Abstract
Sodium samarium borate Na3Sm(BO3)2, was prepared by a flux method and structurally characterized by single-crystal structure analysis for the first time. The results show that it crystallizes in the monoclinic system P21/n, with a = 6.5667(3) Å, b = 8.7675(4) Å, c = 10.1850(5), β = 90.86°, V = 586.32(5) Å3 and Z = 4. The structure contains NaO7, NaO6, NaO5, SmO8, and BO3 units, which are interconnected via corner- or edge-sharing O atoms into a three-dimensional structure. The excitation spectra, emission spectra, decay time, and Commission International de l’Éclairage (CIE) chromaticity index of Na3Sm(BO3)2 were studied. Under near light excitation (406 nm), the powdered Na3Sm(BO3)2 shows the orange-red emission, which originates from the 4G5/2→6H9/2 and 4G5/2→6H7/2 transformation of Sm3+ ion.
1. Introduction
In recent years, developing new luminescent materials has become a hot topic for new lighting and display technology such as phosphor-converted white light emitting diodes (LED) because of their excellent advantages of eco-friendliness, high efficiency, high power efficiency, long lifetime, and low cost [1,2,3]. The most commercially utilized kind of white LED is the combination of a blue LED chip with a yellow phosphor (Y3Al5O12: Ce3+), which blends the blue light from the chip and yellow light from the phosphor resulting in white light. However, this kind of white LED has the poor color rendering index and high Correlated Color Temperature (CCT) for the lack of red component. Another way is to directly use a near-ultraviolet (NUV) LED to excite red, green, and blue (RGB) multiphase phosphors to create warm-white light, which results in a great interest in searching for novel phosphors for white LEDs. The crystal matrix of host materials is one of the most important roles to determine the performance of phosphors. In recent years, a large amount of research work has been devoted to explore new phosphors in various host materials with high performance, good stability, and easily preparation [4,5].
Among various host materials, rare earth borates have been paid intense attention for a wide range of applications due to their significant advantages, such as a low sintering temperature, low cost, broad band gap, high luminous efficiency, and high chemical stability [6,7]. The boron atoms can coordinate to three and four oxygen atoms forming a BO3 triangle and a BO4 tetrahedron, respectively, and these units can further polymerize to complicated BxOy architectures. Meanwhile, the rare earth ions within these borates possess a unique optical behavior and have paved the way for the development of optical phosphors. For these compounds, the electronic transitions originate from the partially filled 4f energy shell of rare earth ions, which are not influenced by the 5s and 5p electrons. These transitions lead to narrow and intense emission bands, which are useful sources of individual colors in multicolor light emitting devices.
The crystal structure of sodium rare-earth borates Na3Ln(BO3)2 (Ln = rare-earth metals) was first reported by Mascetti et al. for La and Nd species through single-crystal X-ray diffraction (SC-XRD), revealing the monoclinic space group P21/c of this family [8]. Later on, the structure of three isotype compounds Na3Ln(BO3)2 (Ln = Pr, Sm Eu) were studied by Wang et al., through powder XRD [9]. However, no single crystal data and luminescent properties were given for the ternary compound Na3Sm(BO3)2 until now. We deem that single crystals of Na3Sm(BO3)2 can be obtained by using a facile high temperature flux method, which is thought to be an effective and powerful tool in solid-state chemistry. In this method, the reactant materials are completely dissolved in molten salt to obtain a uniform liquid, and a single crystal with large size can then be obtained by limiting the number of nuclei formed during cooling. Up to now, many mixed-metal oxides, sulfides, and complex intermetallics have been successfully obtained using this method [10,11,12,13]. We think the Na2O‒B2O3 system can be used as a good flux for the low melting point (700~900 °C) and powerful dissolving capacity for many oxides, including refractory rare-earth oxides. Moreover, Na2O‒B2O3 mixed salt can easily be removed by washing with water after the reaction. Herein, we report the powder synthesis, single crystal preparation, and luminescent properties of Na3Sm(BO3)2.
2. Experimental Section
2.1. Material and Methods
All of the chemicals Na2CO3 (≥99.0%, Sinopharm Chemical Reagent Co., Ltd, Shanghai, China), Sm2O3 (≥99.9%, Sinopharm Chemical Reagent Co.,Ltd, Shanghai, China) and H3BO3 (≥99.0%, Sinopharm Chemical Reagent Co., Ltd, Shanghai, China) were used without further purification. X-ray powder diffraction (XRD) patterns were collected on a Rigaku DMax-2500 diffractometer (Rigaku Corporation, Tokyo, Japan) by using graphite-monochromated Cu Kα radiation in the angular range 2θ = 5−75° with a step size of 0.02°. TGA studies were all carried out with NETZSCH STA 449C instruments (NETZSCH-Gerätebau GmbH, Selb, Germany). The sample and reference (Al2O3) were enclosed in a platinum crucible and heated at a rate of 15 °C·min−1 from room temperature to 1000 °C under a nitrogen atmosphere. Photoluminescence (PL) spectra and the lifetime test were carried out using an FLS920 Edinburgh Analytical Instrument (Edinburgh Instruments Company, Edinburgh, Britain) apparatus. A standard Xe900 continuous-wave xenon lamp (450 W) was used as the excitation source for steady-state measurements (stimulation slit width: 2.0 nm, emission slit width: 2.0 nm). The PL excitation and emission spectra was recorded within 350–450 nm and 475–750 nm, respectively, with a step width of 1 nm and an integration time of 0.2 s. A standard microsecond flash lamp μF920H was used for excitation in lifetime measurements using the time-correlated single-photon counting (TCSPC) technique. The flash lamp operated at a pulse frequency of 2000 Hz with a pulse width of 2 μs. The decay was measured with a range of 10 μs, and counts at the maximum were 2000.
2.2. Synthetic Procedures
Powder sample of Na3Sm(BO3)2 can be obtained in quantitative yield by the solid-state reaction of a mixture of Na2CO3 (2.000 g, 18.87 mmol), Sm2O3 (2.193 g, 6.289 mmol), and H3BO3 (1.555 g, 25.16 mmol) in a molar ratio of 3:1:4, which is similar to Wang’s method [9]. The mixture was ground with an agate mortar and then pressed into a pellet to ensure optimal homogeneity and reactivity. The paste was transferred to a 20 mL platinum crucible, which was placed into a muffle furnace heated to 830 °C in the open air for 40 h. In this stage, intermediate grindings were performed every 10 h to improve the completeness of reaction. The purity of the powder pattern was confirmed by powder XRD studies on a Rigaku DMax-2500/PC powder diffractometer (Rigaku Corporation, Tokyo, Japan) (Figure 1). The syntheses can be expressed by the following equation:
3Na2CO3 + Sm2O3 + 4H3BO3 →2Na3Sm(BO3)2 + 3CO2 + 6H2O.
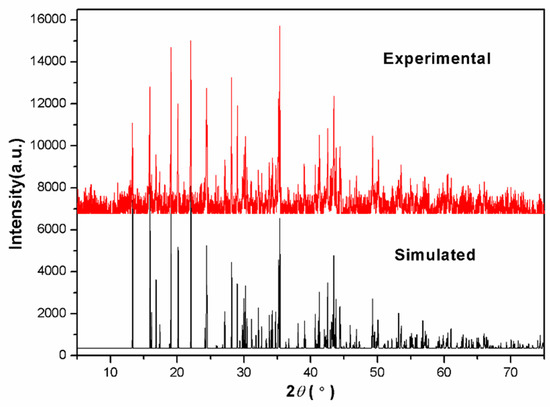
Figure 1.
Experimental and simulated X-ray powder diffraction patterns of Na3Sm(BO3)2 in the 2θ range of 5–75°.
Single crystal of Na3Sm(BO3)2 can be prepared using a high-temperature molten salt method using excess mixture of Na2O(Na2CO3)‒B2O3 as flux, which is the component of the compound Na3Sm(BO3)2 to avoid dopant contamination. The mixture of initial reagents Na2CO3 (2.000 g, 18.87 mmol), Sm2O3 (0.4390 g, 1.258 mmol), and H3BO3 (1.944 g, 31.45 mmol), with a molar ratio of 15:1:25, was thoroughly ground in an agate mortar. The mixture was placed in a 20 mL platinum crucible that was heated in a programmable temperature muffle furnace at 900 °C and held at this temperature for 40 h in the open air until the solution became transparent and clear. The homogenized solution was then slowly cooled to 700 °C at a rate of 4 °C∙h−1 to induce the growth of single crystals. Surprisingly, colorless prism-shaped crystals of Na3Sm(BO3)2 were obtained in a yield of about 55% (based on Sm2O3), and a high quality crystal with dimensions 0.20 × 0.10 × 0.03 mm was selected for SC-XRD analysis. Some millimeter-sized single crystals with a maximum size of 1.50 × 0.30 × 0.20 mm can be manually selected, washed with hot water, and ground into powder for luminescent property studies.
2.3. Structure Solution
A suitable single crystal with dimensions of 0.20 × 0.10 × 0.03 mm was selected for the single-crystal X-ray diffraction (SC-XRD) experiments. A set of intensity data was collected using a Bruker Smart Apex2 CCD single-crystal diffractometer system equipped with a graphite-monochromated Mo Kα radiation source (λ = 0.71073 Å) with a tube power of 50 kV and 30 mA. The frames were collected at an ambient temperature of 296 K with a scan width of 0.5° in ω and integrated with the Bruker Saint software package using a narrow-frame integration algorithm. The unit cell was determined and refined by the least square method upon the refinement of the XYZ centroid of reflections above 20 σ(I). No weak satellite reflections were observed and thus no structure modulation was considered in the structure model. Then, the date was scaled for absorption using the SADABS program of Apex2 package [14]. Intensities of all measured reflections were corrected for Lorentz–polarization (Lp) and crystal absorption effects. The crystal structure of Na3Sm(BO3)2 was solved by the charge-flipping method using the Superflip program [15] and subsequently refined by the Jana2006 crystallographic computing system [16]. All atoms in the structure were refined using harmonic anisotropic atomic displacement parameters (ADPs). The details of the data collection and structure refinement are summarized in Table 1, important bond lengths and angles are listed in Table 2, and atomic coordinates and ADPs are given as supporting information (Tables S1 and S2). Further details of the crystal structure investigations can be obtained from the Fachinformationszentrum Karlsruhe, 76344 Eggenstein-Leopoldshafen, Germany (e-mail: crysdata@fiz-karlsruhe.de), on quoting the depository number of CSD-432527.

Table 1.
Summary of crystal data and structure refinement of Na3Sm(BO3)2.

Table 2.
Selected bond distances (Å) and angles (°) of Na3Sm(BO3)2.
3. Results and Discussion
3.1. Single Crystal Structure
SC-XRD analysis reveals that the compound Na3Sm(BO3)2 crystallizes in the Monoclinic system with space group P21/n. The crystallographic asymmetric unit contains three unique sodium atoms, one unique samarium atom, two unique boron atoms, and six unique oxygen atoms. Each B atom is coordinated by three oxygen atoms into nearly flat BO3 tetrahedron geometry with three B–O bond lengths of 1.352(4)–1.399(4) Å and bond angles of 116.5(3)–123.2(3)°, which is the common value in borate compounds [17,18]. All BO3 triangles are isolated in two types of linear array, whereas Na+ and Sm3+ atoms reside among the BO3 groups and connect them through the columbic action of Na−O and Sm−O to form the structure of Na3Sm(BO3)2, as shown in Figure 2. The coordination environments of three Na atoms are different. Na1 atom is coordinated by eight O atoms into Na1O8 polyhedron with Na1−O bond distances ranging from 2.428(3) Å to 2.658(3) Å, whereas Na2 atom connects with six O atoms to form Na2O6 octahedron with Na2−O bond distances ranging from 2.225(3) Å to 2.666(3) Å. The coordination environment Na3 atom is surrounded by five O atoms into a Na3O5 polyhedron with three shorter Na3−O bond distances (2.301(3) Å, 2.350(3) Å, and 2.375(3) Å) and two longer Na3−O bond distances (2.893(4) Å and 3.071(3) Å), which can be considered as secondary coordination bonds to complete the extended coordination spheres of the large Na+ ion. The coordination mode of Na3O5 shows a big difference from those of Na1O7 and Na2O6, and is an uncommon coordination geometry compared with other reported Na(I) oxide compounds [19]. Although the ionic radii of Sm3+ (0.964 Å) is comparable with that of Na+ (0.950 Å), it adopts a Sm1O8 coordination mode with a Sm1−O bond length of 2.338(3)–2.518(2) Å. In detail, each SmO8 polyhedron connects with three BO3 groups via edge-sharing manner, and two BO3 groups via corner-sharing manner.
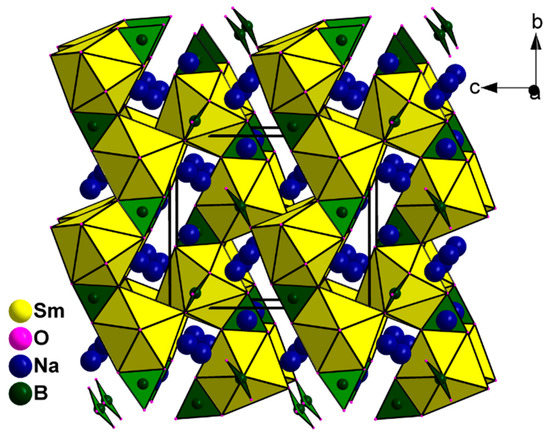
Figure 2.
View of the crystal structure of Na3Sm(BO3)2 containing BO3 planes, Na+ ions, and SmO8 polyhedra.
The coordination environment of B1O3 and B2O3 groups are shown in Figure 3a,b, respectively. Each B1O3 planar triangle connects to three units (Na1O7, Na2O6, and Sm1O8) via edge-sharing O atoms, and seven units (two Na1O7, one Na2O6, two Na3O5, and two Sm1O8) via corner-sharing O atoms. The coordination environment of the B2O3 unit is similar to that of B1O3 unit; each B2O3 unit coordinates with three units (one Na3O5 and two Sm1O8) via edge-sharing O atoms, and seven units (three Na1O7, three Na2O6 and one Na3O5) via corner-sharing O atoms. Hence, the structure of the compound Na3Sm(BO3)2 can be considered a 3D framework that is constructed by NaO7, NaO6, NaO5, SmO8, and BO3 units.

Figure 3.
View of the coordination environment of the B1O3 group (a) and the B2O3 group (b).
3.2. Thermal Stability Study
The analysis of TG/DTA illustrates that the compound Na3Sm(BO3)2 shows a high stable temperature, as shown in Figure 4. There is no obvious exothermic peak below 900 °C. Upon further heating, Na3Sm(BO3)2 started to decompose at 960 °C, and no weight lost was observed until 1000 °C. This result demonstrates a good thermal stability of the compound Na3Sm(BO3)2.

Figure 4.
TG and DTA curves for Na3Sm(BO3)2.
3.3. UV-Vis Spectroscopic
Figure 5 shows the UV-Vis absorption spectrum of Na3Sm(BO3)2 in the range from 240 to 800 nm. The broad absorption band from 240 to 330 nm may correspond to the band-gap transition of Na3Sm(BO3)2, whereas the rest sharp absorption from 340 to 500 nm corresponds to typical intra-4f forbidden transitions of the Sm3+ ions.
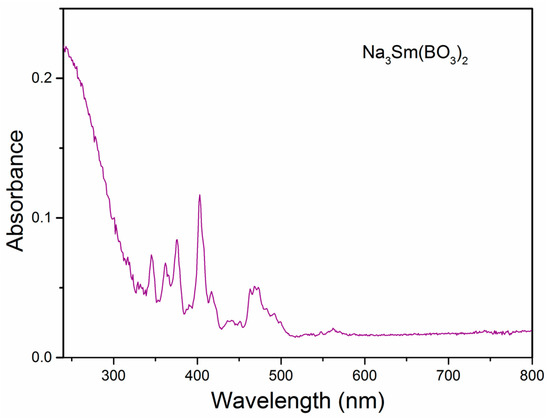
Figure 5.
UV-Vis absorption spectrum of the compound Na3Sm(BO3)2.
3.4. Luminescent Properties
Although with 100% concentration of Sm3+, the compound Na3Sm(BO3)2 exhibits an orange-red emission of Sm3+ ion upon excitation with a xenon lamp at near-ultraviolet light. Figure 6a shows the excitation spectrum monitored at the 4G5/2→6H7/2 transition of Sm3+ with a 605 nm red emission in the range of 350–450 nm. The excitation spectrum consists of a series of peaks centered at 364, 377, 392, 406, 419, and 442 nm, corresponding to the 6H5/2→4L15/2, 6H5/2→6P7/2, 6H5/2→4K11/2, 6H5/2→4F7/2, 6H5/2→4F7/2, and 6H5/2→(4G9/2+4I15/2) intra-configurational 4f→4f transition of Sm3+ ions, respectively [20,21]. Among these, the 6H5/2→4F7/2 transition at 406 nm was the highest intensity, indicating that phosphor Na3Sm(BO3)2 can be effectively activated by near-ultraviolet light.
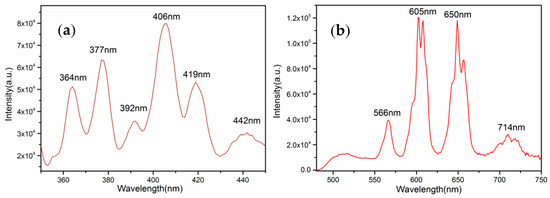
Figure 6.
Excitation (a) and emission (b) spectra of Na3Sm(BO3)2.
Upon excitation at 406 nm, the emission spectrum is composed of several distinct groups of peaks in the range of 475–750 nm (Figure 6b). There are two strong emissions in the orange-red region coming from positions at 605 nm and 650 nm, corresponding to the 4G5/2→6H7/2 and 4G5/2→6H9/2 transitions of Sm3+, respectively [22]. Besides, there are two weak emissions centered at 566 nm and 714 nm, which can be assigned to the 4G5/2→6H5/2 and 4G5/2→6H11/2 transitions of Sm3+, respectively. It is well-known that the emission spectrum of Sm3+ activated phosphor strongly depends on the symmetry of the Sm3+ site in the host lattice [23,24]. The 4G5/2→6H5/2 transition is the magnetic dipole transition, which scarcely changes the crystal field strength around the Sm3+ ions, leading to the independence of the symmetry, whereas the transition of 4G5/2→6H9/2 is the electric dipole transition, whose intensity is very sensitive to the site symmetry of the Sm3+ activators in the host matrix. Although the 4G5/2→6H7/2 emission corresponds to the magnetic dipole transition, its magnetic dipole character is usually very low and dominated by the electric dipole (ED) transition. Hence, if the Sm3+ occupies the site in a low symmetry, the 4G5/2→6H9/2 or 4G5/2→6H7/2 emission is frequently the strongest, but in a site with an inversion center, the 4G5/2→6H5/2 transition becomes dominant. For Na3Sm(BO3)2, it is obviously that the intensity of 4G5/2→6H9/2 and 4G5/2→6H7/2 emissions is stronger than the 4G5/2→6H5/2 emission, which is in good agreement with the crystallographic study that all Sm3+ atoms occupy a general site without an inversion center. The quantum efficiency (QE) of a phosphor is an important parameter to be considered for practical applications. The QE can be measured and calculated according to the equation: , in which the LS represents the emission spectrum, ES represents the excitation spectrum, and ER represents the background. Upon excitation at 406 nm, the corresponding QE of Na3Sm(BO3)2 is calculated to be a low value, about 12%. For the most part, the concentration quenching occurs in 100% Sm3+ phosphor Na3Sm(BO3)2, and the other affecting factors include preparing temperature, the morphology, and the size of single crystals. Therefore, doping La3+ or Gd3+ ions into Na3Sm(BO3)2 may be an effective method to improve the luminescent QE of Na3Sm(BO3)2. This work is still in process.
These three colors are usually referred to as the 1931 color coordinates, the current standard for lighting specifications on the market [25]. In general, the color of any light source in this color space can be represented as an (x,y) coordinate. The location of the color coordinates of phosphor Na3Sm(BO3)2 on the Commission International de l’Éclairage (CIE) chromaticity diagram is presented in Figure 7. Under the excitation at 406 nm, the calculated CIE chromaticity coordinate is (0.660, 0.340), falling in the orange-red region. We may expect that the compound Na3Sm(BO3)2 can be used as a good orange-red phosphor.
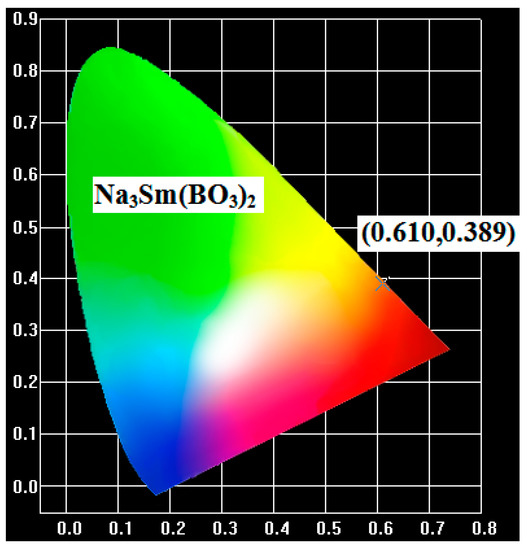
Figure 7.
The Commission International de l’Éclairage (CIE) chromaticity diagram for Na3Sm(BO3)2 with excitation at 406 nm.
Moreover, we studied the luminescence decay curve for the 4G5/2→6H7/2 emission of Sm3+, as shown in Figure 8. The decay curve cannot be well fitted with a single-exponential but can be well fitted with a bi-exponential function according to the equation [26,27]:
where I(t) and I(0) are the luminescence intensities at time 0 and t; A1* and A2* are the fitting parameters; t is the time; τ1 and τ1 are the rapid and slow lifetimes for exponential components, respectively. The double exponential behavior of Na3Sm(BO3)2 may originate from energy loss by ion–ion interactions. Then, the average decay time can be calculated to be 1.654 μs to represent the lifetime by the equation [28]:
I(t) = A1* exp(−t/τ1) + A2* exp(−t/τ2) + I(0)

Figure 8.
Fluorescent decay (black) and fitting (red) curves of Na3Sm(BO3)2.
4. Conclusions
A new type of sodium samarium borate Na3Sm(BO3)2 was prepared using a high temperature molten salt (flux) method and its structure was determined by SC-XRD analyses. Compound Na3Sm(BO3)2 crystallizes in the monoclinic P-centered space group P21/n. The structure of the compound Na3Sm(BO3)2 can be considered as a 3D framework that is constructed by NaO7, NaO6, NaO5, SmO8, and BO3 units. Even with the 100% Sm3+ ion concentration, Na3Sm(BO3)2 shows intense luminescent emission behavior. The excitation spectra cover a wide range from 350 to 450 nm, which suggests that the phosphor Na3Sm(BO3)2 can be effectively excited by a near-UV light source. The emission spectrum excited at 406 nm shows two strong emission bands in the orange-red region: 605 nm (4G5/2→6H9/2) and 650 nm (4G5/2→6H7/2). The CIE chromaticity color coordinates (x = 0.660, y = 0.340) were found to be in the orange-red region of the chromaticity diagram. The decay curve excited by 406 nm was measured and the decay time was fitted to be 1.654 μs. We think Na3Sm(BO3)2 can be potentially used as an orange-red phosphor under near-UV light excitation.
Supplementary Materials
The following are available online at www.mdpi.com/2073-4352/7/5/129/s1, Table S1: fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2) of Na3Sm(BO3)2, Table S2: atomic displacement parameters (Å2) of Na3Sm(BO3)2.
Acknowledgments
We are grateful to the National Natural Science Foundation of China (Project 21201056 and 21307028) and the Program for Innovative Research Team of Henan Polytechnic University (No. T2017-2).
Author Contributions
Dan Zhao conceived and designed the experiments, and wrote the paper; Fa-Xue Ma, Cong-Kui Nie and Lei Zhang performed the experiments; Jian Wang analyzed the data; Yunchang Fan contributed reagents/materials/analysis tools.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kim, K.Y.; Yoon, S.J.; Park, K. Synthesis and photoluminescence properties of red-emitting Ca3-3x/2(VO4)2:XEu3+ phosphors. J. Lumin. 2015, 160, 78–84. [Google Scholar] [CrossRef]
- Zhao, D.; Ma, F.-X.; Zhang, R.-J.; Li, F.-F.; Zhang, L.; Yang, J.; Fan, Y.-C.; Xin, X. Structure modulation, band structure, density of states and luminescent properties of columbite-type ZnNb2O6. Crystengcomm 2016, 18, 2929–2936. [Google Scholar] [CrossRef]
- Barsukova, M.; Goncharova, T.; Samsonenko, D.; Dybtsev, D.; Potapov, A. Synthesis, crystal structure, and luminescent properties of new zinc(ii) and cadmium(ii) metal-organic frameworks based on flexible bis(imidazol-1-yl) alkane ligands. Crystals 2016, 6, 132. [Google Scholar] [CrossRef]
- Li, H.L.; Liu, Y.J.; Zheng, R.; Chen, L.J.; Zhao, J.W.; Yang, G.Y. Trigonal pyramidal {AsO2(OH)} bridging tetranuclear rare-earth encapsulated polyoxotungstate aggregates. Inorg. Chem. 2016, 55, 3881–3893. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.-L.; Lin, C.-S.; He, Z.-Z.; Zhang, H.; Luo, Z.-Z.; Cheng, W.-D. Syntheses of three members of A(ii)M(iv)(PO4)2: Luminescence properties of PbGe(PO4)2 and its Eu3+-doped powders. Crystengcomm 2013, 15, 7089–7094. [Google Scholar] [CrossRef]
- Kalidasan, M.; Asokan, K.; Dhanasekaran, R. Luminescence properties of 100 mev W8+ ion irradiated GdCa4O(BO3)3:Eu3+ and GdCa4O(BO3)3:Tb3+ phosphors. J. Lumin. 2016, 180, 241–250. [Google Scholar] [CrossRef]
- Yang, L.; Wan, Y.; Huang, Y.; Chen, C.; Seo, H.J. Development of YK3B6O12:Re (Re = Eu3+, Tb3+, Ce3+) tricolor phosphors under near-uv light excitation. J. Alloys Compd. 2016, 684, 40–46. [Google Scholar] [CrossRef]
- Mascetti, J.; Vlasse, M.; Fouassier, C. The Crystal Chemistry of the New Rare-Earth Sodium Borates Na3Ln(BO3)2(Ln = La, Nd). J. Solid State Chem. 1981, 39, 288–293. [Google Scholar] [CrossRef]
- Wang, Z.X.; Li, H.K.; Cai, G.M.; Jin, Z.P. Synthesis, crystal structure, and thermal stability of new borates Na3REB2O6(RE = Pr, Sm, Eu). Powder Diff. 2016, 31, 110–117. [Google Scholar] [CrossRef]
- Wang, S.; Ye, N.; Poeppelmeier, K.R. Flux growth and crystal structure refinement of calcite type borate GaBO3. Crystals 2015, 5, 252–260. [Google Scholar] [CrossRef]
- Yu, N.; Wang, S.; Ye, N.; Liang, F.; Lin, Z.; Luo, M.; Poeppelmeier, K.R. A deep-ultraviolet nonlinear optical crystal: Strontium beryllium borate fluoride with planar Be(O/F)3 groups. Chem. Mater. 2016, 28, 4563–4571. [Google Scholar] [CrossRef]
- Abudoureheman, M.; Han, S.; Lei, B.-H.; Yang, Z.; Long, X.; Pan, S. KPb2(PO3)5: A novel nonlinear optical lead polyphosphate with a short deep-UV cutoff edge. J. Mater. Chem. C 2016, 4, 10630–10637. [Google Scholar] [CrossRef]
- Zhen, N.; Nian, L.; Li, G.; Wu, K.; Pan, S. A high laser damage threshold and a good second-harmonic generation response in a new infrared NLO material: LiSm3SiS7. Crystals 2016, 6, 121. [Google Scholar] [CrossRef]
- Bruker, APEX2 and SAINT; Version 2013.11-0; Bruker AXS Inc.: Madison, WI, USA, 2013.
- Palatinus, L.; Chapuis, G. Superflip—A computer program for the solution of crystal structures by charge flipping in arbitrary dimensions. J. Appl. Crystallogr. 2007, 40, 786–790. [Google Scholar] [CrossRef]
- Petricek, V.; Dusek, M.; Palatinus, L. Crystallographic computing system Jana2006: General features. Z. Kristallogr. 2014, 229, 345–352. [Google Scholar]
- Yu, H.; Young, J.; Wu, H.; Zhang, W.; Rondinelli, J.M.; Halasyamani, P.S. Electronic, crystal chemistry, and nonlinear optical property relationships in the dugganite A3B3Cd2O14 family. J. Am. Chem. Soc. 2016, 138, 4984–4989. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Yu, H.; Wu, H.; Halasyamani, P.S. Crystal growth and associated properties of a nonlinear optical crystal-Ba2Zn(BO3)2. Crystals 2016, 6, 68. [Google Scholar] [CrossRef]
- Zhao, D. Heptasodium tetraaluminium tetrakis (diphosphate) orthophosphate, Na7Al4(P2O7)4(PO4). Acta Crystallogr. Sect. 2011, 67, I64. [Google Scholar] [CrossRef] [PubMed]
- Vishwakarma, A.K.; Jayasimhadri, M. Pure orange color emitting Sm3+ doped BaNb2O6 phosphor for solid-state lighting applications. J. Lumin. 2016, 176, 112–117. [Google Scholar] [CrossRef]
- Sun, J.F.; Ding, D.B.; Sun, J.Y. Synthesis and photoluminescence properties of a novel reddish orange-emitting Sm3+-doped strontium borosilicate phosphor. Opt. Mater. 2016, 58, 188–195. [Google Scholar] [CrossRef]
- Yawalkar, M.M.; Zade, G.D.; Dabre, K.V.; Dhoble, S.J. Luminescence study of Eu3+-doped Li6Y(BO3)3 phosphor for solid-state lighting. Luminescence 2016, 31, 1037–1042. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.; Wang, L.X.; Xu, M.J.; Jia, D.Z. Photoluminescence characteristics of Sm3+ doped Sr2P2O7 as new orange-red emitting phosphor. J. Alloys Compd. 2015, 647, 136–140. [Google Scholar] [CrossRef]
- Yang, Z.; Xu, D.; Sun, J.; Sun, Y.; Du, H. Characterization and luminescence properties of Sr3Gd(PO4)3: Sm3+ orange-red phosphor. Opt. Eng. 2015, 54, 105102. [Google Scholar] [CrossRef]
- Shionoya, S.; Yen, W.M. Phosphor Handbook; Phosphor Research Society, CRC Press: Boca Raton, FL, USA, 1998; pp. 459–465. [Google Scholar]
- Zeng, C.; Huang, H.W.; Hu, Y.M.; Miao, S.H.; Zhou, J. A novel blue-greenish emitting phosphor Ba3LaK(PO4)3F:Tb3+ with high thermal stability. Mater. Res. Bull. 2016, 76, 62–66. [Google Scholar]
- Zhang, D.J.; Wang, X.M.; Qiao, Z.A.; Tang, D.H.; Liu, Y.L.; Huo, Q.S. Synthesis and Characterization of Novel Lanthanide(III) Complexes-Functionalized Mesoporous Silica Nanoparticles as Fluorescent Nanomaterials. J. Phys. Chem. C 2010, 114, 12505–12510. [Google Scholar] [CrossRef]
- Annadurai, G.; Kennedy, S.M.M. Synthesis and photoluminescence properties of Ba2CaZn2Si6O17:Eu3+ red phosphors for white LED applications. J. Lumin. 2016, 169, 690–694. [Google Scholar] [CrossRef]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).