Structural and Textural Properties of Al/Cu- and Al/Zn-Pillared Clays for Ethanol Conversion
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Pillared Clays
2.2. Characterization Study
2.3. Catalysis Test in the Conversion of Ethanol
3. Results and Discussions
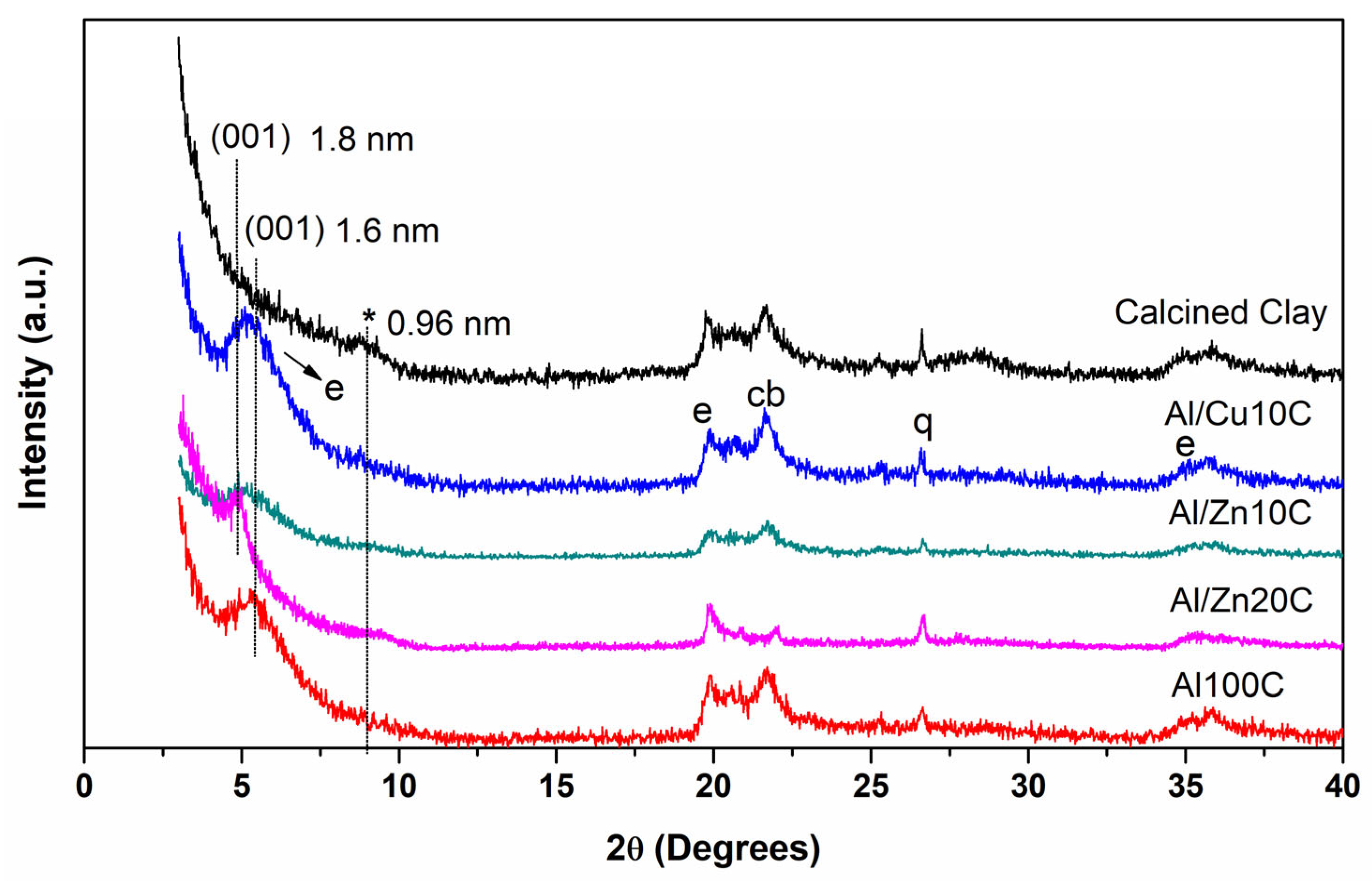
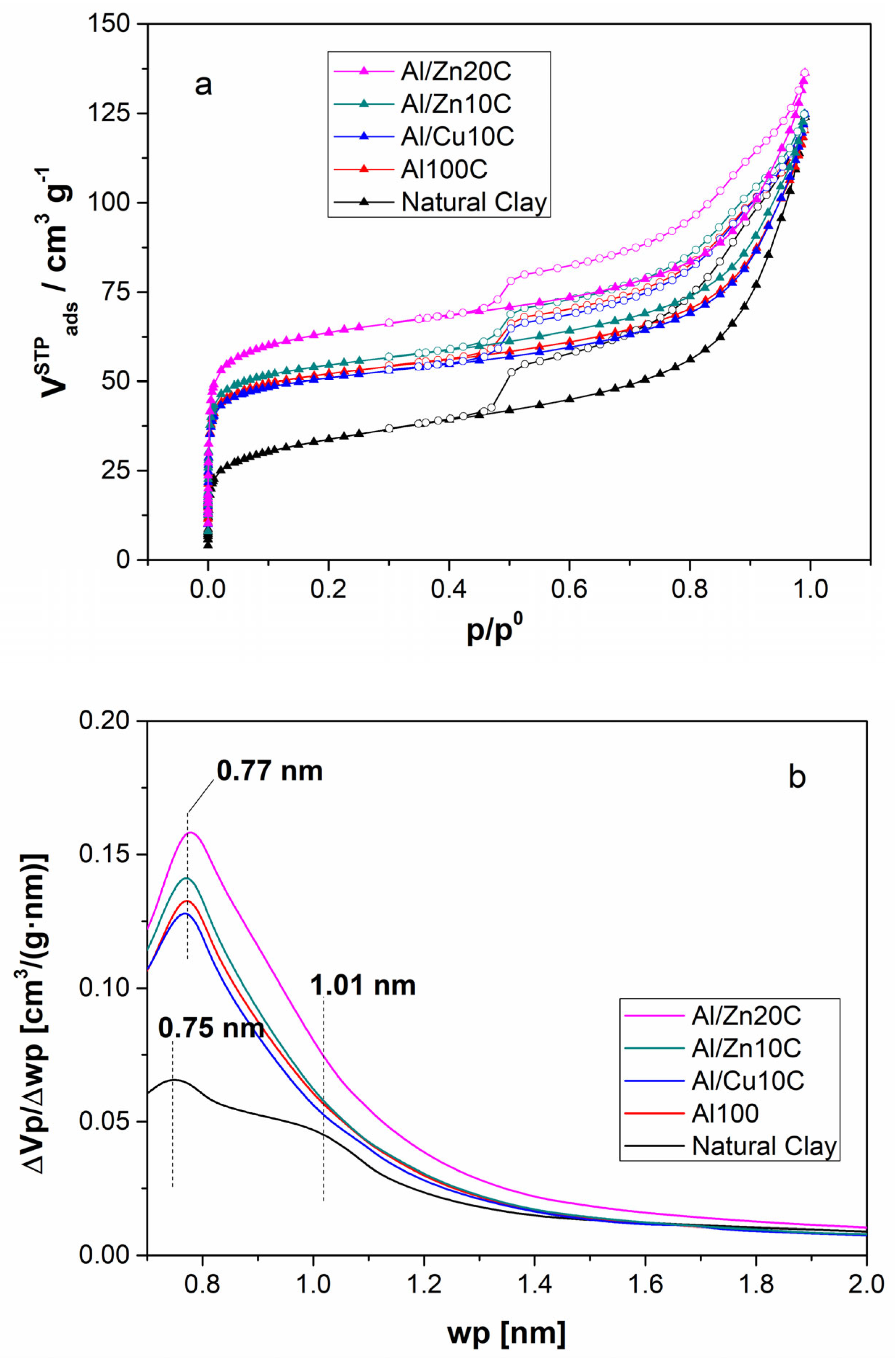
3.1. Pillared Clays with Mixed Al/Cu and Al/Zn Solution
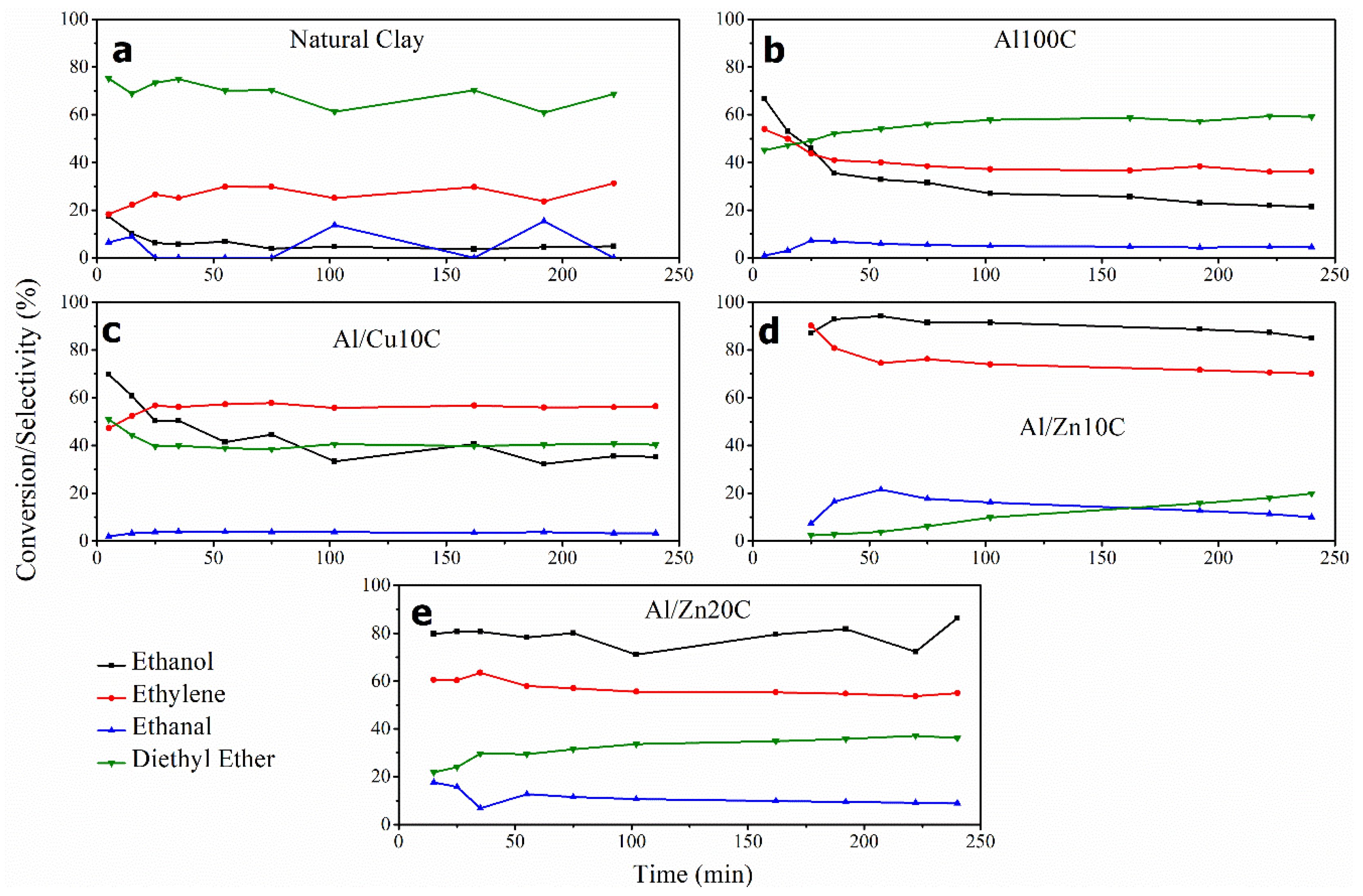
3.2. Catalysis Test in the Conversion of Ethanol
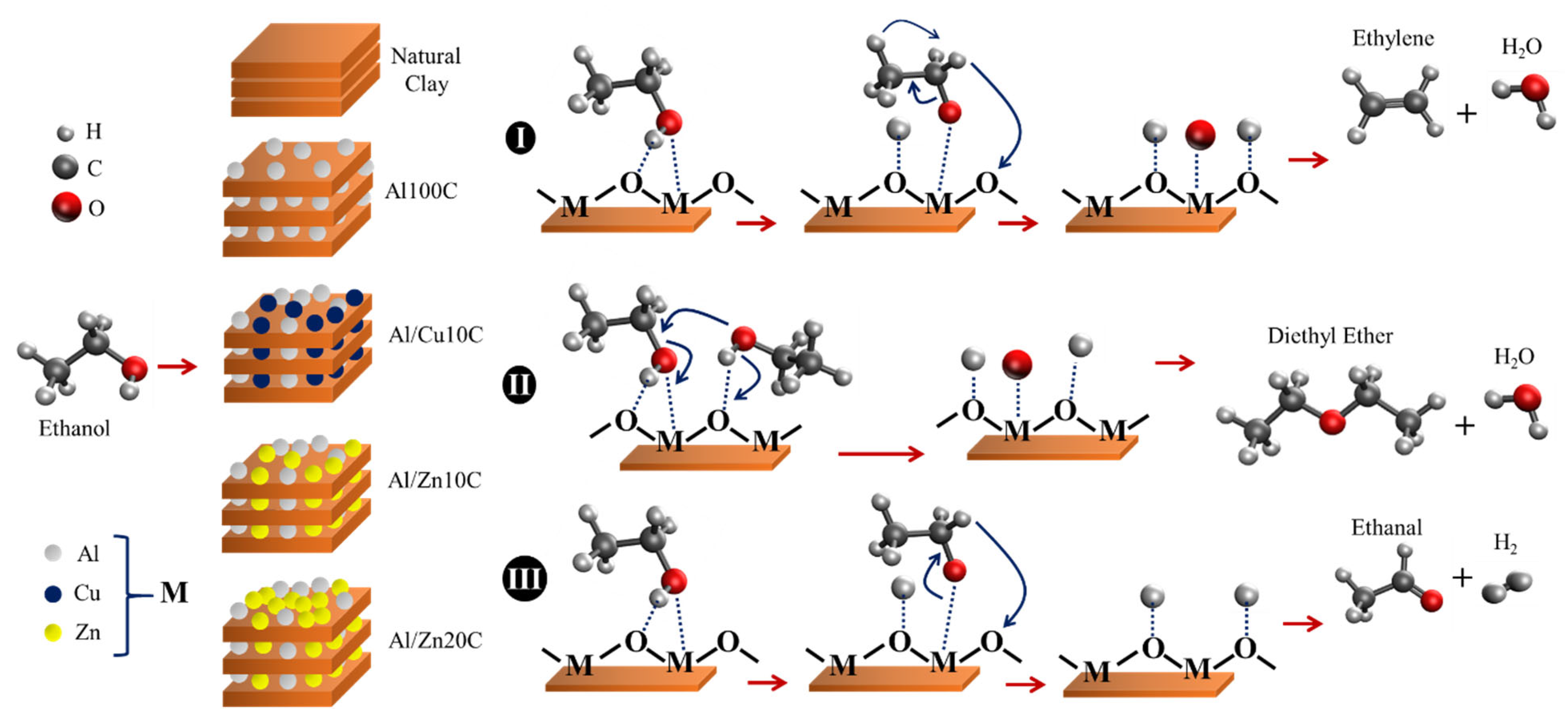
3.3. Mechanism Proposal Conversion of Ethanol Use Pillared Clay
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Salerno, P.; Mendioroz, S. Preparation of Al-pillared montmorillonite from concentrated dispersions. Appl. Clay Sci. 2002, 22, 115–123. [Google Scholar] [CrossRef]
- Maes, N.; Heylen, I.; Cool, P.; Vansant, E.F. The relation between the synthesis of pillared clays and their resulting porosity. Appl. Clay Sci. 1997, 12, 43–60. [Google Scholar] [CrossRef]
- Pergher, S.B.C.; Sprung, R. Pilarização de uma argila brasileira com poliidroxications de alumínio: Preparação, caracterização e propriedades catalíticas. Quim. Nova 2005, 28, 777–782. [Google Scholar] [CrossRef]
- Chmielarz, L.; Kowalczyk, A.; Skoczek, M.; Rutkowska, M.; Gil, B.; Natkański, P.; Radko, M.; Motak, M.; Dębek, R.; Ryczkowski, J. Porous clay heterostructures intercalated with multicomponent pillars as catalysts for dehydration of alcohols. Appl. Clay Sci. 2018, 160, 116–125. [Google Scholar] [CrossRef]
- Vicente, M.A.; Gil, A.; Bergaya, F. Pillared Clays and Clay Minerals, 2nd ed.; Elsevier Ltd.: Amsterdam, The Netherlands, 2013; Volume 5, ISBN 9780080982588. [Google Scholar]
- Hurtado, L.; Avilés, O.; Brewer, S.; Donkor, K.K.; Romero, R.; Gómez-Espinosa, R.M.; Alvarado, O.; Natividad, R. Al/Cu-PILC as a Photo-Fenton Catalyst: Paracetamol Mineralization. ACS Omega 2022, 7, 23821–23832. [Google Scholar] [CrossRef]
- Xie, Y.; Chen, H.; Mei, B.; Tian, X.; Jia, L.; Zhu, W.; Zhang, Y. Highly efficient iodine capture by polyethyleneimine-impregnated CuAl-pillared montmorillonite. J. Environ. Chem. Eng. 2023, 11, 110204. [Google Scholar] [CrossRef]
- Amaya, M.G.; García Blanco, A.A.; Toncón-Leal, C.; Sapag, K. Incorporation of Co in Different Stages of the Synthesis of Al-PILC and Its Effect as a Fischer–Tropsch Catalyst. Ind. Eng. Chem. Res. 2021, 60, 18929–18937. [Google Scholar] [CrossRef]
- Frini, N.; Crespin, M.; Trabelsi, M.; Messad, D.; Van Damme, H.; Bergaya, F. Preliminary results on the properties of pillared clays by mixed Al-Cu solutions. Appl. Clay Sci. 1997, 12, 281–292. [Google Scholar] [CrossRef]
- Mojović, Z.; Banković, P.; Milutinović-Nikolić, A.; Dostanić, J.; Jović-Jovičić, N.; Jovanović, D. Al,Cu-pillared clays as catalysts in environmental protection. Chem. Eng. J. 2009, 154, 149–155. [Google Scholar] [CrossRef]
- Xie, X.; Li, Z.; Li, B.; Wu, X.; An, X. Novel catalyst PTMA-PILC: Structural properties and catalytic performance for the dehydration of bioethanol to ethylene. RSC Adv. 2015, 5, 46316–46324. [Google Scholar] [CrossRef]
- Krutpijit, C.; Jongsomjit, B. Catalytic ethanol dehydration over different acidactivated montmorillonite clays. J. Oleo Sci. 2016, 65, 347–355. [Google Scholar] [CrossRef] [PubMed]
- Bokade, V.V.; Yadav, G.D. Heteropolyacid supported on montmorillonite catalyst for dehydration of dilute bio-ethanol. Appl. Clay Sci. 2011, 53, 263–271. [Google Scholar] [CrossRef]
- Bertella, F.; Pergher, S.B.C. Pillaring of bentonite clay with Al and Co. Microporous Mesoporous Mater. 2015, 201, 116–123. [Google Scholar] [CrossRef]
- Galeano, L.A.; Gil, A.; Vicente, M.A. Effect of the atomic active metal ratio in Al/Fe-, Al/Cu- and Al/(Fe-Cu)-intercalating solutions on the physicochemical properties and catalytic activity of pillared clays in the CWPO of methyl orange. Appl. Catal. B Environ. 2010, 100, 271–281. [Google Scholar] [CrossRef]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of Gases in Multimolecular Layers. Adsorpt. Gases Multimol. Layers 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Mariani, F.Q.; Villalba, J.C.; Anaissi, F.J. Caracterização Estrutural de Argilas Utilizando DRX com Luz Síncrotron, MEV, FTIR e TG-DTG-DTA. Orbital Electron. J. Chem. 2013, 5, 249–256. [Google Scholar]
- Bertella, F. Síntese e Caracterização de Argilas Pilarizadas com Pilares Mistos Al/Co. Master’s Thesis, Universidade Federal do Rio Grande do Norte, Natal, Brazil, 2014. [Google Scholar]
- Schoonheydt, R.A.; Jacobs, K.Y. Clays: From two to three dimensions. In Studies in Surface Science and Catalysis; Elsevier: Amsterdam, The Netherlands, 2001; Volume 137, pp. 299–343. [Google Scholar] [CrossRef]
- Brindley, G.W.; Brown, G. Crystal Structures of Clay Minerals and Their X-Ray Identification; Mineralogical Society of Great Britain and Ireland: London, UK, 1980; Volume 5. [Google Scholar]
- Barrault, J.; Tatibouët, J.M.; Papayannakos, N. Catalytic wet peroxide oxidation of phenol over pillared clays containing iron or copper species. Chemistry 2000, 3, 777–783. [Google Scholar] [CrossRef]
- Chmielarz, L.; Kuśtrowski, P.; Zbroja, M.; Gil-Knap, B.; Datka, J.; Dziembaj, R. SCR of NO by NH3 on alumina or titania pillared montmorillonite modified with Cu or Co: Part II. Temperature programmed studies. Appl. Catal. B Environ. 2004, 53, 47–61. [Google Scholar] [CrossRef]
- Bergaya, F.; Lagaly, G. General introduction: Clays, clay minerals, and clay science. In Handbook of Clay Science; Elsevier: Amsterdam, The Netherlands, 2006; Volume 1, p. 4352. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Guerra, D.L.; Lemos, V.P.; Airoldi, C.; Angélica, R.S. Influence of the acid activation of pillared smectites from Amazon (Brazil) in adsorption process with butylamine. Polyhedron 2006, 25, 2880–2890. [Google Scholar] [CrossRef]
- Smith, M.E. Application of27Al NMR techniques to structure determination in solids. Appl. Magn. Reson. 1993, 4, 1–64. [Google Scholar] [CrossRef]
- Fripiat, J.J. High resolution solid state NMR study of pillared clays. Catal. Today 1988, 2, 281–295. [Google Scholar] [CrossRef]
- Coelho, A.C.V.; Santos, P.S. Argilas Especiais: Argilas Quimicamente Modificadas-Uma revisão. Quim. Nova 2007, 30, 1282–1294. [Google Scholar] [CrossRef]
- Lambed, J.; Chevalier, S.; Franck, R.; Suquet, H.; Barthomeuf, D. Al-Pillared Saponites Part 2—NMR Studies. J. Chem. Soc. Faraday Trans. 1994, 90, 675–682. [Google Scholar] [CrossRef]
- De Oliveira, M.L.; Pergher, S.B.C.; De Aguiar Pontes, D.; Gonzalez, E.A.U.; Santos, R.C.; Pontes, L.A.M. 2-Methylthiophene reactions on modified KSF clays. Mol. Catal. 2020, 493, 111085. [Google Scholar] [CrossRef]
- Lambert, J.F.; Poncelet, G. Acidity in pillared clays: Origin and catalytic manifestations. Top. Catal. 1997, 4, 43–56. [Google Scholar] [CrossRef]
- Chang, F.; Kuo, W.; Lee, K. Dehydrogenation of ethanol over copper catalysts on rice husk ash prepared by incipient wetness impregnation. Appl. Catal. A Gen. 2003, 246, 253–264. [Google Scholar] [CrossRef]
- Dewilde, J.F.; Chiang, H.; Hickman, D.A.; Ho, C.R.; Bhan, A. Kinetics and Mechanism of Ethanol Dehydration on γ-Al2O3: The Critical Role of Dimer Inhibition. ACS Catal. 2013, 3, 798–807. [Google Scholar] [CrossRef]
- De Oliveira, T.K.R.; Rosset, M.; Perez-Lopez, O.W. Ethanol dehydration to diethyl ether over Cu-Fe/ZSM-5 catalysts. Catal. Commun. 2018, 104, 32–36. [Google Scholar] [CrossRef]
- Sarve, D.T.; Singh, S.K.; Ekhe, J.D. Kinetic and mechanistic study of ethanol dehydration to diethyl ether over Ni-ZSM-5 in a closed batch reactor. React. Kinet. Mech. Catal. 2020, 131, 261–281. [Google Scholar] [CrossRef]
- Elliott, D.J.; Pennella, F. Mechanism of Ethanol Formation from Synthesis Gas Over CuO/ZnO/Al2O3. J. Catal. 1988, 114, 90–99. [Google Scholar] [CrossRef]
- Vohs, J.M.; Barteau, M.A. Dehydration and dehidrogenation of ethanol and 1-propanol on the polar surfaces of zinc oxide. Surf. Sci. 1989, 221, 590–608. [Google Scholar] [CrossRef]
- El-Hakam, S.A. Structure, texture and catalytic activity of ZnO/Al2O3catalysts. Colloids Surfaces A Physicochem. Eng. Asp. 1999, 157, 157–166. [Google Scholar] [CrossRef]
- Jenness, G.R.; Christiansen, M.A.; Caratzoulas, S.; Vlachos, D.G.; Gorte, R.J. Site-Dependent Lewis Acidity of γ-Al2O3 and Its Impact on Ethanol Dehydration and Etherification. J. Phys. Chem. C 2014, 118, 12899–12907. [Google Scholar] [CrossRef]
- Rahman, M.M.; Davidson, S.D.; Sun, J.; Wang, Y. Effect of Water on Ethanol Conversion over ZnO. Top. Catal. 2015, 59, 37–45. [Google Scholar] [CrossRef]


| Calcined Montmorillonite | Al100C | Al/Cu10C | Al/Zn10C | Al/Zn20C | |
|---|---|---|---|---|---|
| Na2O | 0.00 | 0.35 | 0.36 | 1.61 | 1.07 |
| MgO | 2.90 | 2.12 | 2.22 | 2.61 | 2.08 |
| Al2O3 | 16.03 | 19.09 | 19.33 | 20.97 | 22.51 |
| SO3 | 0.10 | 0.06 | 0.06 | 0.30 | 0.33 |
| Cl | 0.11 | 0.09 | 0.08 | 0.09 | 0.09 |
| K2O | 0.36 | 0.25 | 0.24 | 0.37 | 0.29 |
| CaO | 1.24 | 0.00 | 0.00 | 0.09 | 0.08 |
| TiO2 | 0.93 | 0.84 | 0.80 | 0.12 | 0.09 |
| Fe2O3 | 8.12 | 7.08 | 6.73 | 3.60 | 2.89 |
| CuO | 0.00 | 0.00 | 0.06 | 0.00 | 0.00 |
| ZnO | 0.00 | 0.00 | 0.00 | 0.12 | 0.44 |
| AMR (%) Pillared Clay | 1.15 | 1.50 | 4.08 | ||
| Sample | BET | α-Plot | Vt | |
|---|---|---|---|---|
| SBET (m2/g) | St (m2/g) | Vo (cm3/g) | VTP (cm3/g) | |
| Montmorillonite | 123 | 56 | 0.04 | 0.19 |
| Al100C | 198 | 48 | 0.07 | 0.19 |
| Al/Cu10C | 193 | 72 | 0.06 | 0.19 |
| Al/Zn10C | 208 | 71 | 0.07 | 0.19 |
| Al/Zn20C | 241 | 103 | 0.08 | 0.21 |
| Natural Clay | Al100C | Al/Cu10C | Al/Zn10C | Al/Zn20C | |
|---|---|---|---|---|---|
| AlVI/AlIV | 9.9 | 7.7 | 9.2 | 9.1 | 7.3 |
| AlIV/AlV | nd | 5.2 | 4.8 | 2.1 | 4.0 |
| Sample | Conversion (%) | Ethylene (%) | Diethyl Ether (%) | Ethanal (%) |
|---|---|---|---|---|
| Montmorillonite | 8 | 27 | 70 | 3 |
| Al100C | 36 | 42 | 54 | 4 |
| Al/Zn10C | 80 | 81 | 11 | 8 |
| Al/Zn20C | 74 | 60 | 28 | 12 |
| Al/Cu10C | 46 | 54 | 41 | 5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
dos Santos, L.M.; Barbosa, F.F.; Bieseki, L.; Braga, T.P.; Pergher, S.B.C. Structural and Textural Properties of Al/Cu- and Al/Zn-Pillared Clays for Ethanol Conversion. Crystals 2025, 15, 203. https://doi.org/10.3390/cryst15030203
dos Santos LM, Barbosa FF, Bieseki L, Braga TP, Pergher SBC. Structural and Textural Properties of Al/Cu- and Al/Zn-Pillared Clays for Ethanol Conversion. Crystals. 2025; 15(3):203. https://doi.org/10.3390/cryst15030203
Chicago/Turabian Styledos Santos, Lamara M., Felipe F. Barbosa, Lindiane Bieseki, Tiago P. Braga, and Sibele B. C. Pergher. 2025. "Structural and Textural Properties of Al/Cu- and Al/Zn-Pillared Clays for Ethanol Conversion" Crystals 15, no. 3: 203. https://doi.org/10.3390/cryst15030203
APA Styledos Santos, L. M., Barbosa, F. F., Bieseki, L., Braga, T. P., & Pergher, S. B. C. (2025). Structural and Textural Properties of Al/Cu- and Al/Zn-Pillared Clays for Ethanol Conversion. Crystals, 15(3), 203. https://doi.org/10.3390/cryst15030203







