Synthesis, Crystal Structure, and Fluorescent Property of a Zn(II) Complex with N-Nicotinoylglycine Ligand
Abstract
:1. Introduction
2. Results and Discussion
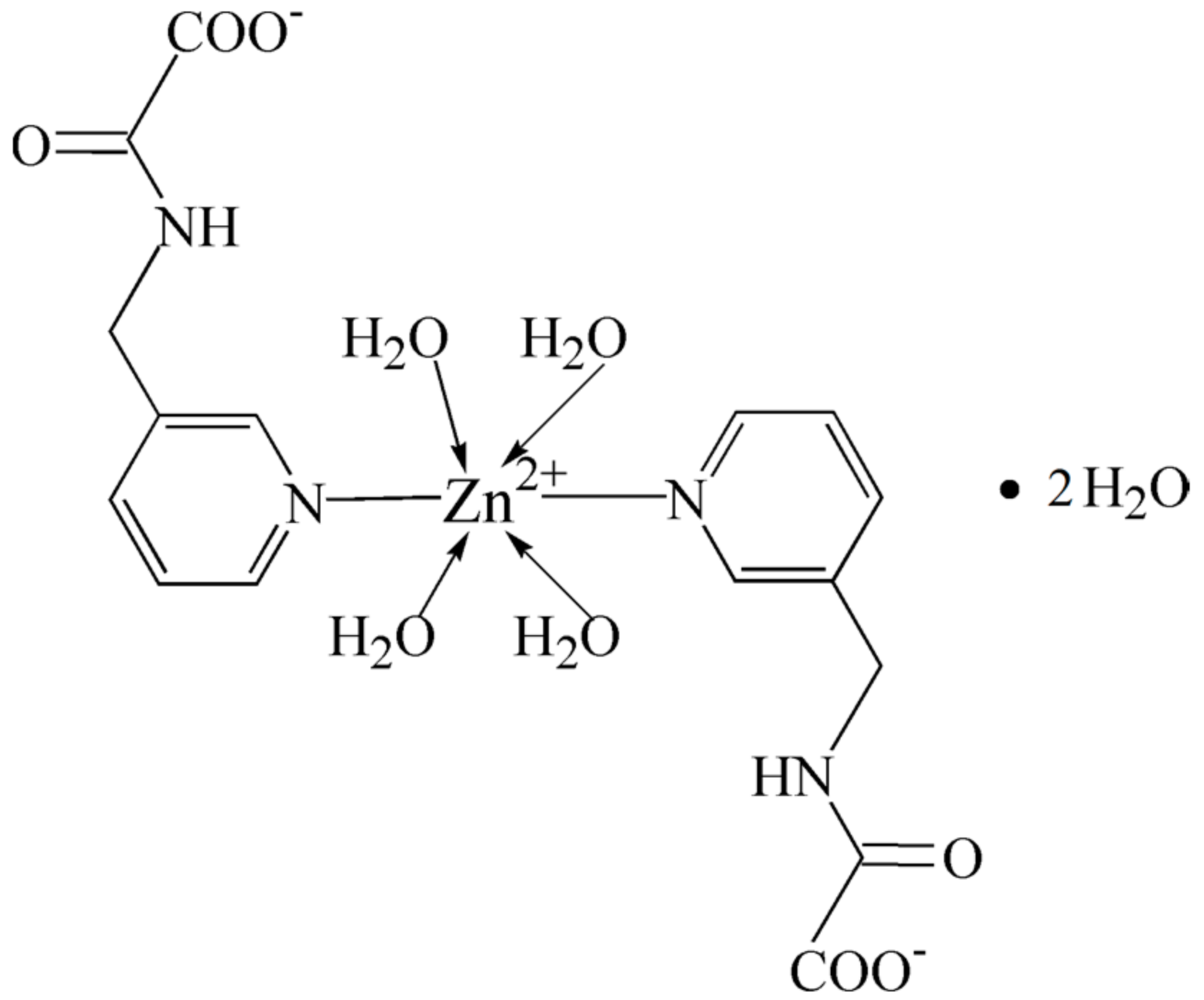
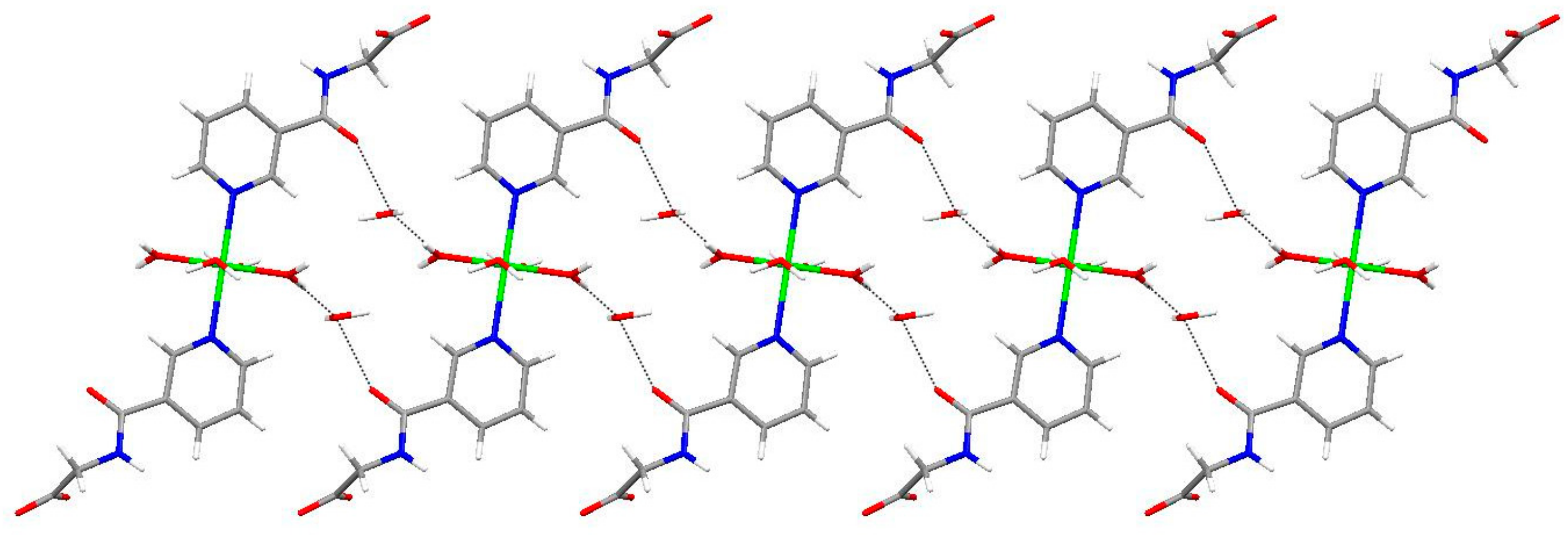
2.1. Structural Description of Zn(II) Complex (1)
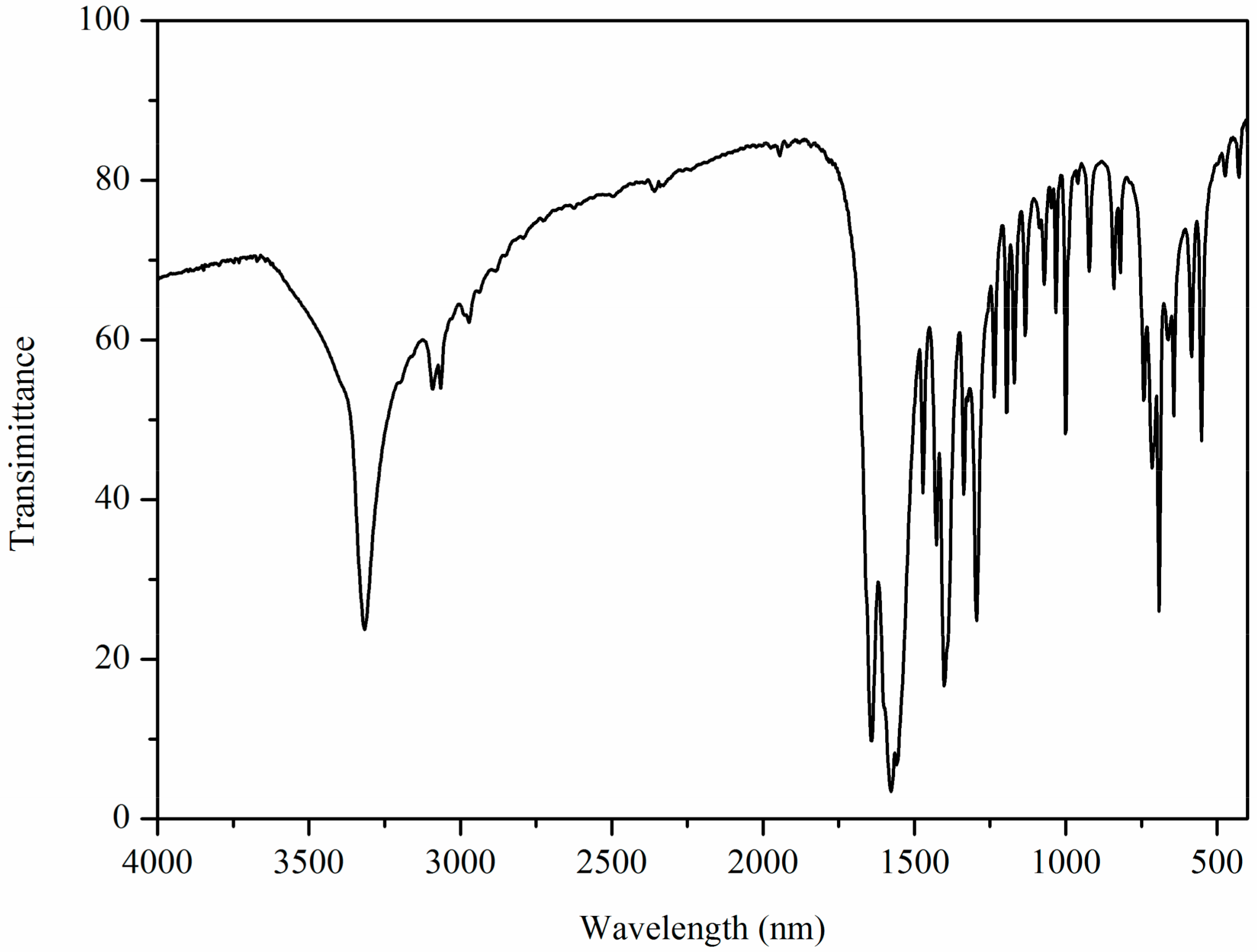
2.2. IR Characterization of Zn(II) Complex
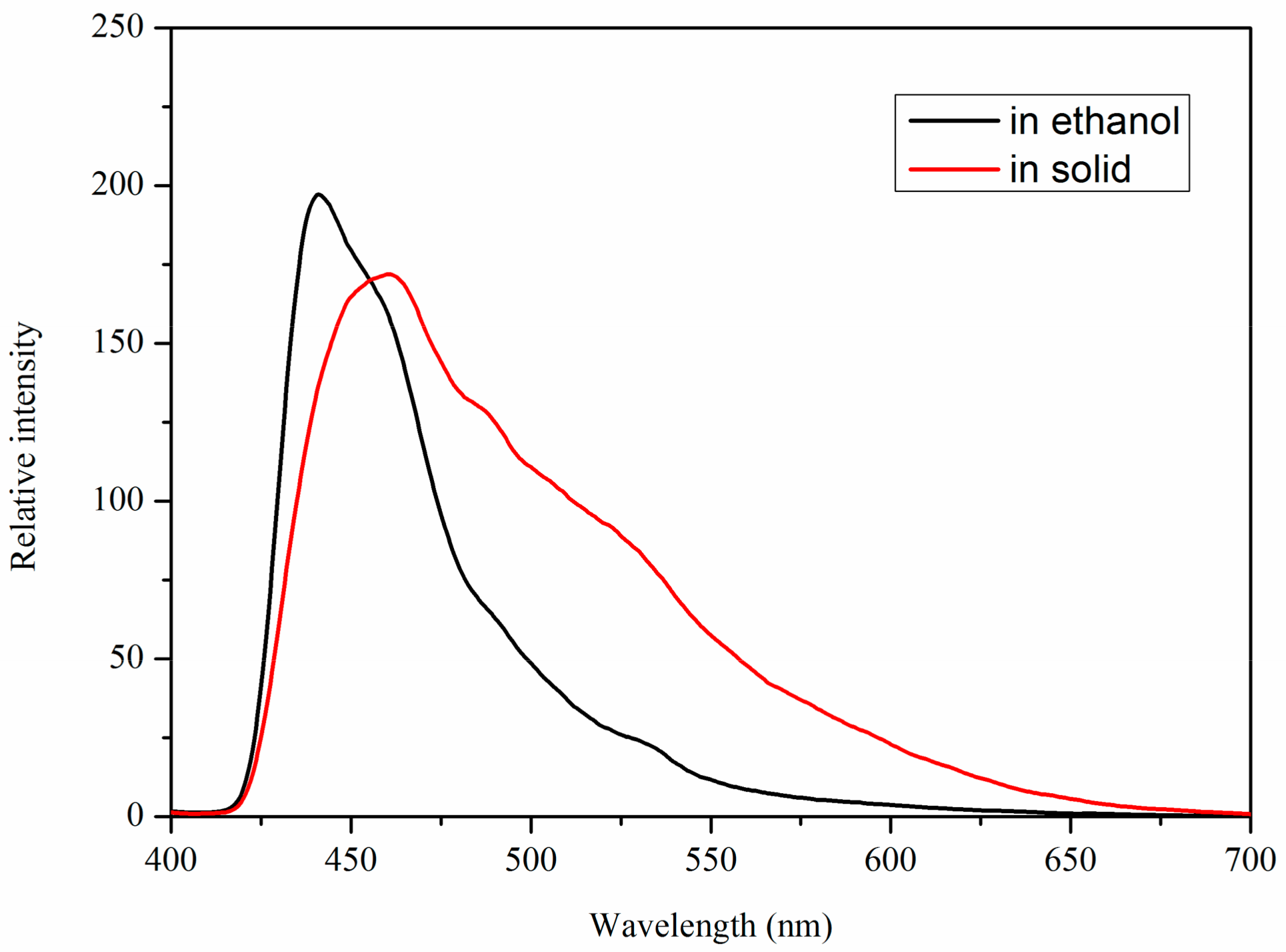
2.3. Fluorescent Properties
3. Experimental Section
3.1. Materials and Instrumentation
3.2. Synthesis of [ZnL2(H2O)4]·H2O (1)
3.3. Crystal Data Collection and Structure Determination
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Yaghi, O.M. Reticular chemistry-construction, properties, and precision reactions of frameworks. J. Am. Chem. Soc. 2016, 138, 15507–15509. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, T.; Yamashiro, C.; Taya, M.; Kure, B.; Tanase, T. Systematic synthesis of di-, tri-, and tetranuclear homo- and heterometal complexes using a mononuclear copper synthon with a tetradentate amino alcohol ligand. Eur. J. Inorg. Chem. 2016, 2016, 2764–2773. [Google Scholar] [CrossRef]
- Lane, A.C.; Vollmer, M.V.; Laber, C.H.; Melgarejo, D.Y.; Chiarella, G.M.; Fackler, J.P.; Yang, X.Z.; Baker, G.A.; Walensky, J.R. Multinuclear copper(I) and silver(I) amidinate complexes: Synthesis, luminescence, and CS2 insertion reactivity. Inorg. Chem. 2014, 53, 11357–11366. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.B.; Xu, S.X.; Wang, J.L.; Zhao, F.; Xia, H.Y.; Wang, Y.B. Four-coordinate N-heterocyclic carbene (NHC) copper(I) complexes with brightly luminescence properties. J. Coord. Chem. 2017, 70, 584–599. [Google Scholar] [CrossRef]
- Tai, X.S.; Wang, X. Synthesis and crystal structure of 1D chained coordination polymer constructed from Ca2+ and 2-[(E)-(2-furoylhydrazono)methyl]benzenesulfonate. Crystals 2015, 5, 458–465. [Google Scholar] [CrossRef]
- He, J.Y.; Liu, Z.X.; Du, G.X.; Fu, Y.X.; Zhang, S.W.; Li, X.F. Chiral Palladium(II) and Nickel(II) Complexes with C2-Symmetrical Tridentate Bis(oxazoline) Ligands: Synthesis, Characterization, and Catalytic Norbornene Polymerization. Organometallics 2014, 33, 6103–6112. [Google Scholar] [CrossRef]
- Hazari, D.; Jana, S.K.; Puschmann, H.; Zangrando, E.; Dalai, S. Three new Coordination polymers of zinc(II) and cadmium(II) with dicarboxylate and bipyridine ligands: Synthesis, structure and luminescence study. J. Inorg. Organomet. Polym. 2015, 25, 1151–1159. [Google Scholar] [CrossRef]
- Wang, L.H.; Liang, L.; Wang, X. Synthesis, structural characterization and catalytic activity of a Cu(II) coordination polymer constructed from 1,4-phenylenediacetic acid and 2,2-bipyridine. Bull. Chem. React. Eng. Catal. 2017, 12, 113–118. [Google Scholar] [CrossRef]
- Feng, L.; Klaus, H. Electrochemistry of dinuclear organometallic [5]trovacenyl derivatives bridged by group 14 elements (Si, Ge, Sn, Pb). Eur. J. Inorg. Chem. 2017, 2017, 82–87. [Google Scholar] [CrossRef]
- Tai, X.S.; Wang, X. Synthesis, structural characterization and antitumor activity of a Ca(II) coordination polymer based on 4-formyl-1,3-benzenedisulfonate-2-furoic acid hydrazide ligands. Crystallogr. Rep. 2017, 62, 242–245. [Google Scholar] [CrossRef]
- Shang, X.F.; Ren, K.; Li, J.; Li, W.L.; Fu, J.J.; Zhang, X.L.; Zhang, J.L. Multi-properties of a Cu(II) complex: Crystal structure, anion binding ability, bioactivity and cell cytotoxicity. Inorg. Chim. Acta 2017, 456, 199–206. [Google Scholar] [CrossRef]
- Cardoso, J.M.S.; Correia, I.; Galvão, A.M.; Marques, F.; Carvalho, M.F.N.N. Synthesis of Ag(I) camphor sulphonylimine complexes and assessment of their cytotoxic properties against cisplatin-resistant A2780cisR and A2780 cell lines. J. Inorg. Biochem. 2017, 166, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Nday, C.M.; Halevas, E.; Tsiaprazi-Stamou, A.; Eleftheriadou, D.; Hatzidimitriou, A.; Jackson, G.; Reid, D.; Salifoglou, A. Synthetic investigation, physicochemical characterization and antibacterial evaluation of ternary Bi(III) systems with hydroxycarboxylic acid and aromatic chelator substrates. J. Inorg. Biochem. 2017, 170, 98–108. [Google Scholar] [CrossRef] [PubMed]
- Yoon, M.Y.; Moon, D.H. New Zr (IV) based metal-organic framework comprising a sulfur-containing ligand: Enhancement of CO2 and H2 storage capacity. Micropor. Mesopor. Mat. 2015, 215, 116–122. [Google Scholar] [CrossRef]
- Yang, L.Z.; Wang, J.; Kirillov, A.M.; Dou, W.; Xu, C.; Fang, R.; Xu, C.L.; Liu, W.S. 2D lanthanide MOFs driven by a rigid 3,5-bis(3-carboxy-phenyl)pyridine building block: Solvothermal syntheses, structural features, and photoluminescence and sensing properties. Cryst. Eng. Commun. 2016, 18, 6425–6436. [Google Scholar] [CrossRef]
- Liu, W.; Huang, X.; Xu, C.; Chen, C.Y.; Yang, L.Z.; Dou, W.; Chen, W.M.; Yang, H.; Liu, W.S. A multi-responsive regenerable europium-organic framework luminescent sensor for Fe3+, CrVI anions, and picric acid. Chem. Eur. J. 2016, 22, 18769–18776. [Google Scholar] [CrossRef] [PubMed]
- Nawrot, I.; Czerwińska, K.; Machura, B.; Kruszynski, R. Synthesis, structural diversity and luminescent properties of cadmium(II) coordination assemblies with 2-(2-aminophenyl)-1H-benzimidazole and pseudohalide ions. J. Lumin. 2017, 181, 103–113. [Google Scholar] [CrossRef]
- Liu, Y.L.; Chen, F.Y.; Di, Y.Q.; Cao, J.; Di, Y.Y.; Zhou, C.S. Two coordination polymers based on a flexible tritopic pyridyldicarboxylate ligand: Structures and magnetic properties. Z. Anorg. Allg. Chem. 2016, 642, 246–249. [Google Scholar] [CrossRef]
- Ma, D.Y.; Hu, P.; Qin, L.; Yan, J.J.; Lin, W.J.; Ding, W.Q.; Lu, H.S.; Lin, D.T.; Sakiyama, H.; Liang, F.L. Synthesis, characterization, and magnetic properties of two transition metal coordination polymers based on 2,5-furandicarboxylic acid and N-donor ligands. J. Inorg. Organomet. Polym. Mater. 2016, 26, 1053–1060. [Google Scholar] [CrossRef]
- Wang, L.H.; Wang, X.; Tai, X.S. Synthesis, crystal structure and catalytic activity of a 1D chained Ca(II) coordination polymer with 3,5-bis(4-pyridylmethoxy)benzoate ligand. Crystals 2017, 7, 72. [Google Scholar] [CrossRef]
- Wang, X.; Chen, B.Q.; He, M. Synthesis, crystal structure of a novel Mn(II) complex with nicotinoyl-glycine. Crystals 2017, 7, 3. [Google Scholar] [CrossRef]
- Nakamoto, K. Infrared and Ramen Spectra of Inorganic and Coordination Compounds, 3rd ed.; John Wiley and Sons: New York, NY, USA, 1978; Volume 1, pp. 359–368. ISBN 9780471163947. [Google Scholar]
- Tai, X.S.; Wang, X.; Li, P.F. Synthesis, crystal structure and luminescent property of a Cd(II) coordination polymer with a N-nicotinoylglycine ligand. Crystals 2017, 7, 33. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXL-97 and SHELXS-97; University of Göttingen: Göttingen, Germany, 1997. [Google Scholar]


| Bond | d | Bond | d |
|---|---|---|---|
| Zn1-O1W | 2.067 (2) | Zn1-O2W | 2.069 (2) |
| Zn1-N1 | 2.254 (3) | C7-N2 | 1.445 (4) |
| C6-O1 | 1.227 (4) | O2-C8 | 1.234 (4) |
| C8-O3 | 1.259 (4) | C1-N1 | 1.338 (5) |
| C5-N1 | 1.342 (4) | N2-C6 | 1.338 (4) |
| Angle | ω | Angle | ω |
| O1WA-Zn1-O1W | 180.00 (14) | O1WA-Zn1-O2WA | 89.89 (10) |
| O2WA-Zn1-O1W | 90.11 (10) | O2W-Zn1-O2WA | 180.0 |
| O1WA-Zn1-N1 | 88.36 (11) | O1W-Zn1-N1 | 91.64 (11) |
| O2WA-Zn1-N1 | 89.84 (10) | O2W-Zn1-N1 | 90.16 (10) |
| N1A-Zn1-N1 | 180.00 (1) |
| Donor-H…Acceptor | D-H | H…A | D…A | ∠D-H…A | Symmetry Transformation |
|---|---|---|---|---|---|
| O1W-H1WB…O3 | 0.96 | 1.95 | 2.699 (4) | 133 | −x + 1, −y + 1, −z |
| O1W-H1WC…O3 | 0.96 | 2.05 | 2.676 (4) | 122 | x, y + 1, z − 1 |
| O2W-H2WA…O2 | 0.86 | 1.81 | 2.649 (4) | 164 | x, y + 1, z − 1 |
| O2W-H2WB…O3W | 0.86 | 1.82 | 2.672 (4) | 168 | x + 1, y + 1, z − 1 |
| Empirical formula | C16H26N4O12Zn |
| Formula weight | 531.78 |
| Temperature/K | 293 (2) |
| Crystal system | Triclinic |
| Space group | P |
| a/Å | 7.7490 (15) |
| b/Å | 8.8265 (18) |
| c/Å | 8.9520 (18) |
| α/° | 82.84 (3) |
| β/° | 64.89 (3) |
| γ/° | 80.80 (3) |
| Volume/Å3 | 546.18 (19) |
| Z | 1 |
| ρcalcmg/mm3 | 1.617 |
| μ/mm−1 | 1.195 |
| S | 1.125 |
| F(000) | 276 |
| Index ranges | −9 ≤ h ≤ 9, −10 ≤ k ≤ 10, −10 ≤ l ≤ 10 |
| Reflections collected | 4227 |
| Independent reflections | 1910 [R (int) = 0.0669] |
| Data/restraints/parameters | 1910/3/151 |
| Goodness-of-fit on F2 | 1.097 |
| Final R indexes [I > = 2σ (I)] | R1 = 0.0551, wR2 = 0.1362 |
| Final R indexes [all data] | R1 = 0.0563, wR2 = 0.1369 |
| Largest diff. peak/hole/e Å−3 | 0.641/−1.296 |
© 2017 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.-C. Synthesis, Crystal Structure, and Fluorescent Property of a Zn(II) Complex with N-Nicotinoylglycine Ligand. Crystals 2017, 7, 151. https://doi.org/10.3390/cryst7060151
Zhang Y-C. Synthesis, Crystal Structure, and Fluorescent Property of a Zn(II) Complex with N-Nicotinoylglycine Ligand. Crystals. 2017; 7(6):151. https://doi.org/10.3390/cryst7060151
Chicago/Turabian StyleZhang, Yun-Chen. 2017. "Synthesis, Crystal Structure, and Fluorescent Property of a Zn(II) Complex with N-Nicotinoylglycine Ligand" Crystals 7, no. 6: 151. https://doi.org/10.3390/cryst7060151
APA StyleZhang, Y.-C. (2017). Synthesis, Crystal Structure, and Fluorescent Property of a Zn(II) Complex with N-Nicotinoylglycine Ligand. Crystals, 7(6), 151. https://doi.org/10.3390/cryst7060151





