Solvent-Mediated Polymorphic Transformation of Famoxadone from Form II to Form I in Several Mixed Solvent Systems
Abstract
1. Introduction
2. Experimental Section
3. Results and Discussion
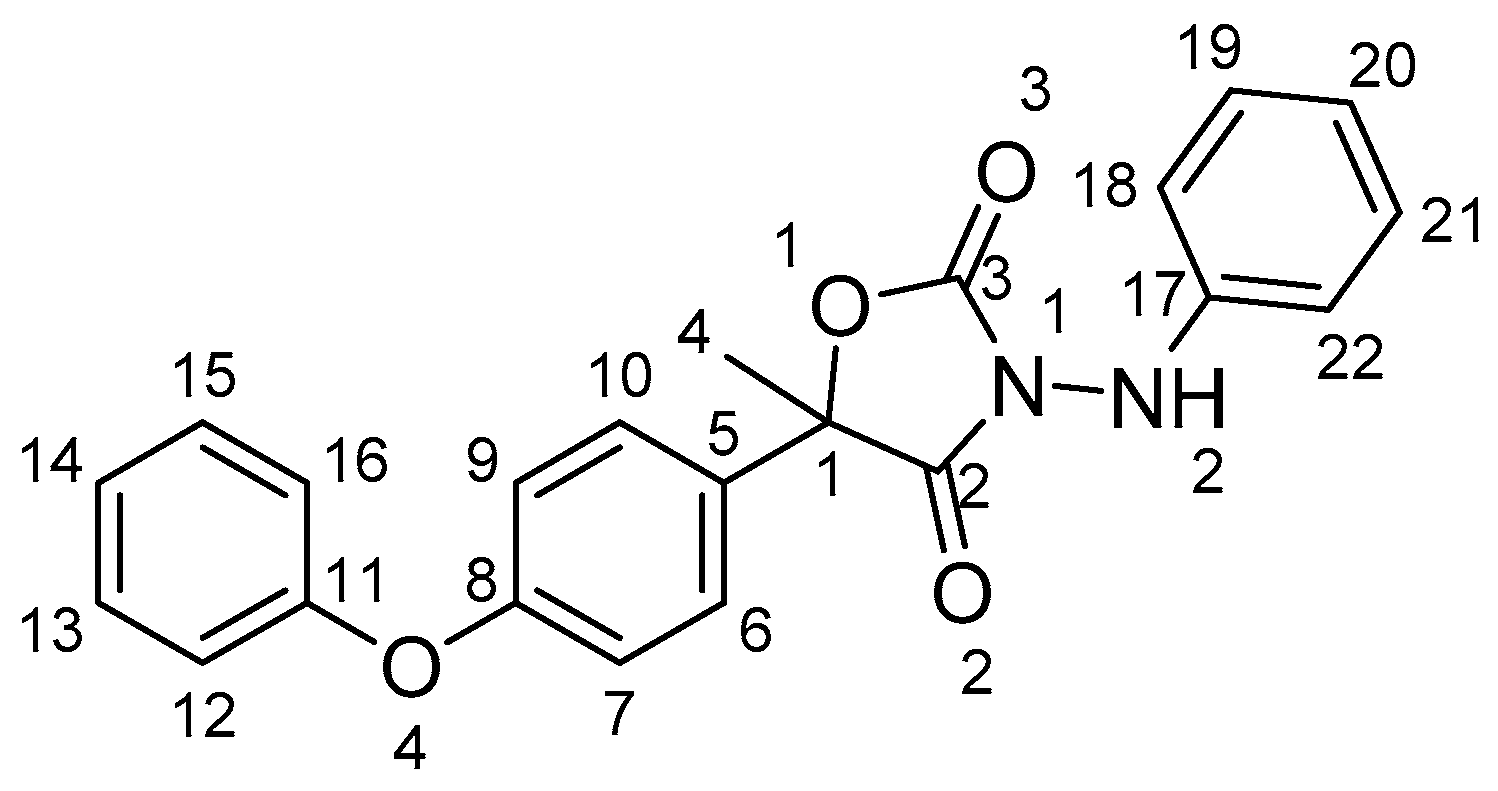
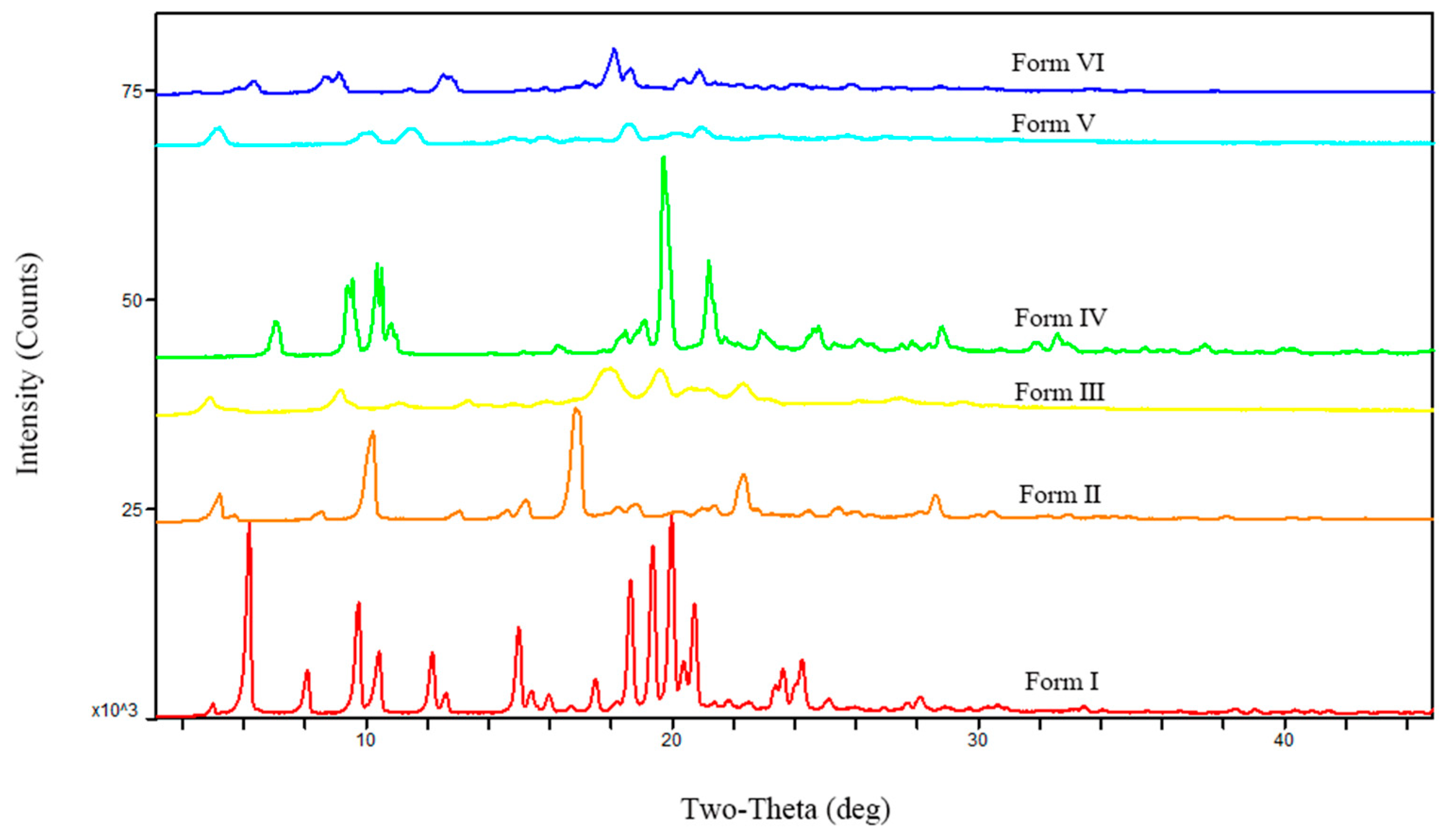
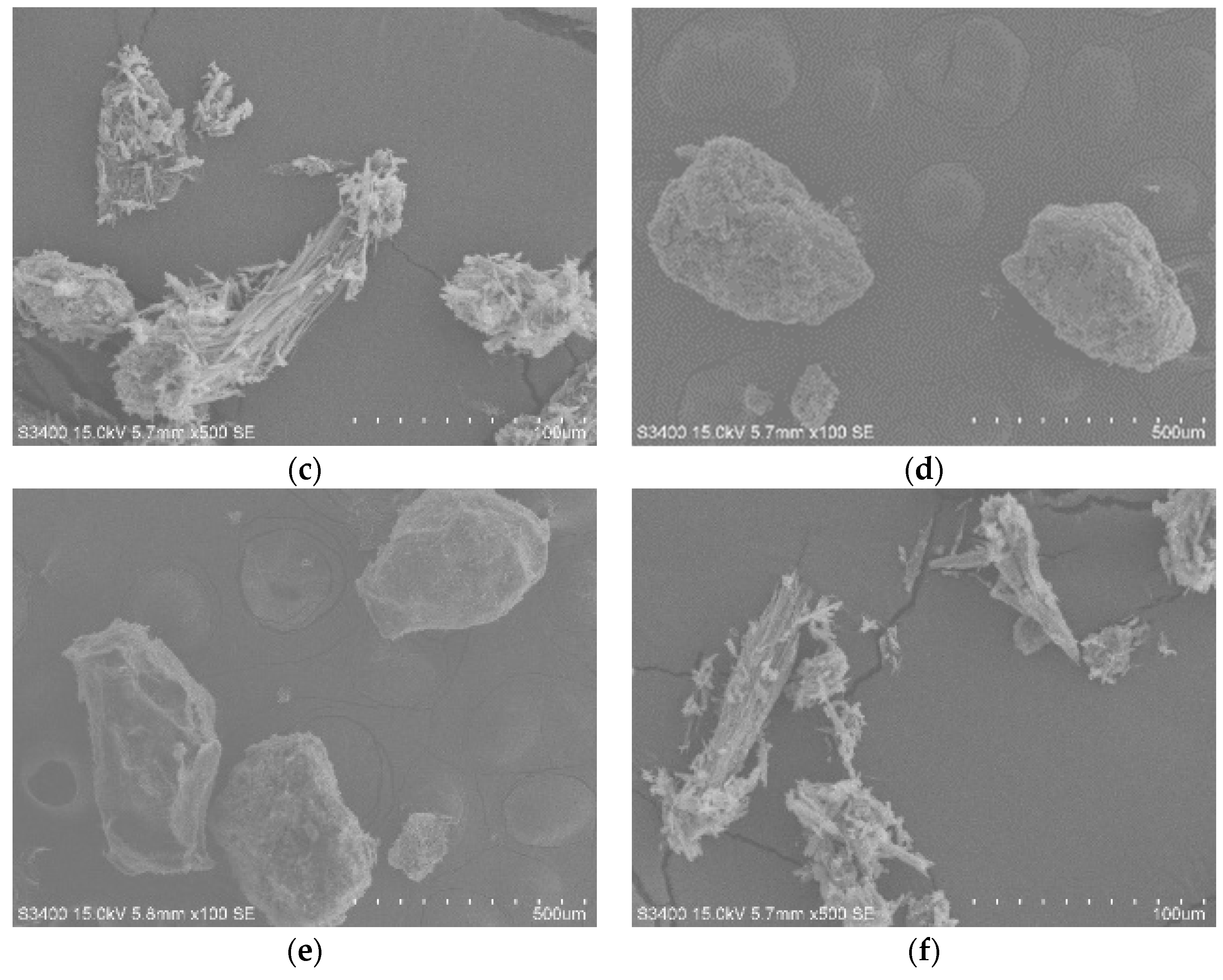
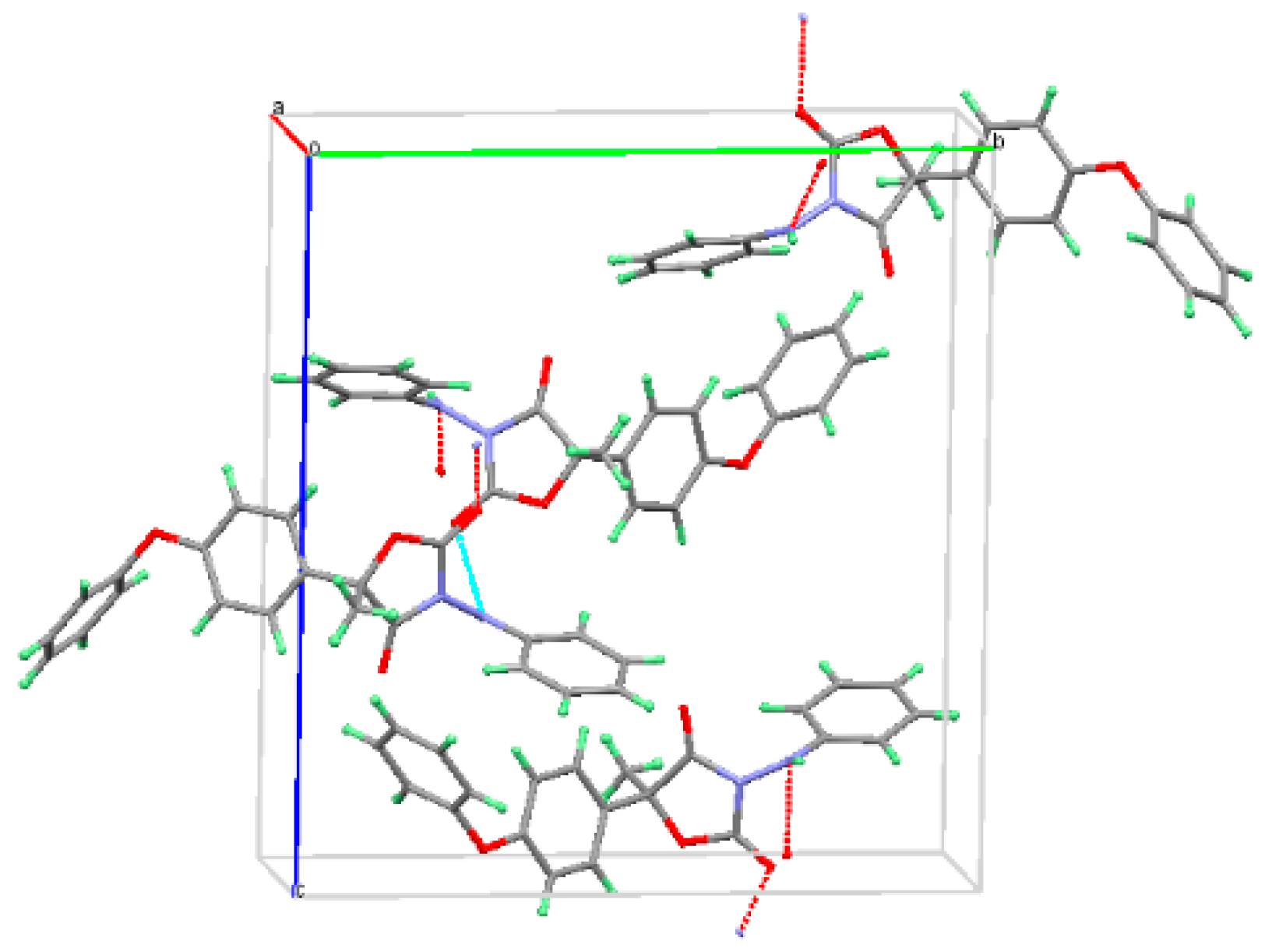
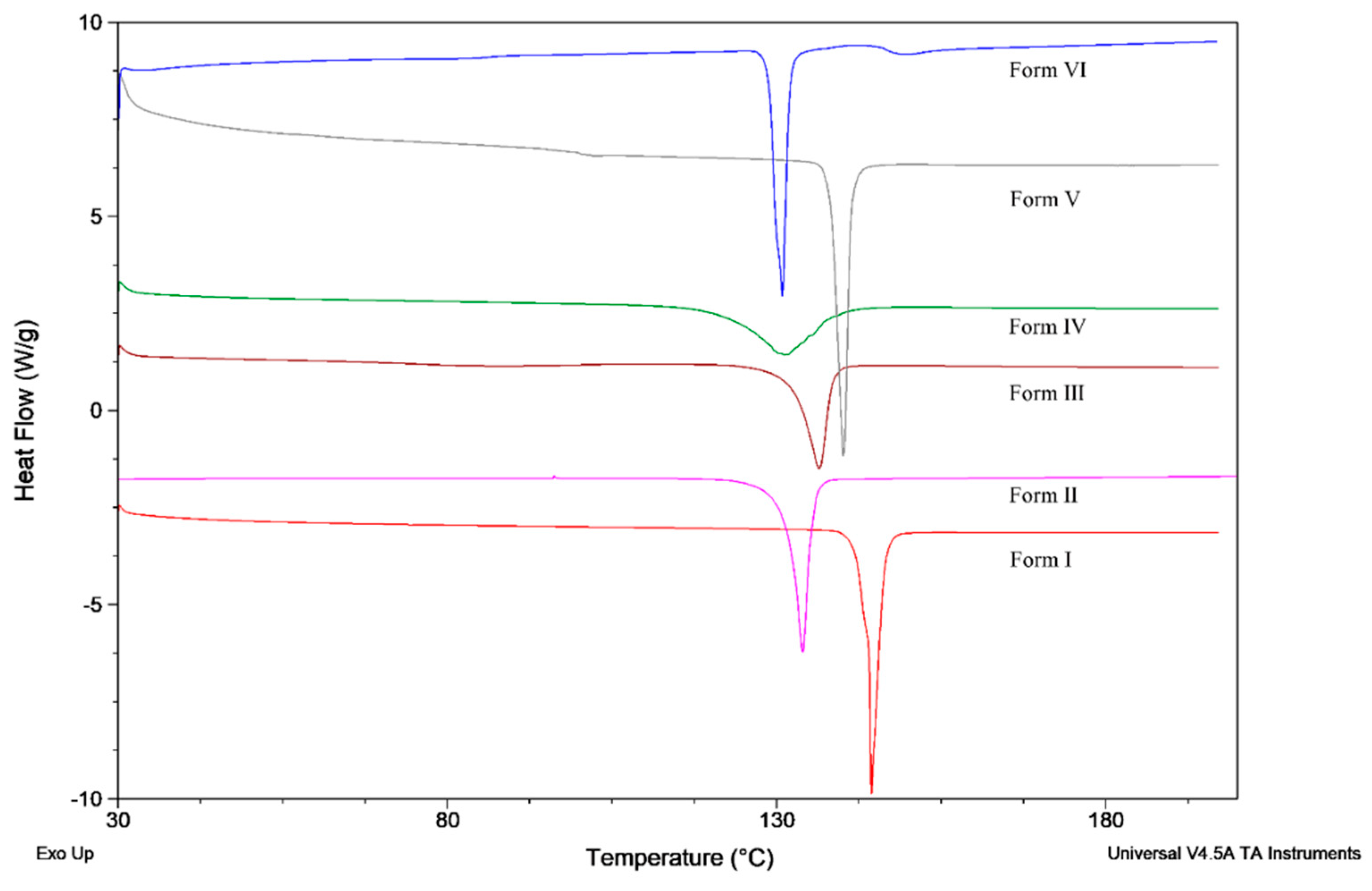
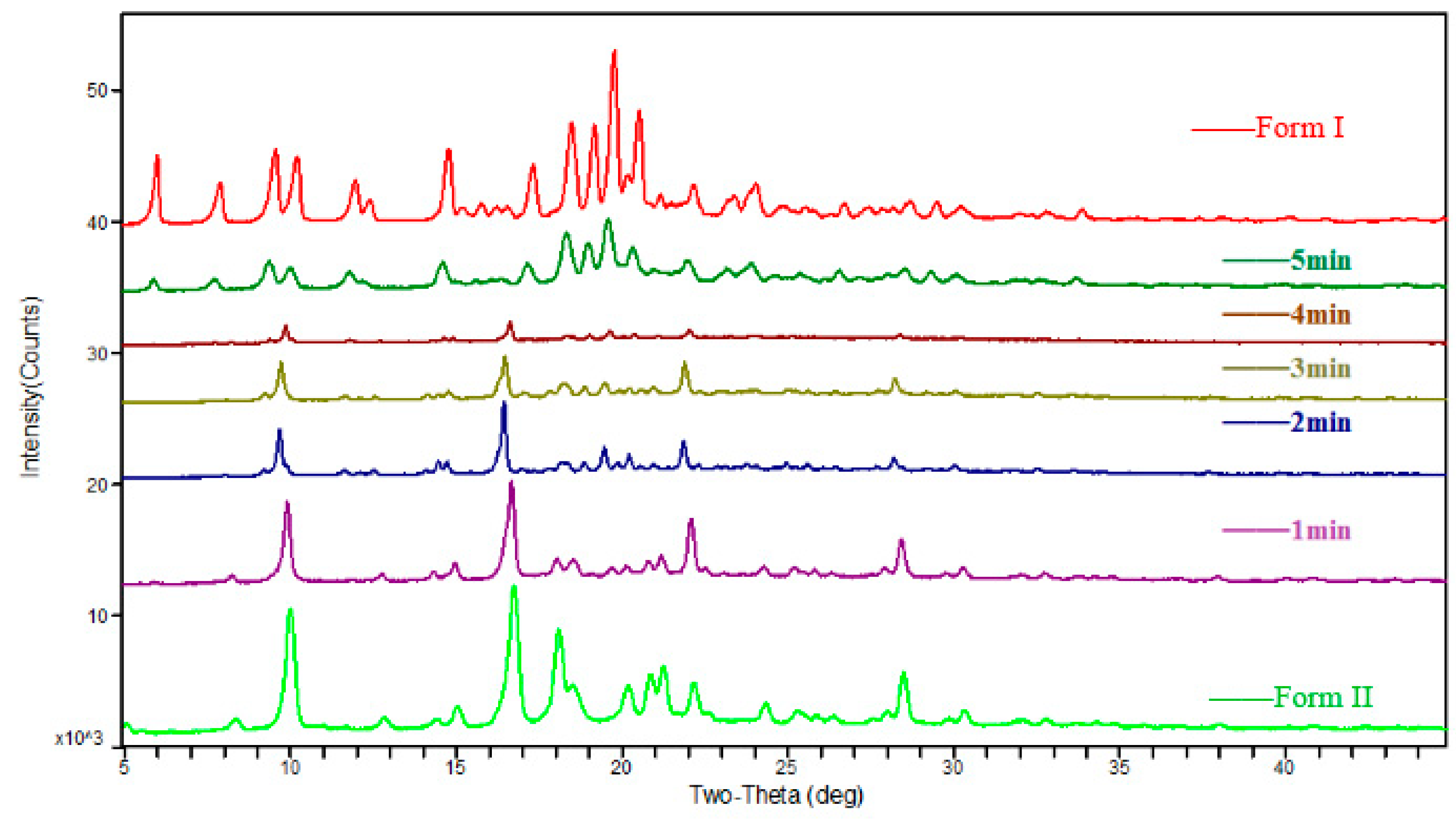
3.1. The Characterization of Starting Materials
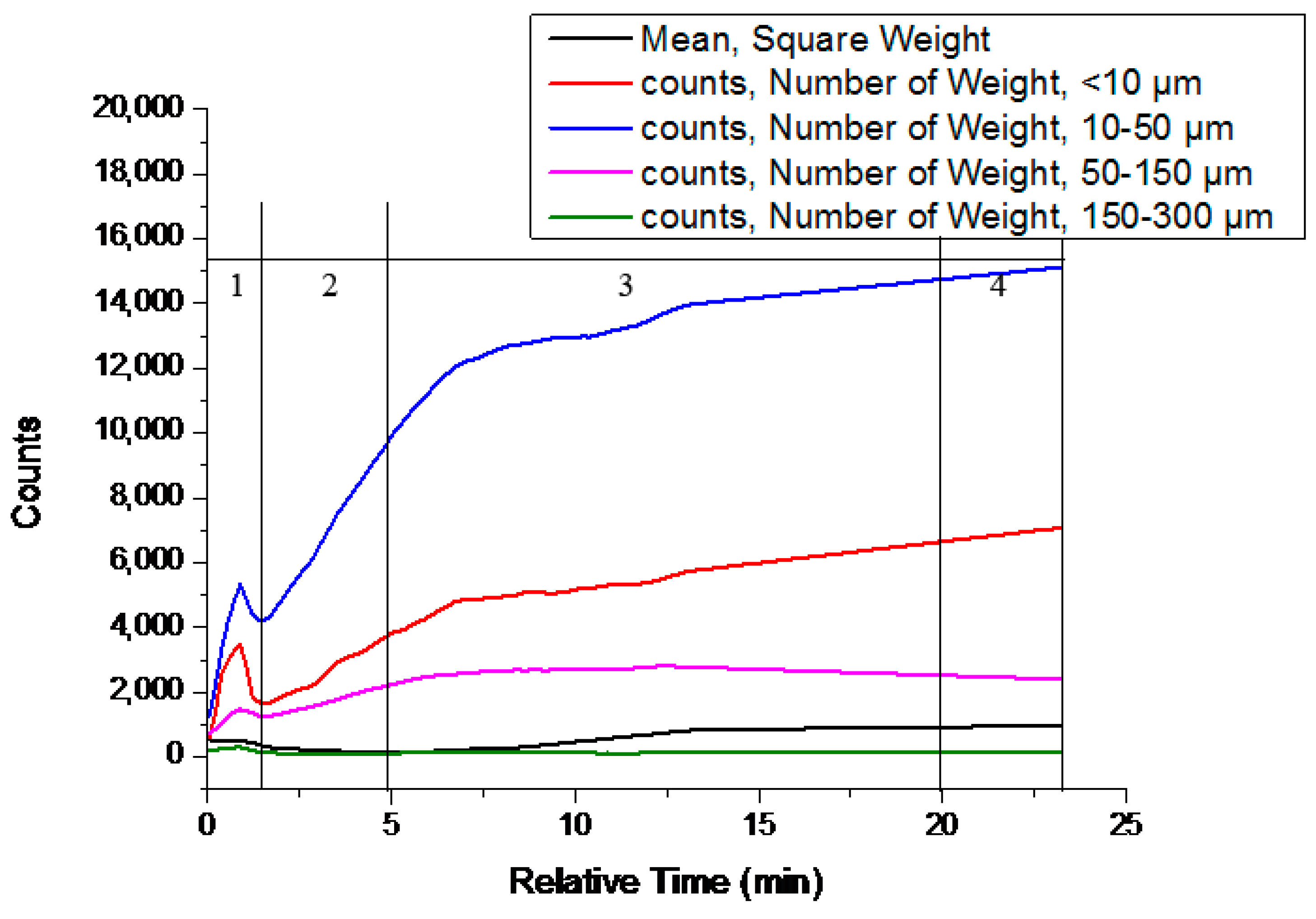
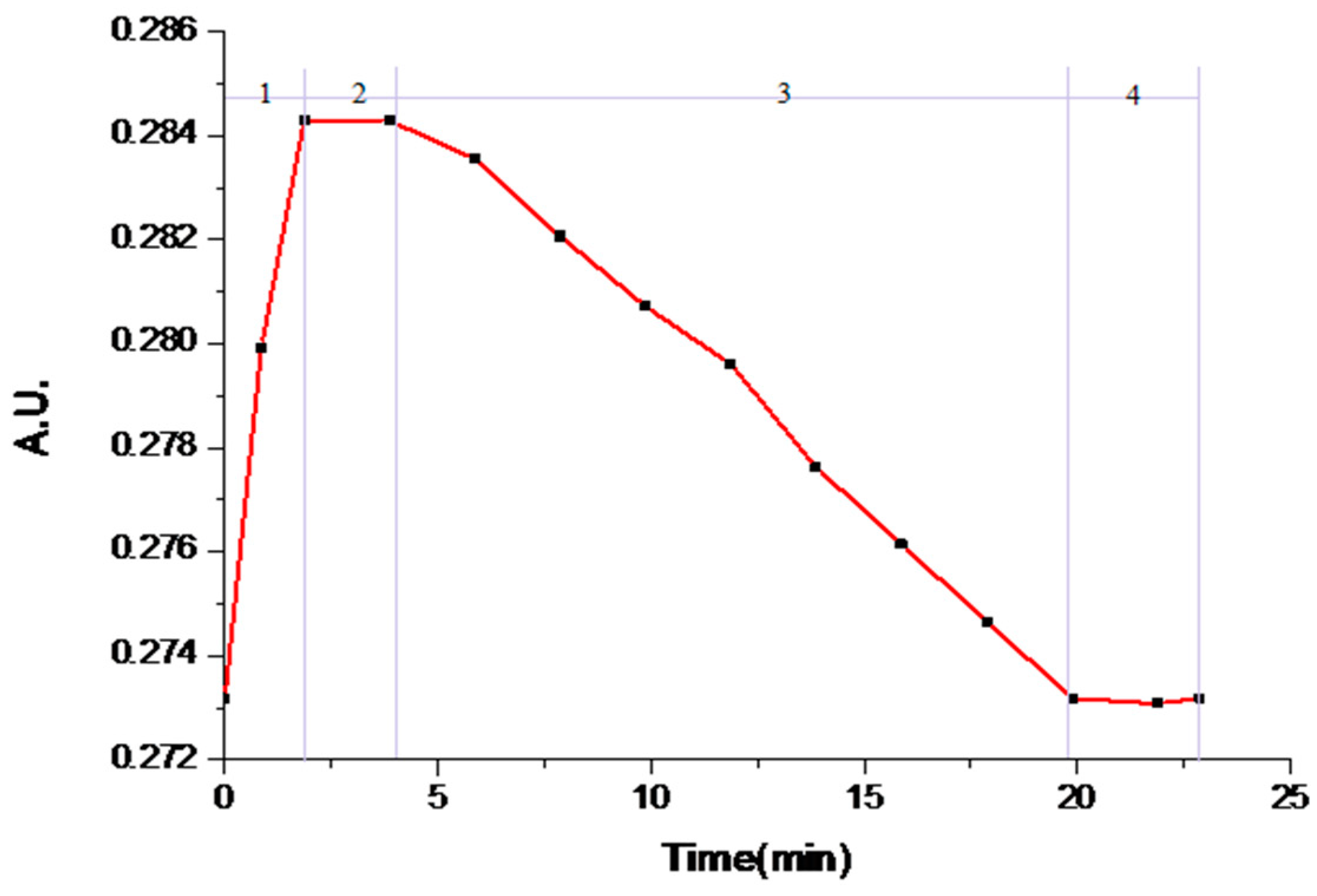
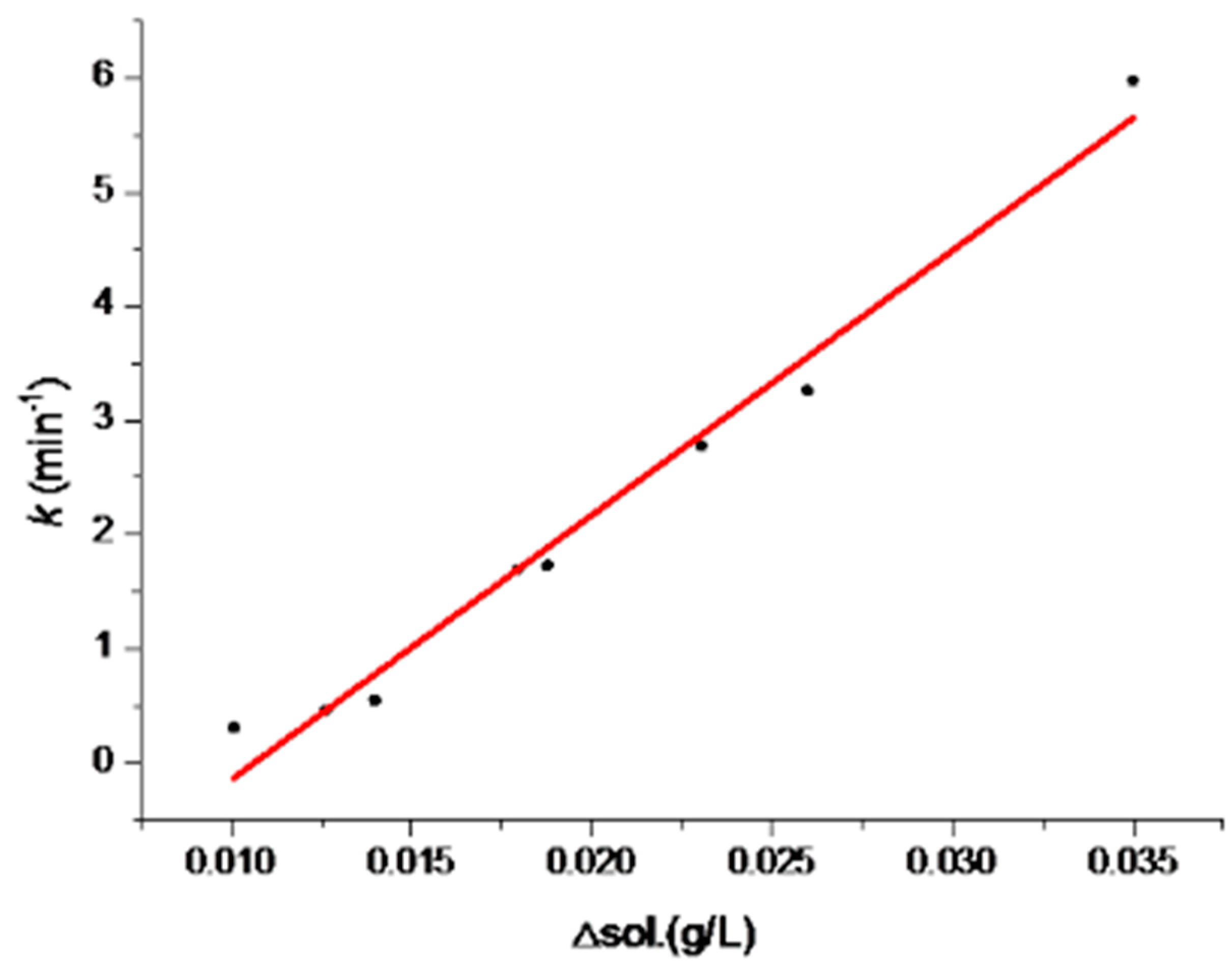
3.2. Solvent-Mediated Polymorphic Transformation Experiments
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Maciej, P.; Piotr, C.; Maciej, P.; Dorota, Z.; Miroslaw, K. On the origin of surface imposed anisotropic growth of salicylic and acetylsalicylic acids crystals during droplet evaporation. J. Mol. Model. 2015, 21, 49. [Google Scholar]
- Gagniere, E.; Mangin, D.; Puel, F. Formation of co-crystals: Kinetic and thermodynamic aspects. J. Cryst. Growth 2009, 311, 2689–2695. [Google Scholar] [CrossRef]
- Cysewski, P.; Przybyłek, M.; Miernik, T.; Kobierski, M.; Ziółkowska, D. On the origin of surfaces-dependent growth of benzoic acid crystal inferred through the droplet evaporation method. Struct. Chem. 2015, 26, 705–712. [Google Scholar] [CrossRef]
- Terry, T. Crystallisation of Polymorphs: Thermodynamic Insight into the Role of Solvent. Org. Process Res. Dev. 2000, 4, 384–390. [Google Scholar]
- Bhugra, C.; Pikal, M.J. Role of thermodynamic, molecular, and kinetic factors in crystallization from the amorphous state. J. Pharm. Sci. 2008, 97, 1329–1349. [Google Scholar] [CrossRef] [PubMed]
- Clavaguera, N.; Clavagueramora, M.T.; Crespo, D.; Pradell, T. Thermodynamic and kinetic factors driving primary crystallization. In Proceedings of the Fifth International Workshop on Non-Crystalline Solids, Santiago de Compostela, Spain, 2–5 July 2015; pp. 272–277. [Google Scholar]
- Stoica, C.; Tinnemans, P.; Meekes, H.; Vlieg, E.; Hoof, P.J.C.M.V.; Kaspersen, F.M. Epitaxial 2D Nucleation of Metastable Polymorphs: A 2D Version of Ostwald’s Rule of Stages. Cryst. Growth Des. 2005, 5, 975–981. [Google Scholar] [CrossRef]
- Chen, C.; Cook, O.; Nicholson, C.E.; Cooper, S.J. Leapfrogging Ostwald’s Rule of Stages: Crystallization of Stable γ-Glycine Directly from Microemulsions. Cryst. Growth Des. 2011, 11, 2228–2237. [Google Scholar] [CrossRef]
- Murphy, D.; Rodrı́Guez-Cintrón, F.; Langevin, B.; Kelly, R.C.; Rodrı́Guez-Hornedo, N. Solution-mediated phase transformation of anhydrous to dihydrate carbamazepine and the effect of lattice disorder. Int. J. Pharm. 2002, 246, 121. [Google Scholar] [CrossRef]
- Nguyen, D.L.T.; Kim, K.J. Solvent-Mediated Polymorphic Transformation of α-Taltirelin by Seeded Crystallization. Chem. Eng. Technol. 2016, 39, 1281–1288. [Google Scholar] [CrossRef]
- Liu, Y.; Gao, H.; Xu, H.; Ren, F.; Ren, G. Influence of Temperature, Solvents, and Excipients on Crystal Transformation of Agomelatine. Org. Process Res. Dev. 2016, 20, 1559–1565. [Google Scholar] [CrossRef]
- Yang, L.; Hao, H.; Zhou, L.; Wei, C.; Hou, B.; Xie, C.; Yin, Q. Crystal Structures and Solvent-Mediated Transformation of the Enantiotropic Polymorphs of 2,3,5-Trimethyl-1,4-diacetoxybenzene. Ind. Eng. Chem. Res. 2013, 52, 17667–17675. [Google Scholar] [CrossRef]
- Gu, C.H.; Young, V.; Grant, D.J.W. Polymorph screening: Influence of solvents on the rate of solvent-mediated polymorphic transformation. J. Pharm. Sci. 2001, 90, 1878–1890. [Google Scholar] [CrossRef] [PubMed]
- Schöll, J.; Bonalumi, D.; Lars Vicum, A.; Mazzotti, M.; Müller, M. In Situ Monitoring and Modeling of the Solvent-Mediated Polymorphic Transformation of l-Glutamic Acid. Cryst. Growth Des. 2006, 6, 881–891. [Google Scholar] [CrossRef]
- Sternberg, J.A.; Geffken, D.; Adams, J.B.; Pöstages, R.; Sternberg, C.G.; Campbell, C.L.; Moberg, W.K. Famoxadone: The discovery and optimisation of a new agricultural fungicide. Pest Manag. Sci. 2001, 57, 143–152. [Google Scholar] [CrossRef]
- Zheng, Y.-J.; Shapiro, R.; Marshall, W.J.; Jordan, D.B. Synthesis and structural analysis of the active enantiomer of famoxadone, a potent inhibitor of cytochrome bc1. Bioorg. Med. Chem. Lett. 2000, 10, 1059–1062. [Google Scholar] [CrossRef]
- Macaluso, R.T.; Wells, B.; Wangeline, C.; Cochran, K.; Greve, B.K. Powder X-ray diffraction and electron microscopy studies of polycrystalline Au2PrIn. Polyhedron 2016, 114, 313–316. [Google Scholar] [CrossRef]
- Oishi, R.; Yonemura, M.; Nishimaki, Y.; Torii, S.; Hoshikawa, A.; Ishigaki, T. Rietveld analysis software for j-parc. Nucl. Instrum. Methods Phys. Res. 2009, 600, 94–96. [Google Scholar] [CrossRef]
- Fedushkin, I.L.; Nevodchikov, V.I.; Bochkarev, M.N.; Dechert, S.; Schumann, H. Reduction of 2,5-di-tert-butylcyclopentadienone and pyridine with thulium diiodide. Structures of the complexes TmI2(THF)2[η5-But2C5H2O]TmI2(THF)3 and [TmI2(C5H5N)4]2(μ2-N2C10H10). Russ. Chem. Bull. 2003, 52, 154–159. [Google Scholar] [CrossRef]
- Beghidja, C.; Rogez, G.; Kortus, J.; Wesolek, M.; Welter, R. Very strong ferromagnetic interaction in a new binuclear mu-methoxo-bridged Mn(III) complex: Synthesis, crystal structure, magnetic properties, and DFT calculations. J. Am. Chem. Soc. 2006, 128, 3140–3141. [Google Scholar] [CrossRef]
- Powell, D.R. Review of X-Ray Crystallography. In X-Ray Crystallography; Girolami, G.S., Ed.; University Science Books: Mill Valley, CA, USA, 2015; 300p, ISBN 9781891389771. (hardcover). [Google Scholar]
- Dieterich, M.; Kutchko, B.; Goodman, A. Characterization of Marcellus Shale and Huntersville Chert before and after exposure to hydraulic fracturing fluid via feature relocation using field-emission scanning electron microscopy. Fuel 2016, 182, 227–235. [Google Scholar] [CrossRef]
- Xu, X.; Abhay, G.; Sayeed, V.A.; Khan, M.A. Process analytical technology to understand the disintegration behavior of alendronate sodium tablets. J. Pharm. Sci. 2013, 102, 1513–1523. [Google Scholar] [CrossRef]
- Kennemur, J.G.; Desousa, J.D.; Martin, J.D.; Novak, B.M. Reassessing the Regioregularity of N-(1-Naphthyl)-N′-(n-octadecyl)polycarbodiimide Using Solution Infrared Spectroscopy. Macromolecules 2015, 44, 5064–5067. [Google Scholar] [CrossRef]
- Costa, I.C.R.; Leite, S.G.F.; Leal, I.C.R.; Miranda, L.S.M.; Souza, R.O.M.A.D. Thermal effect on the microwave assisted biodiesel synthesis catalyzed by lipases. J. Braz. Chem. Soc. 2011, 22, 1993–1998. [Google Scholar] [CrossRef]
- Hamilton, D.L.; Oxtoby, S. Solubility of Water in Albite-Melt Determined by the Weight-Loss Method. J. Geol. 1986, 94, 626–630. [Google Scholar] [CrossRef]
- Chen, J.; Sarma, B.; Evans, J.M.B.; Myerson, A.S. Pharmaceutical Crystallization. Cryst. Growth Des. 2011, 11, 887–895. [Google Scholar] [CrossRef]
- Näther, C.; Jess, I.; Seyfarth, L.; Bärwinkel, K.; Senker, J. Trimorphism of Betamethasone Valerate: Preparation, Crystal Structures, and Thermodynamic Relations. Cryst. Growth Des. 2015, 15, 366–373. [Google Scholar] [CrossRef]
- Cardew, P.T.; Davey, R.J. The Kinetics of Solvent-Mediated Phase Transformations. Proc. R. Soc. Lond. 1985, 398, 415–428. [Google Scholar] [CrossRef]
- Skrdla, P.J.; Robertson, R.T. Dispersive kinetic models for isothermal solid-state conversions and their application to the thermal decomposition of oxacillin. Thermochim. Acta 2007, 453, 14–20. [Google Scholar] [CrossRef]
- Liu, W.; Dang, L.; Wei, H. Thermal, phase transition, and thermal kinetics studies of carbamazepine. J. Therm. Anal. Calorim. 2013, 111, 1999–2004. [Google Scholar] [CrossRef]
- Skrdla, P.J. Crystallizations, solid-state phase transformations and dissolution behavior explained by dispersive kinetic models based on a Maxwell-Boltzmann distribution of activation energies: Theory, applications, and practical limitations. J. Phys. Chem. A 2009, 113, 9329. [Google Scholar] [CrossRef]
- Liu, M.; Pourquie, M.J.B.M.; Fan, L.; Halliop, W.; Cobas, V.R.M.; Verkooijen, A.H.M.; Aravind, P.V. The Use of Methane-Containing Syngas in a Solid Oxide Fuel Cell: A Comparison of Kinetic Models and a Performance Evaluation. Fuel Cells 2013, 13, 428–440. [Google Scholar] [CrossRef]
- Takane, Y.; Young, F.W.; Leeuw, J.D. Nonmetric individual differences multidimensional scaling: An alternating least squares method with optimal scaling features. Psychometrika 1977, 42, 7–67. [Google Scholar] [CrossRef]
- Khoshkhoo, S.; Anwar, J. Crystallization of polymorphs: The effect of solvent. J. Phys. D Appl. Phys. 1993, 26, B90. [Google Scholar] [CrossRef]
- Blagden, N.; Davey, R.J.; Lieberman, H.F.; Williams, L.; Payne, R.; Roberts, R.; Rowe, R.; Docherty, R. Crystal chemistry and solvent effects in polymorphic systems Sulfathiazole. J. Chem. Soc. Faraday Trans. 1998, 94, 1035–1044. [Google Scholar] [CrossRef]
- Gidalevitz, D.; Feidenhans’L, R.; Matlis, S.; Smilgies, D.M.; Christensen, M.J.; Leiserowitz, L. Monitoring in situ growth and dissolution of molecular crystals: Towards determination of the growth unit. Angew. Chem. Int. Ed. Engl. 1997, 36, 955–959. [Google Scholar] [CrossRef]
- Marcus, Y. ChemInform Abstract: The Properties of Organic Liquids that are Relevant to Their Use as Solvating Solvents. Cheminform 1994, 25, 409–416. [Google Scholar] [CrossRef]
- A braham, M.H. ChemInform Abstract: Scales of Solute Hydrogen-Bonding: Their Construction and Application to Physicochemical and Biochemical Processes; National Academy of Sciences-National Research Council: Washington, DC, USA, 1993; pp. 277–283. [Google Scholar]
- Heijnen, J.J.; Riet, K.V. Mass transfer, mixing and heat transfer phenomena in low viscosity bubble column reactors. Chem. Eng. J. 1984, 28, B21–B42. [Google Scholar] [CrossRef]
- Aimar, P.; Field, R. Limiting flux in membrane separations: A model based on the viscosity dependency of the mass transfer coefficient. Chem. Eng. Sci. 1992, 47, 579–586. [Google Scholar] [CrossRef]

| Polymorph | Form I |
|---|---|
| Crystal system | Orthorhombic |
| Space group | P212121 |
| a (Å) | 5.8880 (5) |
| b (Å) | 16.9635 (16) |
| c (Å) | 18.4218 (17) |
| α (°) | 90 |
| β (°) | 90 |
| γ (°) | 90 |
| Volume (Å3) | 1840.0(3) |
| Z | 4 |
| CCDC No. | 1560380 |
| Temperature | 130 K |
| Crystal size | 0.22 × 0.08 × 0.05 mm3 |
| Theta range for data collection | 1.632 to 30,565° |
| Reflections collected | 18,744 |
| Absorption correction | Semi-empirical from equivalents |
| Final R indices [I>2σ (I)] | R1 = 0.0395, ωR2 = 0.0932 |
| Polymorph | Tonset (°C) | Tpeak (°C) | Hf (kJ/mol) |
|---|---|---|---|
| Form I | 141.86 | 144.07 | 93.43 |
| Form II | 129.78 | 133.95 | 91.27 |
| Form III | 130.63 | 136.51 | 71.89 |
| Form IV | 119.99 | 131.2 | 80.23 |
| Form V | 137.12 | 140.22 | 88.02 |
| Form VI | 128.72 | 130.87 | 86.10 |
| No. | Mixed Solvent System | T (°C) | Approximate Form II Added at t = 0 min (g) | Approximate ttrans (min) |
|---|---|---|---|---|
| #1 | Nitromethane/toluene (1:1) | 30 | 2.06 | 1 |
| #2 | Nitromethane/isopropylbenzene (1:1) | 30 | 1.51 | 2 |
| #3 | Acetone/m-xylene (1:1) | 30 | 0.49 | 3 |
| #4 | Acetone/toluene (1:1) | 30 | 5.57 | 5 |
| #5 | Acetone/o-xylene (1:1) | 30 | 5.72 | 15 |
| #6 | Acetone/p-xylene (1:1) | 30 | 5.31 | 19 |
| #7 | Acetone/mesitylene (1:1) | 30 | 1.23 | 26 |
| No. | Mixed Solvent System | Rate Constant k (min-1) | SForm I (g/L) | SForm II (g/L) |
|---|---|---|---|---|
| #1 | nitromethane/toluene (1:1) | 5.98 | 0.15555 | 0.15609 |
| #2 | nitromethane/isopropylbenzene (1:1) | 3.26 | 0.29251 | 0.29278 |
| #3 | acetone/m-xylene (1:1) | 2.78 | 0.26269 | 0.26293 |
| #4 | acetone/toluene (1:1) | 1.73 | 0.19559 | 0.19577 |
| #5 | acetone/o-xylene (1:1) | 0.55 | 0.28751 | 0.28755 |
| #6 | acetone/p-xylene (1:1) | 0.46 | 0.26279 | 0.26281 |
| #7 | acetone/mesitylene (1:1) | 0.31 | 0.49958 | 0.49959 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Du, D.; Ren, G.-B.; Qi, M.-H.; Li, Z.; Xu, X.-Y. Solvent-Mediated Polymorphic Transformation of Famoxadone from Form II to Form I in Several Mixed Solvent Systems. Crystals 2019, 9, 161. https://doi.org/10.3390/cryst9030161
Du D, Ren G-B, Qi M-H, Li Z, Xu X-Y. Solvent-Mediated Polymorphic Transformation of Famoxadone from Form II to Form I in Several Mixed Solvent Systems. Crystals. 2019; 9(3):161. https://doi.org/10.3390/cryst9030161
Chicago/Turabian StyleDu, Dan, Guo-Bin Ren, Ming-Hui Qi, Zhong Li, and Xiao-Yong Xu. 2019. "Solvent-Mediated Polymorphic Transformation of Famoxadone from Form II to Form I in Several Mixed Solvent Systems" Crystals 9, no. 3: 161. https://doi.org/10.3390/cryst9030161
APA StyleDu, D., Ren, G.-B., Qi, M.-H., Li, Z., & Xu, X.-Y. (2019). Solvent-Mediated Polymorphic Transformation of Famoxadone from Form II to Form I in Several Mixed Solvent Systems. Crystals, 9(3), 161. https://doi.org/10.3390/cryst9030161





