Abstract
There is an increasing demand for cold-adapted enzymes in a wide range of industrial branches. Nevertheless, structural information about them is still scarce. The knowledge of crystal structures is important to understand their mode of action and to design genetically engineered enzymes with enhanced activity. The most difficult task and the limiting step in structural studies of cold-adapted enzymes is their crystallization, which should provide well-diffracting monocrystals. Herein, we present a combination of well-established crystallization methods with new protocols based on crystal seeding that allowed us to obtain well-diffracting crystals of two cold-adapted β-d-galactosidases (βDGs) from Paracoccus sp. 32d (ParβDG) and from Arthrobacter sp. 32cB (ArthβDG). Structural studies of both βDGs are important for designing efficient and inexpensive enzymatic tools for lactose removal and synthesis of galacto-oligosaccharides (GOS) and hetero-oligosaccharides (HOS), food additives proved to have a beneficial effect on the human immune system and intestinal flora. We also present the first crystal structure of ArthβDG (PDB ID: 6ETZ) determined at 1.9 Å resolution, and compare it to the ParβDG structure (PDB ID: 5EUV). In contrast to tetrameric lacZ βDG and hexameric βDG from Arthrobacter C2-2, both of these βDGs are dimers, unusual for the GH2 family. Additionally, we discuss the various crystallization seeding protocols, which allowed us to obtain ParβDG and ArthβDG monocrystals suitable for diffraction experiments.
1. Introduction
β-d-Galactosidases (EC 3.2.1.23) are widely used in the food industry as they catalyze the hydrolysis of terminal non-reducing β-d-galactose residue in β-d-galactosides. They are especially relevant in the dairy industry due to their ability to catalyze the hydrolysis of lactose, a natural substrate. Enzymatically hydrolyzed lactose, especially in milk, whey, or whey derivatives, is broadly used due to its higher sweetness, which ameliorates product taste, and to application in specialized food production, for people with lactose malabsorption [1,2,3]. Administration of products with depleted levels of lactose and other digestible oligosaccharides, disaccharides, monosaccharides, and polyols instead of common food is beneficial in the prevention of irritable bowel syndrome (IBS) [4,5,6,7,8,9]. Cold-active β-d-galactosidases (βDGs) have become a focus of attention because of their ability to eliminate lactose from refrigerated milk, convert lactose to glucose and galactose (decreasing its hygroscopicity), and eliminate lactose from dairy industry pollutants associated with environmental problems. Moreover, in contrast to commercially available mesophilic β-d-galactosidase from Kluyveromyces lactis, the cold-active enzyme could make it possible to reduce the risk of mesophilic contamination and save energy during the industrial process connected with lactose hydrolysis [10,11,12].
In addition to hydrolytic activity, some β-d-galactosidases exhibit also a secondary transglycosylation activity, therefore they can be used for the synthesis of oligosaccharides (e.g., GOS and HOS) that are desirable functional food additives [13]. Such an activity is exhibited when there is a high concentration of a substrate and the galactose unit may be transferred onto the substrate, e.g., lactose. The oligosaccharides are built form d-galactose, d-glucose, N-acetylglucosamine, l-fucose, and sialic acid residues linked via O-glycosidic bonds. A vast majority of them carry lactose at their reducing end [14]. GOS are polymers of 2–10 d-galactose units, which are virtually not degraded by human digestive enzymes. Since they reach the colon practically intact and promote the growth of beneficial bacteria (Bifidobacteria and Lactobacilli), they are classified as prebiotics [15,16,17,18]. The importance of GOS as additive to infant formula-milk has been widely discussed as it has been proven not only to promote intestine colonization by beneficial bacteria but also to prevent bacterial adhesion in early stages of infection [14,16,19,20,21,22,23,24]. Moreover, some oligosaccharides are a rich source of sialic acid (essential for brain development) [25]. GOS and HOS are also valued additives to adult food, as recent research shows that they may increase mineral absorption [26,27,28], increase the rate of flu recovery, reduce stress-induced gastrointestinal disfunctions [29], as well as prevent cancer formation, benefit lipid metabolism, prevent hepatic encephalopathy, glycemia/insulinemia, and immunomodulation [30,31].
A typical oligomerization state of βDGs from GH2 is tetrameric [11,32] or hexameric [33]. However, large GH2 βDGs were reported to be active as functional dimers, based on biochemical investigations [34,35]. The crystal structure of the first dimeric GH2 βDG was recently published by us [36]; however, that enzyme is much smaller than typical GH2 βDGs (a monomer of only 70 kDa) and exhibits a different shape and orientation of domain 5, called wind-up domain.
Despite extensive efforts and application of different methods for the crystallization of cold adapted proteins, the process is still challenging, as in the Protein Data Bank (PDB) only around 40 crystal structures of cold-adapted enzymes are available, which is a small percentage of 132,000 total structures deposited in the PDB. Whereas the structures of multiple mesophilic βDGs are known, only two structures of cold-adapted βDGs have been previously deposited in the PDB [33,36] and the obtained results show that the investigated enzyme differs in tertiary and quaternary structure from the previously described ones. Here we describe the crystallization methods used to ameliorate and control crystallization of cold adapted ParβDG and ArthβDG, as well as crystal structure determination of ArthβDG, the second cold-active βDG from the GH2 family identified as dimeric up to a year ago.
2. Materials and Methods
2.1. ArthβDG Production
The heterologous expression of the recombinant β-d-galactosidase from Arthrobacter sp. 32cB was performed in the E. coli LMG 194 cells transformed with pBAD-Bgal 32cB plasmid under the control of PBAD promoter (Table 1). For the production of ArthβDG, the E. coli cells were grown at 30 °C in Luria-Bertani (LB) medium supplemented with 100 µg/mL ampicillin, until an OD600 of 0.5 was reached. Overexpression was induced by addition of 20% L-arabinose solution to the final concentration of 0.02%. The culture was further cultivated for 15 h to OD600 of 3.8 ± 0.2 and harvested by centrifugation (6000 × g, 15 min, 4 °C) [37].

Table 1.
Arthrobacter sp. 32cB production information.
The extraction of intracellular protein was carried out by two separate methods. Method 1: The cells were resuspended in buffer A containing: 20 mM K2HPO4/KH2PO4 (pH 6.0), 50 mM KCl and the cell suspension was disrupted by sonication on an ice bath using 20 repetitions of 15 s impulses with 60 s pauses to avoid sample overheating. The lysate was clarified by centrifugation at 4 °C for 30 min at 9000 × g [37]. Method 2: The cell pellet was ground into a fine powder in a mortar and pestle under liquid nitrogen, with addition of silicone beads. The powder was resuspended in buffer A, and the sample was clarified by centrifugation at 4 °C for 30 min at 9 000 × g.
2.2. Purification of ArthβDG
ArthβDG was purified by two ion-exchange chromatography steps (weak anion exchanger and strong anion exchanger), followed by a size-exclusion chromatography step. The cell-free supernatant was loaded onto a DEAE (BioRad, Hercules, CA, USA) column equilibrated with buffer A (20 mM K2HPO4/KH2PO4 (pH 6.0), 50 mM KCl). The recombinant ArthβDG was eluted using a linear gradient of potassium chloride (20–1020 mM) in the same buffer. The fractions containing ArthβDG were determined and dialyzed against buffer A. In the second step, the protein sample was loaded onto HiPrep Q Sepharose 16/10 column (GE Healthcare, Little Chalfont, UK) equilibrated with buffer A and eluted with a linear gradient of potassium chloride (20–820 mM) in the same buffer. Fractions containing ArthβDG were once again determined and dialyzed against buffer C (20 mM K2HPO4/KH2PO4 (pH 7.5), 150 mM KCl). The concentrated sample was injected onto a Superdex 200 column (GE Healthcare, Little Chalfont, UK), previously equilibrated with buffer C.
The fractions containing ArthβDG were identified by SDS-PAGE electrophoresis run at 10% SDS-polyacrylamide gel and by enzymatic activity assay, in parallel. The determination of fractions containing active βDG may be readily validated by enzymatic activity assay: 10% ortho-nitrophenyl-β-d-galactopyranoside (ONPG) was added to the sample (1:4 ratio). Sample color change into intense yellow was observed for samples containing βDG. The sample of buffer coming from the chromatography column was changed into 0.05 M HEPES pH 7.0 and the samples were concentrated using 50 kDa cutoff membrane Vivaspin filters (Sartorius, Göttingen, Germany).
2.3. ParβDG and ArthβDG Crystallization
All crystallizations were performed in 24-well plates (Hampton Research, Aliso Viejo, CA, USA) using hanging drop vapor-diffusion method at 18 °C. The 1 μL drop of protein was placed on siliconized glass cover slide, covered with an equal volume of reservoir solution and left to equilibrate against 500 μL of crystallization buffer.
First crystallization conditions for ParβDG were found using PEG/Ion ScreenTM HR2-126 and Index ScreenTM HR2-144 (Hampton Research, Aliso Viejo, CA, USA). Initial optimization of crystallization conditions was performed using varying concentrations of precipitants (PEG MME 2K and ammonium acetate), as well as various pHs. To further improve the crystal morphology, various additives were tried from commercially available Additive Screen (Hampton Research, Aliso Viejo, CA, USA).
Initial crystal screenings used for ArthβDG crystallization were: Index ScreenTM HR2-144, PEG/Ion ScreenTM HR2-126, PEG/Ion2 ScreenTM HR2-089 (Hampton Research, Aliso Viejo, CA, USA), and Morpheus® HT-96 (Molecular Dimensions, Suffolk, UK).
2.3.1. Streak Seeding
The seed stock was prepared using the previously obtained hair-type crystals. The crystals were transferred to a tube containing a small volume of reservoir solution (around 20 μL) and the crystals were crushed with a pipette tip. Subsequently, the concentrated seed stock was diluted several times by addition of a larger volume of reservoir solution (~100 μL). The crystallization plate was set up using the previously optimized crystallization conditions. The protein solution was slightly diluted (to 13 mg/mL), premixed with dichloromethane at the final concentration of 0.025% (v/v) and the drops were set in a 1:1 ratio. The cover slides with the drops were sealed and the plate was left for a short pre-equilibration time intact. The hair was run through the seed stock and then through the freshly set drops.
2.3.2. Seed Stock Preparation
The obtained ArthβDG crystals were crushed within a crystallization drop with a CrystalProbe (Hampton Research, Aliso Vieja, CA, USA). Next, they were carefully transferred into 50 µL of cool reservoir solution and the solution was vortexed with addition of SeedBead (Hampton Research, Aliso Vieja, CA, USA), kept cool all the time. A series of dilutions of such prepared seed stock was performed and systematic (10×) dilution was used for the rMMS crystallization experiment.
2.3.3. Random Microseed Matrix Screening Crystallization
The procedure rMMS was applied for screening co-crystallization conditions of ArthβDG complexes with ligands such as lactose and IPTG. The screening was performed at 18 °C using the sitting drop vapor diffusion technique with an automated sample handling robotic system Oryx4 (Douglas Instruments Ltd., Hungerford, UK) [38]. The drop was composed of: 0.20 μL of protein, 0.07 μL of seed solution and 0.13 μL of reservoir, and placed over 50 μL of reservoir solution. The screens such as PEG/Ion ScreenTM HR2-126, PEG/Ion2 ScreenTM HR2-098 (Hampton Research, Aliso Vieja, CA, USA), and Morpheus® HT-96 (Molecular Dimensions, Suffolk, UK) were tested for alternative co-crystallization conditions for complexes of ArthβDG.
2.4. Data Collection and Processing
Initial X-ray diffraction measurement of the crystals was performed at our home source SuperNova (Rigaku Oxford Diffraction, Tokyo, Japan). High-resolution data were collected using a synchrotron source on beamline BL14.2 at BESSY, Berlin, Germany. For some crystals, 1.8 M sodium malonate, 60% TacsimateTM (both with appropriate pH) or 50% glycerol solution was used as cryoprotectant during the data collection. Generally cryoprotectants containing only salts of carboxylic acids worked better than those containing glycerol [39]. The diffraction data were processed with XDSapp [40]. The details for the data collection and processing of ArthβDG are presented in Table 2.

Table 2.
The diffraction data collection and processing statistics for ArthβDG crystal PDB ID: 6ETZ.
2.5. Structure Solution and Refinement
The Matthews value calculation showed that a monomer of protein is present in the asymmetric unit. The structure of ArthβDG was solved by molecular replacement using a monomer of the closest homologue structure (PDB ID: 1YQ2): βDG from Arthrobacter C2-2 [33] with the program PHASER [41]. The model after rebuilding in COOT [42], which was possible due to the significant 2Fo-Fc electron density map for the missing fragments, after first cycle of refinement in REFMAC5 [43] gave Rwork and Rfree values of 19.6% and 23.1%, respectively. That model was further refined in REFMAC5 using maximum-likelihood targets, including TLS parameters [44] defined for each domain, yielding the final Rwork and Rfree of 16.1% and 19.8%, respectively (Table 3).

Table 3.
ArthβDG crystal structure solution and refinement parameters.
3. Results and Discussion
3.1. Crystallization of Cold-Adapted βDGs
3.1.1. Crystallization of Cold-Adapted ParβDG
The crystal structure of ParβDG has been already reported in our previous article [36] that focused on its structural analysis (PDB ID: 5EUV). The crystallization process was not discussed there in detail. Therefore, here we describe all steps that were necessary to obtain monocrystals with good diffraction properties.
Since ParβDG purification has already been described [36], it will be mentioned only briefly. The protein was expressed in E. coli and purified using a two-step protocol employing ion exchange chromatography: the first step was carried out using Fractogel EMD DEAE column (Merck, Darmstadt, Germany) and was followed by a protein separation on a Resource Q column (Merck, Darmstadt, Germany). The active fractions of ParβDG were dialyzed against a buffer composed of 0.02 M sodium phosphate, pH 7.3. The protein was concentrated to 15 mg/mL.
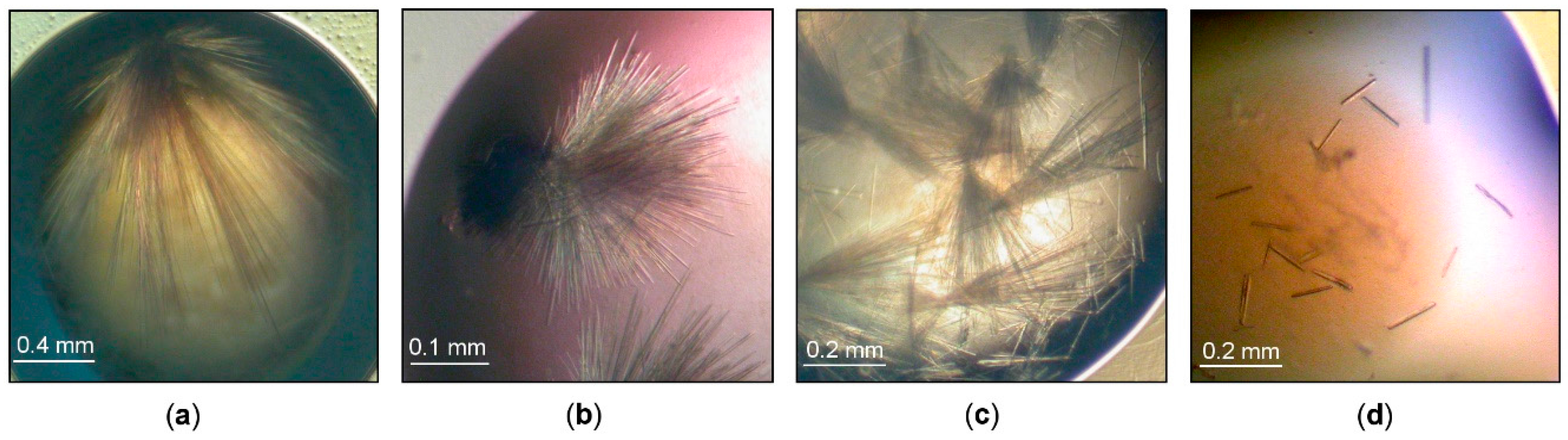
The standard crystallization screening performed for ParβDG gave clustered hair-like crystals in 24% PEG MME 2K, 0.1 M ammonium acetate, 0.1 M Bis-Tris, pH 6.0 (Figure 1a). After an optimization of the crystallization conditions, a decrease of pH to 5.5 improved slightly the morphology of the ParβDG crystals (Figure 1b). Several crystals present in the cluster became thin needles. However, it was still difficult to separate them from the cluster.
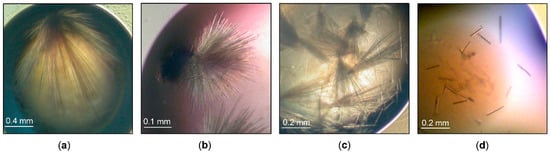
Figure 1.
ParβDG crystals: (a) initial screening (pH 6); (b) initial optimization (pH 5.5); (c) the optimization with Additive Screen; (d) crystals obtained with streak seeding.
Various additives, 96 in total, were tested (Additive Screen, Hampton Research, Aliso Vieja, CA, USA), and the best results were obtained using the previously optimized crystallization conditions and dichloromethane at a final concentration of 0.025% (v/v) as an additive premixed with protein. Some of the crystals still formed hair-type clusters; however, a number of separate needles could be observed in the drops (Figure 1c).
The single needles were obtained by a combination of crystallization with the additive and streak seeding, where the hair was run through a seed stock and then through the freshly set drops. After the second round of seeding, we obtained crystals that grew as separate needles (Figure 1d). The final protein concentration used for setting the drops was 11 mg/mL. The decrease of the protein concentration and introduction of the seeds to the drops enabled significantly improved crystal growth.
3.1.2. Crystallization of Cold-Adapted ArthβDG
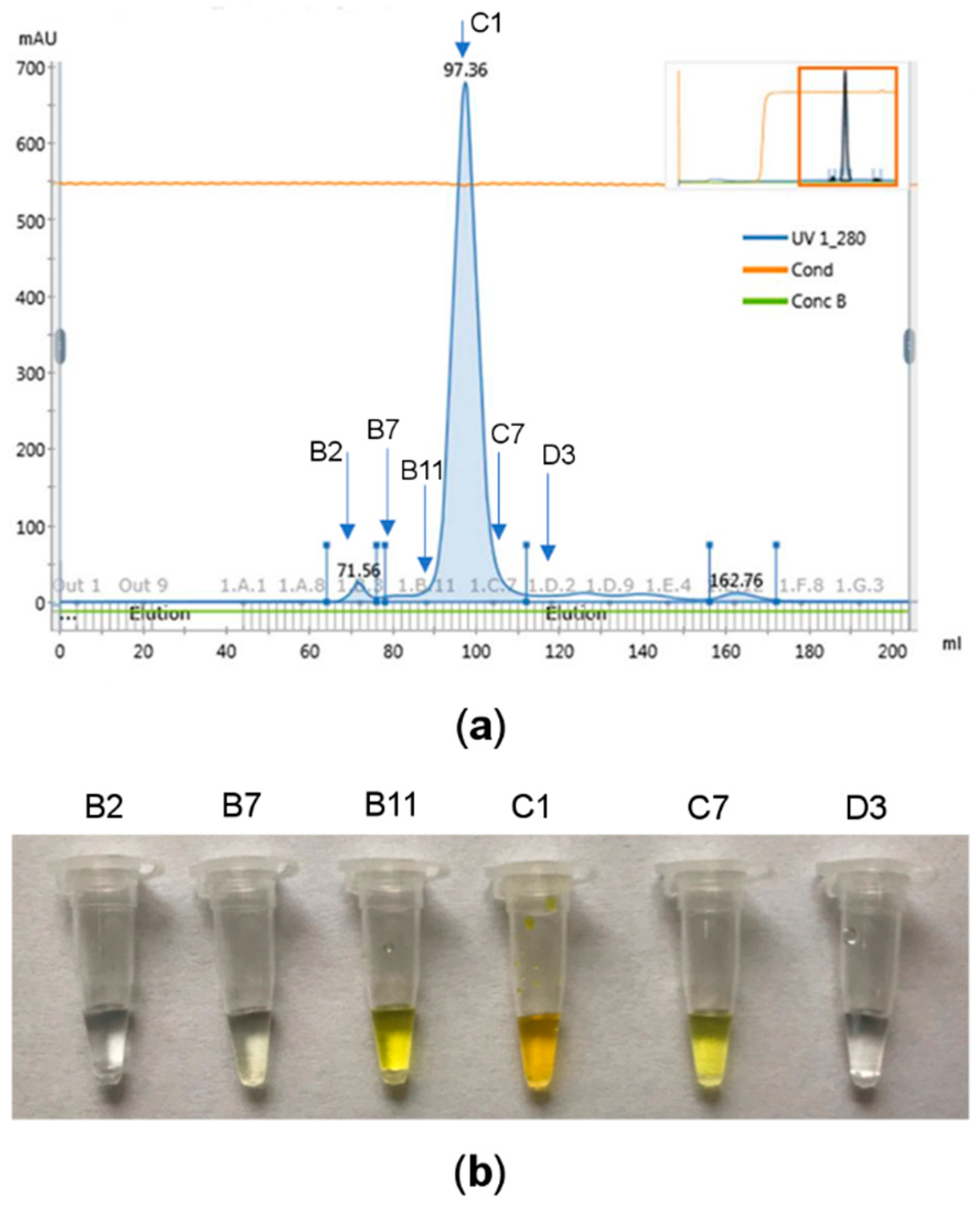
ArthβDG was produced in E. coli as a soluble, intracellular recombinant protein. For its extraction from the cells two methods were used in parallel. Although extraction with mortar and pestle under liquid nitrogen is a time and energy consuming process, it proved to be more beneficial for subsequent protein crystallization than classical sonication. Not only a higher yield of purification was obtained for this extraction method, but the growth of native crystals was more rapid. The first crystals were observed after 3 days, whereas for the protein extracted using sonication the first crystals occurred after 5 days (under corresponding crystallization conditions). Subsequent to extraction, ArthβDG was purified using two steps of ion-exchange and the third step was size-exclusion chromatography. The whole purification procedure was conducted at 4 °C, as the protein samples purified at higher temperature (e.g., 18 °C) produced no crystals even under previously determined crystallization conditions. The cold-adapted enzymes exhibit higher propensity towards thermal denaturation [45], the resulting denaturation or unfolding negatively affects subsequent crystallization. The eluted fractions were tested using an enzymatic assay [46], and the ones exhibiting hydrolytic activity versus ONPG were further analyzed by SDS-PAGE electrophoresis. The recombinant ArthβDG migrated on an SDS-polyacrylamide gel with a molecular weight of ~110 kDa, which was in agreement with the calculated molecular mass of its monomer based on a cloned construct. The observation of a sharp peak of protein during the last step of purification and the presence of a sole band on an electrophoretic gel proved that the sample was highly purified (Figure 2).
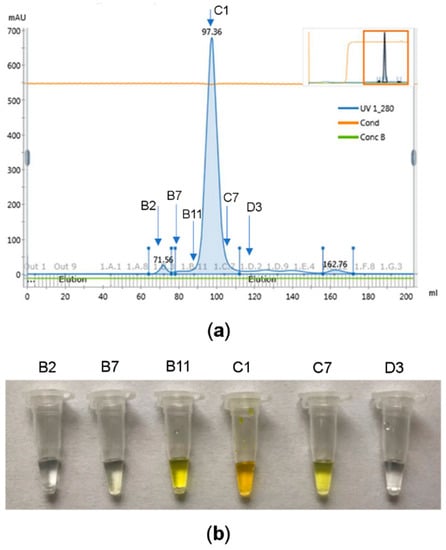
Figure 2.
ArthβDG purification results: (a) protein peak purified and concentrated by size-exclusion chromatography, the fractions indicated with arrows were used for enzymatic assay; (b) results of the enzymatic assay. The selected protein samples were added to 10% ortho-Nitrophenyl-β-d-galactopyranoside (ONPG) solution. The yellow color is produced by ortho-nitrophenol obtained by enzymatic hydrolysis of ONPG, thus indicating fractions with βDG activity (B11, C1, C7), the other tested fractions exhibited no βDG activity (B2, B7, D3); all the fractions from range B11–C7 were combined and used for crystallization experiments.
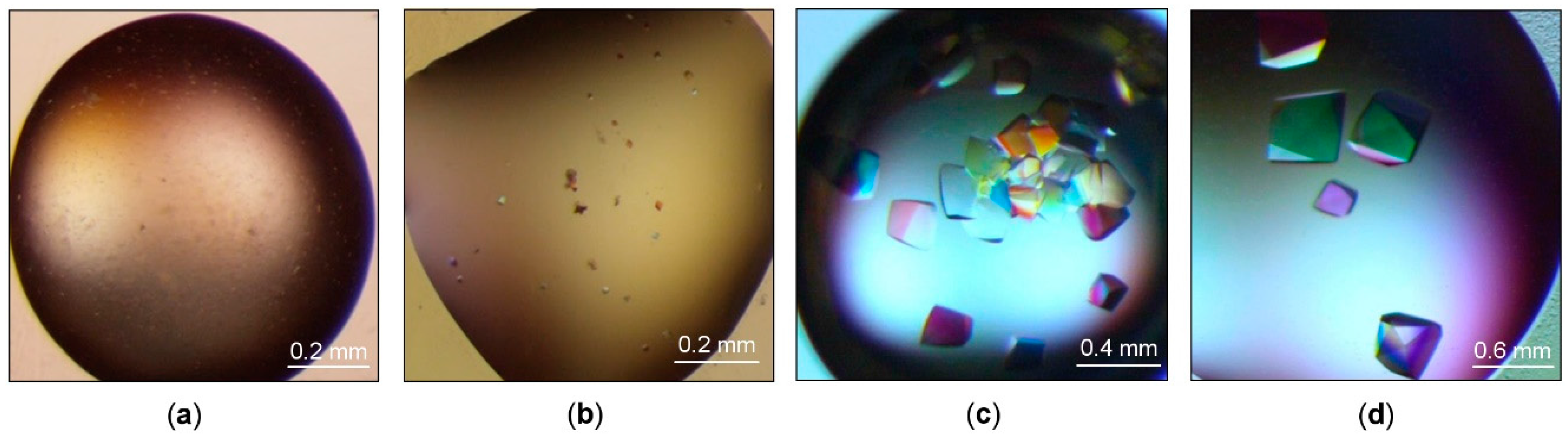
The initial crystallization screening was performed using the same range of ArthβDG concentration (6–12 mg/mL) as we used previously to obtain crystals of cold-adapted aminotransferase from Psychrobacter sp. B6 (PsyArAT) [46]. No crystals were observed for a sample concentration below 10 mg/mL. For the protein concentration exceeding 10 mg/mL, a few conditions yielded small crystals, whose size did not exceed 0.02 × 0.02 × 0.02 mm, or microcrystalline precipitate. However, none of them were directly suitable for diffraction experiments. Thus, for crystallization optimization, a sample concentration of 10 mg/mL was used. The optimized crystallization conditions included precipitants such as: sodium malonate, sodium phosphate, potassium phosphate, ammonium sulfate, pentaerythriol etoxylate, L-proline, sodium citrate, PEG 3350, Jeffamine-2001, and TacsimateTM (Hampton Research, Aliso Vieja, CA, USA). As a result, we obtained larger (up to 0.3 × 0.2 × 0.2 mm) ArthβDG crystals in a tetragonal or bipyramidal form in conditions containing 1.4 M sodium malonate, 1.5% Jeffamine, and 0.1 M HEPES pH 7.0 (Figure 3d), and smaller (up to 0.2 × 0.2 × 0.15 mm) crystals of the same morphology from 35% TacsimateTM pH 8.0 (Figure 3c). The optimization of precipitating solutions containing PEG 3350 and inorganic salts yielded no monocrystals. No better quality crystals of ArthβDG were obtained for crystallization held at 4 °C, regardless of the concept that lowering the experiment temperature, thus thermal energy, should aid crystallization of highly flexible proteins. It might have been caused by its relatively high, as for cold-adapted protein, thermal optimum of 28 °C [37].
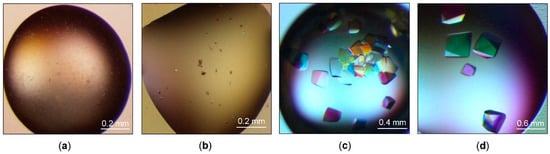
Figure 3.
ArthβDG crystals. The best results of the initial screening: (a) crystallization conditions containing 1.1 M sodium malonate pH 7.0, 0.1 M HEPES pH 7.0, 0.5% Jeffamine® ED-2001 pH 7.0; (b) 30% TacsimateTM pH 7.0; results of the optimization: (c) crystallization conditions containing 35% Tacsimate pH 8.0; (d) 1.4 M sodium malonate, 0.1 M HEPES pH 7.0, 1.5% Jeffamine® ED-2001 pH 7.0.
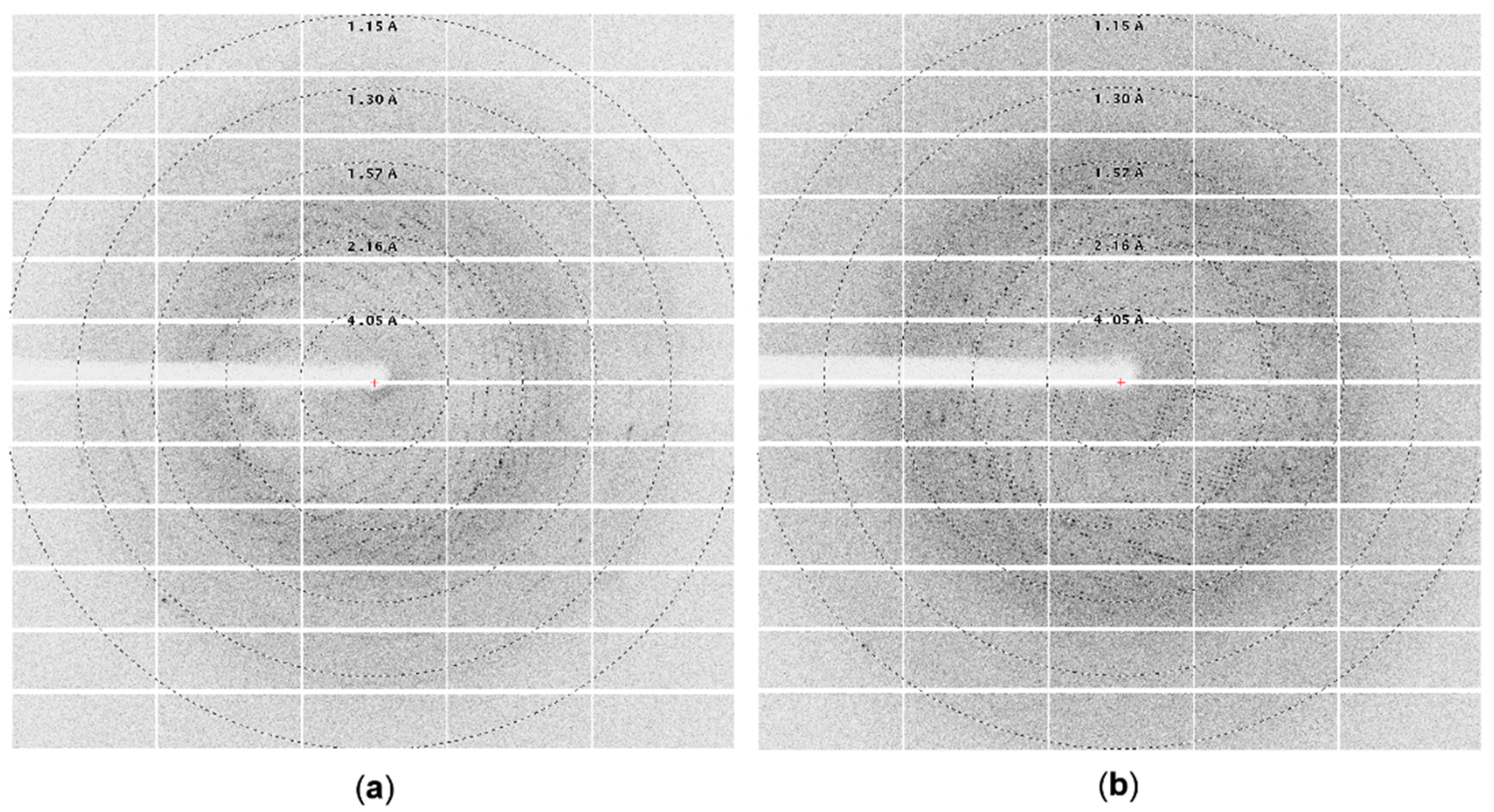
The diffraction experiment performed on initial larger crystals (Figure 3b) using a home source SuperNova diffractometer proved that they were of protein; however, their diffraction, due to the small size, was very poor (~8 Å). Further optimization of crystallization conditions and subsequent measurements utilizing synchrotron radiation allowed us to collect complete diffraction datasets with a resolution up to 2.2 Å for large crystals (Figure 3d) (Figure 4a) and up to 1.8 Å for a little smaller (but still big) crystals (Figure 3c) (Figure 4b). Even though the sizes of crystals from 35% TacsimateTM pH 8.0 were smaller, the resulting diffraction data had higher resolution.
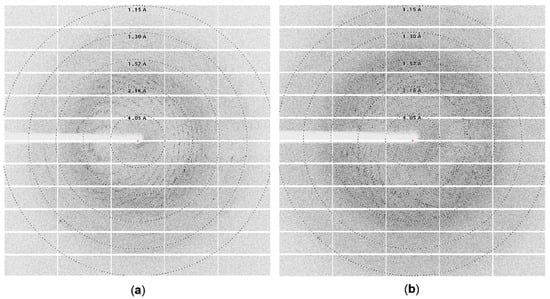
Figure 4.
Diffraction images collected on BL 14.2 line BESSY synchrotron : (a) using ArthβDG crystal crystallized in the presence of 1.4 M sodium malonate, 1.5% Jeffamine, and 0.1 M HEPES pH 7.0; (b) using ArthβDG crystal crystallized in the presence of 35% TacsimateTM pH 8.0.
Since the crystallization conditions included a high concentration of organic acids that might have been preventing ligand binding, the search for alternative crystallization conditions for complexes of ArthβDG with isopropyl β-d-1-thiogalactopyranoside (IPTG) and lactose was performed using the random Microseed Matrix Screening (rMMS) procedure. The introduction of seed stock to crystallization drops allowed us to determine multiple crystallization conditions, containing a minimal concentration of TacsimateTM (introduced with seeds). It is of note that the hits were partially different depending on the used ligand (Figure 5).
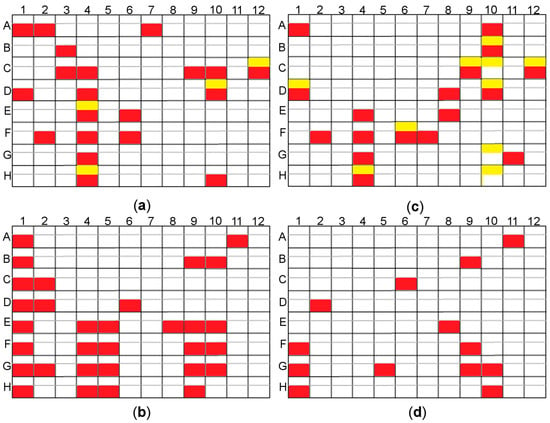
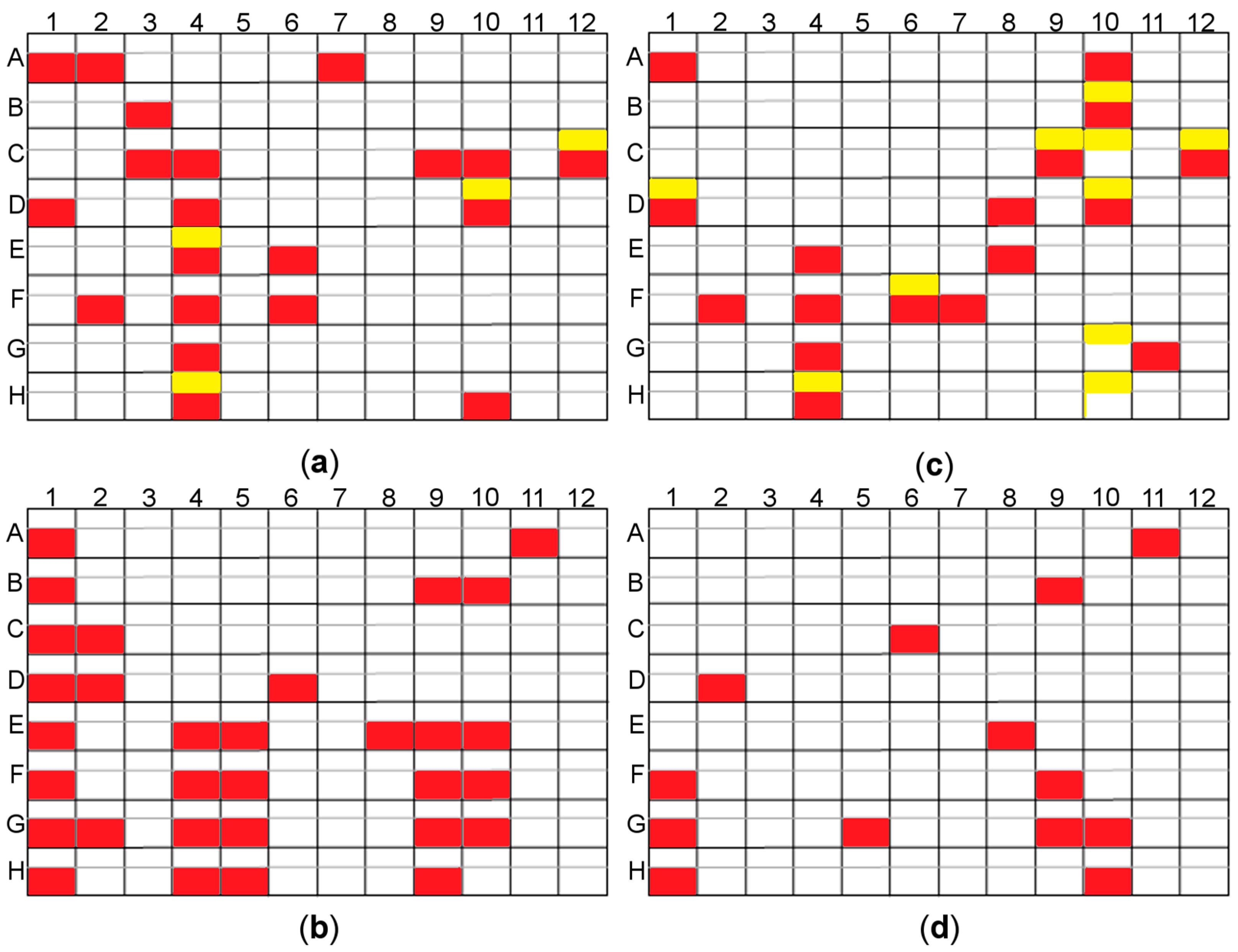
Figure 5.
The results of the rMMS experiments for two screen sets depending on the ligand added; yellow–crystals obtained with no seeding (control), red–crystals obtained by seeding; (a) PEG/Ion and PEG/Ion2 screen ArthβDG co-crystallized with lactose; (b) Morpheus and Morpheus II screen ArthβDG co-crystallized with isopropyl β-d-1-thiogalactopyranoside (IPTG); (c) PEG/Ion and PEG/Ion2 screen ArthβDG co-crystallized with IPTG; (d) Morpheus and Morpheus II screen ArthβDG co-crystallized with lactose. Some of the obtained crystals were big enough for diffraction experiment, e.g., plate (a) A7, plate (b) D8, however most of the obtained crystal hits needed further optimization. Use of microseeding enabled the picking up of a considerable amount of new hits.
Another issue with ArthβDG crystals obtained using the classical hanging drop vapor diffusion method was that crystals, which grew in the same drop possessing the same morphology and of a similar size, were randomly diffracting well (~2 Å) or very poorly (~10 Å). To ensure the required quantity of well diffracting crystals, the seeding of the known crystallization conditions was performed. Different seed stock dilutions, 10×, 100×, 1000×, and different protein concentrations, 6 mg/mL, 8 mg/mL, and 10 mg/mL were examined. The use of 8 mg/mL protein concentration and 1000× diluted seed stock yielded formation of ~10 diffracting crystals per drop with average dimensions of 0.25 × 0.2 × 0.2 mm. The adaptation of the seeding procedure for setting up crystallization manually was performed: 6% v/v of cool seed stock was added to the cooled protein solution directly, right before drop setting. The sample was kept on ice for the time of operations. The crystallization was then set up using the standard hanging drop procedure. Introduction of altered crystallization seeding and lowering the protein concentration to 8 mg/mL proved to reproducibly yield diffracting quality crystals. The number of crystals in a drop could be controlled by the use of different dilutions of readily available, pre-prepared and frozen seed stock.
3.2. Structure of ArthβDG
3.2.1. Overall Fold
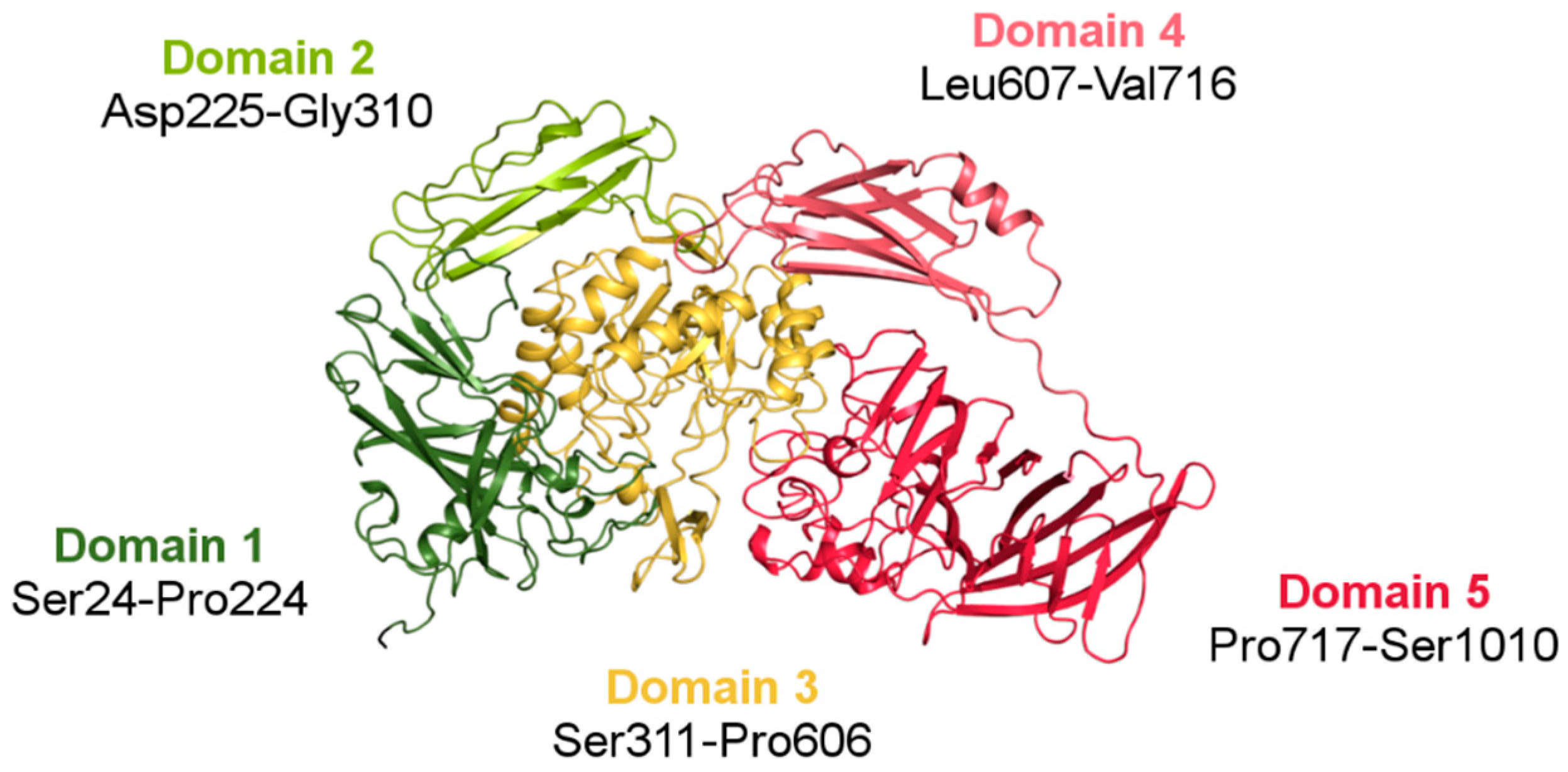
The crystal structure of ArthβDG (PDB ID: 6ETZ) revealed that the protein consists of five domains: (Domain 1 (Ser24-Pro224), Domain 2 (Asp225-Gly310), Domain 3 (Ser311-Pro606), Domain 4 (Leu607-Val716), and Domain 5 (Pro717-Ser1010)), with the catalytic one being TIM-barrel, which is typical for glycoside hydrolases. The other four domains, each with an immunoglobulin-like fold, surround Domain 3 and form the outer surface of the functional dimer. Domain 1, Domain 2, and Domain 4 are jelly-roll type barrels. Domain 5 (Pro717-Ser1010) is a large β-sandwich domain (Figure 6). The long linker between Domain 4 and Domain 5, comprising a high number of proline residues, provides some rigidity to this highly solvent exposed fragment. Regardless of low sequence similarity (35%) (Figure S1), ArthβDG monomer has an overall fold similar to the characteristics for this group of enzymes E. coli lacZ βDG. The intriguing questions, why nature produced such a big multi-domain enzyme to perform a relatively simple reaction, and what is the function of the surrounding domains, still remain open.
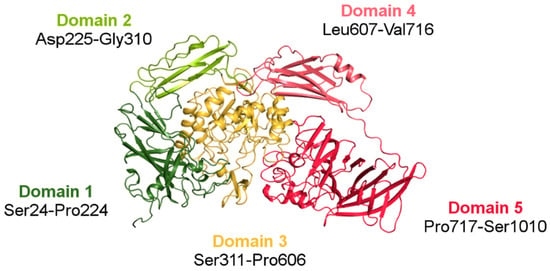
Figure 6.
A monomer of ArthβDG (PDB ID: 6ETZ) colored by domain. The central catalytic Domain 3 (gold) is typical for the GH2 family TIM-barrel fold. The Domains 1 (dark green) and 5 (red) are responsible for intramolecular contacts forming a biologically active dimer.
3.2.2. Architecture of the Functional Dimer
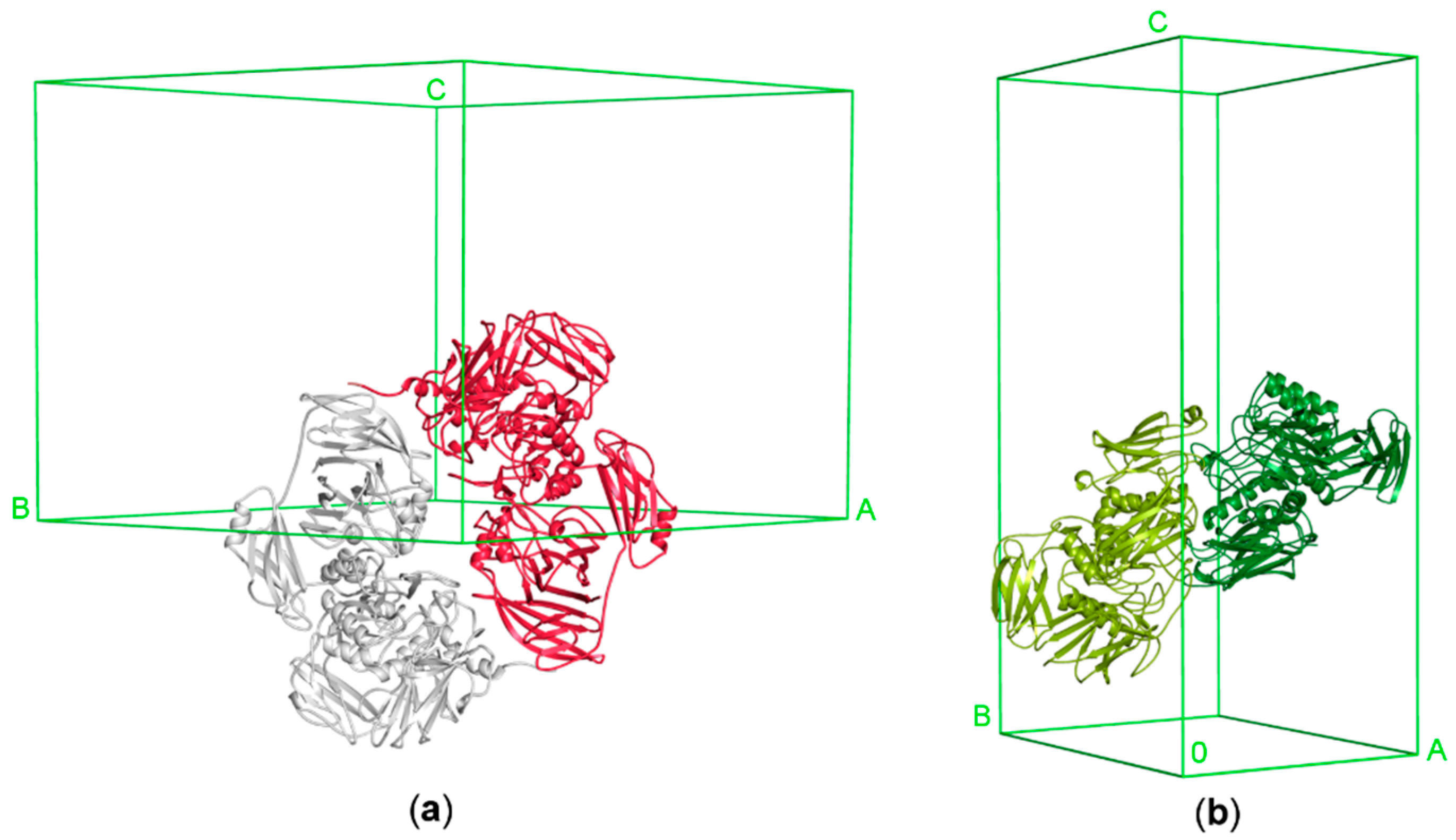
ArthβDG acts as a head-to-tail dimer, with two independent active sites. Even though only a monomer is present in the asymmetric unit of ArthβDG crystal, the functional dimer is mapped by the symmetry operations. The ArthβDG dimer interface is made by extensive contacts between Domain 1 interacting with Domain 5 of an adjacent monomer (Figure 7a). ParβDG acts also as a functional dimer, which is present in the asymmetric unit of the crystal [36]. The active site of these enzymes is created by residues belonging to both monomers of the dimer. The presence of the dimers in solution was confirmed by gel filtration chromatography (Figure S2), for both enzymes [36]. The ParβDG dimer is more elongated and its interface is made by extensive contacts between Domain 3 interacting with Domain 4 of an adjacent monomer and Domains 5 from both monomers (Figure 7b).
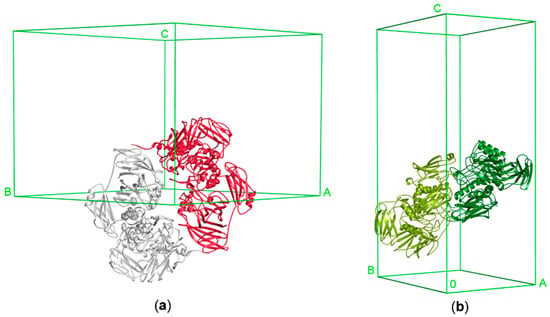
Figure 7.
The functional dimers of ArthβDG and ParβDG in unit cells: (a) ArthβDG (PDB ID: 6ETZ); (b) ParβDG (PDB ID: 5EUV).
The architecture of the functional dimer of ArthβDG differs significantly from the functional dimer of ParβDG (Figure 7). The buried area of the ParβDG dimer is 7180 Å2, while of the substantially larger ArthβDG, 6150 Å2, the assemblies analysis was performed using a PISA server (Table 4) [47].

Table 4.
PISA assembly analysis of ParβDG and ArthβDG.
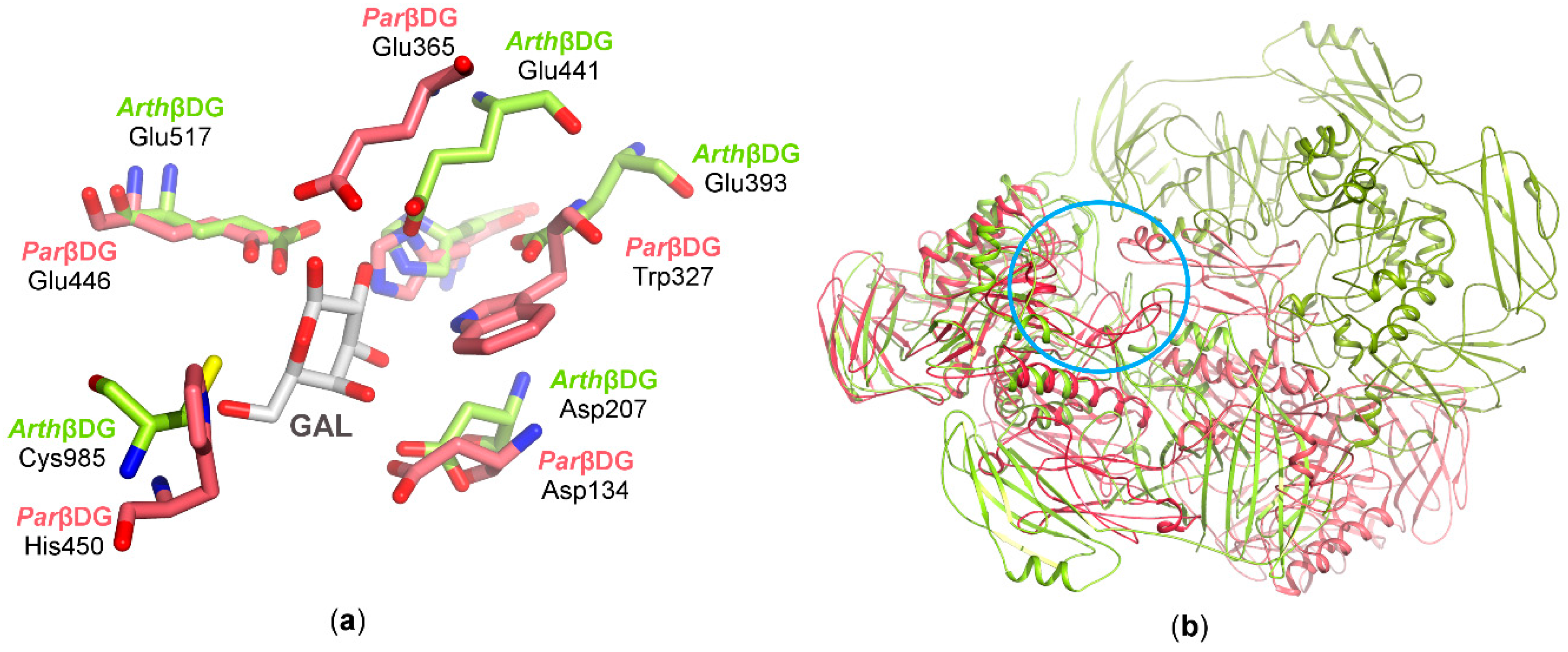
Crystal structures of these two enzymes indicate that the biological assembly arrangement is an important factor as to why ParβDG exhibits low transglycosylation activity. The cramped vicinity of ParβDG active site, and its deep location limits possible galactosyl group acceptors to primarly water molecules. By contrast, the more spacious and easily substrate-accessible active site of ArthβDG allows molecules such as galactose, fructose, or salicin, to be acceptors for transfer of the galactosyl group (Figure 8b).
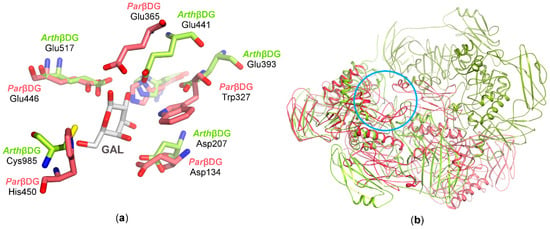
Figure 8.
The superposition of ArthβDG and ParβDG in complex with galactose: (a) residues in active sites involved in galactose binding; (b) the superposition of biologically active dimers with superposed active sites (marked with a blue circle) of monomers A (ParβDG presented in shades of red and ArthβDG in shades of green).
3.2.3. Catalytic Center
In both enzymes the catalytic domain is a highly conserved TIM-barrel, which contains eight parallel β-strands and seven α-helices. The catalytic residues were determined to be Glu441, Glu517 in ArthβDG and Glu365, Glu446 in ParβDG. A superposition of the active sites of ArthβDG with the other two cold-adapted βDGs, as well as their superpositions with homologous mesophilic GH2 βDG, revealed that the catalytic center is highly conserved in this group of enzymes.
A superposition of ArthβDG with ParβDG shows extensive similarity in the region of the catalytic amino acids; however, other parts of the catalytic center exhibit large differences (Figure 8a). Superimposed catalytic centers of both βDGs show that, besides identical catalytic amino acid residues stabilizing galactose binding, His302/His368 and Asp134/Asp207 are also conserved. However, differences in some residues, His450/Cys985 and Trp327/Glu393, result in alteration of active sites shape, volume, and character. The overlay of the biological dimers indicates how the differences in their assemblies influence the substrate selectivity.
The presence of the non-proline cis-peptide bonds in the close vicinity of active sites is a very intriguing observation, as such bonds are extremely rare. Two of them are crucial for the creation of an active site, as they constitute its bottom, and therefore regulate its volume. A comparison with other GH2 βDGs revealed that all three observed non-proline cis-peptides are present in the same places in this family of enzymes. Moreover, they are common for all glycoside hydrolases that catalyze the reaction with the retaining configuration on anomeric carbon of hydrolyzed β-glycoside.
4. Conclusions
Generally, nucleation is a critical stage in the crystallization of proteins, and for those possessing conformationally labile fragments, as e.g., cold adapted enzymes, it is a bottleneck of crystal structure determination, and it is necessary to implement factors lowering the nucleation barrier.
By implementation of different seeding techniques, such as streak seeding, in situ random microseeding, and streak microseeding, crystallization of two cold-adapted βDGs was successful. Further, the microseeding allowed us to obtain diffraction quality crystals in a routine manner, which significantly simplified further structural studies of these proteins and reduced uncertainties connected with the crystallization stage. Additionally, the used techniques of microseeding allowed growing crystals after nucleation (coming from seeds) without achieving oversaturation.
In the case of cold-adapted proteins, which are more prone to thermal denaturation then mesophilic enzymes, the temperature factor must be taken into account at all stages of protein preparation. In the case of ArthβDG, we were able to obtain more protein sample from the same volume of biomass when we used mortar and pestle under liquid nitrogen instead of sonication. The sonication produces a large amount of heat, even though extensive sample cooling was introduced into the protocol, the cold-adapted ArthβDG was too sensitive for this method. The next step, chromatographic purification, was even more susceptible to temperature elevation due to its long overall time. We were able to obtain well crystallizing protein samples only when the whole purification procedure was conducted at 4 °C.
The crystal structure of ArthβDG, as well as establishing purification and crystallization protocols, gives a good starting point for further crystallographic analysis of this enzyme aiming at its activity engineering [48]. The comparison of its catalytic center, with other βDGs indicates putative residues involved in the transglycosylation reaction.
Supplementary Materials
The following are available online at www.mdpi.com/2073-4352/8/1/13/s1. Figure S1: The sequence alignment of ArthβDG and ParβDG performed using EMBOSS Needle Pairwise Sequence Alignment. The sequence similarity is 35%, and identity only 17.6% with 47.3% gaps. Catalytic amino acids marked with red boxes; Figure S2: Biochemical oligomerization assays of ArthβDG. (a) SDS-PAGE analysis stained with Coomasie Brillant Blue G; lane 1 protein molecular-weight markers (Thermo Fisher Scientific), lane 2 ArthβDG; (b) Ve/V0 versus log MW calibration curve for separation of proteins on a Superdex 200 10/200 GL column with ArthβDG marked red (Ve, elution volume; V0, void volume); (c) chromatographic separation of the fraction containing active ArthβDG by gel filtration on a Superdex 200 10/300 GL column.
Acknowledgments
This research was supported by grant 2016/21/B/ST5/00555 (A.B.) from the National Science Centre, Poland. We thank Patrick Shaw Stewart for providing a possibility to test Oryx4 (Douglas Instruments Ltd., East Garston, UK) using our samples. We thank the HZB BESSY Berlin, Germany for providing access to BL 14.2 beamline and MX staff for providing support on beamline. We are grateful to A. Wlodawer, NCI for help with editing the manuscript.
Author Contributions
Maria Rutkiewicz-Krotewicz and Agnieszka J. Pietrzyk-Brzezinska performed crystallization of βDGs; Maria Rutkiewicz-Krotewicz and Anna Bujacz performed synchrotron diffraction data collection, processing, structure solving and carried out structural analysis. Maria Rutkiewicz-Krotewicz purified enzyme, refined the structure and mostly wrote the paper to which Anna Bujacz and Agnieszka J. Pietrzyk-Brzezinska also contributed. Marta Wanarska and Hubert Cieslinski performed enzymes expression in E. coli and determined the purification protocol. Anna Bujacz coordinated the project.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Pritzwald-Stegmann, B.F. Lactose and some of its derivatives. Int. J. Dairy Technol. 1986, 39, 91–97. [Google Scholar] [CrossRef]
- Khan, M.; Husain, Q.; Bushra, R. Immobilization of β-galactosidase on surface modified cobalt/multiwalled carbon nanotube nanocomposite improves enzyme stability and resistance to inhibitor. Int. J. Biol. Macromol. 2017, 105, 693–701. [Google Scholar] [CrossRef] [PubMed]
- Traffano-Schiffo, M.V.; Castro-Giraldez, M.; Fito, P.J.; Santagapita, P.R. Encapsulation of lactase in Ca(II)-alginate beads: Effect of stabilizers and drying methods. Food Res. Int. 2017, 1000, 296–303. [Google Scholar] [CrossRef] [PubMed]
- Borghini, R.; Donato, G.; Alvaro, D.; Picarelli, A. New insights in IBS-like disorders: Pandora’s box has been opened; a review. Gastroenterol. Hepatol. Bed Bench 2017, 10, 79–89. [Google Scholar] [PubMed]
- Rossi, M.; Aggio, R.; Staudacher, H.M.; Lomer, M.C.; Lindsay, J.O.; Irving, P.; Probert, C.; Whelan, K. Volatile organic compounds in feces associate with response to dietary intervention in patients with irritable bowel syndrome. Clin. Gastroenterol. Hepatol. 2017, S1542–S3565, 31201–31216. [Google Scholar] [CrossRef] [PubMed]
- Staudacher, H.M.; Lomer, M.C.E.; Farquharson, F.M.; Louis, P.; Fava, F.; Franciosi, E.; Scholz, M.; Tuohy, K.M.; Lindsay, J.O.; Irving, P.; et al. A diet low in fodmaps reduces symptoms in patients with irritable bowel syndrome and a probiotic restores Bifidobacterium species: A randomized controlled trial. Gastroenterology 2017, 153, 936–947. [Google Scholar] [CrossRef] [PubMed]
- Cozma-Petrut, A.; Loghin, F.; Miere, D.; Dumitraşcu, D.L. Diet in irritable bowel syndrome: What to recommend, not what to forbid to patients! World J. Gastroenterol. 2017, 23, 3771–3783. [Google Scholar] [CrossRef] [PubMed]
- Yuce, O.; Kalayci, A.G.; Comba, A.; Eren, E.; Caltepe, G. Lactose and fructose intolerance in Turkish children with chronic abdominal pain. Indian Pediatr. 2016, 53, 394–407. [Google Scholar] [CrossRef] [PubMed]
- Pawłowska, K.; Umławska, W.; Iwańczak, B. Prevalence of lactose malabsorption and lactose intolerance in pediatric patients with selected gastrointestinal diseases. Adv. Clin. Exp. Med. 2015, 24, 863–871. [Google Scholar] [CrossRef] [PubMed]
- Wierzbicka-Wos, A.; Cieslinski, H.; Wanarska, M.; Kozlowska-Tylingo, K.; Hildebrandt, P.; Kur, J. A novel cold-active β-d-galactosidase from the Paracoccus sp. 32d-gene cloning, purification and characterization. Microb. Cell Fact. 2011, 10, 108–119. [Google Scholar] [CrossRef] [PubMed]
- Bialkowska, A.M.; Cieslinski, H.; Nowakowska, K.M.; Kur, J.; Turkiewicz, M. A new beta-galactosidase with a low temperature optimum isolated from the Antarctic Arthrobacter sp. 20B: Gene cloning, purification and characterization. Arch. Microbiol. 2009, 191, 825–835. [Google Scholar] [CrossRef] [PubMed]
- Cavicchioli, R.; Charlto, T.; Ertan, H.; Mohd Omar, S.; Siddiqui, K.S.; Williams, T.J. Biotechnological uses of enzymes from psychrophiles. Microb. Biotechnol. 2011, 4, 449–460. [Google Scholar] [CrossRef] [PubMed]
- Harju, M. Milk sugars and minerals as ingredients. Int. J. Dairy Technol. 2001, 54, 61–63. [Google Scholar] [CrossRef]
- Kunz, C.; Rudloff, S. Biological functions of oligosaccharides in human milk. Acta Paediatr. 1993, 82, 903–912. [Google Scholar] [CrossRef] [PubMed]
- Boehm, G.; Fanaro, S.; Jelinek, J.; Stahl, B.; Marini, A. Prebiotic concept for infant nutrition. Acta Paediatr. 2003, 92, 64–67. [Google Scholar] [CrossRef]
- Chierici, R.; Fanaro, S.; Saccomandi, D.; Vigi, V. Advances in the modulation of the microbial ecology of the gut in early infancy. Acta Paediatr. 2003, 92, 56–63. [Google Scholar] [CrossRef]
- Bujacz, A.; Jędrzejczak-Krzepkowska, M.; Bielecki, S.; Redzynia, I.; Bujacz, G. Crystal structures of the apo form of β-fructofuranosidase from Bifidobacterium longum and its complex with fructose. FEBS J. 2011, 278, 1728–1744. [Google Scholar] [CrossRef] [PubMed]
- Salminen, S.; Endo, A.; Scalabrin, D. Early gut colonization with Lactobacilli and Staphylococcus in infants: The hygiene hypothesis extended. J. Pediatr. Gastroenterol. Nutr. 2016, 62, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, D.L.; Wilbey, R.A.; Grandison, A.S.; Roseiro, L.B. Milk oligosaccharides: A review. Diary Technol. 2015, 68, 305–321. [Google Scholar] [CrossRef]
- Lee, L.Y.; Bharani, R.; Biswas, A.; Lee, J.; Tran, L.-A.; Pecquet, S.; Steenhout, P. Normal growth of infants receiving an infant formula containing Lactobacillus reuteri, galacto-oligosaccharides, and fructo-oligosaccharide: A randomized controlled trial. Matern. Health Neonatol. Perinatol. 2015, 1, 9. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.T.P.; Bhandari, B.; Cichero, J.; Parakash, S. A comprehensive review on in vitro digestion of infant formula. Food Res. Int. 2015, 76, 373–386. [Google Scholar] [CrossRef] [PubMed]
- Musilova, S.; Rada, V.; Vlkova, E.; Bunesova, V. Beneficial effects of human milk oligosaccharides on gut microbiota. Benef. Microbes 2014, 5, 273–283. [Google Scholar] [CrossRef] [PubMed]
- Orrhage, K.; Nord, C.E. Factors controlling the bacterial colonization of the intestine in breastfed infants. Acta Paediatr. 1999, 88, 47–57. [Google Scholar] [CrossRef]
- Li, M.; Monaco, M.H.; Wang, M.; Comstock, S.S.; Kuhlenschmidt, T.B.; Donovan, S.M. Human milk oligosaccharides shorten rotavirus-induced diarrhea and modulate piglet mucosal immunity and colonic microbiota. ISME J. 2014, 8, 1609–1620. [Google Scholar] [CrossRef] [PubMed]
- McVeagh, P.; Miller., J.B. Human milk oligosaccharides: Only the breast. Acta Paediatr. 1997, 33, 281–286. [Google Scholar] [CrossRef]
- Wallace, T.C.; Marzorati, M.; Spence, L.; Weaver, C.M.; Williamson, P.S. New frontiers in fibers: Innovative and emerging research on the gut microbiome and bone health. J. Am. Coll. Nutr. 2017, 36, 218–222. [Google Scholar] [CrossRef] [PubMed]
- Whisner, C.M.; Martin, B.R.; Schoterman, M.H.; Nakatsu, C.H.; McCabe, L.D.; Wastney, M.E.; van den Heuvel, E.G.; Weaver, C.M. Galacto-oligosaccharides increase calcium absorption and gut Bifidobacteria in young girls: A double-blind cross-over trial. Br. J. Nutr. 2013, 110, 1292–1303. [Google Scholar] [CrossRef] [PubMed]
- Weaver, C.M.; Martin, B.R.; Nakatsu, C.H.; Armstrong, A.P.; Clavijo, A.; McCabe, L.D.; McCabe, G.P.; Duignan, S.; Schoterman, M.H.; van den Heuvel, E.G. Galactooligosaccharides improve mineral absorption and bone properties in growing rats through gut fermentation. J. Agric. Food Chem. 2011, 59, 6501–6510. [Google Scholar] [CrossRef] [PubMed]
- Hughes, C.; Davoodi-Semiromi, Y.; Colee, J.C.; Culpepper, T.; Dahl, W.J.; Mai, V.; Christman, M.C.; Langkamp-Henken, B. Galactooligosaccharide supplementation reduces stress-induced gastrointestinal dysfunction and days of cold or flu: A randomized, double-blind, controlled trial in healthy university students. Am. J. Clin. Nutr. 2011, 93, 1305–1311. [Google Scholar] [CrossRef] [PubMed]
- Mussatto, S.I.; Mancilha, I.M. Non-digestible oligosaccharides: A review. Carbohydr. Polym. 2007, 68, 587–597. [Google Scholar] [CrossRef]
- Swennen, K.; Courtin, C.M.; Delcour, J.A. Non-digestible oligosaccharides with prebiotic properties. Crit. Rev.. Food Sci. Nutr. 2006, 459–471. [Google Scholar] [CrossRef] [PubMed]
- Juers, D.H.; Heightman, T.D.; Vasella, A.; McCarter, J.D.; Mackenzie, L.; Withers, S.G.; Matthews, B.W. A structural view of the action of Escherichia coli (lacZ) β-galactosidase. Biochemistry 2001, 40, 14781–14794. [Google Scholar] [CrossRef] [PubMed]
- Skalova, T.; Dohnalek, J.; Spiwok, V.; Lipovova, P.; Vondrackova, E.; Petrokova, H.; Duskova, J.; Strnad, H.; Kralova, B.; Hasek, J. Cold-active beta-galactosidase from Arthrobacter sp. C2-2 forms compact 660 kDa hexamers: Crystal structure at 1.9 Å resolution. J. Mol. Biol. 2005, 353, 282–294. [Google Scholar] [CrossRef] [PubMed]
- Faning, S.; Leahy, M.; Sheehan, M. Nucleotide and deduced amino acid sequences of Rhizobium meliloti 102F34 lacZ gene: Comparison with prokaryotic beta-galactosidases and human beta-glucuronidase. Gene 1994, 141, 91–96. [Google Scholar] [CrossRef]
- Burchhardt, G.; Bahl, H. Cloning and analysis of the beta-galactosidase-encoding gene from Clostridium thermosulfurogenes EM1. Gene 1991, 106, 13–19. [Google Scholar] [CrossRef]
- Rutkiewicz-Krotewicz, M.; Pietrzyk-Brzezinska, A.J.; Sekula, B.; Cieslinski, H.; Wierzbicka-Wos, A.; Kur, J.; Bujacz, A. Structural studies of a cold-adapted dimeric β-d-galactosidase from Paracoccus sp. 32d. Acta Crystallogr. D 2016, 72, 1049–1061. [Google Scholar] [CrossRef] [PubMed]
- Pawlak-Szukalska, A.; Wanarska, M.; Popinigis, A.T.; Kur, J. A novel cold-active β-d-galactosidase with transglycosylation activity from the Antarctic Arthrobacter sp. 32cB-gene cloning, purification and characterization. Process. Biochem. 2014, 49, 2122–2133. [Google Scholar] [CrossRef]
- Shaw Stewart, P.D.; Kolek, S.A.; Briggs, R.A.; Chayen, N.E.; Baldock, P.F.M. Random microseeding: A theoretical and practical exploration of seed stability and seeding techniques for successful protein crystallization. Cryst. Growth Des. 2011, 11, 3432–3441. [Google Scholar] [CrossRef]
- Bujacz, G.; Wrzesniewska, B.; Bujacz, A. Cryoprotection properties of salts of organic acids: A case study for a tetragonal crystal of hew lysozyme. Acta Crystallogr. D 2010, 66, 789–796. [Google Scholar] [CrossRef] [PubMed]
- Sparta, K.M.; Krug, M.; Heinemann, U.; Mueller, U.; Weiss, M.S. Xdsapp2.0. J. Appl. Crystallogr. 2016, 49, 1085–1092. [Google Scholar] [CrossRef]
- McCoy, A.J.; Grosse-Kunstleve, R.W.; Adams, P.D.; Winn, M.D.; Storoni, L.C.; Read, R.J. Phaser crystallographic software. J. Appl. Cryst. 2007, 40, 658–674. [Google Scholar] [CrossRef] [PubMed]
- Emsley, P.; Cowtan, K. Coot: Model-building tools for molecular graphics. Acta Crystallogr. D 2004, 60, 2126–2132. [Google Scholar] [CrossRef] [PubMed]
- Murshudov, G.N.; Vagin, A.A.; Dodson, E.J. Refinement of macromolecular structures by the maximum-likelihood method. Acta Crystallogr. D 1997, 53, 240–255. [Google Scholar] [CrossRef] [PubMed]
- Winn, M.D.; Isupov, M.N.; Murshudov, G.N. Use of TLS parameters to model anisotropic displacements in macromolecular refinement. Acta Crystallogr. D 2001, 57, 122–133. [Google Scholar] [CrossRef] [PubMed]
- Gerday, C. Psychrophily and catalysis. Biology 2013, 2, 719–741. [Google Scholar] [CrossRef] [PubMed]
- Bujacz, A.; Rutkiewicz-Krotewicz, M.; Nowakowska-Sapota, K.; Turkiewicz, M. Crystal structure and enzymatic properties of a broad substrate-specificity psychrophilic aminotransferase from the Antarcticsoil bacterium Psychrobacter sp. B6. Acta Crystallogr. D 2015, 71, 632–645. [Google Scholar] [CrossRef] [PubMed]
- Krissnel, E.; Henrick, K. Interference of macromolecular assemblies from crystalline state. J. Mol. Biol. 2007, 372, 774–797. [Google Scholar] [CrossRef] [PubMed]
- Talens-Perales, D.; Polaina, J.; Marín-Navarro, J. Enzyme Engineering for Oligosaccharide Biosynthesis. In Frontier Discoveries and Innovations in Interdisciplinary Microbiology; Springer: New Delhi, India, 2016; Chapter 2; pp. 9–31. [Google Scholar]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).