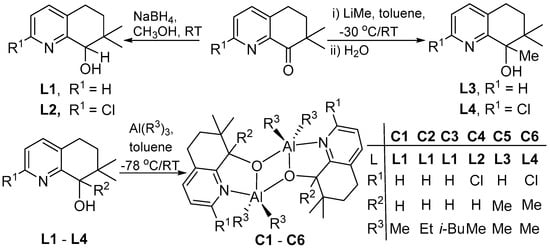
Scheme 1.
Synthetic routes to L1–L4 and aluminum complexes C1–C6.
Single crystals of C1 and C4 suitable for the X-ray determinations were grown by the slow diffusion of n-hexane into their toluene solutions at room temperature under a nitrogen atmosphere. Perspective views of the structures of C1 and C4 are shown in Figure 1 and Figure 2; selected bond lengths and bond angles are collected in Table 2. Both C1 and C4 have been generated by symmetry and adopt similar arrangements and will be discussed together. Their structures consist of two aluminum centers that are each chelated by an N^O-bound 5,6-dihydro-7,7-dimethylquinolin-8-olate which also acts as a bridging ligand via O1. The five-coordinate geometry of each metal center is completed by two methyl ligands. The main difference between the structures relates to the substituent located on the 2-position of the N^O-chelate viz. H (C1) and Cl (C4). Indeed, this dimeric arrangement is similar to that observed in the unsubstituted 5,6-dihydroquinolin-8-olates M (Chart 1) [37]. The Al-N bond length in C1 [2.122(13) Å] is slightly shorter than that in C4 [2.194(3) Å] likely reflecting the steric properties of the more bulky chloride substituent positioned at C1. There are no intermolecular contacts of note.
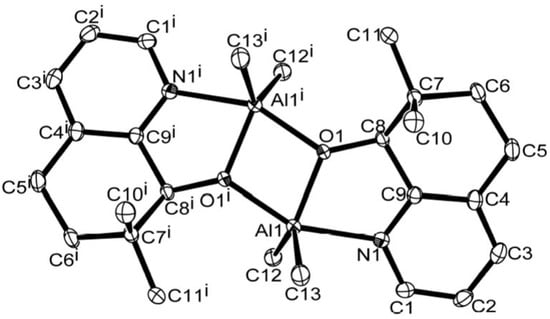
Figure 1.
ORTEP representation of C1. Thermal ellipsoids are shown at 30% probability while hydrogen atoms have been omitted for clarity.
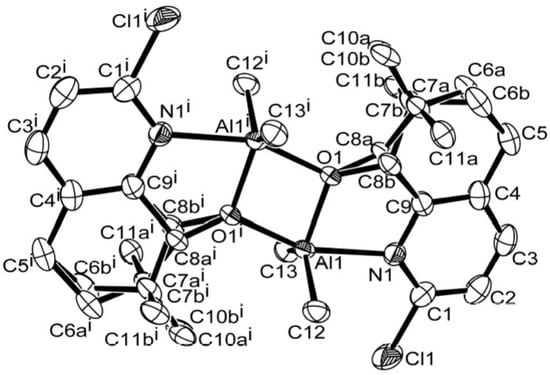
Figure 2.
ORTEP representation of C4 showing the disorder in the saturated sections of the rings. Thermal ellipsoids are shown at 30% probability while hydrogen atoms have been omitted for clarity.

Table 2.
Selected bond lengths (Å) and angles (°) for C1 and C4.
The 1H NMR spectra of C1–C6 reveal characteristic peaks for the corresponding 5,6-dihydro-7,7-dimethylquinolin-8-olate ligands with the inequivalent gem-dimethyl groups giving rise to two distinct signals. Likewise, the Al-CH3 (C1, C4–C6) or Al-CH2 (C2, C3) resonances are seen, in most cases, as separate signals and appear most upfield in their spectra. Interestingly, the 1H NMR spectrum of C1, recorded in three different deuterated solvents, indicates that the solvent polarity has some effect on the chemical shift of the resonances (Table 3). In an apolar solvent such as toluene-d8 and benzene-d6, each proton displays very similar chemical shifts. However, the shift in chloroform-d is greatly affected, which is best exemplified by the most upfield Cy-CH3 signal which appears at δ 0.58 in C6D6 while in CDCl3 at δ 0.82. In addition, the number of Al-CH3 signals shows some variation with two peaks (δ −0.12, −0.26) in C6D6 and three peaks (δ −0.62, −0.66, −0.67) in CDCl3. It is uncertain as to the origin of these differences but it may be due to the ability of the more polar chloroform to interrupt the apparent monomeric/dimeric equilibrium in solution (vide infra).

Table 3.
The 1H NMR chemical shifts for C1 recorded in different deuterated solvents.
3.2. Ring Opening Polymerization of ε-Caprolactone by C1–C6
To explore the capacity of C1–C6 to serve as pro-initiators for the ring-opening polymerization of ε-CL, C1 was firstly selected as the test system; the results of the catalytic evaluations are collected in Table 3. In the first instance, the study focused on establishing the optimal temperature by performing the polymerization runs between 25 and 110 °C. Typically, each run was conducted in toluene with one equivalent of PhCH2OH (BnOH) as activator and 250 equivalents of the monomer over a run time of 10 min (runs 1–7, Table 4). Below 40 °C, there was no monomer consumption after 10 min. However, on raising the temperature from 60 to 90 °C, the conversion gradually increased from 47% at 60 °C to a maximum of >99% at 90 °C (runs 4, 5, Table 4). Further increasing the temperature up to 110 °C led to a steady decrease in the conversion. These findings contrast with observations seen for M (Chart 1) in which only 20% conversion was observed at 80 °C and 95% at 90 °C [37].

Table 4.
The ROP of ε-CL using C1 a.
With the ε-CL:Al:BnOH ratio fixed at 250:1:1 and the run temperature at 90 °C, the influence of reaction time on the polymerization was explored (runs 5, 8–10, Table 4). Monitoring the polymerization by 1H NMR spectroscopy at intervals of 3, 5, 7 and 10 min saw the conversion rise from 43% to approaching 100% while the molecular weight of polymer increased from 1.04 × 104 to 1.89 × 104 g/mol. By contrast, the TOF decreased with time implying gradual deactivation of the active species over longer run times. To investigate the importance of BnOH in the activation of C1, the polymerization runs were performed with and without benzyl alcohol over different run times but with the temperature maintained at 90 °C; the results are collected in Table 4 (runs 11–17). Without benzyl alcohol, C1 showed no activity after 20 min while after 30 min 15% conversion was observed, highlighting the induction period needed when using C1 alone. This result contrasts with the virtually 100% conversion observed after 10 min using an equimolar ratio of C1 to BnOH (run 5, Table 4). On increasing the BnOH/Al ratio from 1 to 10, the conversion gradually decreased from 99 to 69% (runs 5, 14–17, Table 4) while the Mn of the PCL lowered from 1.89 × 104 to 0.28 × 104 g/mol, suggesting the possible role of BnOH as a chain transfer reagent. An alternative explanation could be due to the likely intermediate ‘L1Al(OCH2Ph)2’ undergoing disproportionation and dimerization to form an inactive species in a manner similar to that previously reported [41].
In order to gain some information on the structural features of the PCLs, all the samples obtained by employing different amounts of BnOH were analyzed by 1H NMR spectroscopy and by MALDI-TOF mass spectrometry. In the case where no BnOH was employed, the MALDI-TOF spectrum indicated that the polymer was comprised of linear polymers capped by two different types of end-group (α and β in Figure 3). In α (the major species) the capping group is CH3 while in β it is L1. These findings are supported by the 1H NMR data that reveal aromatic signals characteristic of L1 in β and a distinct singlet peak at δ 2.14 that can be assigned to the acetyl end group (CH3C=O) in α (Figure 4). Therefore, it would seem probable that this polymerization proceeded by a coordination-insertion mechanism in which the Al-Me and Al-L1 independently initiated the ring opening polymerization by pathways I and II (Scheme 2) [42,43,44,45].
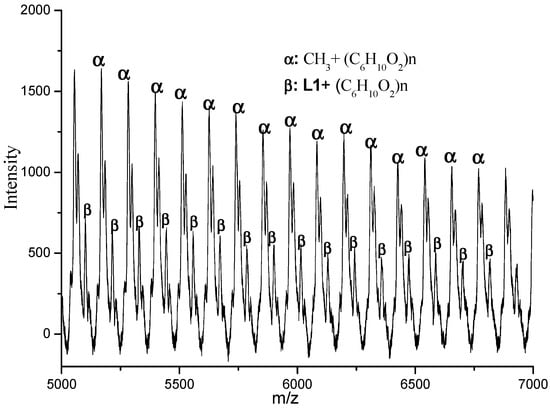
Figure 3.
The MALDI-TOF mass spectrum of the PCL obtained using C1 (run 13, Table 3).
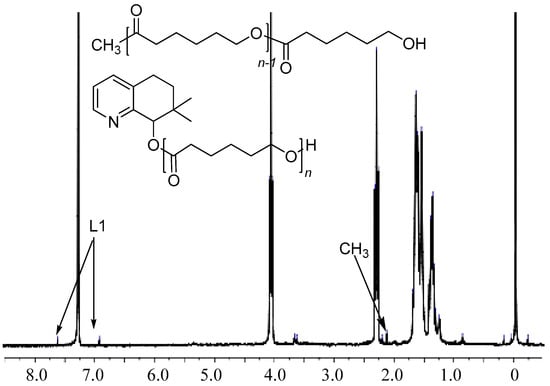
Figure 4.
The 1H NMR spectrum of the PCL obtained using C1 as initiator (run 13, Table 3).
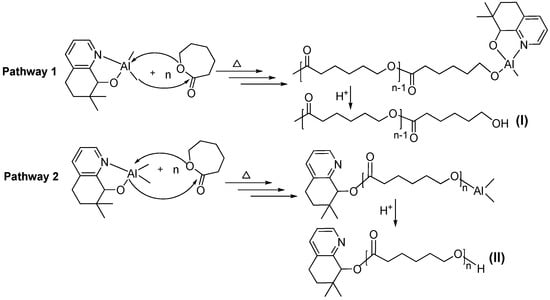
Scheme 2.
Possible mechanistic pathways for the ring opening polymerization of ε-CL using C1.
On the other hand, when one equivalent or more of BnOH was employed in combination with C1, the 1H NMR spectra of the PCLs showed only signals typical of BnO end groups (e.g., δ 5.0 (OCH2Ph) in Figure 5). This observation was corroborated by the MALDI-TOF spectrum that showed solely peaks corresponding to a linear chain polymer capped by a BnO group (Figure 6). Similarly, the polymers obtained by using larger amounts of BnOH possessed linear structures with BnO end groups, which were again confirmed by 1H NMR spectroscopy and MALDI-TOF mass spectrometry (Figures S1–S4). As an additional feature of the spectra, it was evident that the more BnOH employed the lower the molecular weight of the polymer formed (runs 5, 14–17, Table 4).
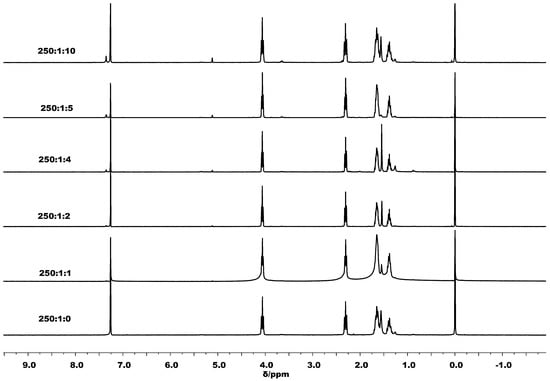
Figure 5.
The 1H NMR spectra of the PCL obtained using C1 with different amounts of BnOH (runs 5, 13–17, Table 4).
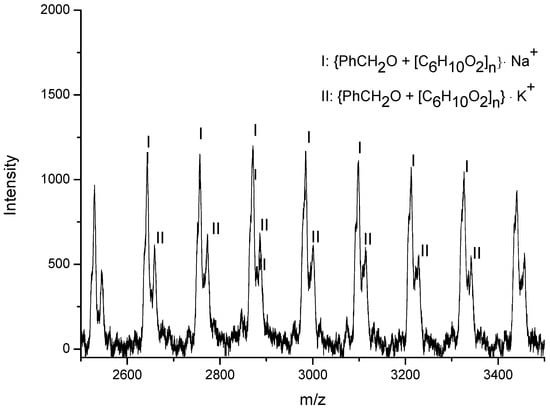
Figure 6.
The MALDI-TOF mass spectrum of the PCL obtained using C1/BnOH as initiator (run 5, Table 4).
With the amount of benzyl alcohol maintained at one molar equivalent, the effect of changing the amount of ε-CL on the performance of C1 was then examined. On increasing the molar ratio of ε-CL:Al from 100 to 300, all the conversions were greater than 90% with the TOF showing an upward trend (runs 5, 18, 19, Table 4). However, on further increasing the ε-CL:Al ratio between 400 and 750, a dramatic decrease of both the conversion and TOF was evident (runs 20–22, Table 4). On the other hand, the molecular weight of the PCL was found to gradually increase from 0.75 × 104 to 2.51 × 104 g/mol as the ε-CL:Al molar ratio was raised from 100 to 750 (runs 5, 18–22, Table 4), which can be explained by the faster rate of coordination and rate of propagation at higher monomer concentration.
The effect of solvent on the polymerizations using C1 was also studied. As a bulk polymerization was conducted at 90 °C, almost 100% conversion was observed after 10 min. The molecular weight was, however, much lower than that obtained in toluene (run 23, Table 4). When using n-hexane, dichloromethane or THF, the activity dramatically decreased and only trace amounts of polymer could be obtained (runs 24–26, Table 4). It is worthy to note that the above findings are significantly different to that found using analogue M (Chart 1). In that case, no activity was observed in bulk polymerization and moderate conversion was observed in other solvents [37]. One reason to account for these differences may be due to the improved solubility of C1 leading to more facile reactivity.
Based on the optimal conditions established for C1, the remaining five complexes, C2–C6, were also investigated for ROP; the results are collected in Table 4 alongside the data for C1. In terms of the steric properties of the aluminum-R3 group, replacing Me with an i-Bu group slightly decreased the conversion from 99 to 91% and the molecular weight from 1.89 × 104 to 1.21 × 104 g/mol (runs 1–3, Table 5); the increased reactivity of an Al-Me over an Al-i-Bu towards BnOH may be a contributing factor. With a chloride present at the 2-position of the ligand, the conversion decreased rapidly as exemplified by C1 > C4 and C5 > C6. The reason for this observation is likely due to the electron withdrawing properties of a chloride resulting in decreased electron density at aluminum and in-turn slower reaction; steric factors could no doubt also be influential. In addition, the molecular weight of the PCLs generated by C5 and C6 (R2 = Me) are slightly lower than that seen with C1–C4 (R2 = H), suggesting the detrimental effect of the Cy-CMeO methyl group on chain propagation. Similar to C1, the MALDI-TOF mass spectra of the PCL generated by C2–C5 showed linear polymers capped with BnO groups as the unique polymer class. Likewise, the 1H NMR spectra of PCLs generated by C2–C5 also clearly showed signals characteristic of a BnO group (Figures S5–S9). Indeed, these results are consistent with the polymerizations proceeding by ‘coordination-insertion’ route with ‘LAl-OCH2Ph’ presumed as the active species. Unexpectedly, the structural analysis of the PCL obtained by C6/BnOH indicated that two polymer families were present, the major one being a linear structure capped with a PhCH2O group and the other a linear one capped with a methoxy group. These results suggest that the different R1 and R2 substituents have little effect on the polymer structure but have large effect on their reactivity.

Table 5.
The ROP of ε-CL promoted by C1–C6/BnOH a.
3.3. Mechanistic Analysis
On account of the differences in polymerization behavior between C1–C6 and its unsubstituted counterpart M (Chart 1), the mode of activation was subject to a new 1H and 27Al NMR spectroscopic investigation. In particular, toluene-d8 solutions of C1, C1/BnOH and C1/BnOH in the presence of 10 molar equivalents of ε-CL, were examined at various temperatures.
Firstly, the variable temperature 1H NMR experiment was performed on C1 alone; the stacked spectra are shown in Figure 7. The data reveal the signals for the ligand show some modest shift to lower field with increasing temperature. On the other hand, two separate singlet peaks (δ −0.11, −0.24) for the Al-Me’s at room temperature significantly shifted to lower field and became progressively broader on increasing the temperature. When the temperature reached 100 °C, two peaks effectively collapsed at ca. δ 0, which may be due to the onset of an exchange process at high temperature [46]. In the 27Al NMR spectrum of C1, one broad peak around δ 176.83 (a) was evident at room temperature. However, on raising the temperature from 25 to 100 °C, a second more upfield peak (b) (δ 127.9) gradually grew in intensity (Figure 8). Based on these findings, we presume that C1 exists mainly as a dimeric species at low temperature which is partly transformed into a monomeric species at higher temperature in a manner similar to that previously reported [37].
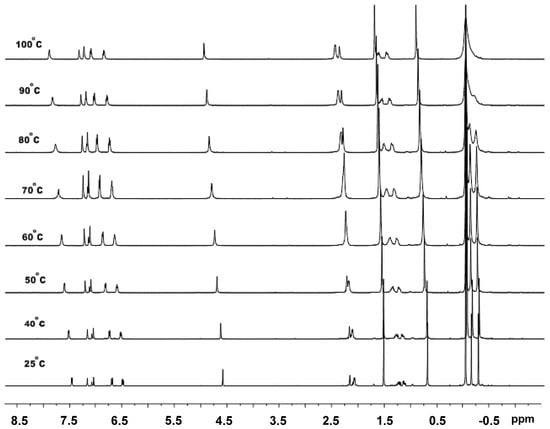
Figure 7.
VT-1H NMR spectra of C1 recorded between 25 and 100 °C (in toluene-d8).
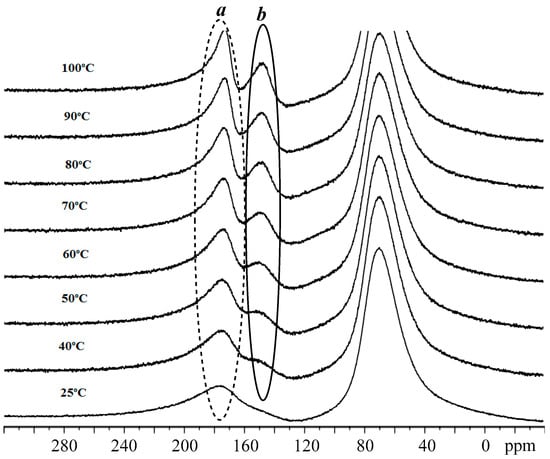
Figure 8.
VT-27Al NMR spectra of C1 recorded between 25 and 100 °C (in toluene-d8); the broad signal at δ 68.0 derives from the aluminum alloy present in the NMR probe.
We also monitored the variable temperature 1H and 27Al NMR spectra of a 1:1 mixture of C1 and BnOH; the corresponding spectra recorded between 25 and 100 °C are collected in Figure 9 and Figure 10. The 1H NMR spectrum of C1/BnOH shows multiple upfield peaks for the Al-Me resonances at room temperature which converged into two sharp peaks on raising the temperature. By contrast, there were no major shifts of the downfield Haryl protons over the temperature range. At room temperature, the coordinated benzyl alcohol methylene protons are inequivalent leading two mutually coupled doublets, this splitting pattern is maintained up to 70 °C above which the signals start to merge and broaden. Unexpectedly, the ratio of the PhCH2O:Al-CH3 protons is close to 2:6 at each temperature, which would appear to rule out a species of the type L1Al(OCH2Ph)(CH3). In the 27Al NMR spectra of C1/BnOH, two broad peaks were observed in the temperature range 25 to 80 °C. Further increasing the temperature to 90 °C led to apparent coalescence and then at 100 °C the formation of a single broad peak. By analogy with the NMR findings for C1 alone, we propose an equilibrating mixture of dimeric and monomeric species each coordinated by intact BnOH ligands that becomes time-averaged at high temperature.
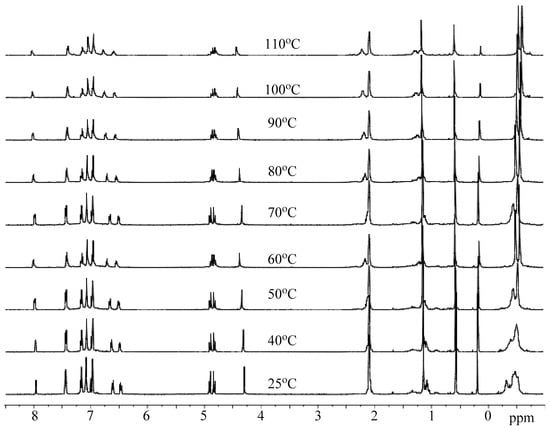
Figure 9.
VT-1H NMR spectra of C1/BnOH recorded between 25 and 110 °C (in toluene-d8).
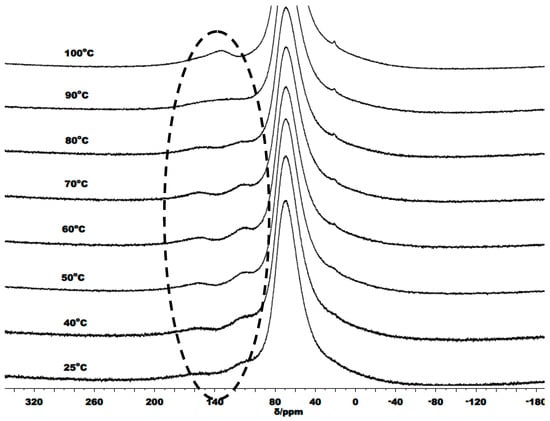
Figure 10.
VT-27Al NMR spectra of C1/BnOH recorded between 25 and 100 °C (in toluene-d8); the broad signal at δ 68.0 derives from the aluminum alloy present in the NMR probe.
In order to gain information about the catalyst when in the presence of monomer, the 1H and 27Al NMR spectra of C1/BnOH in the presence of 10 equivalents of ε-CL were also conducted over the 25 to 100 °C temperature range; the spectra are shown in Figure 11. In all the VT 1H NMR spectra the PhCH2O resonance appeared as a singlet (around δ 5.0), which is indicative of a PhCH2O end group in PCL. Hence, it is apparent that the polymerization occurred rapidly when the monomer was mixed with C1/BnOH even at room temperature. As the temperature was raised, the peaks for PCL (δ 3.94, 1.52) gradually increased as the resonance for ε-CL (δ 3.58) reduced, in accord with the higher polymerization rate at higher temperature. On the other hand, the VT-27Al NMR spectra /BnOH/10 ε-CL gave a single broad resonance at δ 69.0 across the temperature range that we tentatively assign to the active species; unfortunately, this chemical shift also coincides with the probe signal. Nevertheless, related aluminum complexes containing bound α-alkoxy esters, that have been considered as the active species in the ROP, have also shown resonances around δ 70 in their 27Al NMR spectra [13].
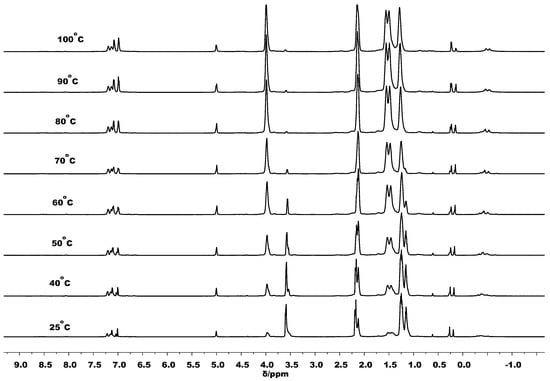
Figure 11.
VT-1H NMR spectra of C1/BnOH/10 ε-CL recorded at temperatures between 25 and 100 °C (in toluene-d8).
4. Conclusions
A series of soluble aluminum 5,6-dihydro-7,7-dimethylquinolin-8-olates, C1–C6, have been successfully prepared and fully characterized. The molecular structures of C1 and C4 indicate that they adopt dimeric forms in the solid state. In the absence of BnOH, C1 showed only low catalytic efficiency for the ROP of ε-CL with the spectroscopic and spectrometric data for the resulting polymer in agreement with a linear structure capped with either a methyl or a L1 group. By contrast, in the presence of BnOH, C1 exhibited excellent efficiency for the ROP of ε-CL with essentially 100% conversion in only 10 min at 90 °C. The chloro-substituted complexes C4 and C6 showed lower activity than that seen with C1–C3 and C5, highlighting the importance of both electronic and steric factors on initiator performance. All polymers displayed linear structures that were capped with PhCH2O groups, which was confirmed by 1H NMR spectroscopy and by MALDI-TOF mass spectrometry. The solution state properties of C1 and C1/BnOH over various temperatures have been investigated by multinuclear NMR spectroscopy and highlight the importance of both monomeric and dimeric species. In the presence of 10 molar equivalents of monomer, the active initiator generated from C1/BnOH, could be monitored by 27Al NMR spectroscopy while the chain propagation by 1H NMR spectroscopy.
Supplementary Materials
The following are available online at http://www.mdpi.com/2073-4360/10/7/764/s1, Figures S1–S4: The 1H NMR and MALDI-TOF spectrum of PCL obtained by C1 with different amount BnOH, Figures S5–S9: The MALDI-TOF spectrum of PCL obtained by C2–C6/BnOH.
Author Contributions
W.-H.S. and W.Z. conceived and designed the experiments; Q.Z. performed the experimental work and the analysis of structural data; T.L. took the single crystal determination. W.Z., W.-H.S. contributed regents/materials/analysis. W.Z., W.-H.S. and G.A.S. wrote the paper.
Acknowledgments
This research was funded by National Natural Science Foundation of China (Nos 51473170, 51273202 and U1362204). G.A.S. thanks the Chinese Academy of Sciences for a President’s International Fellowship for Visiting Scientists.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Reddy, M.M.; Vivekanandhan, S.; Misra, M.; Bhatia, S.K.; Mohanty, A.K. Biobased plastics and bionanocomposites: Current status and future opportunities. Prog. Polym. Sci. 2013, 38, 1653–1689. [Google Scholar] [CrossRef]
- Singhvi, M.; Gokhale, D. Biomass to biodegradable polymer (PLA). RSC Adv. 2013, 3, 13558–13568. [Google Scholar] [CrossRef]
- Rhim, J.-W.; Park, H.-M.; Ha, C.-S. Bio-nanocomposites for food packaging applications. Prog. Polym. Sci. 2013, 38, 1629–1652. [Google Scholar] [CrossRef]
- Armentano, I.; Bitinis, N.; Fortunati, E.; Mattioli, S.; Rescignano, N.; Verdejo, R.; Lopez-Manchado, M.A.; Kenny, J.M. Multifunctional nanostructured PLA materials for packaging and tissue engineering. Prog. Polym. Sci. 2013, 38, 1720–1747. [Google Scholar] [CrossRef]
- Terzopoulou, Z.; Baciu, D.; Gounari, E.; Steriotis, T.; Charalambopoulou, G.; Bikiaris, D. Biocompatible Nanobioglass Reinforced Poly(ɛ-Caprolactone) Composites Synthesized via In Situ Ring Opening Polymerization. Polymers 2018, 10, 381. [Google Scholar] [CrossRef]
- Wei, Y.; Wang, S.; Zhou, S. Aluminum alkyl complexes: Synthesis, structure, and application in ROP of cyclic esters. Dalton Trans. 2016, 45, 4471–4485. [Google Scholar] [CrossRef] [PubMed]
- Arbaoui, A.; Redshaw, C. Metal catalysts for ɛ-caprolactone polymerization. Polym. Chem. 2010, 1, 801–826. [Google Scholar] [CrossRef]
- Spassky, N.; Wisniewski, M.; Pluta, C.; Le Borgne, A. Highly stereoelective polymerization of rac-(d,l)-lactide with a chiral Schiff’s base/aluminium alkoxide initiator. Macromol. Chem. Phys. 1996, 197, 2627–2637. [Google Scholar] [CrossRef]
- Ovitt, T.M.; Coates, G.W. Stereoselective ring-opening polymerization of meso-Lactide: Synthesis of syndiotactic poly(lactic acid). J. Am. Chem. Soc. 1999, 121, 4072–4073. [Google Scholar] [CrossRef]
- Tang, Z.H.; Chen, X.S.; Pang, X.; Yang, Y.K.; Zhang, X.F.; Jing, X.B. Stereoselective polymerization of rac-lactide using a monoethylaluminum schiff base complex. Biomacromolecules 2004, 5, 965–970. [Google Scholar] [CrossRef] [PubMed]
- Radano, C.P.; Baker, G.L.; Smith, M.R. Stereoselective polymerization of a racemic monomer with a racemic catalyst: Direct preparation of the polylactic acid stereocomplex from racemic lactide. J. Am. Chem. Soc. 2000, 122, 1552–1553. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, Y.; Sun, W.-H.; Wang, L.; Redshaw, C. Dimethylaluminium aldiminophenolates: Synthesis, characterization and ring-opening polymerization behavior towards lactides. Dalton Trans. 2012, 41, 11587–11596. [Google Scholar] [CrossRef] [PubMed]
- Kan, C.; Ge, J.L.; Ma, H.Y. Aluminum methyl, alkoxide and α-alkoxy ester complexes supported by 6,6′-dimethylbiphenyl-bridged salen ligands: Synthesis, characterization and catalysis for rac-lactide polymerization. Dalton Trans. 2016, 45, 6682–6695. [Google Scholar] [CrossRef] [PubMed]
- Iwasa, N.; Fujiki, M.; Nomura, K. Ring-opening polymerization of various cyclic esters by Al complex catalysts containing a series of phenoxy-imine ligands: Effect of the imino substituents for the catalytic activity. J. Mol. Catal. A Chem. 2008, 292, 67–75. [Google Scholar] [CrossRef]
- Iwasa, N.; Katao, S.; Liu, J.Y.; Fujiki, M.; Furukawa, Y.; Nomura, K. Notable effect of fluoro substituents in the imino group in ring-opening polymerization of ε-caprolactone by Al complexes containing phenoxyimine ligands. Organometallics 2009, 28, 2179–2187. [Google Scholar] [CrossRef]
- Meduri, A.; Fuoco, T.; Lamberti, M.; Pellecchia, C.; Pappalardo, D. Versatile copolymerization of glycolide and rac-lactide by dimethyl(salicylaldiminato)aluminum compounds. Macromolecules 2014, 47, 534–543. [Google Scholar] [CrossRef]
- García-Valle, F.M.; Tabernero, V.; Tomas, C.; Mosquera, M.E.G.; Cano, J.; Milione, S. Biodegradable PHB from rac-β butyrolactone: Highly controlled ROP mediated by a pentacoordinated aluminum complex. Organometallics 2018, 37, 837–840. [Google Scholar] [CrossRef]
- Shi, T.; Luo, W.L.; Liu, S.F.; Li, Z.B. Controlled random copolymerization of rac-lactide and caprolactone by well-designed phenoxyimine Al complexes. J. Polym. Sci. Part A Polym. Chem. 2018, 56, 611–617. [Google Scholar] [CrossRef]
- Shi, T.; Zheng, Q.; Zuo, W.; Liu, S.; Li, Z. Bimetallic aluminum complexes supported by bis(salicylaldimine) ligand: Synthesis, characterization and ring-opening polymerization of lactide. Chin. J. Polym. Sci. 2018, 36, 149–156. [Google Scholar] [CrossRef]
- Maisse-François, A.; Azora, L.; Schmitta, A.-L.; Coquela, A.; Brelotc, L.; Welter, R.; Bellemin-Laponnaz, S.; Dagorne, S. Structural diversity and versatility for organoaluminum complexes supported by mono- and di-anionic aminophenolate bidentate ligands. J. Organomet. Chem. 2012, 696, 4248–4256. [Google Scholar] [CrossRef]
- Wang, Y.; Ma, H.Y. Aluminum complexes of bidentate phenoxy-amine ligands: Synthesis, characterization and catalysis in ring-opening polymerization of cyclic esters. J. Organomet. Chem. 2013, 731, 23–28. [Google Scholar] [CrossRef]
- Chen, L.; Li, W.; Yuan, D.; Zhang, Y.; Shen, Q.; Yao, Y. Syntheses of Mononuclear and Dinuclear Aluminum Complexes Stabilized by Phenolato Ligands and Their Applications in the Polymerization of ε Caprolactone: A Comparative Study. Inorg. Chem. 2015, 54, 4699–4708. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Ouyang, H.; Chen, L.J.; Yuan, D.; Zhang, Y.; Yao, Y. A Comparative Study on Dinuclear and Mononuclear Aluminum Methyl Complexes Bearing Piperidyl–Phenolato Ligands in ROP of Epoxides. Inorg. Chem. 2016, 55, 6520–6524. [Google Scholar] [CrossRef] [PubMed]
- Roymuhury, S.K.; Chakraborty, D.; Ramkumar, V. Aluminium complexes bearing N,O-aminophenol ligands as efficient catalysts for the ring opening polymerization of lactide. Eur. Polym. J. 2015, 70, 203–214. [Google Scholar] [CrossRef]
- Zhang, W.; Wang, Y.; Wang, L.; Redshaw, C.; Sun, W.-H. Dialkylaluminium 2-imidazolylphenolates: Synthesis, characterization and ring-opening polymerization behavior towards lactides. J. Organomet. Chem. 2014, 750, 65–73. [Google Scholar] [CrossRef]
- Sumrit, P.; Chuawong, P.; Nanok, T.; Duangthongyou, T.; Hormnirun, P. Aluminum complexes containing salicylbenzoxazole ligands and their application in the ring-opening polymerization of rac-lactide and ε-caprolactone. Dalton Trans. 2016, 45, 9250–9266. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Dong, W.S.; Liu, J.Y.; Li, Y.S. Living ring-opening homo- and copolymerisation of ε-caprolactone and L-lactide by cyclic β-ketiminato aluminium complexes. Dalton Trans. 2014, 43, 2244–2251. [Google Scholar] [CrossRef] [PubMed]
- Bouyahyi, M.; Roisnel, T.; Carpentier, J.-F. Aluminum Complexes of Bidentate Fluorinated Alkoxy-Imino Ligands: Syntheses, Structures, and Use in Ring-Opening Polymerization of Cyclic Esters. Organometallics 2012, 31, 1458–1466. [Google Scholar] [CrossRef]
- Liang, L.-C.; Chen, F.-Y.; Huang, M.-H.; Cheng, L.-C.; Li, C.-W.; Lee, H.M. Aluminium complexes of bidentate N,O- and N,N-ligands derived from oxidative functionalization of amido phosphines: Synthesis, structure and reactivity. Dalton Trans. 2010, 39, 9941–9951. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.-L.; Lin, Y.-F.; Jiang, M.-T.; Lu, W.-Y.; Vandavasi, J.K.; Wang, L.-F.; Lai, Y.-C.; Chiang, M.Y.; Chen, H.-Y. Improvement in aluminum complexes bearing schiff bases in ring opening polymerization of ε caprolactone: A five-membered-ring system. Organometallics 2017, 36, 1936–1945. [Google Scholar] [CrossRef]
- Francis, J.A.; Bott, S.G.; Barron, A.R. Aluminium compounds containing bidentate ligands: Chelate ring size and rigid conformation effects. J. Chem. Soc. Dalton Trans. 1998, 3305–3310. [Google Scholar] [CrossRef]
- Francis, J.A.; Bott, S.G.; Barron, A.R. Sterically crowded aryloxides of aluminum: Intramolecular coordination of bidentate ligands. J. Organomet. Chem. 2000, 597, 29–37. [Google Scholar] [CrossRef]
- Galvez-Ruiz, J.C.; Noth, H.; Flores-Parra, A. Organometallic Aluminum Compounds Derived from 2-(1,3,5-Dithiazinan-5-yl)ethanol Ligands. Inorg. Chem. 2003, 42, 7569–7578. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Han, S.Y.; Kim, J.H.; Kang, Y.Y.; Lee, J.; Kim, Y. Monomeric or dimeric aluminum complexes as catalysts for cycloaddition between CO2 and epoxides. Eur. J. Inorg. Chem. 2015, 2323–2329. [Google Scholar] [CrossRef]
- Li, M.; Chen, M.; Chen, C.L. Ring-opening polymerization of rac-lactide using anilinotroponebased aluminum complexes-sidearm effect on the catalysis. Polymer 2015, 64, 234–239. [Google Scholar] [CrossRef]
- Sun, W.-H.; Shen, M.; Zhang, W.J.; Huang, W.; Liu, S.F.; Redshaw, C. Methylaluminium 8-quinolinolates: Synthesis, characterization and use in ring-opening polymerization (ROP) of ε-caprolactone. Dalton Trans. 2011, 40, 2645–2653. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Zhang, W.; Rajendran, N.; Liang, T.; Sun, W.-H. Thermo-enhanced ring-opening polymerization of ε-caprolactone: The synthesis, characterization, and catalytic behavior of aluminum hydroquinolin-8-olates. Dalton Trans. 2017, 46, 7833–7843. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal-structure determination. Acta Crystallogr. Sect. A Found. Adv. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Save, M.; Schappacher, M.; Soum, A. Controlled Ring-Opening Polymerization of Lactones and Lactides Initiated by Lanthanum Isopropoxide, General Aspects and Kinetics. Macromol. Chem. Phys. 2002, 203, 889–899. [Google Scholar] [CrossRef]
- Tseng, H.-C.; Chiang, M.Y.; Lu, W.-Y.; Chen, Y.-J.; Lian, C.-J.; Chen, Y.-H.; Tsai, H.-Y.; Lai, Y.-C.; Chen, H.-Y. A closer look at ε-caprolactone polymerization catalyzed by alkyl aluminum complexes: The effect of induction period on overall catalytic activity. Dalton Trans. 2015, 44, 11763–11773. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, D.; Chen, E.Y.-X. Neutral, three-coordinate, chelating diamide aluminum complexes: Catalysts/initiators for synthesis of telechelic oligomers and high polymers. Organometallics 2002, 21, 1438–1442. [Google Scholar] [CrossRef]
- Yu, R.-C.; Hung, C.-H.; Huang, J.-H.; Lee, H.-Y.; Chen, J.-T. Four- and five-Coordinate aluminum ketiminate complexes: Synthesis, characterization, and ring-opening polymerization. Inorg. Chem. 2002, 41, 6450–6455. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Wang, S.; Zhu, X.; Zhou, S.; Mu, X.; Huang, Z.; Hong, D. Aluminum Complexes Bearing N-Protected 2-Amino- or 2-Imino-Functionalized Pyrrolyl Ligands: Synthesis, Structure, and Catalysis for Preparation of Pyrrolyl-End-Functionalized Polyesters. Organometallics 2016, 35, 2621–2629. [Google Scholar] [CrossRef]
- Li, H.; Debuigne, A.; Jrome, R.; Lecomte, P. Synthesis of Macrocyclic poly(ɛ-caprolactone) by intramolecular cross-Linking of unsaturated end groups of chains precyclic by the initiation. Angew. Chem. Int. Ed. 2006, 45, 2264–2267. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Sierra, M.L.; Oliver, J.P. Synthesis and Spectroscopic Studies of Alkylaluminum Alkoxides Derived from Optically Active Alcohols. Crystal and Molecular Structure of Monomeric Dimethylaluminum (2S,3R)-(+)-4-(Dimethylamino)-l,2-diphenyl-3-methyl-2-butanoxide. Organometallics 1994, 13, 4285–4293. [Google Scholar] [CrossRef]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).