Abstract
This study is focused on the hydrothermal synthesis of iron–manganese oxide nanostructures, focusing on the influence of Fe:Mn precursor ratios, temperature, and reaction time on phase formation, morphology, and structural characteristics. Three molar ratios (Fe:Mn = 2:1, 1:1, and 1:2) were explored under variable conditions (80 °C, 120 °C, and 200 °C; 4, 12, and 24 h). X-ray diffraction (XRD) analysis revealed distinct phase selectivity depending on precursor composition: FeMn2O4 was obtained with 1:2 ratio, Fe3Mn3O8 with 1:1, and Fe2MnO4 with 2:1, each without phase mixing. Scanning electron microscopy (FESEM) showed a pronounced effect of temperature and time on nanoparticle morphology, ranging from compact agglomerates to well-defined rod-like structures at 200 °C/24 h. Dynamic light scattering (DLS) indicated narrow size distributions for samples synthesized at 120 °C/12 h, with hydrodynamic diameters between 20 and 50 nm. Raman spectroscopy confirmed the presence of characteristic vibrational modes of spinel-type structures and validated structural integrity. High-resolution transmission electron microscopy (HRTEM) evidenced well-ordered lattice fringes with interplanar spacings of ~0.48–0.52 nm, consistent with spinel phases and indicative of high crystallinity. These findings demonstrate that controlled atomic binding and thermal parameters enable selective synthesis of pure iron–manganese oxide phases with tailored morphologies, offering a scalable route for designing advanced functional materials in catalysis, energy, and biomedical applications.
1. Introduction
Transition metal oxides have garnered significant interest in the scientific community due to their unique magnetic [1], catalytic [2], and electronic properties [3], which make them suitable candidates for applications in energy storage [4], environmental remediation [5], magnetic devices [6], and biomedicine [7]. Among these, mixed metal oxides, particularly those involving iron (Fe) and manganese (Mn), exhibit enhanced physicochemical characteristics arising from synergistic interactions between the two metal centers [8,9]. The spinel-type iron–manganese oxides (MnFe2O4, FeMn2O4), for instance, have been reported to display favorable magnetic behavior, high stability, and promising redox activity, rendering them attractive for applications such as magnetic hyperthermia, lithium-ion batteries, and photocatalytic degradation of pollutants [10,11,12,13]. In recent years, nanostructuring of these oxides has become a focal point in materials research. Reducing particle size to the nanometer scale not only increases the surface area-to-volume ratio but also significantly alters surface energy, crystal structure, and defect density [14,15]. These nanoscale effects critically influence the functionality of manganese-iron oxide nanoparticles [16]. As such, the synthesis method, along with its specific parameters—particularly temperature—plays a pivotal role in defining the final properties of these nanostructures. Among various synthesis approaches, the hydrothermal method stands out due to its versatility, simplicity, and ability to produce highly crystalline nanostructures with controlled size, shape, and phase composition under relatively mild conditions [16,17]. In hydrothermal synthesis, aqueous chemical reactions are conducted in sealed vessels (autoclaves) at elevated temperatures and autogenous pressures. The reaction environment facilitates enhanced solubility and reactivity of precursors, enabling the formation of metastable and novel crystalline phases that may not be accessible via conventional methods such as co-precipitation or sol–gel routes [18]. Temperature, as one of the most critical parameters in hydrothermal synthesis, has a multifaceted influence on the nucleation and growth mechanisms of nanoparticles [19]. It not only affects the thermodynamic stability and kinetics of phase formation but also governs the solubility of metal precursors, diffusion rates, and crystal growth rates [20,21]. For instance, lower temperatures tend to favor the formation of amorphous or poorly crystalline phases due to reduced energy input for atomic rearrangement, while higher temperatures can influence the attainment of better crystalline ordering, phase transformation, and growth of larger crystallites [22]. Moreover, temperature variations can influence the oxidation states of manganese and iron, altering the stoichiometry and magnetic properties of the resulting oxides [23]. Previous studies have demonstrated that manganese and iron can form various oxide phases depending on synthesis conditions. Iron oxides, such as magnetite (Fe3O4), maghemite (γ-Fe2O3), and hematite (α-Fe2O3), are sensitive to pH, redox potential, and temperature. Similarly, manganese exhibits a wide range of oxidation states (Mn2+, Mn3+, Mn4+), leading to a variety of oxide structures such as MnO, Mn2O3, Mn3O4, and MnO2 [16,24]. In bimetallic systems, the formation of spinel structures like MnFe2O4 is particularly desirable due to their thermal and chemical stability and ferrimagnetic ordering [25]. However, despite the substantial body of work on manganese-iron oxides, a detailed understanding of how hydrothermal temperature alone affects the structural evolution, crystallinity, and phase composition remains limited. Most studies address multiple parameters simultaneously, making it difficult to isolate the specific influence of temperature. Furthermore, inconsistencies in precursor selection, reaction times, and post-synthesis treatments hinder the establishment of systematic correlations between synthesis temperature and nanoparticle properties. In this context, the present study aims to elucidate the role of temperature in governing the structural evolution of nanoparticles synthesized via the hydrothermal method.
Our work contributes to the optimization of synthesis parameters for the tailored design of nanomaterials. By systematically exploring the temperature-dependent structural features of these nanoparticles, we provide valuable insights for their targeted application in energy storage systems, catalysis, and biomedical devices. Additionally, this investigation may support the development of predictive models for the synthesis of other multimetallic oxide systems where temperature is a key factor in phase selection and crystallinity control. Overall, this study not only deepens the understanding of temperature-controlled hydrothermal synthesis mechanisms for nanoparticles but also opens avenues for future research on thermally tunable nanostructured materials with application-specific properties. Please note that in this work, “iron–manganese oxides” is used to refer to the broader family of oxides containing both Fe and Mn, and the specific term “manganese ferrites” is used exclusively when referring to spinel-type phases such as MnFe2O4.
2. Materials and Methods
2.1. Materials and Reagents
The synthesis of manganese-iron oxide nanoparticles was carried out using commercially available reagents, all of which were employed as received without additional purification. The metal precursors used were iron (III) chloride hexahydrate (FeCl3·6H2O, Sigma-Aldrich, St. Louis, MO, USA) and manganese (II) chloride tetrahydrate (MnCl2·4H2O, Sigma-Aldrich), chosen for their high solubility in aqueous media. To initiate the co-precipitation reaction and control the pH of the system, sodium hydroxide (NaOH, Sigma-Aldrich, St. Louis, MO, USA) was used as a strong base. To assist in directing the morphology of the nanoparticles, sodium dodecyl sulfate (SDS, Sigma-Aldrich, St. Louis, MO, USA) served as a surfactant during the hydrothermal process. Absolute ethanol (C2H5OH, ≥99.8%) and deionized water were utilized in the cleaning stages following synthesis, which involved ultrasonic dispersion and centrifugation to ensure removal of any remaining unreacted precursors or byproducts. All reagents were of analytical grade and used under ambient laboratory conditions. Deionized water was produced in-lab using a purification system with a resistivity of 18.2 MΩ·cm.
2.2. Synthesis of Iron–Manganese Nanostructures
Iron–manganese oxide nanostructures were synthesized using a hydrothermal method [16] to investigate the influence of temperature, reaction time, and precursor ratio on structural and morphological development. Three different molar ratios of Fe to Mn precursors were evaluated: 2:1, 1:1, and 1:2. For each composition, the corresponding amounts of iron (III) chloride hexahydrate and manganese (II) chloride tetrahydrate were dissolved in deionized water under constant magnetic stirring. A surfactant, sodium dodecyl sulfate (SDS), was added to regulate particle formation and growth during the process. The pH of the resulting solution was adjusted by the gradual addition of sodium hydroxide (NaOH) until a uniform dispersion was obtained. Each prepared mixture was transferred into a Teflon-lined stainless steel autoclave and subjected to hydrothermal processing at different temperatures: 80 °C, 120 °C, 180 °C, and 200 °C, for fixed reaction durations of 4, 8, 12, or 24 h, depending on the experimental condition. Following the heat treatment, the autoclaves were allowed to cool to room temperature without external intervention. The solid products were separated by centrifugation and washed repeatedly with ethanol and deionized water, using ultrasonic assistance between washes to ensure the removal of residual ions and surfactant molecules. This cycle was repeated until a neutral pH was achieved in the supernatant. Finally, the cleaned nanoparticles were air-dried and stored in dry conditions for subsequent analysis. Note that the samples synthesized at 180 °C did not exhibit any significant morphological or structural distinctions compared to those synthesized at 200 °C. In all observed cases, the trends in crystal growth, particle faceting, and phase formation at 180 °C closely paralleled those at the highest temperature studied, particularly in terms of anisotropic growth and crystallinity. Given the focus of the manuscript on identifying representative and contrasting synthesis conditions that highlight key morphological transitions (e.g., low vs. high temperature, short vs. long time), we opted to present only the most relevant and distinct cases to ensure clarity and reduce redundancy in the Results Section.
2.3. Physical and Chemical Characterization
A combination of analytical techniques was employed to investigate the structural, morphological, and vibrational features of the synthesized iron–manganese oxide nanostructures. X-ray diffraction (XRD) analysis was carried out using a Bruker D8 Advance diffractometer (Bruker, Karlsruhe, Germany) equipped with a Cu Kα radiation source (λ = 1.5406 Å). Diffraction patterns were collected in the 2θ range from 10° to 80°, using a step size of 0.02° and a scan speed of 0.5 s/step. The obtained data were used to identify the crystalline phases present and to assess the effect of precursor ratios on phase formation. Scanning electron microscopy (SEM) was performed to assess nanoparticle morphology and size distribution. The images were acquired using a JEOL JSM-7401F field-emission SEM (JEOL Ltd., Tokyo, Japan), operating at an accelerating voltage of 15 kV. Samples were deposited on carbon-coated copper stubs and gold-sputtered for improved conductivity prior to analysis. This technique enabled visualization of the particle shapes, surface textures, and size uniformity as a function of synthesis parameters. On the other hand, dynamic light scattering (DLS) measurements were performed to estimate the hydrodynamic diameter of selected samples dispersed in absolute ethanol. The analyses were conducted on a Litesizer 500 instrument (Anton Paar GmbH, Graz, Austria), operating at a wavelength of 658 nm with automatic angle adjustment. Measurements were taken at 25 °C, and the particle size distribution was calculated based on the intensity of scattered light using cumulant and distribution fitting methods. Raman spectroscopy was employed to investigate the vibrational properties of the nanoparticles and to verify the formation of spinel ferrite structures. The spectra were recorded using a Horiba LabRAM HR Evolution system (Horiba Scientific, Paris, France) equipped with a 633 nm He–Ne laser, a 1200 grooves/mm diffraction grating, and an optical microscope with a 50× objective. Spectra were collected in the 200–800 cm−1 range with an acquisition time of 10 s per accumulation, averaged over multiple scans. Transmission electron microscopy (TEM) and high-resolution TEM (HRTEM) were conducted on representative samples to visualize internal structure and lattice fringes. A JEOL ARM200F transmission electron microscope (JEOL Ltd., Japan), operating at 200 kV, was used. All measurements were performed at room temperature unless otherwise noted, and the characterization was conducted on at least two independently synthesized batches for reproducibility.
3. Results and Discussion
3.1. Effect of Concentration on Iron–Manganese Phase
The crystalline phases formed in iron–manganese oxide nanostructures were strongly dependent on the Fe:Mn molar ratio used during hydrothermal synthesis. Figure 1 shows the XRD patterns corresponding to samples prepared at three different precursor ratios: Fe:Mn = 2:1 (blue curve), 1:1 (red curve), and 1:2 (black curve). For the sample synthesized with an Fe:Mn ratio of 2:1, the diffraction peaks match the cubic spinel structure of Fe2MnO4 (PDF reference: COD-98-012-9099). The most prominent reflections were indexed to the (022), (113), (222), (004), (224), (115), and (044) planes, characteristic of the inverse spinel configuration [16,26]. The well-defined peak positions and intensities indicate a high degree of crystallinity and phase homogeneity. This structure arises when iron is present in excess, promoting the stabilization of the Fe-rich spinel phase where Mn ions preferentially occupy octahedral sites, and Fe ions are distributed between tetrahedral and octahedral coordination environments. The presence of Fe in a higher oxidation state (Fe3+) under alkaline hydrothermal conditions further facilitates the formation of Fe2MnO4, which is thermodynamically favored under iron-rich environments [27]. In contrast, when the Fe and Mn concentrations were equimolar (Fe:Mn = 1:1), the resulting phase corresponded to Fe3Mn3O8, a less common but structurally ordered oxide compound. The XRD pattern for this sample (red curve) reveals a distinct set of reflections indexed to the (022), (113), (222), (004), (224), (115), and (044) planes, in agreement with a monoclinic or orthorhombic framework derived from mixed-valence iron and manganese species [28]. The symmetric and narrow peaks suggest a well-ordered lattice, and the absence of any additional peaks confirms the exclusive formation of this ternary oxide [29]. The formation of Fe3Mn3O8 under equimolar conditions is attributed to the balanced redox interaction between Fe3+/Fe2+ and Mn2+/Mn3+ ions during the hydrothermal reaction. For the sample prepared with a Fe:Mn ratio of 1:2, the dominant crystalline phase was identified as FeMn2O4, as seen in the XRD pattern shown in black. This structure corresponds to a normal spinel ferrite, with Mn occupying the majority of the octahedral sites and Fe confined primarily to tetrahedral positions. The diffraction peaks in this pattern match well with the standard reference for FeMn2O4 (PDF: COD-98-012-2844), confirming the formation of a manganese-rich spinel [30]. The peaks are sharp and symmetrical, indicative of a highly crystalline phase with no detectable impurities or secondary phases. This composition is commonly associated with increased magnetic responsiveness due to enhanced Mn2+ content and the associated cation distribution, which may modify superexchange interactions between metal centers.
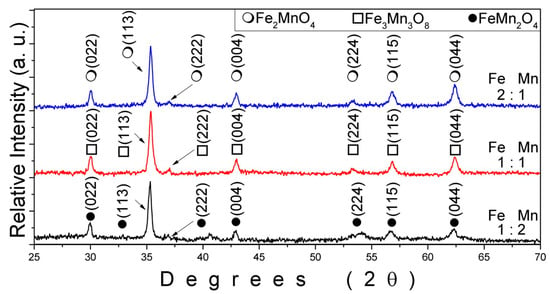
Figure 1.
XRD patterns of Mn–Fe oxide nanoparticles synthesized with different Fe:Mn ratios, showing phase transitions from FeMn2O4 (●) to Fe3Mn3O8 (□) and Fe2MnO4 (○) as Fe content increases.
Importantly, the observed evolution from FeMn2O4 to Fe2MnO4 across the three compositions indicates a clear and controllable route for tuning the final crystal structure through stoichiometric design. The results highlight that precursor ratio plays a decisive role in directing phase formation, even more than other factors such as time or temperature under the synthesis conditions explored. From a crystallographic standpoint, the systematic shift in peak position and intensity across the samples may also reflect subtle changes in lattice parameter and atomic site occupancy as the Fe:Mn ratio varies. Although Rietveld refinement was not performed in this study, the distinct phase identity of each sample and the consistency with reference patterns allow for confident phase assignment. Additionally, the uniformity of the peaks and their lack of broadening suggest relatively low internal strain and well-developed crystalline domains. These findings are significant because different phases of iron–manganese oxides exhibit distinct physicochemical behaviors. For instance, FeMn2O4 is known for its soft magnetic properties and catalytic activity, while Fe2MnO4 may offer greater thermal stability and corrosion resistance. The intermediate Fe3Mn3O8 phase may possess unique mixed-valence characteristics that can be exploited in electrochemical or sensing applications.
Thus, controlling the Fe:Mn ratio during synthesis offers a powerful tool for tailoring the functional properties of Mn–Fe oxides to meet the demands of specific applications in catalysis, magnetic storage, or biomedical fields. Thus, the XRD results confirm that hydrothermal synthesis allows for precise phase control of iron–manganese oxides by simply adjusting the Fe:Mn molar ratio in the precursor solution. The formation of FeMn2O4, Fe3Mn3O8, and Fe2MnO4 as distinct and phase-pure products demonstrates the robustness of the approach. From a crystallographic perspective, FeMn2O4, Fe3Mn3O8, and Fe2MnO4 are all mixed-metal oxides that can adopt spinel-type or spinel-related structures, but their crystal symmetries, cation distributions, and oxidation states differ significantly. FeMn2O4 typically crystallizes in a normal spinel structure (space group Fd3̅m), where Fe2+ ions preferentially occupy the tetrahedral (A) sites, and Mn3+ ions occupy the octahedral (B) sites. In contrast, Fe2MnO4 is also a spinel-type oxide but with a different Fe:Mn ratio, which influences the degree of cation disorder and may lead to mixed valency of Mn (Mn2+/Mn3+) and Fe (Fe2+/Fe3+), resulting in variable site occupancies and Jahn–Teller distortions at the B sites. Meanwhile, Fe3Mn3O8 crystallizes in a non-spinel structure typically indexed in a monoclinic or orthorhombic system, associated with layered arrangements of Fe and Mn cations in distinct coordination environments. This phase is commonly formed under specific thermal or stoichiometric conditions where charge balance is achieved through ordered cation sublattices rather than the typical spinel framework. These differences directly impact the observed XRD patterns, crystallite sizes, and growth orientations during synthesis. Thus, although the phases may share similar compositional elements, their structural frameworks and internal symmetries are markedly distinct.
3.2. Influence of Temperature and Time on Morphology
The morphological evolution of iron–manganese oxide nanostructures synthesized via hydrothermal treatment was investigated as a function of synthesis temperature (80 °C, 120 °C, and 200 °C) and reaction time (4, 12, and 24 h). Figure 2 presents a comprehensive matrix of FESEM micrographs for three distinct Fe:Mn molar ratios: Fe20Mn80, Fe50Mn50, and Fe80Mn20. Each row corresponds to increasing synthesis temperatures, while each column reflects increasing reaction times. This design allows the direct correlation of morphological changes with synthesis parameters and provides insights into the nucleation and growth processes dominating each condition.
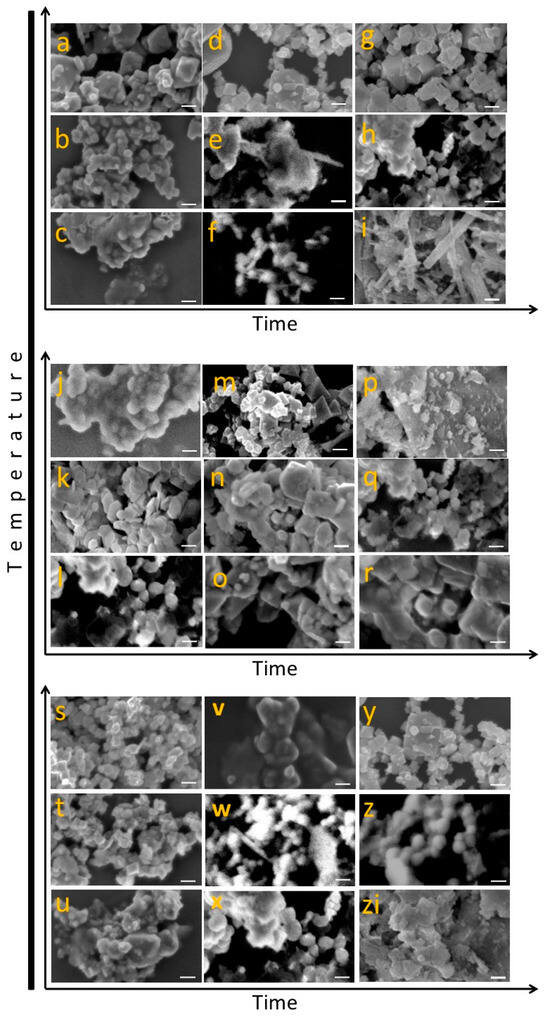
Figure 2.
SEM micrographs of MnFe2O4 nanoparticles synthesized under different hydrothermal conditions, illustrating the morphological evolution with temperature (80–200 °C) and time (4–24 h). Nanoparticles exhibit diverse structures ranging from flake-like to well-defined polyhedral morphologies as synthesis conditions vary. Scale bar equals to 100 nm.
3.2.1. Fe20Mn80—Mn-Rich System
The top set of micrographs (a–i) corresponds to the Fe20Mn80 composition. At the lowest temperature (80 °C) and shortest time (4 h, image a), the sample exhibits agglomerated rounded particles with smooth surfaces, suggestive of early-stage nucleation and low diffusion rates. Increasing the synthesis time to 12 h (image b) results in more compact aggregates, but individual particles remain indistinct. By 24 h (image c), growth continues without clear crystallite definition, indicating that low-temperature conditions limit anisotropic development. At 120 °C (images d–f), the surface morphology becomes increasingly rough with elongated features emerging at longer synthesis durations. Specifically, after 24 h (image f), flower-like or dendritic structures begin to appear, indicating a transition toward directional crystal growth enabled by increased thermal energy. At the highest temperature (200 °C), significant morphological transformation is observed. Image g (4 h) shows well-defined, faceted particles of cubic or polyhedral geometry, marking the onset of crystalline growth. After 12 h (image h), the structures become porous and branched, while at 24 h (image i), needle-like and rod-shaped particles dominate, indicating extensive anisotropic growth along preferential axes. This confirms that Mn-rich environments at elevated temperatures and extended times favor the formation of high-aspect-ratio structures.
3.2.2. Fe50Mn50—Equimolar Composition
The middle panel (j–r) shows the morphological progression for the equimolar Fe50Mn50 composition. At 80 °C, all time intervals (images j–l) display dense aggregates with poorly defined individual grains, similar to the low-temperature trend observed in the Mn-rich system. However, a slightly coarser texture is evident, possibly due to higher iron content accelerating early coarsening processes. At 120 °C (images m–o), an evident transformation occurs. For 4 h (m), small polyhedral particles begin to emerge from amorphous aggregates. By 12 h (n), the surface becomes granular and more organized, while after 24 h (o), faceted nanocrystals predominate, suggesting that intermediate thermal energy promotes crystal nucleation and moderate growth. The most notable morphology is found at 200 °C (images p–r). At 4 h (p), particles are faceted and densely packed, indicating a high degree of crystallinity even at short times. Increasing to 12 h (q), the particles evolve into hierarchical structures with surface roughness indicative of secondary growth. At 24 h (r), uniform polyhedral particles dominate the field, forming dense, interconnected networks. This morphology may reflect optimal co-nucleation and co-growth of Fe and Mn oxides under balanced stoichiometry and elevated thermal input.
3.2.3. Fe80Mn20—Fe-Rich System
The bottom set (s–zi) illustrates the morphological evolution for the Fe80Mn20 ratio. At 80 °C (s–u), particle aggregation is significant across all times. Image s (4 h) shows loosely aggregated clusters, which begin to compact slightly at 12 h (t), but maintain an amorphous texture. At 24 h (u), the structures remain irregular and indistinct, indicating insufficient driving force for crystallization at this temperature, despite the high Fe content. At 120 °C (v–x), significant phase separation is visible. Image v (4 h) presents granular particles with moderate uniformity. At 12 h (w), the contrast in the image indicates possible porosity or phase heterogeneity, which could arise from differential solubility or growth kinetics between Fe and Mn oxides. By 24 h (x), the material exhibits cauliflower-like morphology, suggestive of self-assembled nanoclusters. At 200 °C (y–zi), pronounced morphological features emerge early. Image y (4 h) shows agglomerated nanocrystals with a tendency to form porous networks. After 12 h (z), the system evolves toward elongated particles interlinked by grain boundaries. At 24 h (zi), compact and densely intergrown faceted particles dominate, likely indicating the predominance of iron oxide crystallization, favored by the high Fe content and thermal energy.
Overall, the results reveal clear trends across all compositions. First, increasing synthesis temperature significantly enhances crystallinity and structural definition, which aligns with thermally driven nucleation and growth kinetics. At 80 °C, the energy input appears insufficient for significant crystal formation, regardless of the Fe:Mn ratio. At 120 °C, intermediate features emerge, such as surface coarsening, polyhedral faceting, and partial alignment. The most distinct morphologies are achieved at 200 °C, particularly in Mn-rich systems that produce elongated or acicular structures, and in Fe-rich systems where compact crystalline domains prevail. Reaction time also plays a critical role, albeit secondary to temperature. Prolonged reaction durations at high temperatures allow for complete growth and recrystallization, as seen in images i, r, and zi. Conversely, extended times at low temperatures mainly promote aggregation without significant improvement in particle definition. The Fe:Mn ratio influences the dominant morphological features by affecting the growth kinetics and phase stability of the system. Mn-rich systems favor needle-like and dendritic morphologies under high-temperature conditions, likely due to the preferential growth of Mn-rich phases with anisotropic crystal habits. In contrast, Fe-rich systems tend to yield polyhedral and densely packed crystals, reflecting the faster nucleation and growth of iron oxide structures under hydrothermal conditions.
3.3. Structure and Size of Nanoparticles
The structural integrity and particle size of the synthesized iron–manganese oxide nanoparticles were thoroughly examined through Dynamic Light Scattering (DLS) and Raman spectroscopy, as shown in Figure 3. These complementary techniques allowed the identification of morphological homogeneity and crystallographic fingerprints associated with distinct phases, providing insights into how synthesis parameters influence final nanoparticle characteristics. Figure 3a displays DLS measurements corresponding to the most morphologically uniform nanoparticles observed across the synthesis conditions. These results were selected from the complete morphological matrix presented in Figure 2. DLS analysis revealed that all three samples exhibit a sharp unimodal distribution, indicating a high degree of homogeneity in particle size. The intensity peaks are narrowly distributed around 50–100 nm, which confirms the efficiency of the hydrothermal method in controlling growth kinetics when optimized parameters are employed. The top panel of Figure 3a (red line) corresponds to a sample with a Fe:Mn ratio of 1:2 synthesized at 120 °C for 12 h. This sample displays the narrowest size distribution with a peak centered at approximately 55 nm, suggesting a highly regulated nucleation process under these conditions. The second panel (blue line), representing a 1:1 stoichiometry prepared under similar thermal conditions, also shows a single peak near 65 nm, albeit with a slightly broader distribution. This broadening may be attributed to competitive growth among nuclei with varying diffusion rates due to the equimolar cationic content. Finally, the bottom panel (gray line), which reflects the Fe:Mn 2:1 composition synthesized at 200 °C for 24 h, shows a well-defined peak around 75 nm. While still narrow, the size distribution is comparatively broader than the other two, possibly due to agglomeration tendencies or less constrained crystal growth at higher iron content and longer reaction time. These DLS results are consistent with the field-emission scanning electron microscopy (FESEM) images, which demonstrate an evident morphological regularity in these samples compared to others synthesized under suboptimal thermal or temporal conditions. DLS data also reinforce the hypothesis that manganese-rich compositions promote tighter control over nucleation, likely due to their stronger Lewis acidity and their role in moderating crystallization rates [31]. Table 1 shows the size distribution of the entire sample studied in this work.
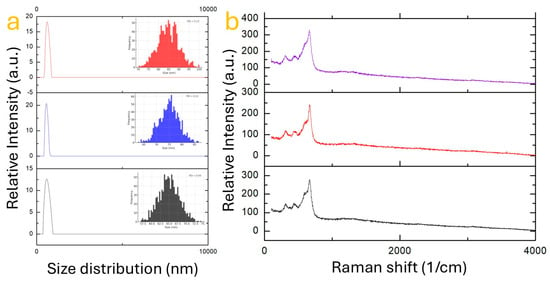
Figure 3.
(a) Size distribution of selected iron–manganese oxide nanoparticle samples measured by dynamic light scattering (DLS). (b) Corresponding Raman spectra of the same samples, showing characteristic vibrational modes of the spinel ferrite structure.

Table 1.
Size distribution of the explored conditions measured experimentally by DLS.
Complementary to size analysis, Raman spectroscopy (Figure 3b) was performed to confirm the chemical structure and local ordering of the synthesized materials. Each panel corresponds to the same samples represented in the DLS plots and further supports the phase homogeneity identified in the XRD and FESEM analyses. Raman spectroscopy is particularly sensitive to metal-oxygen vibrational modes, which makes it an ideal tool to distinguish spinel-type and mixed-valence oxide structures often formed in Fe-Mn systems. In the upper spectrum (purple), corresponding to the FeMn2O4-dominant sample, the strongest band is located near 640 cm−1, which is attributed to the symmetric stretching mode of the Mn–O bond in tetrahedral sites of the spinel structure. Additional minor bands observed around 480 cm−1 and 300 cm−1 are associated with bending vibrations and support the presence of a highly ordered inverse spinel structure. The sharpness and intensity of these peaks confirm the crystallinity of the sample and the stability of this manganese-rich phase at moderate synthesis temperatures. The middle spectrum (red), corresponding to the Fe3Mn3O8 phase, shows a Raman signature with a major peak near 610 cm−1, which is typical for mixed-valence manganese–iron oxides where Fe3+ and Mn3+ coexist. The broader nature of this peak suggests slightly lower crystallinity or the presence of lattice distortions caused by equal competition between the two cations for the same lattice positions. Additional features at lower wavenumbers indicate structural complexity, which may be associated with oxygen vacancies or partial disorder within the octahedral framework. In the lower spectrum (black), representing the Fe2MnO4-rich sample, the main peak appears at approximately 590 cm−1, accompanied by broader features at 480 cm−1 and 320 cm−1 [16]. The relative intensity and broadening of these peaks indicate a less ordered lattice, possibly due to excess iron incorporation leading to defect formation or heterogeneous coordination environments. This is in line with the previously observed broader size distribution and the more irregular morphology seen under FESEM. The combined analysis of particle size and structural fingerprints illustrates a clear correlation between synthesis parameters and final nanoparticle properties. Mn-rich compositions tend to favor smaller and more monodisperse nanoparticles with highly ordered spinel-type crystal lattices, as seen in the FeMn2O4 sample. This behavior is attributed to the strong crystal field stabilization and lower reduction potential of Mn3+ [32], which may promote controlled nucleation and suppress rapid particle growth or agglomeration. In contrast, the Fe-rich sample exhibits both a larger particle size and less defined Raman features, suggesting that excess iron disrupts the ordered spinel structure. The mixed Fe:Mn ratio, however, leads to intermediate characteristics—uniform size with slightly disordered structural features—highlighting the delicate balance between composition and thermal parameters in tailoring nanoparticle properties. Importantly, the absence of secondary Raman bands or significant fluorescence background across all spectra suggests high phase purity and minimal contamination [33]. This observation supports the earlier XRD findings, which identified single-phase materials under well-optimized conditions and validates the robustness of the synthesis methodology.
3.4. Implication of Atomic Bindings in the Phase Formation
The atomic structure of nanoparticles is a fundamental factor that governs their crystallographic phase, stability, and physicochemical properties. Figure 4 presents two key transmission electron microscopy (TEM) images that serve to explore the relationship between local atomic arrangements and the development of distinct crystallographic phases in the synthesized iron–manganese oxide nanomaterials. These images provide direct evidence of how synthesis parameters influence atom-level organization, favoring particular bonding motifs and ultimately dictating the emergence of specific structural phases such as FeMn2O4, Fe3Mn3O8, or Fe2MnO4, as described in prior sections. Figure 4a displays a bright-field low-magnification TEM image of a representative sample selected based on its high uniformity and structural order—synthesized at 120 °C for 12 h using a Fe:Mn precursor ratio of 1:2. The image shows a semi-agglomerated cluster of nanocrystals, where the particles exhibit clear contrast variations indicative of multiple crystalline grains and interfaces. The aggregation of particles appears dense but not overly fused, suggesting a degree of electrostatic stabilization during the hydrothermal process, potentially due to surface hydroxylation or the intrinsic charge of the oxide surfaces in aqueous media [34]. From this overview, it is possible to infer that the particles present coherent growth and crystalline faceting rather than amorphous morphology, supporting conclusions previously obtained by FESEM and DLS. The interparticle boundaries are sharp and well-defined, indicating limited sintering or phase diffusion between nanograins, which is advantageous for preserving distinct crystallographic domains. More critically, Figure 4b shows the high-resolution TEM (HRTEM) image from the square-marked region in panel (a), zooming in on a lattice-resolved portion of a single nanoparticle. This image is pivotal for understanding the atomic-scale ordering and identifying the nature of atomic bonds that stabilize specific crystalline phases. A regular arrangement of bright spots can be clearly observed, representing atomic columns viewed along a crystallographic zone axis. The observed periodicity corresponds to interplanar distances in the range of 0.48–0.52 nm, which match well with the (113) and (222) planes of FeMn2O4 and Fe3Mn3O8 spinel structures, as identified by X-ray diffraction. The intensity and clarity of the fringes further suggest a high degree of crystallinity. The continuity of lattice planes over the field of view indicates that this particle comprises a single crystallographic domain or at most a twinned region without grain boundary disruptions, which is typical in well-annealed spinel-type nanocrystals. This high-resolution visualization confirms that the atoms in the synthesized nanostructures occupy well-defined lattice positions, forming periodic three-dimensional arrays governed by metal–oxygen–metal (M–O–M’) bridging, where M and M’ represent Fe3+ and Mn3+/Mn2+ cations. The M–O–M’ bonding network is a signature of the inverse spinel structure often adopted by transition-metal oxides [35]. In this framework, oxygen atoms form a close-packed FCC lattice, with Fe and Mn ions occupying octahedral and tetrahedral interstices. The alternating Fe–O–Mn bonds introduce distortions in bond lengths and angles, leading to slight deviations from ideal symmetry that are detectable in the subtle curvature of the lattice fringes. The observed lattice ordering supports the notion that the formation of specific phases—such as FeMn2O4—is highly dependent on the precise atomic coordination environment. The stabilization of this phase is likely driven by two factors: (i) the preferential occupation of Mn3+ in octahedral sites and (ii) the partial reduction of Mn4+ to Mn2+ under hydrothermal conditions, promoting the spinel-type configuration [16,36]. This results in a robust network of corner-sharing MO6 octahedra and tetrahedra, as expected for spinel materials. Samples with excess iron content tend to adopt more complex mixed-valence phases, such as Fe3Mn3O8, where charge imbalance leads to oxygen vacancy formation or disordered site occupancy. These structural complexities typically manifest in the form of irregular or strained lattice fringes, which were not observed in the current HRTEM image, further confirming that the examined particle belongs to a structurally simpler, energetically favorable configuration—most likely FeMn2O4. The atomic-level evidence suggests that the hydrothermal method not only promotes phase-selective nucleation but also favors energetically stable atomic arrangements due to gradual reduction-oxidation equilibria.
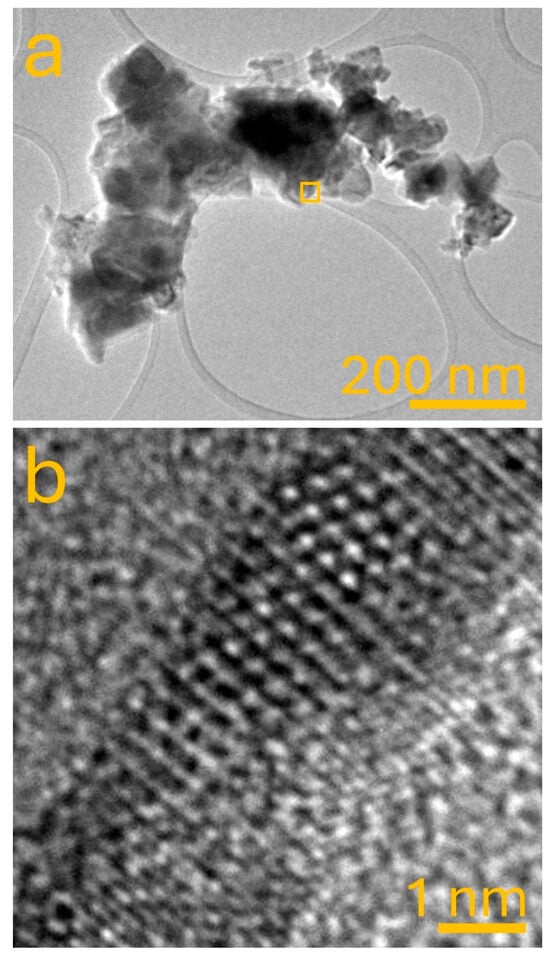
Figure 4.
(a) TEM image of iron manganese oxide nanoparticles synthesized via the hydrothermal method. (b) High-resolution TEM (HRTEM) image of the region marked in (a), revealing clear lattice fringes corresponding to the crystalline structure of iron manganese oxide nanoparticles.
The slow ramping of temperature and prolonged reaction time allow atoms to rearrange into their thermodynamically preferred sites, thereby minimizing lattice strain and maximizing crystal integrity. Furthermore, the bonding flexibility between Fe–O and Mn–O linkages supports the coexistence of multiple oxidation states [37].
While core@shell nanostructures are commonly observed in multicomponent oxide systems, particularly those synthesized via hydrothermal methods under phase-separating conditions, our experimental evidence suggests that such architecture is not present in the nanoparticles obtained in this study. High-resolution transmission electron microscopy (HRTEM) imaging, although limited to a representative lattice-resolved region, reveals uniform lattice fringe continuity throughout individual nanoparticles, with no observable contrast or interface that would indicate a discrete core or shell region. Furthermore, the lack of concentric contrast variations in the bright-field TEM images—often a hallmark of core@shell morphologies—supports the conclusion that the particles exhibit homogenous phase distribution. This observation is corroborated by the morphological and compositional consistency observed across synthesis parameters (Fe:Mn ratios, temperature, and time), as presented in the FESEM and DLS results. The systematic evolution from amorphous aggregates to faceted crystalline particles occurs without evidence of phase demixing or preferential shell growth. Additionally, no secondary phase growth or encapsulation tendency was detected in the analyzed conditions, which would otherwise suggest heterogeneous nucleation pathways typical of core@shell systems. The synthesis parameters employed in this work promote co-precipitation and co-crystallization of Fe and Mn oxides, favoring solid solution formation over phase segregation [38].
The morphological diversity observed across varying Fe:Mn precursor ratios can be partly attributed to the electronic structure and bonding interactions inherent to each compositional system. In Mn-rich environments (Fe20Mn80), the dominance of Mn3+/Mn2+ species with higher ionic radii and lower crystal field stabilization energy tends to promote anisotropic growth and dendritic structures, especially at higher synthesis temperatures. In contrast, Fe-rich systems (Fe80Mn20) show more compact and faceted morphologies, likely due to the greater prevalence of Fe3+ ions, which exhibit stronger Fe–O bonding and favor isotropic nucleation and crystal growth. The intermediate Fe50Mn50 composition appears to balance these behaviors, resulting in homogeneously distributed polyhedral particles and well-ordered surface textures [39].
This compositional dependence aligns with prior theoretical insights into spinel-type oxides, where the nature and occupancy of cationic sites significantly affect the lattice parameters, electron distribution, and growth energetics. For example, Xu et al. [40] performed a theoretical analysis of ZnO·nAl2O3 spinels and demonstrated that varying the metal-oxide ratio modulates the electronic structure and local bonding environment, ultimately guiding phase stability and crystal habit. Analogously, in the Fe–Mn–O system, variations in Fe:Mn ratios influence the degree of inversion in the spinel structure, affect charge compensation mechanisms, and alter the nucleation/growth kinetics through local strain and oxygen vacancy distribution.
4. Conclusions
This study demonstrates the successful phase-selective synthesis of iron–manganese oxide nanostructures through hydrothermal methods by controlling Fe:Mn molar ratios, temperature, and reaction time. Three distinct crystalline phases—FeMn2O4, Fe3Mn3O8, and Fe2MnO4—were obtained without phase mixing, directly influenced by the precursor ratio (1:2, 1:1, and 2:1, respectively), as confirmed by XRD. Morphological evolution was clearly dependent on thermal and temporal parameters. FESEM analysis revealed that low-temperature and short-time conditions (e.g., 80 °C/4 h) produced irregular, aggregated particles, while higher temperatures (200 °C) and longer reaction times (24 h) led to well-defined, faceted, and elongated nanostructures. This trend suggests thermally promoted anisotropic growth and enhanced recrystallization dynamics. DLS measurements on selected samples synthesized at 120 °C for 12 h showed narrow size distributions centered around 50–75 nm, indicating good control over particle size under optimized conditions. These results were consistent with FESEM observations and supported the formation of uniform nanostructures. On the other hand, Raman spectroscopy verified the characteristic vibrational modes of spinel-type and mixed-valence oxides. Additionally, HRTEM images confirmed high crystallinity, with lattice fringes corresponding to interplanar distances of ~0.48–0.52 nm, matching the (113) and (222) planes of spinel structures. The controlled variation in synthesis parameters enables the tailored formation of pure iron–manganese oxide phases with tunable morphology and structural order. These insights offer a solid foundation for the rational design of nanomaterials for technological applications requiring phase purity and defined surface characteristics, such as catalysis, energy conversion, and magnetic systems.
Author Contributions
Conceptualization, O.E.C.-M., I.T.-S., M.d.R.M.-F. and Y.M.H.-R.; methodology, O.E.C.-M., I.T.-S., M.d.R.M.-F. and Y.M.H.-R.; software, O.E.C.-M., I.T.-S., M.d.R.M.-F. and Y.M.H.-R.; validation, O.E.C.-M., I.T.-S., M.d.R.M.-F. and Y.M.H.-R.; formal analysis, O.E.C.-M., I.T.-S., M.d.R.M.-F. and Y.M.H.-R.; investigation, O.E.C.-M., I.T.-S., M.d.R.M.-F. and Y.M.H.-R.; resources, O.E.C.-M. and Y.M.H.-R.; data curation, I.T.-S. and M.d.R.M.-F.; writing—original draft preparation, O.E.C.-M.; writing—review and editing, Y.M.H.-R.; visualization, O.E.C.-M., I.T.-S., M.d.R.M.-F. and Y.M.H.-R.; supervision, I.T.-S., M.d.R.M.-F. and Y.M.H.-R.; project administration, I.T.-S., M.d.R.M.-F. and Y.M.H.-R.; funding acquisition, I.T.-S., M.d.R.M.-F. and Y.M.H.-R. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Secretaría de Educación, Ciencia, Tecnología e Innovación de la CDMX (SECTEI) and the Secretaría de Investigación y Posgrado del IPN (SIP-IPN), and the APC was funded by the Secretaría de Investigación y Posgrado del IPN.
Data Availability Statement
All data related to this work is available under request to the corresponding author.
Acknowledgments
The authors acknowledge Secretaría de Educación, Ciencia, Tecnología e Innovación de la CDMX (SECTEI) for the economic support for this research. The authors also thank the Secretaría de In-vestigación y Posgrado of the Instituto Politécnico Nacional (IPN) for the partial economic support given to this research. The research was carried out in the Laboratorio de Sistemas para Diagnóstico y Tratamiento de Cáncer, UPII-TA-IPN.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Wang, S.; Hui, S.; Li, Z.; Shi, Z.; Wang, Y.; Zhang, L.; Wu, H. Transition metal ions induce pH-dependent coordination bonds, ionic conductivity and in-situ magnetic particles for tailoring microwave absorption of gels. J. Mater. Sci. Technol. 2025, 239, 288–298. [Google Scholar] [CrossRef]
- Balakrishnan, P.D.; Sekar, C.; Ramesh, R.; Premkumar, T.; Kanchana, P. Solid-State synthesis of transition nanometal oxides (MnO2, Co3O4, NiO, and ZnO) for catalytic and electrochemical applications. J. Ind. Eng. Chem. 2024, 140, 434–453. [Google Scholar] [CrossRef]
- Rakshit, A.; Biswas, D.; Mondal, R.; Kabi, S.; Roy, D. Electrical conduction and optical characteristics of Li2O and Bi2O3 Co-doped zinc-phosphate glassy nanocomposites: A critical observation of mixed ionic electronic effect. Phys. B Condens. Matter 2024, 695, 416587. [Google Scholar] [CrossRef]
- Mahendra, G.; Roy, R.; Singh, A.K. VO2·xH2O nanoribbons as high-capacity cathode material for aqueous zinc-ion batteries: Electrolyte selection and performance optimization. J. Power Sources 2024, 624, 235515. [Google Scholar] [CrossRef]
- Huang, X.-T.; Sun, Y.-J.; Zhang, F.; Bai, C.-W.; Chen, F. Commercial nanomaterials in wastewater treatment: A case study on manganese oxide-activated periodate systems. Sep. Purif. Technol. 2024, 351, 127837. [Google Scholar] [CrossRef]
- Zhang, X.; Zou, J.; Yan, L.; Wang, G.; Li, Q.; Zhou, L. Ti3O MOenes: Quantum Spin Hall Effect and Promising Semiconductors for Light-Harvesting. J. Phys. Chem. Lett. 2024, 15, 8360–8366. [Google Scholar] [CrossRef]
- Gaona-Esquivel, A.; Hernandez-M, D.S.; Hernández-Rodríguez, Y.; Cigarroa-Mayorga, O. The role of Nd as a dopant in Mn3O4NPs on the heat induction of artificial breast tissue due to the irradiation of microwaves. Mater. Chem. Phys. 2022, 292, 126822. [Google Scholar] [CrossRef]
- Wu, R.; Gao, R.; Li, Z.; Wang, J.; Wang, H.; Wu, Y.; Jiang, S.; Zhang, X.; Wang, X.; Liu, X.; et al. Nanoporous high-entropy Mn-Fe-Co-Ni amorphous oxide: Boosting oxygen evolution efficiency through electron spin-polarization. J. Mater. Sci. Technol. 2025, 238, 266–275. [Google Scholar] [CrossRef]
- Erdem, Ü.; Sarı, K.A.; Dogan, D.; Gungunes, H.; Arıcan, G.O.; Sarı, U. Fabrication and characterization of ferromagnetic FeMnCo/nanofibers for broadband electromagnetic wave absorption. J. Alloy. Compd. 2025, 1036, 181958. [Google Scholar] [CrossRef]
- Teymoori, S.M.; Alavi, S.M.; Rezaei, M. Catalytic oxidation of CO over the MOx − Co3O4 (M: Fe, mn, cu, ni, cr, and Zn) mixed oxide nanocatalysts at low temperatures. Sci. Rep. 2025, 15, 25808. [Google Scholar] [CrossRef]
- Fetohi, A.E.; Khater, D.Z.; Amin, R.; El-Khatib, K. Nickel sulfide–transition metal sulfides bi-electrocatalyst supported on Nickel Foam for water splitting. J. Phys. Chem. Solids 2025, 207, 112906. [Google Scholar] [CrossRef]
- Zhang, D.; Li, X.; Sun, J.; Huo, Z.; Zhang, C.; Cheng, Z.; Cao, F.; Luo, B.; Liu, G.; Liu, X.; et al. A general and convenient strategy to construct spinel A Fe3-O4/porous carbon nanosheet (A = Co, Cu, Mn, Mg, Fe) composites as anodes for lithium ion batteries. Electrochim. Acta 2025, 538, 146927. [Google Scholar] [CrossRef]
- Lin, L.-Y.; Huang, J.-I.; Tsai, H.-Y. Redox-induced engineering of amorphous/crystalline MnFeOx catalyst enables H2O/SO2-tolerant NOx abatement at ultra-low temperatures. J. Hazard. Mater. 2025, 489, 137618. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhang, S.; Yao, W.; Zhu, Y.; Qian, J.; Yang, J.; Yang, N. Design and synthesis of hierarchical MnO–Fe3O4@C/expanded graphite composite for sensitive electrochemical detection of bisphenol A. Talanta 2024, 269, 125453. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Zhu, D.; Peng, L.; Yang, Y.; Qi, K.; Yuan, D. Bimetal heterostructured nanoparticles anchored hierarchical carbon nanospheres with abundant active sites for high-performance liquid/flexible zinc-air batteries. J. Energy Storage 2025, 131, 117513. [Google Scholar] [CrossRef]
- Cigarroa-Mayorga, O.E. Tuning the size stability of MnFe2O4 nanoparticles: Controlling the morphology and tailoring of surface properties under the hydrothermal synthesis for functionalization with myricetin. Ceram. Int. 2021, 47, 32397–32406. [Google Scholar] [CrossRef]
- Montalto, M.; Freitas, W.d.S.; Mastronardo, E.; Ficca, V.C.; Placidi, E.; Baglio, V.; Mosca, E.; Vecchio, C.L.; Gatto, I.; Mecheri, B.; et al. Spinel-type high-entropy oxides for enhanced oxygen evolution reaction activity in anion exchange membrane water electrolyzers. Chem. Eng. J. 2025, 507, 160641. [Google Scholar] [CrossRef]
- Kogulakrishnan, K.; Nithiyanantham, S.; Mohan, R.; Giridharan, N.; Silambarasan, S.; Venkadesh, A.; Latha, V.; Gunasekaran, B.; Palaniappan, L. Structural, electrical and magnetic studies on Zn doped MnFe2O4 nanoparticles with via Sol-Gel approach. J. Mol. Struct. 2024, 1321, 140026. [Google Scholar] [CrossRef]
- Yu, Y.; Chen, M.; Lei, Y.; Niu, H. Unconventional ice nucleation pathway induced by irregular silver iodide surfaces. Commun. Phys. 2025, 8, 7. [Google Scholar] [CrossRef]
- Guo, S.; Yu, M.; Lee, J.-K.; Qiu, M.; Yuan, D.; Hu, Z.; Zhu, C.; Wu, Y.; Shi, Z.; Ma, W.; et al. Separating nanobubble nucleation for transfer-resistance-free electrocatalysis. Nat. Commun. 2025, 16, 919. [Google Scholar] [CrossRef]
- Zürbes, K.R.; Bruns, T.M.; Mani, E.; Bandyopadhyay, S. Seed-mediated synthesis of gold nanorods with tannic acid as the reducing agent-A kinetic study. Colloids Surfaces A Physicochem. Eng. Asp. 2025, 725, 137483. [Google Scholar] [CrossRef]
- Kumar, N.; Kesari, S.; Krylova, S.; Rao, R.; Surovtsev, N.; Ishchenko, D.; Pryanichnikov, S.; Govorkova, T.; Bobin, S.; Lonchakov, A.; et al. Structural phase transition in crystalline HgSe: Low-temperature and high-pressure Raman spectroscopic investigation. J. Phys. Chem. Solids 2025, 207, 112977. [Google Scholar] [CrossRef]
- Li, X.; Zhong, L.; Li, Z.; Fu, Y.; Dong, M.; Min, X.; Zhao, S. Oxalic acid induced synthesis of renewable Ce-MnFe2O4 nanocatalysts with hybrid crystallinity for enhanced ozone decomposition. Environ. Res. 2025, 283, 122111. [Google Scholar] [CrossRef]
- Narayanaswamy, V.; Jagal, J.; Al-Omari, I.A.; Haider, M.; Gopi, C.V.M.; Obaidat, I.M.; Issa, B. Chitosan-PEG functionalized cube-shaped MnFe2O4 nanoparticles for curcumin delivery and magnetic hyperthermia against MDA-MB-231 cancer cells. Mater. Chem. Phys. 2025, 345, 131235. [Google Scholar] [CrossRef]
- Zaka, A.; Aftab, M.; Fatima, N.; Mashood, K.; Asghar, M.A.; Numan, A.; Ahmad, M.S.; Haider, A.; Iqbal, M.; Shah, W.A.; et al. MnFe2O4 thin film electrodes via AACVD: A facile route for enhanced oxygen evolution reaction. Fuel 2025, 395, 135179. [Google Scholar] [CrossRef]
- Majani, S.S.; Veena, M.A.; Hemanth Kumar, C.M.; Setty, P.B.S.; Iqbal, M.; Shivamallu, C.; Cull, C.A.; Hales, K.E.; Broadway, P.R.; Amachawadi, R.G. Sustainable synthesis of iron-doped manganese oxide nanoparticles for effective photo-accelerated detoxification of tetracycline. Sci. Rep. 2025, 15, 18081. [Google Scholar] [CrossRef]
- Collins-Martinez, V.H.; Cazares-Marroquin, J.F.; Salinas-Gutierrez, J.M.; Pantoja-Espinoza, J.C.; Lopez-Ortiz, A.; Melendez-Zaragoza, M.J. The thermodynamic evaluation and process simulation of the chemical looping steam methane reforming of mixed iron oxides. RSC Adv. 2021, 11, 684–699. [Google Scholar] [CrossRef]
- Mungse, P.; Saravanan, G.; Rayalu, S.; Labhsetwar, N. Mixed Oxides of Iron and Manganese as Potential Low-Cost Oxygen Carriers for Chemical Looping Combustion. Energy Technol. 2015, 3, 856–865. [Google Scholar] [CrossRef]
- Xie, H.; Chen, X.; Zhang, C.; Lao, Z.; Liu, X.; Xie, X.; Semiat, R.; Zhong, Z. Identifying the Fe3Mn3O8 phase as a superior catalyst for low-temperature catalytic oxidation of formaldehyde in air. Environ. Sci. Nano 2022, 9, 767–780. [Google Scholar] [CrossRef]
- Nguyen, T.D.; Phan, N.H.; Do, M.H.; Ngo, K.T. Magnetic Fe2MO4 (M:Fe, Mn) activated carbons: Fabrication, characterization and heterogeneous Fenton oxidation of methyl orange. J. Hazard. Mater. 2011, 185, 653–661. [Google Scholar] [CrossRef]
- Yan, G.; Vlachos, D.G. Impact of Metal Clusters on the Lewis Acidity of Oxide Surfaces: First-Principles Calculations of Pt10/γ-Al2O3(110). J. Phys. Chem. C 2024, 128, 16996–17005. [Google Scholar] [CrossRef]
- Yang, J.; Zhao, Y.; Liang, S.; Zhang, S.; Ma, S.; Li, H.; Zhang, J.; Zheng, C. Magnetic iron–manganese binary oxide supported on carbon nanofiber (Fe3−xMnxO4/CNF) for efficient removal of Hg0 from coal combustion flue gas. Chem. Eng. J. 2018, 334, 216–224. [Google Scholar] [CrossRef]
- Cigarroa-Mayorga, O.; Gallardo-Hernández, S.; Talamás-Rohana, P. Tunable Raman scattering enhancement due to self-assembled Au nanoparticles layer on porous AAO: The influence of the alumina support. Appl. Surf. Sci. 2021, 536, 15099–15106. [Google Scholar] [CrossRef]
- Cihan, A.; Zarzycki, P.; Hao, Z. Surface Hydroxylation-Induced Electrostatic Forces Thicken Water Films on Quartz. Langmuir 2024, 40, 15099–15106. [Google Scholar] [CrossRef]
- Gabal, M.A.; Katowah, D.F.; Hussein, M.A.; Al-Juaid, A.A.; Awad, A.; Abdel-Daiem, A.M.; Saeed, A.; Hessien, M.M.; Asiri, A.M. Structural and Magnetoelectrical Properties of MFe2O4 (M = Co, Ni, Cu, Mg, and Zn) Ferrospinels Synthesized via an Egg-White Biotemplate. ACS Omega 2021, 6, 22180–22187. [Google Scholar] [CrossRef]
- Huan, V.D.; Van Quang, N.; Hanh, N.T.; Van, C.A.; Tu, N.; Trung, D.Q.; Van Du, N.; Hung, N.D.; Viet, D.X.; Bach, T.N.; et al. Dual green and red emitting Mn-doped MgAl2O4 phosphors for WLED and plant growth LED applications. J. Alloys Compd. 2025, 1027, 180621. [Google Scholar] [CrossRef]
- Feng, M.; Xu, Z.; Li, J.; Wang, N.; Lin, K.; Zhang, M. Insight into the role of reactive species on catalyst surface underlying peroxymonosulfate activation by P–Fe2MnO4 loaded on bentonite for trichloroethylene degradation. Chemosphere 2024, 357, 141943. [Google Scholar] [CrossRef]
- Oberdick, S.D.; Abdelgawad, A.; Moya, C.; Mesbahi-Vasey, S.; Kepaptsoglou, D.; Lazarov, V.K.; Evans, R.F.L.; Meilak, D.; Skoropata, E.; van Lierop, J.; et al. Spin canting across core/shell Fe3O4/MnxFe3−xO4 nanoparticles. Sci. Rep. 2018, 8, 3425. [Google Scholar] [CrossRef]
- Xu, P.; Wang, H.; Ren, L.; Tu, B.; Wang, W.; Fu, Z. Theoretical study on composition-dependent properties of ZnO·nAl2O3 spinels. Part I: Optical and dielectric. J. Am. Ceram. Soc. 2021, 104, 5099–5109. [Google Scholar] [CrossRef]
- Xu, P.; Wang, H.; Ren, L.; Tu, B.; Wang, W.; Fu, Z. Theoretical study on composition-dependent properties of ZnO·nAl2O3 spinels. Part II: Mechanical and thermophysical. J. Am. Ceram. Soc. 2021, 104, 6455–6466. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).