Abstract
Duplex stainless-steel grade 2205 (2205 DSS) is the most widely used of the current duplex materials. The duplex steel alloy is characterized by high strength and high corrosion resistance through enhancing nitrogen and molybdenum contents. The activated tungsten inert gas (ATIG) welding technique uses the same equipment as tungsten inert gas (TIG), but prior to the welding operation, a thin layer of flux is deposited. Activation fluxes are known to influence the shape and energy characteristics of the arc. They promote the change in shapes and dimensions of the welds, namely, increasing the depth and narrowing the weld width. This work is dedicated to investigate the influence of the thermophysical properties of individual metal oxide fluxes on 2205 DSS welding morphology. It helps also to identify the recommended flux properties in order to perform full penetrated ATIG welds. Thirteen kinds of oxides (SiO2, TiO2, Fe2O3, Cr2O3, ZnO, Mn2O3, V2O5, MoO3, Co3O4, SrO, ZrO2, CaO, and MgO) have been tested and three current intensity levels (120, 150 and 180 A) have been considered. The results showed that the main input factors affecting the weld depth (D) were the welding current intensity with a contribution of up to 53.36%, followed by the oxides enthalpy energy with 15.05% and then by the difference between the oxides and the base metal of 2205 DSS (BM 2205 DSS) melting points with a contribution of 9.71% of the data variance. The conditions on individual oxides’ thermophysical properties to achieve full penetrated weld beads have been also revealed.
1. Introduction
Stainless steels (SS) are extensively used materials in various industries, owing to their numerous advantages. Their applications are wide and they are commonly used in different industries and pipelines owing to their high weldability and service performance. Duplex stainless steels (DSSs) with austenitic and ferritic phases have been increasingly used for many industrial applications, e.g., in the oil and gas sector, due to their good mechanical properties and corrosion resistance, particularly in marine environments [1,2,3].
For joining, cladding, or repairing DSSs, the welding parameters play a major role in maintaining and controlling the equal balance of austenite and ferrite phases [4,5].
Tungsten inert gas (TIG), also known as gas tungsten arc welding (GTAW), is a process highly used for joining DSS parts. It is able to produce high-quality welds at inexpensive costs, with more flexibility, for any weld configurations, and in any welding positions. The TIG welding process is a widespread industrial process that is used in several engineering applications such as cars, aerospace, shipbuilding, and power plants. TIG also has advanced to the additive manufacturing field, via the construction of thin walls [6]. In addition to its production, TIG has been used for repairing, as seen in a martensitic SS cracked Pelton runner repaired by TIG welding [7]. However, only a maximum of 3 mm penetration is achieved at the highest level of heat input (2.21 kJ/mm), which can be harmful to the duplex microstructure by the formation of deleterious sigma phases [8,9]. These shortcomings restrict the traditional TIG welding applications. ATIG is a variant of conventional TIG welding where the same equipment is used except for prior welding; a thin flux layer of 10 mm width and 0.2 to 0.4 mm thickness is deposited on the surface parts to be joined. Three different methods for the application of the flux layer on the base plates are reported in the literature: (i) using a brush, (ii) by spraying, and (iii) by conveying with a shielding gas [10,11]. ATIG welding has been adopted in many industries owing to its high production efficiency related to its capability to join ticker parts up to 8 mm in one pass without edge preparation or filler metal [12,13,14]. Fluxes used during ATIG welding are usually metal oxides and halides [15,16]. Fluxes have been tested as a single component or as a mixture of two or more different powders in order to achieve the best weld penetration and weld aspect [17,18,19].
The main mechanisms determining the weld morphology are inverse Marangoni convection and arc constriction. In inverse Marangoni convection, the weld bead shape is mostly influenced by surfactant elements such as oxygen. The presence of surfactant elements ensures inward convection. The liquid metal motion in the weld pool has a centripetal convection resulting in a deep and narrow weld morphology [20,21,22]. Howse and Lucas [23] have applied Simonik’s principle [24] to explain the observed constriction of the arc and the increased weld pool penetration. Indeed, the constriction arc increases the current density at the anode spot, which contributes to an increase in the Lorentz force [25]. The mechanisms mentioned above coexist to increase the ATIG weld bead penetration [26,27,28].
The TIG welding process parameters were optimized using an RBF-NN-based model [29], Generic Algorithm (GA) [30], Artificial Neural Networks (ANN) [31], and the Taguchi method [32]. These methods help the understanding of the complex relationship between input such as welding parameters (e.g., welding intensity, welding speed etc.) and output parameters such as mechanical properties [33] or welding bead morphology [34].
The available works in the ATIG welding process have been devoted to the optimization of single [35], bi-component, or tri-component flux. The aim is either to improve the mechanical properties using the Swarm Particle Optimization method [36] or to enhance the depth penetration and weld aspect using the Mixing Design method [37].
In this work, regression analysis is used. The mathematical model that relates the output responses, such as depth and aspect ratio, with the input parameters as the selected thermophysical properties flux combined with three current intensities (120, 150, and 180 A) can be obtained. The expected responses, weld depth and weld width were fitted into Design Expert.
Based on the results obtained, the main thermophysical characteristic which influences the weld bead morphology will be highlighted. Moreover, the obtained results indicate the value ranges of the obtained main input parameters which promote the high weld penetration. The novelty of this work is to facilitate the selection of fluxes for any material to be welded using ATIG welding. Furthermore, the usefulness of the present study could be considered as a guideline for researchers as well as industries to refine the selection of fluxes in order to achieve efficient welding.
2. Materials and Methods
2.1. Material
The DSS grade 2205 in the form of a 6 mm thick plate manufactured by Outokumpu Stainless AB SWEDEN was investigated. The related chemical composition, the melting temperature, and boiling temperature are depicted in Table 1.

Table 1.
The chemical composition of 2205 DSS.
The thermophysical properties were gathered in Table 2, such as boiling point, surface tension, melting point, proportion of oxygen in oxides, oxides first ionization energy, and oxides enthalpy energy.

Table 2.
Oxides input properties data [39,40,41,42].
2.2. Welding Procedure
The 200 mm welding lines were carried out on (200 × 100) mm2 plain rectangular plates of the 2205 DSS material. Before welding, the plates were cleaned with acetone. The used powders have been heated separately in a furnace at 100 °C for 1 h to eliminate the humidity. Flux in the form of powder has been mixed with acetone in the proportion of (1 ÷ 1) and made in the form of paste; a brush was used to apply the mixture on the plain edges to be joined. The strip band of paste of 10 mm wide and an average of 0.24 mm thickness has been deposited on the plain plate. Specimens for morphology study were prepared by the usual metallurgical polishing methods and then etched with a solution of one volume of water, one volume of hydrochloric acid (HCL), one volume of nitric acid (HNO3), and one volume of fluoride acid (HF). The cross-sections of the weld beads were photographed using an optical microscope CAROLINA (CAROLINA, Burlington, VT, USA). Table 3 shows the welding conditions.

Table 3.
Welding conditions.
To make sure that the arc welding was stabilized, specimens were taken at 30 mm from the edges. For each weld, five depth and width bead measurements were taken.
2.3. Mathematical Modeling
The mathematical model that expresses the output variables to the input parameters can be obtained by using regression analysis. By applying the polynomial function, the mathematical formulation has been found. The expression of the second-order polynomial function is represented by Equation (1)
So, the terms of this equation are: Xi denotes the ten input factors such that i ∈ {1, 2, …, 10},
b: denotes the factor corresponding to the specified terms such that
bo: is a constant term,
bi: are coefficients of the linear terms,
bij: are coefficients of the linear interaction terms,
bii: are coefficients of the second-order terms.
Design Expert software (DOE) has been used to find out a mathematical model for Y in terms of input factors.
3. Results and Discussions
3.1. Morphology of Welds
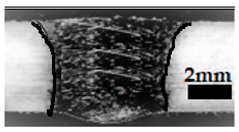
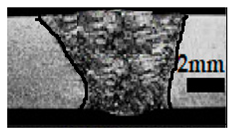
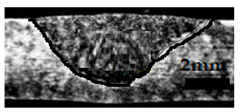
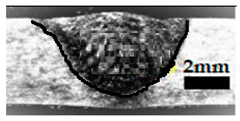
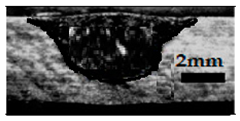
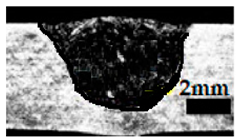
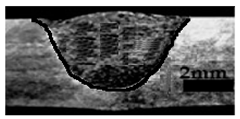
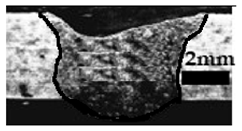
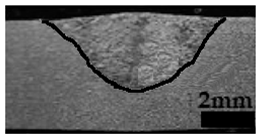
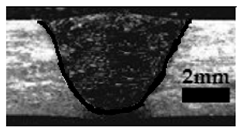
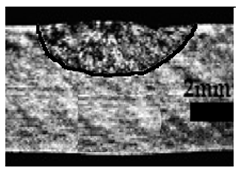
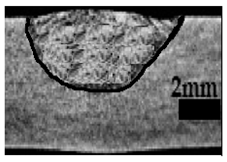
Table 4 shows the cross-sectional macrographs of the conventional TIG and ATIG processed materials. We noticed that for a current intensity of 120 A, the welds were partially penetrated regardless of the type of oxides. However, for 180 A, almost all the oxides tested resulted to full penetration welds except for ZrO2, Cao, SrO, and MgO. ATIG welds performed under 150 A exhibited partially penetrated welds except for welds executed with SiO2 and V2O5. Furthermore, the weld depths in TIG and ATIG welding in the case of 180 A were greater than those performed with 150 A due to the higher heat input, followed by the weld depths performed with 120 A (c.f. Table 5).

Table 4.
Morphology of welds carried out under three current intensity levels (120, 150, and 180 A) of TIG and ATIG welding (ATIG with different single oxides).

Table 5.
The investigated factors and their corresponding responses.
Table 5 shows the inputs (X1, X2, …, X9, and I) and their corresponding responses, depth, and ratio aspect. The results depicted that the partial penetration welds are characterized by all welds carried out with 120 A regardless of the type of oxide used. In the latter welds, the heat input was not sufficient to liberate the oxygen. The highest linear energy of 180 A ensured the highest values of the depth. The oxygen, as a surfactant element, once liberated, contributes to getting centripetal molten metal in the 2205 DSS weld pool, which moves in a centripetal direction, according to inverse Marangoni convection, resulting in a high depth with narrower weld bead [43].
3.2. Weld Depth Penetration Modelling
The response variable D can be represented by Equation (2).
The validity as well as the accuracy of the D mathematical model are shown in Table 6.

Table 6.
Predicted against actual value and residuals of D mathematical model.
Design Expert software with a quadratic model could obtain the best appropriate fit and auto-selection of terms relying on the adjusted R2.
D = 51.3 − 7.13 ×10−3 X2 − 4.7 × 10−5 X3 − 2.66 × 10−3 X4 + 0.298 X7 − 5.02 X8 + 2.29 × 10−3 X9 − 0.396 I + 4.25 × 10−6 X2×3 − 1.2 × 10−5 X3 I − 2.21 × 10−3 X7 I + 3.62 × 10−2 X8 I − 3.10 × 10−5 X9 I + 5.52 × 10−4 I2
Figure 1 shows the performance of the proposed mathematical model, and Table 7 depicts ANOVA results, where F-value = 30.29 is sufficiently high, as well as R2 = 0.94, adjusted-R2 = 0.909, and predicted-R2 = 0.839. The difference between R2 and the adjusted-R2 is less than 0.2, which demonstrates the equation is statistically significant. Furthermore, the S/N = 22.22 is high enough which indicates that the model in representing the data is adequate.
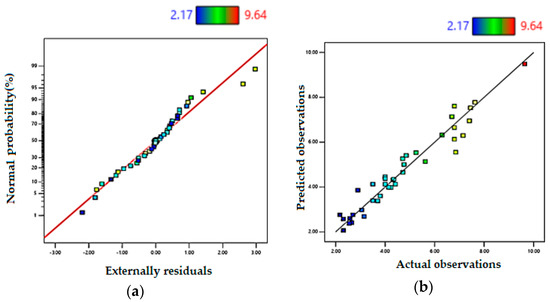
Figure 1.
Performance of the model for depth penetration (D): (a) residuals’ normal plot for D. (b) Predicted vs. actual data for D.

Table 7.
Results of D in terms of X1, X2, …, X9 and I.
Relying on the ANOVA results, one can identify the major factor that affects the weld depth D. The major contributor is the current intensity (I). It accounts for up to 53.36% of the variability in the data with a linear impact. Increasing current intensity increases the heat supplied to the workpiece; it allows the release of oxygen which will play its role as a surfactant element. The second parameter is the oxide enthalpy energy (X9); it contributes to up to 15.05% of the variability in the data through its linear effect. The large negative value suggests that the ionic compound is a much more stable oxide. Consequently, oxygen as a surfactant element is partially or not liberated. Oxygen from the decomposition of the oxide flux in the welding pool alters the surface tension gradients on the weld pool surface, changing the Marangoni convection direction and weld penetration depth. The following contributor term is the temperature range of the oxide (X4); it comes with a percentage of 9.71%. The linear interaction between the current intensity (I) and each of the oxide’s melting points (X3), the proportion of oxygen in the oxides (X7), the oxide’s first ionization energy (X8), and the oxide’s enthalpy energy (X9) show statistically significant evidence as shown in Table 8. The interaction effect of oxide surface tension (X2) and oxide melting point (X3) is statistically significant and is up to 4.37%; however, their linear components (X2 and X3) are statistically insignificant. The effect of the current intensity also shows a nonlinear effect of the term I2. The other factors show a minor effect on the depth of the weld.

Table 8.
Predicted against actual values of D/W and residuals.
Based on Figure 2, the oxide melting point must be up to 2180 °C to achieve the full penetrated weld bead carried out with 180 A. The heat input is sufficient to dissociate the oxides and liberates the oxygen. As a surfactant element, oxygen in the weld pool contributes to the reverse Marangoni convection resulting in deeper weld bead. So, while welding 2205 DSS, the oxides which ensure the full penetrated welds of 6 mm thickness plates are those which are characterized by a melting point between 670 and 2350 °C. Within the tested welding parameters, the more effective action on the depth penetration weld is provided by oxides, which have a melting point in the temperature range 670 to 2350 °C.
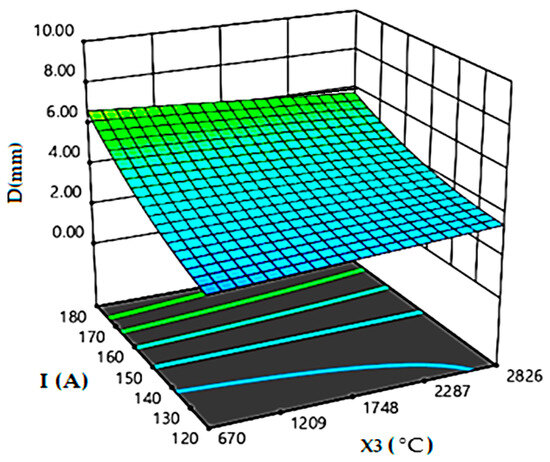
Figure 2.
Effect of oxide melting point.
Figure 3 depicts the effect of the oxide and materials melting points difference in relation with the current intensity on the weld depth. The difference between oxide melting point and BM 2205 DSS melting point must be below 740 °C to achieve a full penetrated weld bead. Moreover, when the melting point of flux is close to that of the material to be welded, the penetration will be more pronounced and reaches the 8 mm of the weld depth.
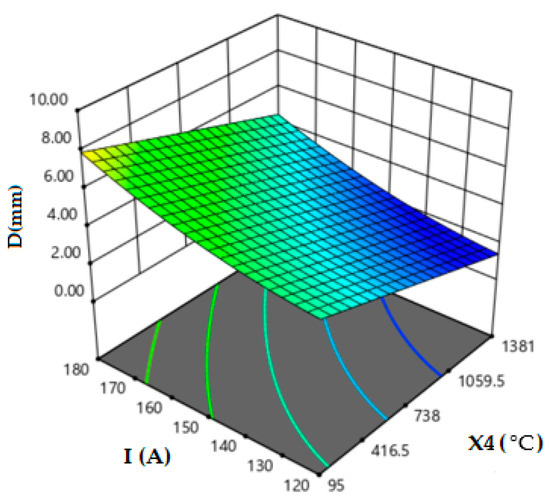
Figure 3.
Effect of the oxide and the base materials melting points difference in relation with the current intensity.
Figure 4 depicts the effect of oxygen proportion in the oxide with the current intensity on the weld depth. The oxygen percentages must be in the range of 30 to 52% to ensure the fully penetrated weld bead for welds performed with 170 to 180 A current intensity. Moreover, when the current intensity increases up to 180 A, the oxygen, which is around 30%, is sufficient to ensure 8 mm depth weld in a single pass. We notice that if the oxygen content “O2” is more than 30% in the oxide, it is more effective when the absolute values of the difference between the melting point of the oxides and the melting point of the BM 2205 DSS are below 890 °C. This indicates that the oxygen contained in the flux has a great effect on the morphology of the 2205 DSS welds.
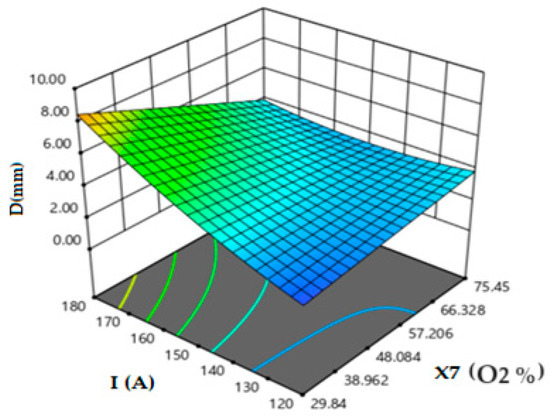
Figure 4.
Effect of oxygen proportion in oxide and current intensity on weld depth.
Based on Figure 5, the influence of the ionization energy (IE) of the oxides in combination with the current intensity on the formation of the welding beads of 2205 DSS reveals the favorable effect of this parameter on weld penetration. This aspect happens when the energy of ionization is beyond 10.6 eV owing to the increase in temperature in the welding arc. This contributes to the increase in welding arc energy associated with the use of flux and with ionization energy potential greater than 10.6 eV, leading to an increase in the total energy of the arc. Furthermore, when IE is 12 eV associated with 180 A, the weld penetration reaches a depth of 8 mm.
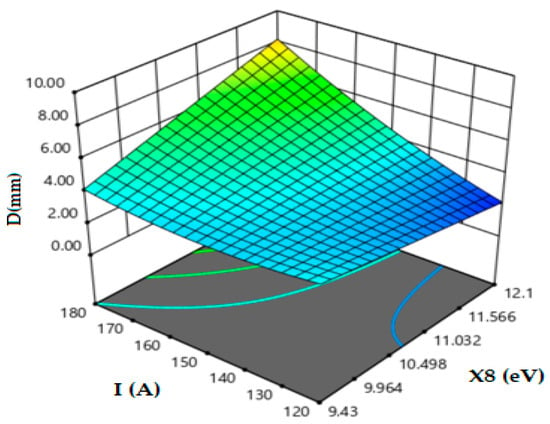
Figure 5.
Effect of oxide energy of ionization and current intensity on weld depth.
Figure 6 reveals that the absolute values of the enthalpy energy of the formation of oxides must be greater than |870| kJ/mol to obtain a fully penetrated weld bead when performed by a current of 160 A. If the current intensity is 180 A, the weld bead reaches 8 mm of depth with the enthalpy of formation of the oxide with |1550| kJ/mol.
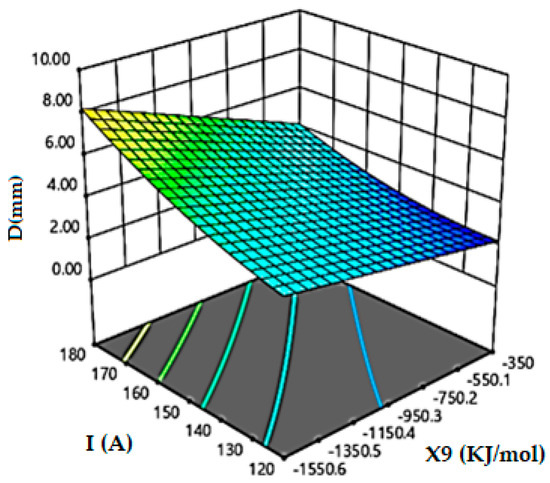
Figure 6.
Effect of enthalpy energy of formation of oxides (KJ/mol) and current intensity on weld depth.
3.3. Modeling of Weld Aspect Ratio (D/W)
Design Expert software was used to obtain the response variable D/W represented by Equation (3).
D/W = − 0.114 + 2.1 × 10−4 X1 + 5.1 × 10−5 X3 − 1.53 × 10−4 X9 + 3.44 × 10−3 I − 1.03 × 10−7 X1 × X3
Table 8 represents the predicted against actual values of D/W and residuals. It clearly demonstrates that the aspect ratio equations, developed based on welding input parameters, align closely with the actual output values.
Figure 7 shows the significance of the proposed model. Table 9 confirms that the ANOVA results confirm the statistical significance of the model. The calculated mathematical model is significant where F-value = 18.83 is sufficiently high, as well as the obtained R2 = 0.74, the adjusted R2 = 0.7, and the predicted R2 = 0.645. The difference between the obtained and the adjusted R2 is small, which indicates that the equation is statistically significant. Furthermore, the ratio S/N = 17.6 is high enough to show the adequacy of the model proposed.
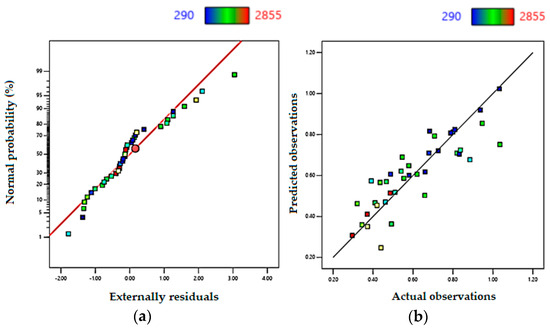
Figure 7.
Model fit for aspect ratio for aspect ratio (D/W). (a) Residuals’ normal plot for D/W. (b) Actual data vs. predicted for D/W.

Table 9.
ANOVA figures for D/W with X1, X2, …, X9 and I.
Based on the ANOVA results, we can identify the major factor that affects the aspect ratio of the weld (D/W). The main contributor factor is the current intensity (I) with a proportion of about 49.0%. The second parameter is the linear interaction between the boiling point (X1) and the melting temperature (X3); its effect contributes to about 21.44%. The third factor is the factor (X3), which comes with a proportion of 19.7%. The influence of enthalpy of formation (X9) is also statistically significant.
4. Conclusions
The objective of this paper is to study the effects of the input thermophysical properties of oxide fluxes in combination with three levels of current intensity, which are 120, 150, and 180 A, on the ATIG weld depth and the weld aspect ratio of a 6 mm 2205 DSS material. In addition, this study makes it possible to select the main thermophysical properties of the flux which affect the weld morphology. Moreover, the value ranges of the main contributed input parameters were indicated. The polynomial mathematical model has effectively been formulated to predict the morphology of the weld bead based on a set of defined inputs, encompassing both depth and aspect ratio as functions. The main conclusions are as follows:
(i) The developed regression model has proven effective in modeling the geometries of ATIG welds on 6 mm thick plates of 2205 DSS. Predicted values for the geometric weld morphology closely matched the actual experimental results.
(ii) The principal input factors that influence the weld depth (D) are the welding current intensity (I) with a contribution of up to 53.36%, followed by the oxide enthalpy energy with 15.05% and then by the difference between oxides and BM 2205 DSS melting points with a percentage of 9.71%. The interaction of the two factors deserves to be highlighted, particularly between intensity current, with several input factors such as oxides melting point, proportion of oxygen in oxides, oxides first ionization energy, and oxides enthalpy energy . This interaction effect contributes to about 1.73%, 4.45%, 4.31%, and 1.62%, respectively of the oxides’ melting points, the proportion of oxygen in oxides, and the oxide’s first ionization energy of the data variance.
(iii) The principal input factors affecting the aspect ratio (D/W) are the current intensity, with a contribution of 48.99%, followed by the oxide melting point with 19.70%, and then by the oxide’s enthalpy energy, with a contribution of up to 6.95%. The combination effect of oxide boiling point (X1) and oxide melting point (X3) affects the aspect ratio of the weld bead with a contribution of up to 21.44%.
(iv) It has been determined that the full penetrated weld of 6 mm thick plates of 2205 DSS can be achieved in a single pass by the following thermophysical properties conditions of individual fluxes oxides:
- ✓ Oxides enthalpy energy formation: > |870| kJ/mol,
- ✓ Oxides melting point Tmo: 670 °C < Tmo < 2350 °C,
- ✓ Oxides ionization energy (IE): IE > 10.6 eV,
- ✓ |Oxides melting point-BM 2205 DSS melting point| < 720 °C,
- ✓ Proportion of oxygen in oxides (%): 30% < O2 % < 52%.
Author Contributions
Conceptualization, K.T. and R.D.; methodology, K.T., R.D. and A.C.H. software, E.A.; validation, R.D. and K.T.; formal analysis, A.O., K.T. and I.A.; investigation, A.C.H., R.D., H.S.A. and K.T.; resources, E.A., R.D., A.O. and A.C.H.; data curation, I.A. and A.O.; writing—original draft preparation, K.T., E.A. and R.D.; writing—review and editing, R.D., E.A. and K.T.; visualization, E.A., I.A. and K.T.; supervision, K.T., R.D. and A.C.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The data used to support the findings of this study are included within the article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Maslak, M.; Stankiewicz, M.; Slazak, B. Duplex Steels Used in Building Structures and Their Resistance to Chloride Corrosion. Materials 2021, 14, 5666. [Google Scholar] [CrossRef] [PubMed]
- Örnek, C.; Davut, K.; Kocaba, M.; Bayatli, A.; Ürgen, M. Understanding corrosion morphology of duplex stainless steel wire in chloride electrolyte. Corros. Mater. Degrad. 2021, 2, 397–411. [Google Scholar] [CrossRef]
- Calderon, U.; Briz, A.E.; Garcia, H.; Matanza, A. The weldability of duplex stainless steel in structural components to withstand corrosive marine environments. Metals 2020, 10, 1475. [Google Scholar] [CrossRef]
- Shyu, S.W.; Huang, H.Y.; Tseng, K.H.; Chou, C. Study of the Performance of Stainless Steel A-TIG Welds. J. Mater. Eng. Perform. 2008, 17, 193–201. [Google Scholar] [CrossRef]
- Kumar, S.M.; Sankarapandian, S.; Shanmugam, N.S. Investigations on mechanical properties and microstructural examination of activated TIG-welded nuclear grade stainless steel. J. Braz. Soc. Mech. Sci. Eng. 2020, 42, 292. [Google Scholar] [CrossRef]
- Elif, K.; Yahya, B. Additive manufacturing method and different welding applications. J. Mater. Res. Technol. 2020, 9, 11424–11438. [Google Scholar]
- Esteban, F.; Nixon, R.; José, A.A.; Alejandro, T.; Jorge, E.G.B. Finite element analysis of the localized post-weld heat treatment of a Pelton runner. Mater. Today Commu. 2023, 36, 106795. [Google Scholar]
- Alrobei, H.; Touileb, K.; Djoudjou, R.; Ouis, A.; Hedhibi, A.; AlBaijan, I.; Malik, R.; Sherif, E. Mechanical and Corrosion Resistant Properties of ATIG welded 2205 Duplex Stainless-Steel via different fluxes. J. Mech. Sci. Technol. 2021, 35, 5469–5476. [Google Scholar] [CrossRef]
- Tathgir, S.; Rathod, D.W.; Batish, A. A-TIG welding process for enhanced-penetration in duplex stainless-steel: Effect of activated fluxes. Mater. Manuf. Process. 2019, 34, 1659–1670. [Google Scholar] [CrossRef]
- Patel, D.; Jani, S. ATIG welding: A small step towards sustainable manufacturing. Adv. Mater. Process. Technol. 2020, 7, 514–536. [Google Scholar] [CrossRef]
- Patel, N.P.; Sharma, D.K.; Upadhyay, G.H. Effect of Activated Fluxes on Weld Penetration and Mechanism Responsible for Deeper Penetration of Stainless Steels—A Review. In Current Advances in Mechanical Engineering; Lecture Notes in Mechanical Engineering; Springer: Singapore, 2021; pp. 737–746. [Google Scholar] [CrossRef]
- Chandrasekar, G.; Kannan, R.; Prabakaran, M.; Ganesamoorthy, P.R. Effect of Activating Flux (Metal Oxide) on the Weld Bead Nomenclature of Tungsten Inert Gas Welding Process—A Review. IOP Conf. Ser. Mater. Sci. Eng. 2020, 988, 12084. [Google Scholar] [CrossRef]
- Touileb, K.; Hedhibi, A.; Djoudjou, R.; Abousoufiane, O.; Abdallah, B.; Albaijan, I.; Hany, S.A.; Mohamed, M.Z.A. Mechanical, Microstructure, and Corrosion Characterization of Dissimilar Austenitic 316L and Duplex 2205 Stainless-Steel ATIG Welded Joints. Materials 2022, 15, 7. [Google Scholar] [CrossRef] [PubMed]
- Stalker, D.S.; Jogi, B.F.; Thorat, S.B.; Chavan, A.A. Activated Pulsed-Tungsten Inert Gas Welding of DSS 2205; Techno-Societal 2018; Springer International Publishing: Cham, Germany, 2020; pp. 511–521. [Google Scholar]
- Sharma, P.; Dwivedi, D.K. A-TIG welding of dissimilar P92 steel and 304H austenitic stainless steel: Mechanisms, microstructure and mechanical properties. J. Manuf. Process. 2019, 44, 166–178. [Google Scholar] [CrossRef]
- Singh, S.R.; Khanna, P. A-TIG (activated flux tungsten inert gas) welding:—A review. Mater. Today Proc. 2021, 44, 808–820. [Google Scholar] [CrossRef]
- Albaijan, I.; Hedhibi, A.C.; Touileb, K.; Djoudjou, R.; Ouis, A.; Alrobei, H. Effect of Binary Oxide Flux on Weld Shape, Mechanical Properties and Corrosion Resistance of 2205 Duplex Stainless Steel Welds. Adv. Mater. Sci. Eng. 2020, 2020, 5842741. [Google Scholar] [CrossRef]
- Venkatesan, G.; Muthupandi, V.; Justine, J. Activated TIG welding of AISI 304L using mono- and tri-component fluxes. Int. J. Adv. Manuf. Technol. 2017, 93, 329–336. [Google Scholar] [CrossRef]
- Rakesh, N.; Mohan, A.; Navaf, P.; Harisankar, M.; Nambiar, S.J.; Harikrishnan, M.; Devadathan, J.; Rameshkumar, K. Effect of fluxes on weld penetration during TIG welding—A review. Mater. Today Proc. 2023, 72, 3040–3048. [Google Scholar] [CrossRef]
- Heiple, C.R.; Roper, J.R. Mechanism for minor element effect on GTA fusion zone geometry. Weld. J. 1982, 61, 97–102. [Google Scholar]
- Pandya, D.; Badgujar, A.; Ghetiya, N. A novel perception toward welding of stainless steel by activated TIG welding: A review. Mater. Manuf. Process. 2021, 36, 877–903. [Google Scholar] [CrossRef]
- Touileb, K.; Ouis, A.; Djoudjou, R.; Hedhibi, A.C.; Alrobei, H.; Albaijan, I.; Alzahrani, B.; Sherif, E.-S.M.; Abdo, H.S. Effects of ATIG welding on weld shape, mechanical properties, and corrosion resistance of 430 ferritic stainless steel alloy. Metals 2020, 10, 404. [Google Scholar] [CrossRef]
- Howse, D.S.; Lucas, W. Investigation into arc construction by active flux for TIG welding. Sci. Technol. Weld. Join. 2000, 5, 189–193. [Google Scholar] [CrossRef]
- Simonik, A.G. The effect of contraction of the arc discharge upon the introduction of electro-negative elements. Weld. Prod. 1976, 3, 49–51. [Google Scholar]
- Suman, S.; Santanu, D. Effect of Polarity and Oxide Fluxes on Weld-bead Geometry in Activated Tungsten Inert Gas (A-TIG) Welding. J. Weld. Join. 2020, 38, 380–388. [Google Scholar]
- Singh, S.R.; Khanna, P. Investigation of A-TIG welded duplex stainless-steel plates. Int. J. Interact. Des. Manuf. 2022, 18, 2225–2235. [Google Scholar] [CrossRef]
- Pandya, D.; Badgujar, A.; Ghetiya, N.; Oza, A.D. Characterization and optimization of duplex stainless steel welded by activated tungsten inert gas welding process. Int. J. Interact. Des. Manuf. 2022, 1–13. [Google Scholar] [CrossRef]
- Chern, T.-S.; Tseng, K.-H.; Sai, H.-L.T. Study of the characteristics of duplex stainless steel activated tungsten inert gas welds. Mater. Des. 2011, 32, 255–263. [Google Scholar] [CrossRef]
- Ahmed, A.N.; Noor, C.W.M.; Allawi, M.F.; Shafie, A.E. RBF-NN-based model for prediction of weld bead geometry in Shielded Metal Arc Welding (SMAW). Neural Comput. Appl. 2018, 29, 889–899. [Google Scholar] [CrossRef]
- Kshirsagar, R.; Jones, S.; Lawrence, J.; Tabor, J. Optimization of TIG Welding Parameters Using a Hybrid Nelder Mead-Evolutionary Algorithms Method. J. Manuf. Mater. Process. 2020, 4, 10. [Google Scholar] [CrossRef]
- Ran, L.; Manshu, D.; Hongming, G. Prediction of Bead Geometry with Changing Welding Speed Using Artificial Neural Network. Materials 2021, 14, 1494. [Google Scholar] [CrossRef]
- Sekar, C.B.; Rajendra Boopathy, S.; Vijayan, S.; Koteswara, S.R. Multi objective optimization of welding parameters using Taguchi based grey relation analysis in activated TIG(ATIG) welding on SAF2507 super duplex stainless steel. In Proceedings of the Recent Trends in Manufacturing Technologies, Materials Processing, and Testing, Chennai, India, 18–19 February 2021; AIP Conference Proceedings. AIP Publishing: Melville, NY, USA, 2021; Volume 2395, p. 30007. [Google Scholar]
- Ramadan, N.; Boghdadi, A. Parametric Optimization of TIG Welding Influence On Tensile Strength of Dissimilar Metals SS-304 And Low Carbon Steel by Using Taguchi Approach. Am. J. Eng. Res. 2020, 9, 7–14. [Google Scholar]
- Las-Casas, M.S.; De Ávila, T.L.D.; Bracarense, A.Q.; Lima, E.J. Weld parameter prediction using artificial neural network: FN and geometric parameter prediction of austenitic stainless steel welds. J. Braz. Soc. Mech. Sci. Eng. 2018, 40, 26. [Google Scholar] [CrossRef]
- Aditya, K.; Akash, S.; Ashwin, A.; Gautam, R.; Baskar, C.S.; Ramachandran, D.; Koteswara, K.S.R. Effect of welding current on properties of activated gas tungsten arc super duplex stainless steel welds. Mater. Test. 2022, 64, 1242–1253. [Google Scholar]
- Chihaoui Hedhibi, A.; Touileb, K.; Ouis, A.; Djoudjou, R.; Ahmed, M.Z. Mechanical Properties and Microstructure of TIG and ATIG Welded 316L Austenitic Stainless Steel with Multicomponent Flux Optimization using Mixing Design method and Particle Swarm Optimization (PSO). Materials 2021, 14, 7139. [Google Scholar] [CrossRef] [PubMed]
- Touileb, K.; Djoudjou, R.; Chihaoui, H.A.; Ouis, A.; Benselama, A.; Ibrahim, A.; Abdo, H.S.; Samad, U.A. Comparative Microstructural, Mechanical and Corrosion Study between Dissimilar ATIG and Conventional TIG Weldments of 316L Stainless Steel and Mild Steel. Metals 2022, 12, 635. [Google Scholar] [CrossRef]
- Mills, K.C. Recommended Values of Thermophysical Properties for Selected Commercial Alloys; National Physical Laboratory and ASM International; Woodhead Publishing: Cambridge, UK, 2002. [Google Scholar]
- Keene, B.J. Review of Data for the Surface Tension of Pure Metals. Int. Mater. Rev. 1993, 38, 157–192. [Google Scholar] [CrossRef]
- Keene, B.J. Review of Data for Surface Tension of Iron and Its Binary Alloys. Int. Mater. Rev. 1988, 33, 1–37. [Google Scholar] [CrossRef]
- Yanhui, L.; Xuewei, L.V.; Chenguang, B.A.I.; Bin, Y.U. Surface Tension of the Molten Blast Furnace Slag Bearing TiO2: Meas & Eval. ISIJ Int. 2014, 54, 2154–2161. [Google Scholar]
- Juan, J.V.; Peter, N.Q. Thermophysical Properties. In ASM Handbook; Casting; ASM International: Almere, The Netherlands, 2008; Volume 15, pp. 468–481. [Google Scholar]
- Vysakh, K.B.; Mathiazhagan, A.; Krishna, P.S. A systematic overview on activated-Tungsten inert gas welding. Weld. Int. 2022, 36, 597–615. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).