Abstract
The synthesis, crystal structure, and conductivity of a solvent-included ternary charge-transfer salt (BEDT-TTF)2GaCl4(C6H5Cl)0.5 (1) is described and interpreted. Electrochemical oxidation of neutral bis(ethyelenedithio)-tetrathiafulvalene (BEDT-TTF) in the presence of (Me4N)Ga(C2O4)Cl2 in a mixture of C6H5Cl and C2H5OH yields crystals of 1. Compound 1 crystallized as a monoclinic C 2/c space group with cell parameters: a = 47.0615(4) Å, b = 6.7895(1) Å, c = 31.6297(4) Å, β = 132.064(1)°, V = 7503.0(2) Å3, Z = 8 at 293 K and a = 46.4767(5) Å, b = 6.7398(1) Å, c = 31.0778(4) Å, β = 131.630(1)°, V = 7267.4(2) Å3, Z = 8 at 120 K. The formal charge of the donor molecule was assigned as +0.5 from bond lengths in the TTF core. The donor molecules stack with C―···S contacts along the c direction and side-to-side S…S contacts along the b direction to form a two-dimensional donor layer on the bc plane. In the anion sheet, C6H5Cl chain is sandwiched by two GaCl4− chains with Cl…Cl contacts. Compound 1 shows semiconductive behavior with Eα = 124 meV between room-temperature and 150 K and σ300K = 1 S·cm−1.
1. Introduction
In a charge-transfer salt, the conductivity is usually controlled by the arrangement of the conducting π-unit, such as TTF (TTF = tetrathiafulvalene), BEDT-TTF, M(dmit)2n− (dmit = 1,3-dithiole-2-thione-4,5-dithiolate), and their derivatives [1]. Recently, it has been found that the conductivity can be influenced by the solvent molecules which exist in the counterion layer [2]. Depending on the crystal structure of the reported binary charge-transfer salts, interesting physical properties of the ternary charge-transfer salts are expected. So the synthesis and characterization of the binary and ternary compounds is necessary in order to achieve a deep understanding of the molecular conductor. The binary charge-transfer salt of GaCl4− has been reported [3,4]. Because FeCl4− and GaCl4− could be replaced arbitrarily, the charge-transfer salt of GaCl4− is an important reference for a molecule-based magnetic conductor [3,4]. A ternary charge-transfer salt of BEDT-TTF, GaCl4− and solvent molecule C6H5Cl was obtained when looking for the charge-transfer salt of [BEDT-TTF] and [Ga(C2O4)Cl2−]. Its crystal structure and conductivity are reported here.
2. Results and Discussion
During the process of electrochemical oxidation of BEDT-TTF in a mixture of C6H5Cl and C2H5OH, the electrolyte (CH3)4NGa(C2O4)Cl2 decomposed into Ga(C2O4)33– and GaCl4−. Ga(C2O4)33– was transferred into GaCl4− when chlorinated alkane existing as κ'-BETS2FeCl4 was obtained from BETS, ((CH3)4N)3Fe(C2O4)3 in C6H5Cl [5]. The ternary charge-transfer salt 1 is composed of BEDT-TTF, GaCl4− and C6H5Cl. We failed to obtain a high quality single crystal of 1 when (CH3)4NGaCl4 was used as electrolyte.
There are two BEDT-TTF molecules (A, B), one GaCl4− and half a molecule of C6H5Cl in an independent unit of 1 as shown Figure 1. The Cl on the solvent molecule is disordered in two positions, and C7 is disordered in two positions at 293 K and 120 K. The formal charge of the BEDT-TTF molecule assigned from bond lengths on TTF cores (Table 2) is +0.5 as in Table 2 [6].
Except for the ethylene groups, all of the atoms on the two BEDT-TTF molecules (A, B) are coplanar with separate deviations of 0.08 and 0.08 Å. The packing is generated by the symmetry operation with the C 2/c space group as shown in Figure 2. BEDT-TTF molecules stack in the sequence of …AABBAABB… with the main planes parallel to each other to form a column along the c axis. There are intermolecular C…S contacts through hydrogen bonds between AA, AB and BB along the c-axis, S···S contacts between AA, BB along the b-axis and between AB along the c-axis in a donor-layer as in Figure 3.
In the anion sheet, the C6H5Cl chain is sandwiched by two GaCl4− chains along the b axis, and the shortest Cl···Cl distance is 3.592 Å between C6H5Cl and GaCl4−, 3.813 Å between two GaCl4− anions as shown in Figure 4. Ga…Ga distances are 6.790 Å along the b axis, 9.368 Å between two GaCl4− chains intercalated by a C6H5Cl chain, and 7.306 Å between neighboring GaCl4− chains. There are short contacts between donor and anion: S…Cl (S7…Cl1 3.554(1) Å, S6…Cl3 3.572(1)) Å, C−H…Cl (C7A―H7A···Cl3 2.86 Å/159.3°, C7B−H7BB…Cl3 2.83 Å /170.2°, C17−H17A…Cl3 2.94 Å /174.6°), and between donor and solvent molecule C18−H18B…Cl5 2.90 Å/124.1°.Compound 1 crystallized as monoclinic with cell parameters: a = 47.0615(4) Å, b = 6.7895(1) Å, c = 31.6297(4) Å, β = 132.064(1)°, V = 7503.0(2) Å3, Z = 8 at 293 K, space group C 2/c. It remained the same until 120 K. The binary compound (BEDT-TTF)2GaCl4 (2) crystallized as triclinic with cell parameters: a = 31.911(6) Å, b = 16.580(4) Å, c = 6.645(2) Å, a = 98.15(2)°, β = 85.60(2)°, γ = 90.55(2)°, V = 3470 Å3, Z = 4 at 298 K, space group P?1 [3]. In 2, a donor layer was formed on the bc plane with BEDT-TTF packed face-to-face along the b axis and side-by-side along the c axis, the S…S contact was observed between donor molecules along the b axis as dimerization. The donor layer and the anion layer were stacked alternatively along the a axis in 1 and 2. The tilted angle of the donor molecule to the anion layer is 80° in 1 and 70° in 2, so the a axis expanded from 31.911 Å in 2 to 47.062 Å in 1. The unit cell parameters of 1 are related to 2 by the transfer matrix (1,0,0; 0,0,−1; 0,1,0). From 2 to 1, the b axis is doubled, α and β angles are shifted to 90°, and are accompanied by a space group change from P?1 to C 2/c. The main difference between 1 and 2 is the dimerization of the donor in 2, which does not exist in 1.

Table 1.
Crystallographic data of 1.
| Compound | 1 | 1 |
|---|---|---|
| formula | C23H18Cl4.50GaS16 | C23H18Cl4.50GaS16 |
| Fw | 1036.58 | 1036.58 |
| F(000) | 4156 | 4156 |
| T, K | 120 | 293 |
| crystal system | monoclinic | monoclinic |
| space group | C 2/c | C 2/c |
| a, Å | 46.4767(5) | 47.0615(4) |
| b, Å | 6.7398(1) | 6.7895(1) |
| c, Å | 31.0778(4) | 31.6297(4) |
| α, ° | 90 | 90 |
| β, ° | 131.630(1) | 132.064(1) |
| γ, ° | 90 | 90 |
| V, Å3 | 7276.4(2) | 7502.99(16) |
| Z | 8 | 8 |
| Dc, g/cm3 | 1.892 | 1.835 |
| μ(Mo Kα), mm−1 | 2.027 | 1.966 |
| crystal size, mm3 | 0.34 × 0.24 × 0.10 | 0.34 × 0.24 × 0.10 |
| Tmin and Tmax | 0.417, 0.916 | 0.556, 0.839 |
| θmin, θmax, ° | 0.994, 27.50 | 0.993, 27.54 |
| no. total reflns. | 55220 | 58492 |
| no. uniq. reflns (Rint) | 8324(0.0863) | 8587(0.0843) |
| no. obs. [I° 2σ(I0)] | 5590 | 4933 |
| no. params | 425 | 425 |
| R1,wR2 [I° 2σ(I0)] | 0.0370,0.0797 | 0.0392, 0.0918 |
| R1,wR2 (all data) | 0.0723,0.0877 | 0.0911, 0.1042 |
| GOF | 0.967 | 0.974 |
| aΔρ, e/Å3 | 0.773/−0.874 | 0.559/−0.501 |
| bMax. and mean Δ/σ | 0.001/0.000 | 0.001/0.000 |
| CCDC | 794827 | 794828 |
The temperature dependent resistance of 1 is shown in Figure 5. It showed semiconductive behavior from 300 K to 150 K with Eα = 0.124 eV and σrt = 1 S.cm−1 for 1, 150 K to 110 K with Eα = 0.041 eV. The conductivity is higher than δ-(BEDT-TTF)2GaCl4 with σrt = 0.1S.cm−1, Eα = 0.2 eV as expected from the donor arrangement as no dimerization was observed [3]. The conductivity is lower than the bilayered magnetic charge-transfer salt δ-(BEDT-TTF)3(FeCl4)2 because the conductivity in δ-(BEDT-TTF)3(FeCl4)2 is contributed to from both layer-A and layer-B with an anisotropic room-temperature conductivity range from 4.6~120 S·cm−1 [7]. This corresponds with the expectancy that the conductivity of the charge-transfers salts is dominated by the arrangement of the BEDT-TTF molecules. The dimerization of the donor disappears when the solvent molecule is included and the conductivity of the crystal increases.

Table 2.
Bond lengths of the tetrathiafulvalene (TTF) core.
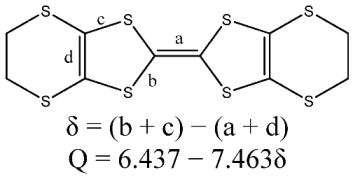

| Donor | a | b | c | d | δ | Q |
|---|---|---|---|---|---|---|
| 120 Κ | ||||||
| ET1 | 1.363(4) | 1.735(3) | 1.744(3) | 1.353(4) | 0.77575 | 0.65 |
| 1.736(3) | 1.744(3) | 1.335(4) | ||||
| 1.737(3) | 1.746(3) | |||||
| 1.738(3) | 1.751(3) | |||||
| ET2 | 1.363(4) | 1.739(3) | 1.745(3) | 1.338(4) | 0.77325 | 0.67 |
| 1.737(3) | 1.745(3) | 1.354(4) | ||||
| 1.739(3) | 1.742(3) | |||||
| 1.736(3) | 1.746(3) | |||||
| 290 K | ||||||
| ET1 | 1.365(4) | 1.730(4) | 1.741(3) | 1.353(4) | 0.7665 | 0.72 |
| 1.734(3) | 1.739(3) | 1.336(4) | ||||
| 1.732(3) | 1.746(3) | |||||
| 1.734(3) | 1.748(3) | |||||
| ET2 | 1.357(4) | 1.744(3) | 1.744(3) | 1.337(4) | 0.786 | 0.57 |
| 1.739(3) | 1.746(3) | 1.344(4) | ||||
| 1.733(3) | 1.746(3) | |||||
| 1.741(3) | 1.741(4) | |||||
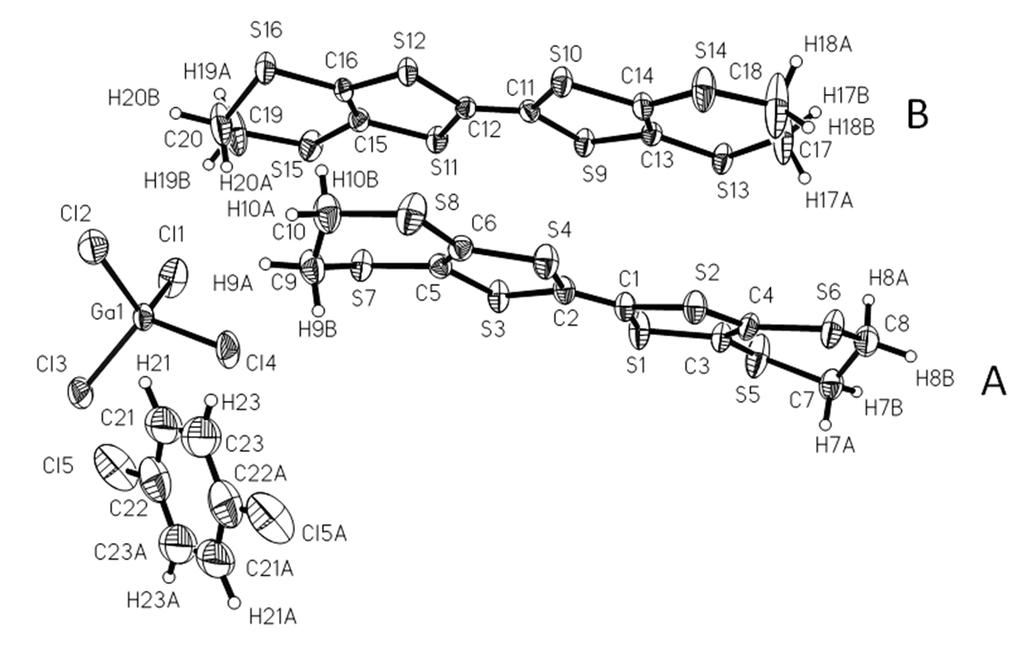
Figure 1.
Oak Ridge Thermal Ellipsoid Plot (ORTEP) drawing of 1 with 30% ellipsoid at 120 K.
Figure 1.
Oak Ridge Thermal Ellipsoid Plot (ORTEP) drawing of 1 with 30% ellipsoid at 120 K.
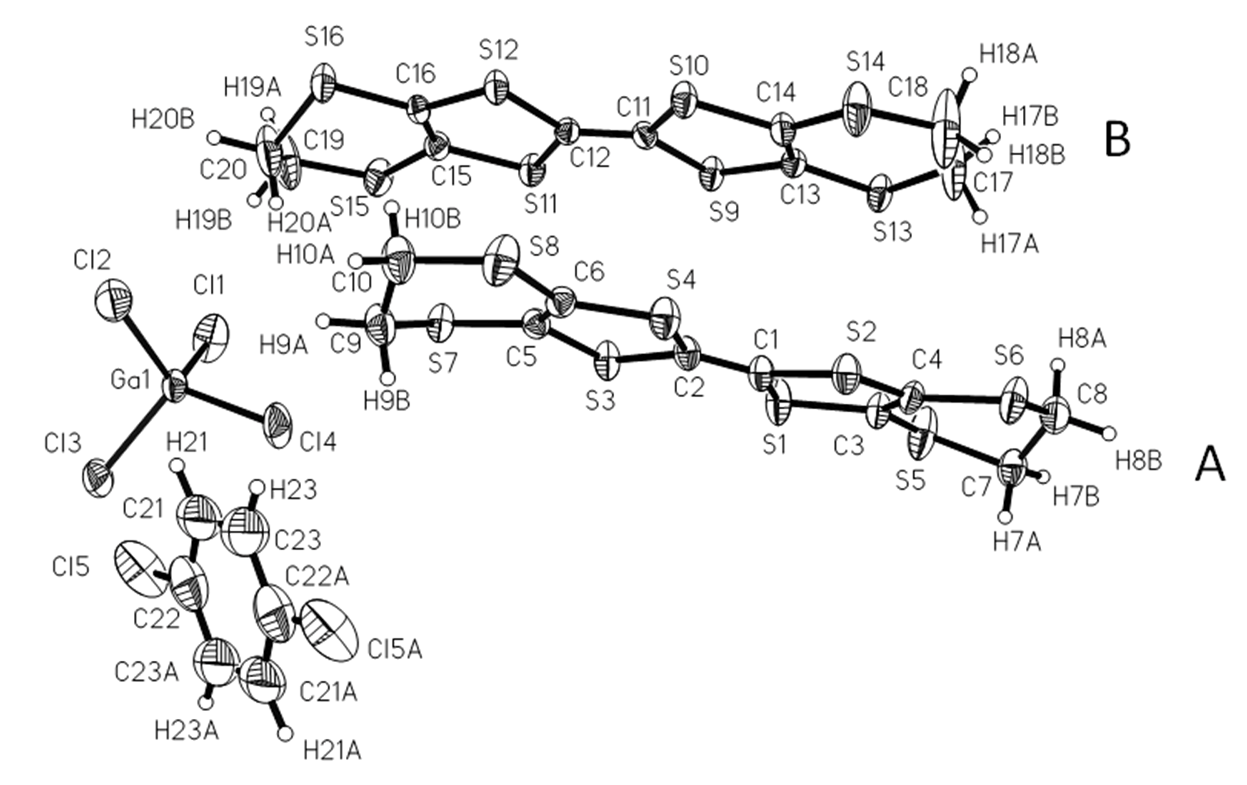
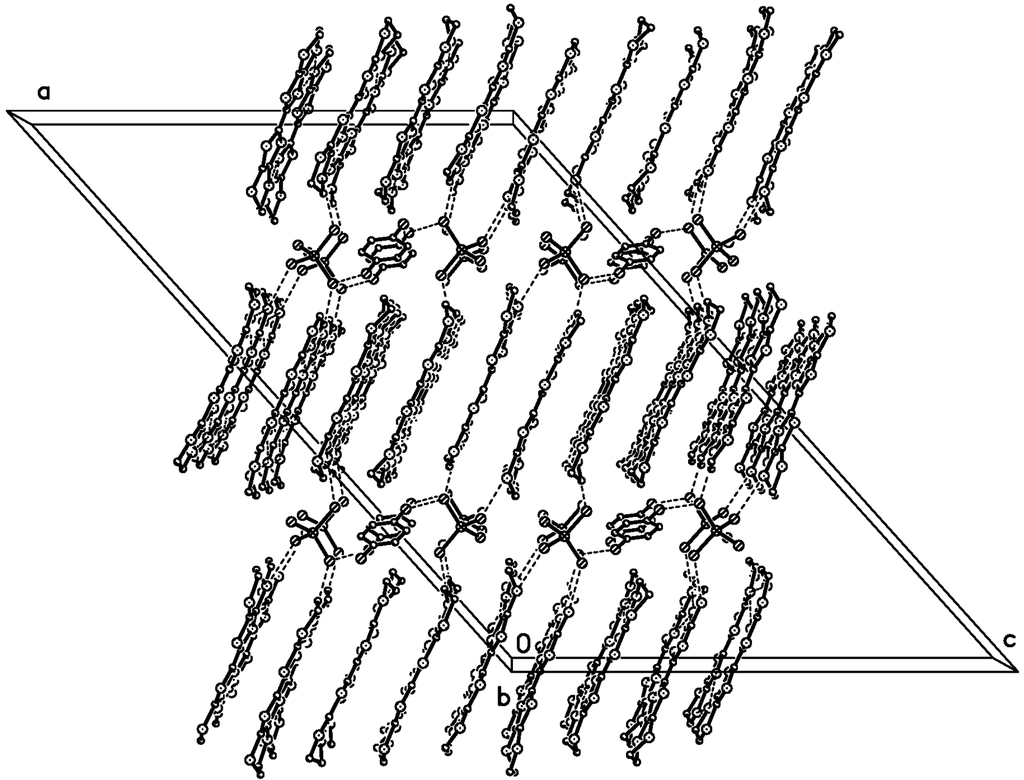
Figure 2.
Packing diagram of 1 along the b axis at 120 K. H and disordered atoms on the donor were omitted for clarity. Dashed lines are C‒H···Cl, Cl···Cl contacts.
Figure 2.
Packing diagram of 1 along the b axis at 120 K. H and disordered atoms on the donor were omitted for clarity. Dashed lines are C‒H···Cl, Cl···Cl contacts.
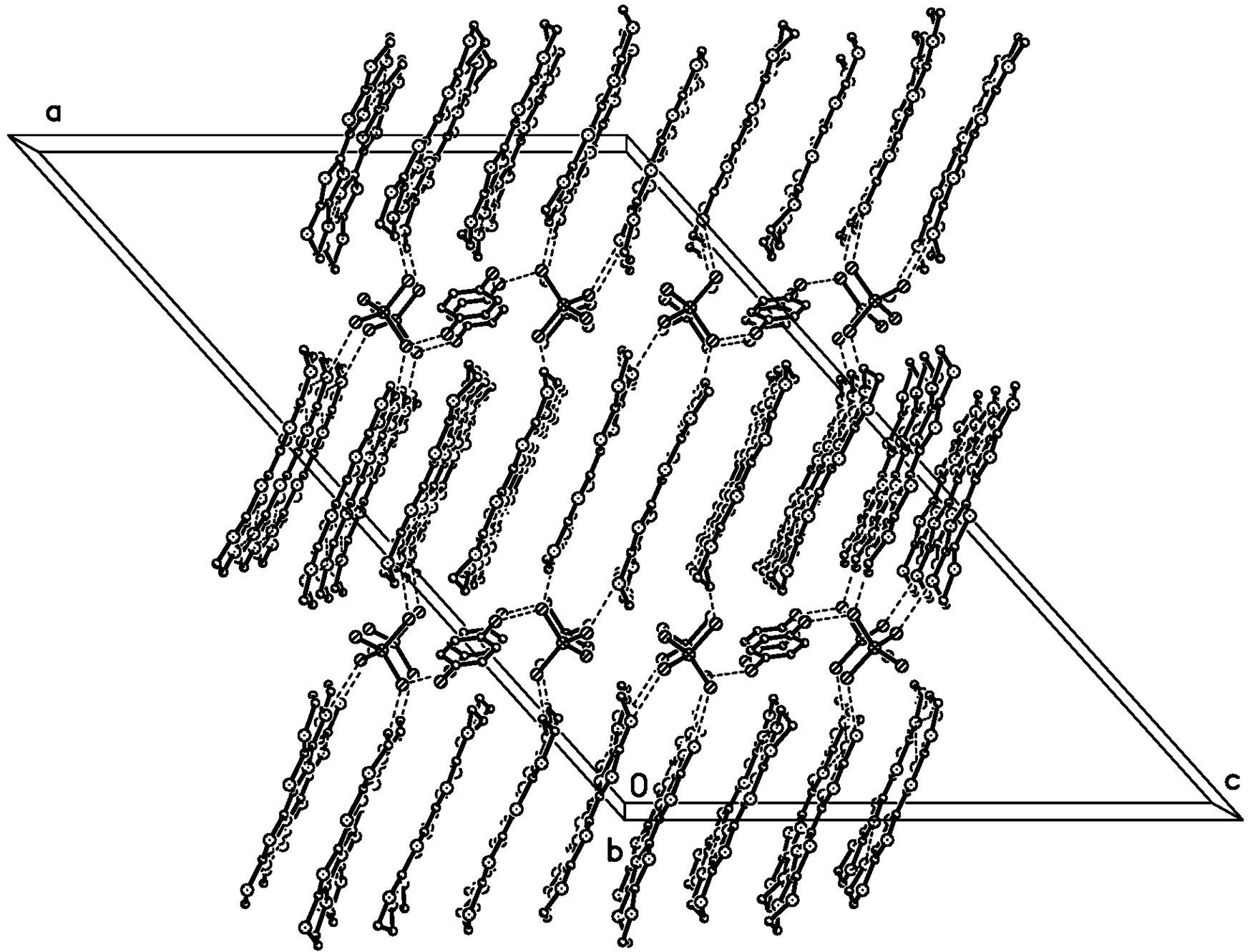
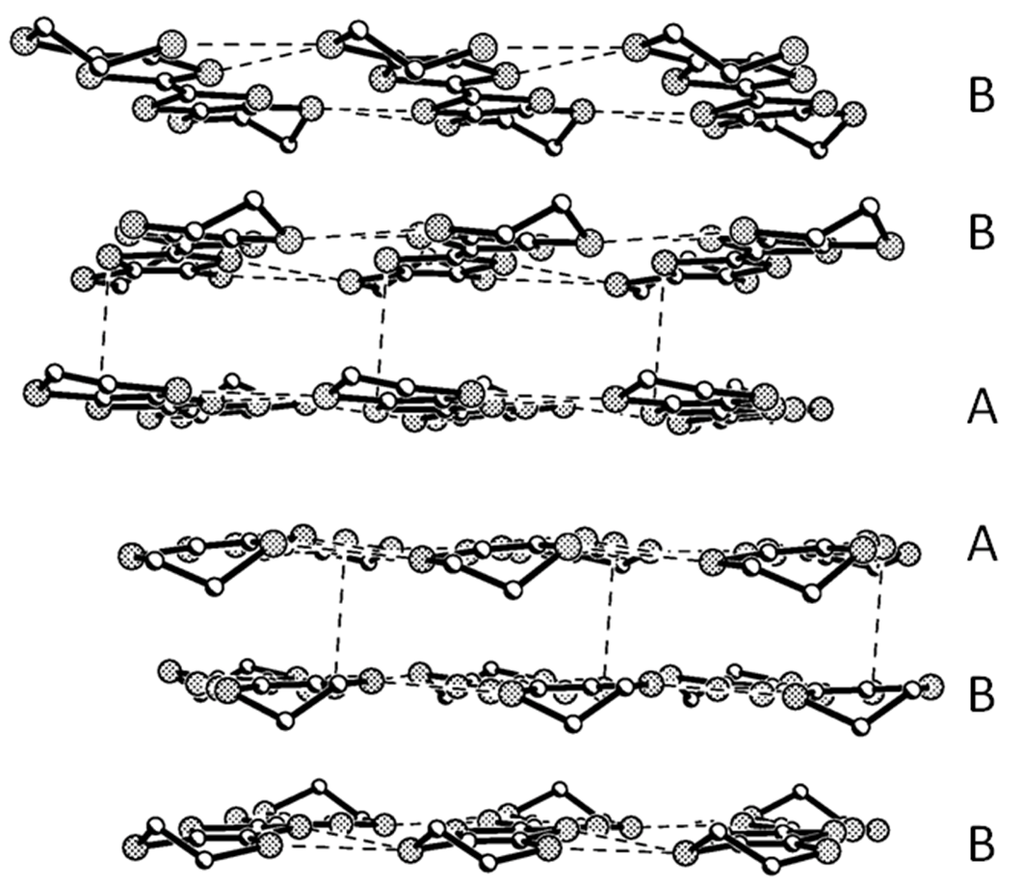
Figure 3.
Donor arrangement on the bc plane. H and disordered atoms were omitted for clarity.
Figure 3.
Donor arrangement on the bc plane. H and disordered atoms were omitted for clarity.
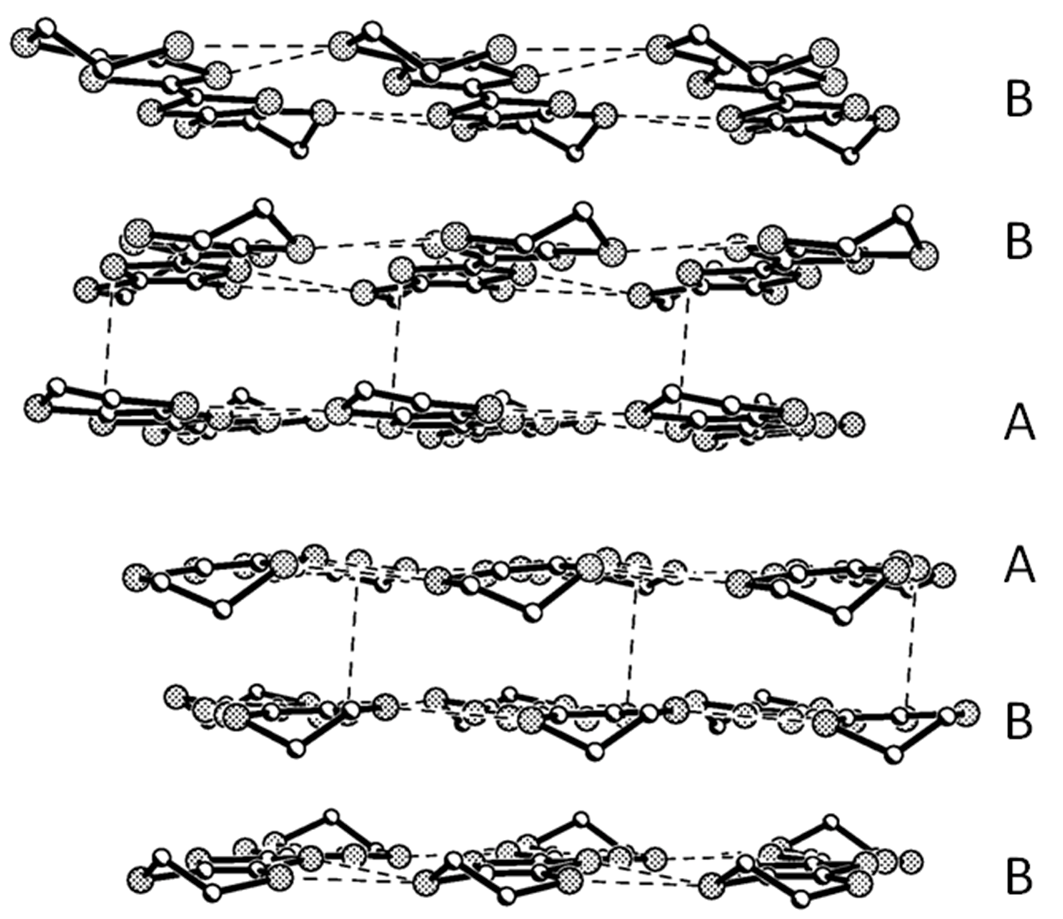
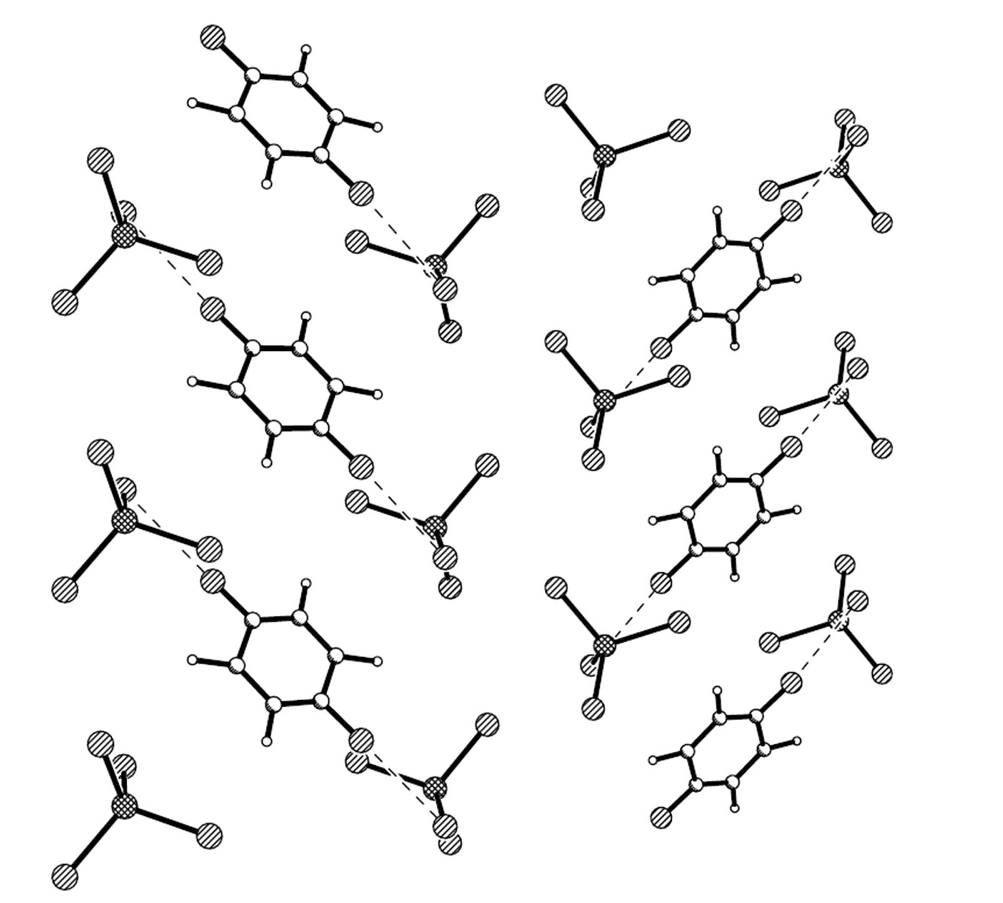
Figure 4.
GaCl4− and C6H5Cl in the anion layer. Dashed lines are Cl···Cl contacts between anion and solvent.
Figure 4.
GaCl4− and C6H5Cl in the anion layer. Dashed lines are Cl···Cl contacts between anion and solvent.
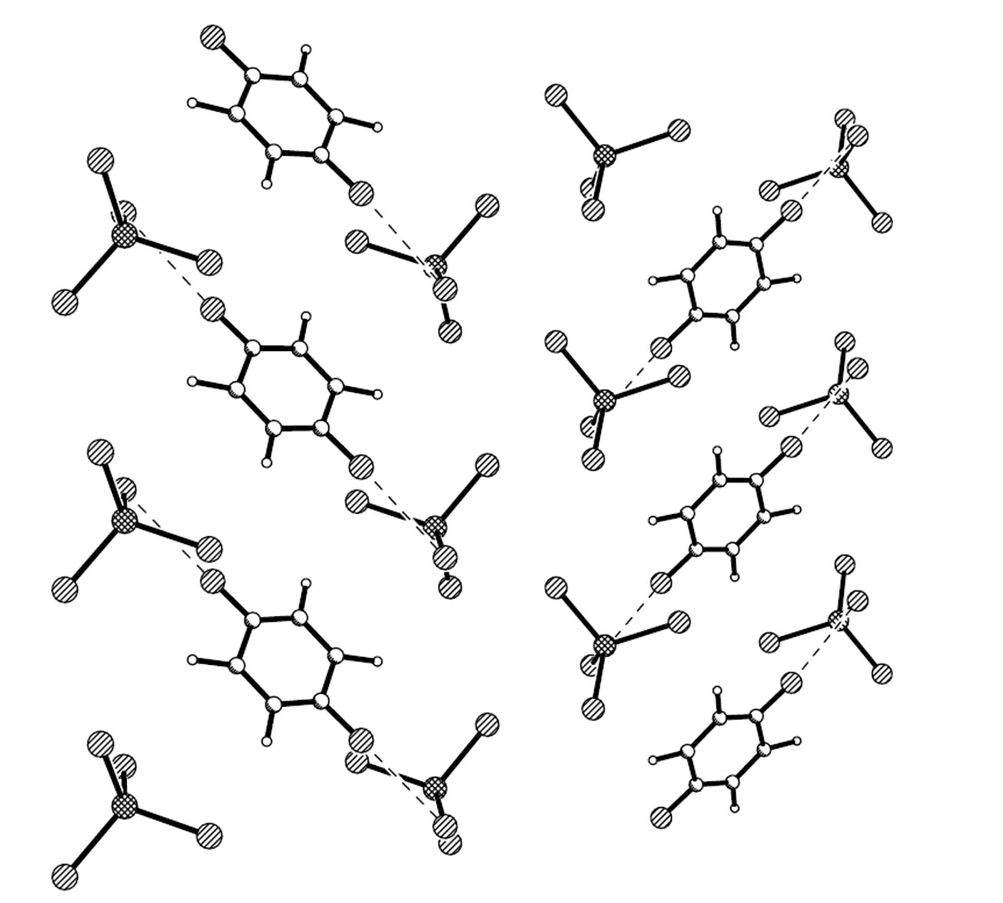
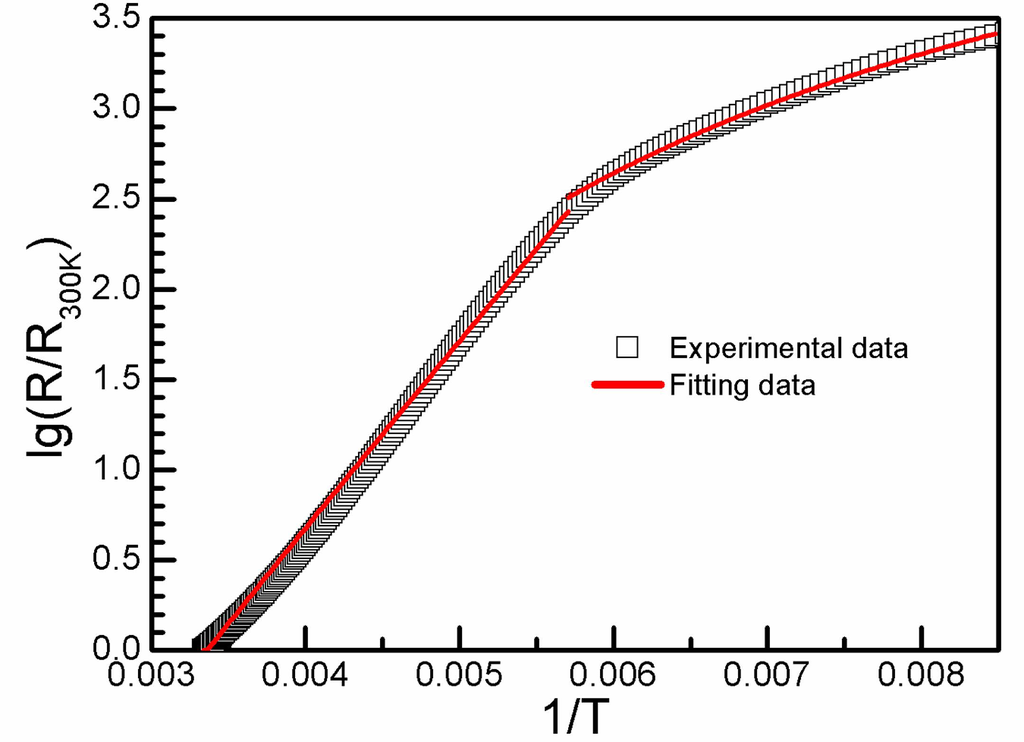
Figure 5.
Temperature-dependent resistivity of 1.
Figure 5.
Temperature-dependent resistivity of 1.
3. Experimental Section
All reagents are commercially available and were used as received without further purification unless otherwise stated. Block colorless crystalline (CH3)4NGa(C2O4)Cl2 was synthesized from 1 mmol [(CH3)4N]3Ga(C2O4)3 and 2 mmol GaCl3 in 10mL H2O. The products were recrystallized twice from ethanol. (CH3)4NFe(C2O4)Cl2 is isostructural as reported [8]. C6H5Cl and C2H5OH were distilled before use.
5.0 mg BEDT-TTF, 15.0 mg (CH3)4NGa(C2O4)Cl2 were dissolved in a mixture of 25.0 mL C6H5Cl and 5.0 mL C2H5OH. The platelike dark brown single crystal of (BEDT-TTF)4 (GaCl4)2·C6H5Cl (1) was obtained by an electrocrystallization method with a Pt cathode at 0.20 μA for two weeks.
X-ray diffraction data of 1 were collected on a Nonius Kappa CCD diffractometer with graphite monochromated Mo Kα (λ = 0.71073 Å) radiation at 290 K and 120 K. Lorentz, polarization, and empirical absorption corrections were carried out [9]. The crystal structure was solved by the direct method and refined by full-matrix least-squares on F2 using the SHELX program, with anisotropic thermal parameters for all non-hydrogen atoms [10]. The hydrogen atoms on the ethyl groups were determined by calculation. Crystallographic data and refinement parameters are summarized in Table 1. Two sets of structure data have been deposited at the Cambridge Crystallographic Data Centre.
The measurement of the resistance as a function of the temperature from 300 K to 2 K was carried out on a Quantum Design PPMS 9 system by a four-probe method. Twenty μm Au wires were attached to a single crystal by gold paste on the best developed surface.
4. Conclusions
When a solvent molecule was included in a binary charge-transfer salt (BEDT-TTF)2GaCl4, the dimerization of the donor molecules disappeared, and the conductivity increased in the ternary charge-transfer salt (BEDT-TTF)2GaCl4(C6H5Cl)0.5. New magnetic conductors can be expected when solvent molecules are intercalated into binary charge-transfer salts of FeCl4− [7].
Acknowledgments
This work was financially supported by the National Natural Science Foundation of China (21173230), MOST (2011CE93202), 973 (2013CB933402).
References
- Williams, J.M.; Ferraro, J.R.; Thorn, R.J.; Carlson, D.; Geiser, U.; Wang, H.H.; Kini, A.M.; Whangbo, M. Organic Superconductors(Including Fullerences); Prentice Hall, Inc.: Englewood Cliffs, NJ, USA, 1992. [Google Scholar]
- Coronado, E.; Curreli, S.; Gimenez-Saiz, C.; Gomez-Garcia, C.J. The Series of Molecular Conductors and Superconductors ET4[AFe(C2O4)3].PhX (ET = bis(ethylenedithio)tetrathiafulvalene; (C2O4)2− = oxalate; A+ = H3O+, K+; X = F, Cl, Br, and I): Influence of the Halobenzene Guest Molecules on the Crystal Structure and Superconducting Properties. Inorg. Chem. 2012, 51, 1111–1126. [Google Scholar]
- Kurmoo, M.; Graham, A.W.; Day, P.; Coles, S.J.; Husthouse, M.B.; Caulfield, J.L.; Singleton, J.; Pratt, F.L.; Hayes, W.; Ducasse, L.; et al. Cystal Structure and Magnetism of (BEDT-TTF)2MCl4 (BEDT-TTF = Bis(ethylenedithio)tetrathiafulvalne; M = Ga, Fe). Inorg. Chem. 1996, 35, 4719–4726. [Google Scholar]
- Coronado, E.; Day, P. Magnetic Molecular Conductors. Chem. Rev. 2004, 104, 5419–5448. [Google Scholar] [CrossRef]
- Zhang, B.; Pratt, F.L.; Kurmoo, M.; Okano, Y.; Kobayashi, H.; Zhu, D.B. A hybrid organic-inorganic conductor κ’-BETS2FeCl4, BETS = bis(ethylenedithio)tetraselenafulvalene). Cryst. GrowthDesign 2007, 7, 2548–2552. [Google Scholar] [CrossRef]
- Guionneau, P.; Kepert, C.J.; Bravic, G.; Chasseau, D.; Truter, M.R.; Kurmoo, M.; Day, P. Determining the Charge Distribution in BEDT-TTF Salt. Synth. Met. 1997, 86, 1973–1974. [Google Scholar] [CrossRef]
- Zhang, B.; Kurmoo, M.; Mori, T.; Zhang, Y.; Pratt, F.L.; Zhu, D.B. Polymorphism in hybrid organic-inorganic bilayered magnetic conductors (BEDT-TTF)3(FeIIICl4)2, BEDT-TTF = bis(ethylenedithio) tetrathia- fulvalene. Cryst. Growth Design 2010, 10, 782–789. [Google Scholar] [CrossRef]
- Xu, H.; Wang, Z.; Liu, T.; Gao, S. Synthesis, Structure, and Magnetic Properties of (A)[FeIII(oxalate)Cl2] (A = Alkyl Ammonium Cations) with Anionic 1D [FeIII(oxalate)Cl2]- Chains. Inorg. Chem. 2007, 46, 3089–3096. [Google Scholar] [CrossRef]
- Nonius, B.V. “Collect”, Data Collection Software, version 1997; Enraf-Nonius: Delft, The Netherlands, 1998. [Google Scholar]
- Sheldrick, G.M. SHELXL-97, PC version; University of Göttingen: Göttingen, Germany, 1997. [Google Scholar]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
