Abstract
In this work we present the continuation of studies carried out on the changes of geometric parameters of the hydrogen bonds in amino acid crystals subjected to temperature or pressure variations. Changes in geometric parameters of the hydrogen bonds are correlated with the temperature behavior of the Raman wavenumber of NH3+ torsional band. Now four monocrystals, L-valine, L-isoleucine, taurine, and L-arginine hydrochloride monohydrate, are studied. Temperature evolution of the Raman wavenumber of NH3+ torsional band, with positive slope (dν/dT = 0.023 cm−1/K) of L-isoleucine, can be related to the stability of the crystal structure and the hydrogen bonds strengths on heating due to different temperature lattice parameters variation.
1. Introduction
A hydrogen bond (HB) is a highly directional interaction notably responsible for molecular conformation, aggregation, and ordering states of a vast number of complex biological molecular systems. Changes in geometric parameters of the HB can be induced by pressure or temperature variation, as the Coulombic interaction between the protons and acceptor atoms is relatively weak compared to intramolecular covalent bonds. This effect is observed in protein dynamics at high pressure [1] and the temperature dependence of the hydrophobic interaction in protein folding [2]. Thus, the study of the stability mechanism of the molecular systems subjected to temperature or pressure variation can be related to changes in geometric parameters of the HBs.
Amino acid crystals, small model organic molecules linked in the solid structure through complex HB networks, have provided a wide canvas to study the pressure and temperature effects in the solid state. For example, L-alanine crystal constitutes an example of a molecular system linked by HB intermolecular interactions where the effect of variation of thermodynamics parameters was investigated. With increasing pressure, changes occur in the HB to maintain the stability of the crystalline structure, and a recent study through X-ray diffraction does not indicate the occurrence of any structural change [3]. Freire et al. [4], using Raman spectroscopy to study the torsional vibration of ammonium group, suggest the effect of high pressure on the HB in L-alanine crystal can be seen as a change in the geometry of the bond originated by a deviation of the linearity, instead of being due to the decreasing of its length. Funnell et al. [3], in the state-of-the-art experimental approaches using high-pressure single-crystal X-ray and neutron powder diffraction measurements in crystals of deuterated L-alanine, confirmed those results providing a complete picture of the effects of pressure on the HB in L-alanine. In addition, some wavelength links were resolved by the Brillouin light scattering method, which is currently actively used [5,6].
Additionally, a few amino acids were investigated as function of temperature in search of polymorphic crystalline phases using the Raman spectroscopy technique. On one hand, for L-valine, taurine, and L-arginine hydrochloride monohydrate, crystals with monoclinic structure, evidences of phase transitions were obtained [7,8,9]. On the other hand, for the monoclinic crystals of L-isoleucine, no evidence of a structural phase transition was found [10].
Beyond providing information about phase transitions, Raman spectroscopy is a powerful experimental tool to obtain information on HB changes through the study of vibrational wavenumber behavior of some modes belonging to amino acid crystals. Interpreting vibrational wavenumbers of amino acid molecules constitutes a complex task because each wavenumber depends on a few force constants of the vibrational modes. Information contained in the Raman spectra of the amino acids can improve the quality of studies regarding HB changes under variation of pressure or temperature, providing new insights to understand the stability of the molecular systems.
The present work investigates the behavior of the torsional vibrational modes of ammonium group from L-valine (VAL), L-isoleucine (ISO), taurine (TAU), and L-arginine hydrochloride monohydrate (AHM) submitted to temperature variation. A comparative study is carried out among these four monocrystals and a discussion related to the behavior of the vibrational modes provides insights about the HB changes and the factors that have influence on the stability of the system.
2. Materials and Methods
Single crystals of good optical quality were grown by slow evaporation of saturated aqueous solution at 294 K. Characterization and crystalline axes were identified from X-ray diffraction data. Stokes-shifted polarized Raman spectra were obtained using a Jobin Yvon Triplemate 64000 micro-Raman system and an argon-ion laser operating at 514.5 nm as the exciting source. A LN2-cooled charge-coupled-device system was employed to record the spectra. The exciting power striking the sample was about 1 mW. The slits were set for a 1 cm−1 spectral resolution. Low-temperature Raman measurements were carried out using a helium closed-cycle refrigeration system. The temperature was monitored by a digital temperature controller unit with a temperature stability of 0.1 K.
3. Results
In Figure 1 we reproduce, for totally symmetric representations, the Raman bands associated with NH3+ torsional vibration recorded at low temperature for the four crystals (VAL, ISO, TAU, and AHM). We remember that the Porto notation z(yy)z, for example, means that light is arriving and leaving the crystal on the z direction, while the polarizations of the incident and scattered beams are parallel to the y axis of the crystal. We also remember that the normal modes can appear at different wavenumber values on different scattering geometries due the fact that they belong to different irreducible representations. However, the important point is that we have chosen a specific scattering geometry and analyzed the NH3+ torsional modes as a function of temperature. Two Raman bands are observed for all the structures; this fact indicates that the NH3+ torsional vibration is non-degenerate at low temperature condition. Similar characteristics are also observed in other amino acid crystals, such as α-glycine [11]. The thermal evolution of the observed wavenumber of the Raman bands is shown in Figure 2.
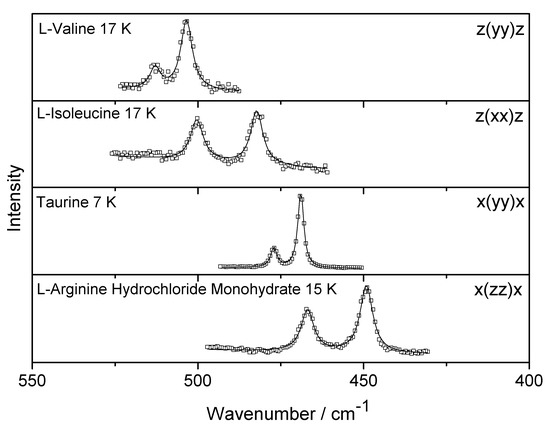
Figure 1.
The Raman bands associated with the NH3+ torsional vibration at low temperature for L-valine (VAL), L-isoleucine (ISO), taurine (TAU), and L-arginine hydrochloride monohydrate (AHM).
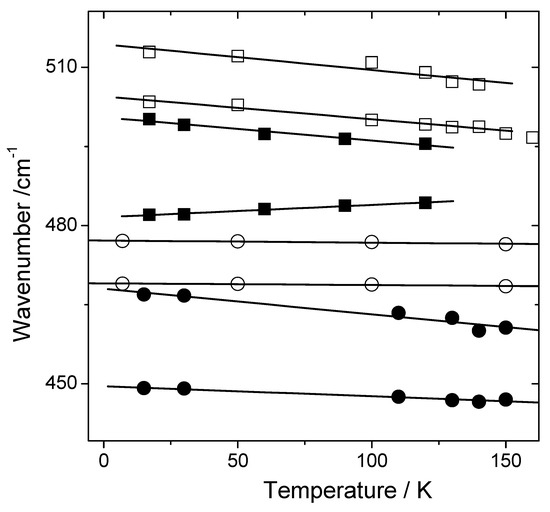
Figure 2.
Linear fitting for wavenumber thermal evolution of the of Raman bands associated with NH3+ torsional vibration. L-valine (□), L-isoleucine (▪), taurine (◦) and L-arginine hydrochloride monohydrate (●) crystals.
Table 1 presents parameters of the linear fitting for wavenumber thermal evolution. The decreasing of the wavenumber of the Raman bands associated with NH3+ torsional vibration is observed in all structures, except for the L-isoleucine (band recorded around 480 cm−1).

Table 1.
Parameters of the linear fitting for wavenumber thermal evolution of the Raman bands, ν (T) (cm−1) and dν (T)/dT (cm−1/K), associated with NH3+ torsional vibration for L-valine, L-isoleucine, taurine, and L-arginine hydrochloride monohydrate crystals.
VAL [12], ISO [13], TAU [14], and AHM [15] crystallizes in monoclinic structure. For VAL, ISO, and AHM, crystals contain two crystallographically independent molecules in the asymmetric unit. These crystals, with one amino group and one carboxylic acid group, crystallize from water as dipolar species known as zwitterions or dipolar ions. Thus, a zwitterion is an organic molecule with a positively charged segment and a negatively charged segment. Zwitterions allow the formation of hydrogen bonds, in the form of N-H+- - -O--C, which are bonds that play a major role in determining the structures of amino acid crystals. Due the fact that HBs are created between zwitterions, the possible structures are confined to a limited number of configurations, i.e., to only energetically stable configurations. Additionally, for AHM crystals the Cl and H2O units work as acceptors. The different molecules and configurations mean that the amino acids have different geometric parameters of their HBs as illustrated in Table 2.

Table 2.
Comparison of geometric parameters of the hydrogen bonds (HBs) for four different amino acid structures: Bond distances (N…O) Å [bond angles (C-N…O) °].
Some HB characteristics, such as equilibrium bond distances, are influenced by temperature and pressure. In other words, the equilibrium bond distance is controlled by a combination of thermodynamics and quantum mechanics effects. In hydrogen-bonded systems, like liquid water, the internal energy associated with a hydrogen bond is a significant fraction of the internal energy of the system [16]. Similarly, the hydrogen bond represents an important fraction of the internal energy of crystals composed by networks of HBs.
The temperature sensitivity of the HBs can be analyzed through the observation of the temperature wavenumber shift of certain phonons by Raman spectroscopy. Several studies in different types of crystalline materials showed that the temperature wavenumber shift of a phonon in a Raman spectrum can be written as the sum of two parcels, e.g., the thermal expansion contribution and the anharmonic contribution [17]. Eventually, these contributions can be inferred from the Raman spectrum with bands associated with modes of low energy. On the other hand, from bands associated with vibrations belonging to CO2 and NH3 groups, one can discover various aspects from conformation of the molecules in the unit cell and the effect of thermodynamics parameters on the hydrogen bonds [18].
Previous studies on the NH3+ torsional mode of amino acids as seen by Raman spectroscopy have revealed interesting aspects of its temperature behavior. In most of the cases, the NH3+ torsional mode is very sensitive to modification of HBs as temperature changes. For α-glycine, the wavenumbers of NH3+ torsional modes remain approximately constant in the temperature range 83–302 K. This fact was interpreted as small or no significant change in intralayer hydrogen bond length in the temperature range investigated [9]. However, the NH3+ group can also play an important role in the dynamic of different amino acid crystals under temperature variation as shown by many studies [19]. Regarding β-glycine, a different behavior compared with the α-glycine example was related with a phase transition observed on cooling at ~250 K [20]. In a study performed on L-alanine crystal, Forss [21] calculated the activation energy for NH3+ reorientation through the analysis of the linewidth of torsional band of the ammonium group. Such a study served as a complementary method to NMR in discovering torsional barrier heights. Additionally, a study presented by reference [22] showed a two-step behavior of the wavenumber of the NH3+ torsional mode as function of temperature and a discontinuity at 220 K. This fact was interpreted as the increase in anharmonicity of the torsional vibrations on heating, as consequence of large amplitude reorientations of the NH3+ group starting from 220 K with switching of the N-H---O HB between two neighboring molecules in the unit cell [22].
From the overview given in the last paragraph, we note the analysis of the torsional vibration of ammonium group can provide information about the HB associated with amino acid crystals. The temperature evolution of the wavenumber of the NH3+ torsional mode in the Raman spectra of VAL, TAU, and AHM observed in Figure 2 and Table 1 (negative angular coefficient dν/dT) reflects the progressive weakening of the HBs with heating due to thermal expansion and anharmonicity. The effect is interpreted in a relatively trivial way—as the bond length increases, there is a reduction of the force constant of the NH3+ torsional vibration. Consequently, the expected effect of weakening of the HBs with temperature is observed.
The presence of the N-H…Cl HBs in L-arginine hydrochloride monohydrate crystals does not change the behavior of the wavenumber of the Raman bands of the NH3+ torsional vibration. This structure does not have truly short HBs. Indeed, the lengths of the HBs in AHM tend to be average or greater, indicating rather weak bonds. Obviously, minimum energy is attained in the crystal by maximizing the number of bonds at the expense of bond strength [15].
The crystal structure of taurine indicates unambiguously that taurine possesses the zwitterion configuration and its structural formula is correctly written as NH+-CH2-CH2-SO3−. The molecules are held together by a three-dimensional network of N-H---O bonds with relatively strong connections [14] (Table 2). This characteristic may be associated with the weak variation observed in the temperature behavior of the Raman wavenumber of NH3+ torsional mode (Table 1).
Now, let us discuss the only crystal among the four previously presented where dν/Dt > 0, i.e., L-isoleucine. In this crystal, different temperature evolution of the Raman wavenumber of NH3+ torsional band occurs, with negative (dν/dT = −0.044 cm−1/K) and positive (dν/dT = 0.023 cm−1/K) slopes as observed in Figure 2 and Table 1. The observation that the Raman bands are shifted to higher wave numbers with increasing temperature can be interpreted as an increase in the force constant of the oscillator from the NH3+ torsional vibration. To explain this effect, we consider that the network of the N-H---O HB of the L-isoleucine structure is very similar to those of the corresponding structure in L-valine [10,11]. However, at room temperature, the L-isoleucine presents a long intermolecular HB with 3.43 Å (145°) (Table 2). The increase in the force constant of the NH3+ torsional vibration may be related to the specific HB changes in the crystalline structure. The long intermolecular HB possibly has important participation in this effect. Long intermolecular HB in L-isoleucine can become most intense on heating due the reduction of the N…O length and the increasing of the bond angles. This effect is possible since amino acid crystals may present contraction in one of their crystallographic axes with temperature increasing, as occurs with L-alanine crystals [23].
4. Conclusions
We have studied the NH3+ torsion vibrational mode of different amino acids addressing the important problem of change of the HBs and structural stability with temperature variation. Our study has provided quantitative information about the temperature evolution of the normal modes associated with the torsion of NH3+ group for four different amino acid crystals. The natural softening of the HBs on heating is observed for L-valine, taurine, and L-arginine hydrochloride monohydrate, as inferred from the negative dν/dT values. From this investigation we were also able to observe that L-isoleucine presents an apparently anomalous behavior, showing a positive dν/dT value. This means that as the temperature is increased, part of the HBs strengthens, possibly as consequence of different temperature lattice parameters variation, which favors decreasing of length of HB on heating.
Author Contributions
Conceptualization, A.L.d.O.C. and R.J.C.L.; methodology, A.L.d.O.C. and R.J.C.L.; software, data curation, A.L.d.O.C., R.J.C.L. and P.T.C.F.; writing—original draft preparation, A.L.d.O.C. and R.J.C.L.; writing—review and editing, P.T.C.F.
Funding
The APC was funded by Universidade CEUMA.
Acknowledgments
Universidade Federal do Maranhão, Universidade Federal do Ceará and Universidade CEUMA.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Heremans, K.; Smeller, L. Protein structure and dynamics at high pressure. Biochim. Biophys. Acta (BBA)-Protein Struct. Mol. Enzymol. 1998, 1386, 353–370. [Google Scholar] [CrossRef]
- Baldwin, R.L. How does protein folding get started? Trends Biochem. Sci. 1989, 14, 291–294. [Google Scholar] [CrossRef]
- Funnell, N.P.; Dawson, A.; Francis, D.; Lennie, A.R.; Marshall, W.G.; Moggach, S.A.; Parsons, S. The effect of pressure on the crystal structure of l-alanine. Cryst. Eng. Comm. 2010, 12, 2573. [Google Scholar] [CrossRef]
- Freire, P.T.C.; Melo, F.E.A.; Mendes Filho, J.; Lima, R.J.C.; Teixeira, A.M.R. The behavior of NH3 torsional vibration of l-alanine, l-threonine and taurine crystals under high pressure: A Raman Spectrosc. Study. Vib. Spectrosc. 2007, 45, 99–102. [Google Scholar] [CrossRef]
- Sadovnikov, A.V.; Grachev, A.A.; Sheshukova, S.E.; Sharaevskii, Y.P.; Serdobintsev, A.A.; Mitin, D.M.; Nikitov, S.A. Magnon straintronics: Reconfigurable spin-wave routing in strain-controlled bilateral magnetic stripes. Phys. Rev. Lett. 2018, 120, 257203. [Google Scholar] [CrossRef] [PubMed]
- Sadovnikov, A.V.; Beginin, N.E.; Sheshukova, S.E.; Sharaevskii, Y.P.; Stognij, A.I.; Novitski, N.N.; Nikitov, S.A. Route toward semiconductor magnonics: Light-induced spin-wave nonreciprocity in a YIG/GaAs structure. Phys. Rev. B 2019, 99, 054424. [Google Scholar] [CrossRef]
- Lima, J.A.; Freire, P.T.C.; Lima, R.J.C.; Moreno, A.J.D.; Mendes Filho, J.; Melo, F.E.A. Raman scattering of L-valine crystals. J. Raman Spectrosc. 2005, 36, 1076–1081. [Google Scholar] [CrossRef]
- Lima, R.J.C.; Freire, P.T.C.; Sasaki, J.M.; Melo, F.E.A.; Mendes Filho, J.; Moreira, R.L. Temperature-dependent Raman study of taurine single crystal. J. Raman Spectrosc. 2001, 32, 751–756. [Google Scholar] [CrossRef]
- Lima, R.J.C.; Freire, P.T.C.; Sasaki, J.M.; Melo, F.E.A.; Mendes Filho, J. Temperature-dependent Raman study of L-arginine hydrochloride monohydrate single crystal. J. Raman Spectrosc. 2002, 33, 625–630. [Google Scholar] [CrossRef]
- Almeida, F.M.; Freire, P.T.C.; Lima, R.J.C.; Remédios, C.M.R.; Mendes Filho, J. Raman spectra of L-isoleucine crystals. J. Raman Spectrosc. 2006, 37, 1296–1301. [Google Scholar] [CrossRef]
- Murli, C.; Thomas, S.; Venkateswaran, S.; M Sharma, S. Raman spectroscopic investigation of α-glycine at different temperatures. Phys. B Condens. Matter 2005, 364, 233–238. [Google Scholar] [CrossRef]
- Torii, K.; Iitaka, Y. The crystal structure of L-valine. Acta Crystallogr. Sect. B Struct. Crystallogr. Cryst. Chem. 1970, 26, 1317–1326. [Google Scholar] [CrossRef]
- Torii, K.; Iitaka, Y. The crystal structure of L-isoleucine. Acta Crystallogr. Sect. B Struct. Crystallogr. Cryst. Chem. 1971, 27, 2237–2246. [Google Scholar] [CrossRef]
- Okaya, Y. Refinement of the crystal structure of taurine, 2-aminoethylsulfonic acid. An example of computer-controlled experimentation. Acta Crystallogr. 1966, 21, 726–735. [Google Scholar] [CrossRef]
- Dow, J.; Jensen, L.H.; Mazumdar, S.K.; Srinivasan, R.; Ramachandran, G.N. Refinement of the structure of arginine hydrochloride monohydrate. Acta Crystallogr. Sect. B Struct. Crystallogr. Cryst. Chem 1970, 26, 1662–1671. [Google Scholar] [CrossRef]
- Dougherty, R.C. Temperature and pressure dependence of hydrogen bond strength: A perturbation molecular orbital approach. J. Chem. Phys. 1998, 109, 7372–7378. [Google Scholar] [CrossRef]
- Carabatos-Nédelec, C.; Becker, P. Order-Disorder and structural phase transitions in solid-state materials by Raman scattering analysis. J. Raman Spectrosc. 1997, 28, 663–671. [Google Scholar] [CrossRef]
- Cavaignac, A.L.O.; Soares, R.A.; Lima, R.J.C.; Façanha-Filho, P.F.; Freire, P.T.C. The behavior of the deformation vibrations of NH3 in semi-organic crystals under high pressure studied by Raman spectroscopy. Crystals 2018, 8, 245. [Google Scholar] [CrossRef]
- Kolesov, B.A.; Boldyreva, E.V. Difference in the dynamic properties of chiral and racemic crystals of serine studied by Raman spectroscopy at 3–295 K. J. Phys. Chem. B 2007, 111, 14387–14397. [Google Scholar] [CrossRef] [PubMed]
- Drebushchak, V.A.; Boldyreva, E.V.; Kovalevskaya, Y.A.; Paukov, I.E.; Drebushchak, T.N. Low-temperature heat capacity of β-glycine and a phase transition at 252 K. J. Therm. Anal. Calorim. 2005, 79, 65–70. [Google Scholar] [CrossRef]
- Forss, S. A Raman spectroscopic temperature study of NH3+ torsional motion as related to hydrogen bonding in the L-alanine crystal. J. Raman Spectrosc. 1982, 12, 266–273. [Google Scholar] [CrossRef]
- Vik, A.F.; Yuzyuk, Y.I.; Barthes, M.; Sauvajol, J.L. Low-wvenumber dynamics of L-alanine. J. Raman Spectrosc. 2005, 36, 749–754. [Google Scholar] [CrossRef]
- Lima, R.J.C.; Santos-Junior, E.C.; Moreno, A.J.D.; Façanha-Filho, P.F.; Freire, P.T.C.; Yoshida, M.I. Thermal study of l-alanine, l-threonine, and taurine crystals related to hydrogen bonding. J. Therm. Anal. Calorim. 2013, 111, 627–631. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).