Abstract
To reduce the hygroscopicity of betaine (BET), three co-crystals were synthesized: betaine-3,5-dihydroxybenzoic acid (BET-3,5-DHB), betaine-2,4-dihydroxybenzoic acid (BET-2,4-DHB), and betaine-3-hydroxybenzoic acid (BET-3-HDB). BET, commonly present in natural products, is used to treat homocysteine methyltransferase and has additional benefits such as hepatoprotective and neuroprotective properties. However, BET is extremely hygroscopic. Three cocrystals are acquired to address the problem. Comprehensive characterization, such as X-ray diffraction analysis, spectral analysis and thermal analysis, were conducted for co-crystals and BET. Based on the evaluation results for dynamic vapor sorption, the three co-crystals, especially BET-3-HDB, significantly reduce BET’s hygroscopicity. At 90% RH, the weight changes of BET-2,4-DHB (0.36%), BET-3,5-DHB (1.79%) and BET-3-HDB (0.32%) are 306.9, 61.7, and 345.3 times greater than those of the raw material BET, respectively. Hygroscopicity results from BET’s ability to easily create hydrogen bonding interactions with water molecules due to its carboxylate ions. Nevertheless, carboxylate ions establish hydrogen bonds with CCFs in co-crystals, lowering BET’s hygroscopicity and minimizing the likelihood of contact with water molecules. The contributions of contacts in the co-crystals are shown by Hirschfeld surface analysis as follows: H-H > O-H > H-O. Coulomb forces are dominant in the co-crystals by 3D energy frameworks.
1. Introduction
Betaine (BET), as a natural product, is widespread in animals, plants, and microorganisms [1]. Individual mean daily BET intake in the human diet ranges from 1 g/day to 2.5 g/day [2]. BET functions primarily as an osmotic agent and methyl donor, helping to maintain intracellular osmolarity because it possesses both N+ and COO− chemical configurations (Scheme 1) [3,4,5]. BET can serve as a substrate for homocysteine methyltransferase, being an efficient methyl donor for homocysteine methylation [6,7]. Therefore, homocystinuria [8,9,10], a metabolic disease that manifests in early childhood and is defined by an excess of homocysteine in the bloodstream [11], can be treated with BET. Furthermore, BET exhibits hepatoprotective properties [12,13], neuroprotective effects [14,15], safeguards myocardial function [16,17], and provides numerous other benefits [18,19,20,21]. BET, however, is hygroscopic [22] and highly soluble in water (160 g/100 g of water).
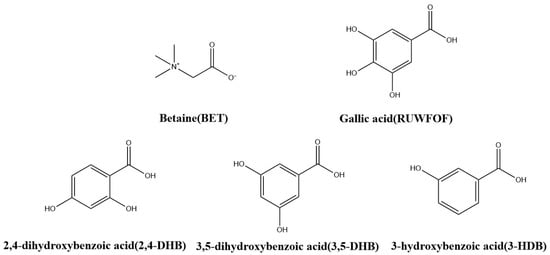
Scheme 1.
Chemical Structures of BET, gallic acid (RUWFOF), 2,4-DHB, 3,5-DHB and 3-HDB.
The ability of a substance to absorb moisture from the air is commonly described as hygroscopicity [23,24]. In the pharmaceutical industry, it is widely recognized that the water content of a drug or excipient in the solid state can have a profound effect on a wide range of physicochemical properties, such as stability (physical and chemical), solubility, dissolution, boiling point and so on [25,26,27,28,29]. The hygroscopicity of solid drugs can lead to the formation of agglomerates, crusts and lamellar forms, potentially resulting in problems during material processing [30,31,32]. The interaction of a solid drug with atmospheric water can also cause an anhydrous-hydrate type phase transition, potentially resulting in changes in the thermodynamic activity of the anhydrous form and affecting the stability, solubility, and bioavailability of the drug [33,34]. Consequently, in the pharmaceutical industry, it is an essential early step to assess the possibility of hydration and/or the hygroscopic behavior of the API in solid form. An assessment of the equilibrium water content of a drug substance is an activity that should be monitored during the entire journey from discovery to drug product development [35,36].
Co-crystals are multi-component systems that consist of two or more individual components of the active pharmaceutical ingredient (API) and co-crystal former (CCF) in stoichiometric ratios, linked by non-ionic and non-covalent interactions [37]. The formation of co-crystals leads to changes in the stacking arrangement and interaction forces in the crystal lattice, which in turn leads to changes in the physicochemical properties of the drug [38,39], such as solubility, dissolution rate, and hygroscopicity. In recent years, it has been recognized that co-crystals have become one of the most commonly used methods to improve the physicochemical properties of active ingredients by altering the crystal structure without changing their therapeutic function. Numerous studies have been published on the development of hygroscopicity by co-crystals. In comparison to isosorbide (ISO) crystals [40], the hygroscopicity of four novel ISO co-crystals has decreased: isosorbide-piperazine (ISO-PZ), isosorbide-hydrochlorothiazide (ISO-HCT), isosorbide-3,5-dihydroxybenzoic acid (ISO-3,5DHBA), and isosorbide-gallic acid (ISO-GA). Epalrestat (EP) and metformin (MET), two medications used to treat diabetes, can produce drug–drug multicomponent crystals that improve the photostability and solubility of EP and reduce the hygroscopicity of MET [41]. Based on the Cambridge Crystallographic Data Centre (CCDC), we find that BET forms co-crystals with small molecule chain organic acids: maleic (NASQED), fumaric (BAHLEC), citric acid (XOBHIF), and hydrogen oxalate (GUKYEQ); benzoic structural organic acids: gallic acid (RUWFOF); and other organic acids: catechol (RUWFIZ), vitamin C (RUWFEV), and p-toluenesulfonic acid (WIQRIX).
This study presents the synthesis of three co-crystals to reduce the hygroscopicity of BET: betaine-2,4-dihydroxybenzoic acid (BET-2,4-DHB), betaine-3,5-dihydroxybenzoic acid (BET-3,5-DHB), and betaine-3-hydroxybenzoic acid (BET-3-HDB). As shown in Scheme 1, the structures of the three CCFs obtained in this study that form co-crystals with BET are similar to those reported for gallic acid. Gallic acid possesses more hydroxyl groups located in the para and meta positions. The co-crystals were characterized using single-crystal X-ray diffraction (SCXRD), powder X-ray diffraction (PXRD), differential scanning calorimetry (DSC), thermogravimetric analysis (TG), and infrared spectroscopy (IR), and their hygroscopicity was evaluated through dynamic vapor sorption (DVS). We examine co-crystal interaction forces through the application of Hirschfeld surface analysis, energy frameworks, and molecular electrostatic potential surfaces (MEPS).
2. Materials and Methods
2.1. Materials
BET (Purity > 99%) raw material and BET monohydrate were purchased from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China) 2,4-DHB (Purity > 99%) and 3,5-DHB (Purity > 99%) were acquired from Hubei Wande Chemical Industry Co., Ltd. (Tianmen, China), and 3-HDB (Purity > 99%) was obtained from Wuhan Yuancheng Gongchuang Technology Co, Ltd. (Wuhan, China). All solvents were sourced from the Beijing Chemical Reagent Factory (Beijing, China).
2.2. Synthesis and Crystallization
2.2.1. BET-2,4-DHB
The co-crystal of BET-2,4-DHB was synthesized by solvent-assisted grinding. A total of 23.4 mg of BET (0.2 mmol) and 30.8 mg of 2,4-DHB (0.2 mmol) were accurately weighed and added to the mortar and pestle. Then, 2 drops of ethanol were added at a time, and the samples were manually ground for 20 min at room temperature. The co-crystal obtained was dissolved in acetonitrile and then left to stand under ambient conditions for approximately 5–10 days, resulting in the formation of colorless crystals.
2.2.2. BET-3,5-DHB
The co-crystal of BET-3,5-DHB was synthesized by solvent-assisted grinding. A total of 23.4 mg of BET (0.2 mmol) and 30.8 mg of 3,5-DHB (0.2 mmol) were accurately weighed and added to the mortar and pestle. Then, 2 drops of ethanol were added at a time, and the samples were manually ground for 20 min at room temperature. The co-crystal obtained was dissolved in ethanol-sec-butanol (1:1) and then left to stand under ambient conditions for approximately 5–10 days, resulting in the formation of colorless crystals.
2.2.3. BET-3-HDB
The co-crystal of BET-3-HDB was synthesized by solvent-assisted grinding. A total of 23.4 mg of BET (0.2 mmol) and 27.6 mg of 3-HDB (0.2 mmol) were accurately weighed and added to the mortar and pestle. Then, 2 drops of ethanol were added at a time, and the samples were manually ground for 20 min at room temperature. The co-crystal obtained was dissolved in methanol-isopropanol (1:1) and then left to stand under ambient conditions for approximately 5–10 days, resulting in the formation of colorless crystals.
2.3. SCXRD
All the diffraction intensity data of single crystals were collected by the Rigaku XtaLAB Synergy four-circle diffractometer (Rigaku, The Woodlands, TX, USA) with Cu-Kα radiation (λ = 1.54178 Å). The SCXRD test was conducted at 293 K. Crystal structures were generated through Olex2 (Version 1.5) [42,43] and Shelx97 [44] software using the direct method and refined by the full-matrix least-squares method on F2 with anisotropic displacement parameters. All the non-hydrogen atoms were refined anisotropically and the positions of all hydrogen atoms were obtained using geometric calculations and the difference Fourier method. Molecular graphs were generated with Mercury (2023.3.0) [45].
2.4. PXRD
PXRD patterns were collected by the Rigaku D/Max-2550 powder X-ray diffractometer (Rigaku, Tokyo, Japan) under a Cu Kα radiation of λ = 1.54178 Å, 2θ scanning range of 3−40°, and a scanning speed of 8°/min. Electron tube voltage and amperage were set to 40 kV and 150 mA, respectively. The collected data were processed using JADE 6.0 software [46,47] and simulated PXRD patterns were obtained using Mercury (2023.3.0) [45] software.
2.5. IR
Measurements of each sample were made by a Spectrum 400 Fourier Transform Infrared (FT-IR) instrument (PerkinElmer, Waltham, MA, USA) with an attenuated total reflection accessory. It was operated at 4000–400 cm−1 with 16 scanning times and a resolution of 4.000 cm−1.
2.6. DSC and TG
A DSC apparatus (Mettler Toledo, Greifensee, Switzerland) was used to measure the DSC curves of APIs, CCFs, and co-crystals. The sample (3–5 mg) was placed in an aluminum crucible with a lid and heated at 10 °C/min. A TG apparatus (Mettler Toledo, Greifensee, Switzerland) was used to measure the TG curves of APIs, CCFs, and co-crystals. A total of 8–10 mg of sample was weighed and heated at 10 °C/min. The plots of DSC and TG were processed using STARe (Evaluation Software 16.30) data processing software.
2.7. DVS
The moisture sorption and desorption measurements of samples were recorded by Surface Measurement Systems (SMS) DVS apparatus (London, UK). Approximately 30–50 mg of sample was placed on a quartz DVS round-bottomed pan. The sample was pre-equilibrated with a continuous flow of dry nitrogen for 5 min. The relative humidity (RH) was then gradually increased from 0% to 90% with a 10% increase. Thereafter, the RH was gradually reduced to 0% in equal increments. The equilibration criterion was maintained at 0.02%/minute at each step throughout the process.
2.8. Theoretical Calculation
The B3LYP-D3/6-31G* level was employed for all the hydrogen atom geometry optimizations. The B3LYP-D3/6-311G** level was used for single-point energy calculations using the Gaussian 16 package. Hirshfeld Surface and energy frameworks were calculated using CrystalExplorer 21.3 software.
3. Results and Discussion
3.1. SCXRD Analysis
Crystals of three co-crystals obtained from the solution using the slow evaporation method were suitable for single-crystal X-ray diffraction (SCXRD) determination, namely the BET-2,4-DHB co-crystal (1:1), the BET-3,5-DHB co-crystal (1:1), and the BET-3-HDB co-crystal (1:1). The crystallographic data of the three co-crystals are summarized in Table 1. BET-2,4-DHB and BET-3,5-HDB are both in the monoclinic space group P21/c, whereas BET-3-HDB is in the orthorhombic crystal system space group Pca21. The detailed formation of hydrogen bonds [48] is listed in Table 1.

Table 1.
Crystal Cell Parameters of the co-crystals.
The crystal structure of BET-2,4-DHB comprises a unit cell with two BET molecules and two 2,4-DHB molecules. As shown in Figure 1a, the O1 of BET forms a hydrogen bond with the O2f of one 2,4-DHB molecule, and the O2 of BET forms a hydrogen bond with the O4f of another 2,4-DHB molecule, resulting in the formation of a “Z”-shaped chain arrangement of BET and 2,4-DHB. On the a-axis, the pink area of Figure 1a shows a “Z”-shaped chain, which extends along the c-axis. However, there are no obvious hydrogen bonding connections among the chains in the b-axis.
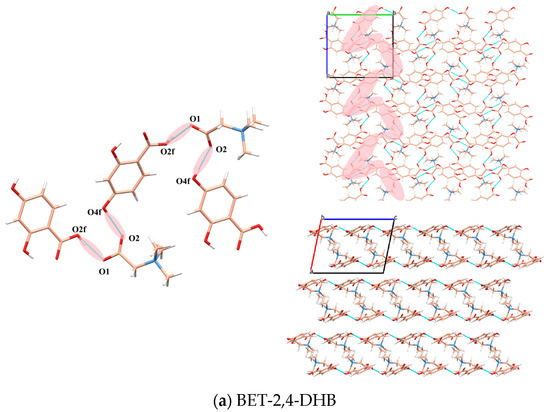
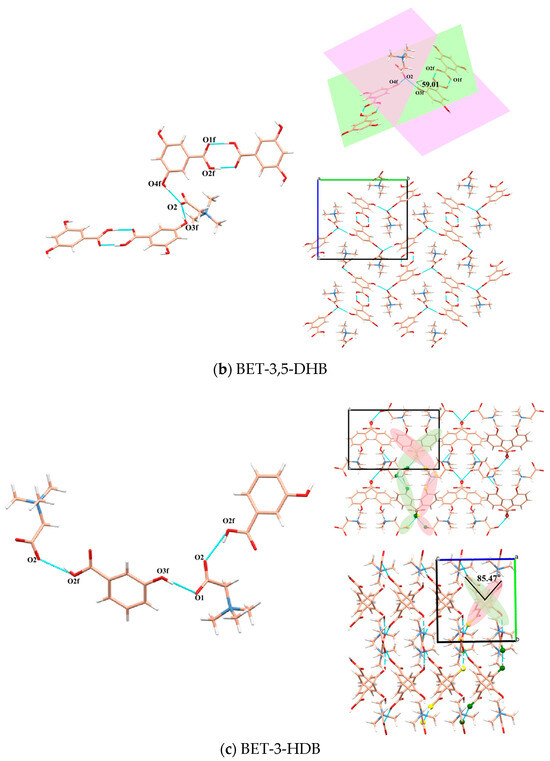
Figure 1.
Hydrogen bond interactions and 3D packing structures of BET-2,4-DHB (a), BET-3,5-DHB (b) and BET-3-HDB (c).
In the BET-3,5-DHB unit cell, there are two BET molecules and two 3,5-DHB molecules. In contrast to the other two crystal structures, O2 is only involved in the formation of hydrogen bonds in the BET molecule. O1, which is not involved in the formation of hydrogen bonds, may be exposed on the surface of the object, increasing the likelihood of hydrogen bonding with water molecules in the air and producing a stronger hygroscopicity than that of the other two co-crystals. As shown in Figure 1b, the O2 of one BET molecule forms two hydrogen bonds with two 3,5-DHB molecules of O4f and O3f, respectively. Additionally, two 3,5-DHB molecules form two hydrogen bonds O2f-H2f…O1f in the same plane. The two planes forming 59.01° are those in which the two 3,5-DHB molecules linked by the same BET are situated. On the a-axis, following the hydrogen bonding linkage of BET and 3,5-DHB described above, BET and 3,5-DHB extend regularly along the b-axis and c-axis in three-dimensional space.
A unit cell of the BET-3-HDB crystal structure has two BET molecules and two 3-HDB molecules. Similar to the structure of BET-2,4-DHB, O1 and O2 of one BET molecule form hydrogen bonds with two 3-HDB molecules of O3f and O2f, respectively, forming a chain structure. On the c-axis, the two chains labeled in pink and green are symmetrically oriented along the a-axis. The two planes in which the 3-DHB molecules on the two chains of symmetry are located form 85.47° on the a-axis.
3.2. PXRD Analysis
A standard method for structure determination and phase identification, PXRD provides kinematic and complete data sets that can be collected easily and quickly [49]. PXRD is widely recognized as being useful for phase identification due to its phase specificity [50]. Figure 2 illustrates the pure phase of BET, CCFs (2,4-DHB, 3,5-DHB and 3-HDB), and the three co-crystal PXRD patterns as well as the theoretical PXRD patterns (labeled with the prefix “Calculated”) of the co-crystals calculated by Mercury. The X-ray diffraction peaks are correlated with the cell parameters and the intensity of the peaks is determined by the distribution of atoms within the lattice [51]. In comparison with API and CCFs, the peak positions and peak intensities of the three co-crystals present remarkable changes, as shown by the specific peak position values in Figure 2.
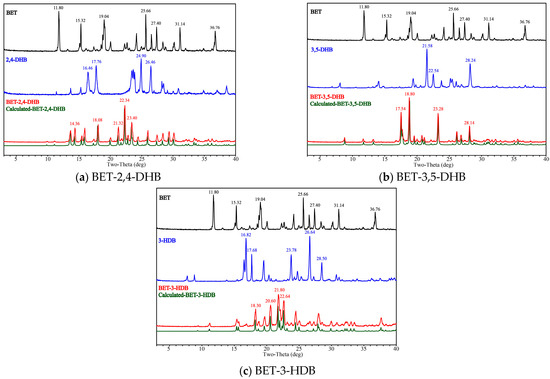
Figure 2.
The PXRD patterns of API, CCFs, the three co-crystals and the corresponding co-crystals.
3.3. IR Analysis
The primary locations in the COO⁻ structure where BET produces hydrogen bonds are indicated by SCXRD analysis. The COO⁻ structure of BET has a multi-electronic π-bonding system, in which the negative charge on the oxygen is not collected on one oxygen but dispersed on two oxygens and one carbon, showing an averaged structure. The IR spectral wavenumber bands of the COO− structure of BET are within the range of 1550–1650 cm−1. The IR spectral wavenumber of -COOH and -OH of CCFs participates in the formation of hydrogen bonds in the range of 3450–3200 cm−1 and 1900–1630 cm−1. When hydrogen bonds are formed, the vibrational frequency shifts towards lower frequencies. In Figure 3, the co-crystals of vibrational frequencies move to lower frequencies or disappear, implying the formation of hydrogen bonds.
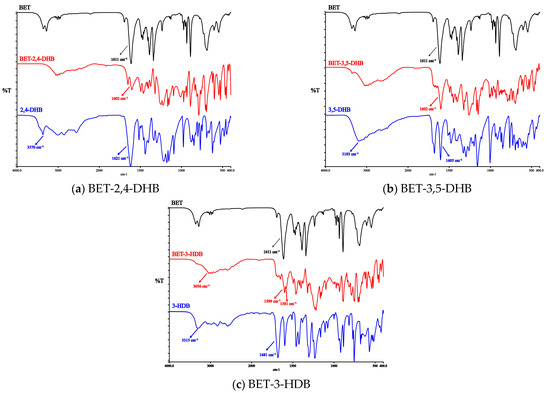
Figure 3.
The IR spectra of API, CCFs and the three co-crystals.
3.4. Thermal Analysis
Thermal analysis includes DSC [52,53] and TG. The DSC curves of all three co-crystals display two endothermic peaks distinct from those of API (303.16 °C) and CCFs in Figure 4 (left). This phenomenon is an indication that a new phase may have been created. TG is a thermal analysis technique that analyzes the change in mass (weight/mass change and rate of weight change) of a substance as a function of temperature. TG can be used to detect the crystallization of solvents, the loss of water molecules, sublimation, and so forth. The TG curves of the three co-crystals are displayed in Figure 4 (right). The TG curves of the three co-crystals do not show any weight loss steps of solvent or water molecules, which exactly corresponds to the DSC curves. There are two phases for weight loss in BET-2,4-DHB and BET-3,5-DHB. A partial structural breakdown of weight loss could occur at BET-2,4-DHB’s first weightless phase. The weight loss range of BET-3,5-DHB (210–380 °C) is compatible with the weight loss range of raw BET (250–360 °C), and the first step is 45.07%, which is near to the theoretical weight loss of 43.19% for BET.
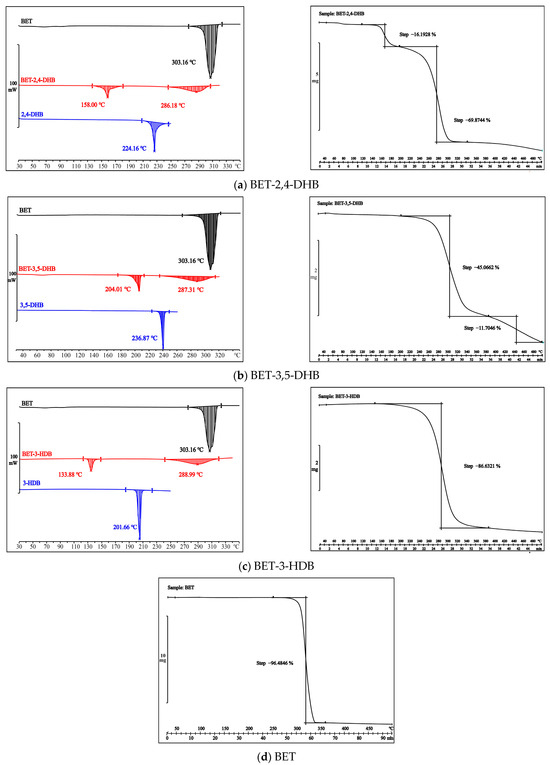
Figure 4.
DSC (left) and TG (right) profiles of API, CCFs and the three co-crystals.
3.5. Hirshfeld Surface and 3D Energy Frameworks
Graphical tools based on Hirshfeld surfaces and two-dimensional (2D) fingerprint maps are valuable for visualizing and analyzing intermolecular interactions. Hirshfeld surface and 2D fingerprint maps, which are developed to visualize the contribution of intermolecular interactions in BET-2,4-DHB (a), BET-3,5-DHB (b), and BET-3-HDB (c) co-crystals, are presented in Figure 5. The dark red spots indicate the locations where hydrogen bonds are formed, and the shade of color corresponds to the strength of the interaction on the Hirshfeld surface. BET mainly acts as a hydrogen bonding acceptor, while CCFs (2,4-DHB, 3,5-DHB and 3-HDB) act as both hydrogen bonding acceptors and hydrogen bonding donors. Meanwhile, BET-3,5-DHB displayed more dark red spots with four than the other two co-crystals with two.
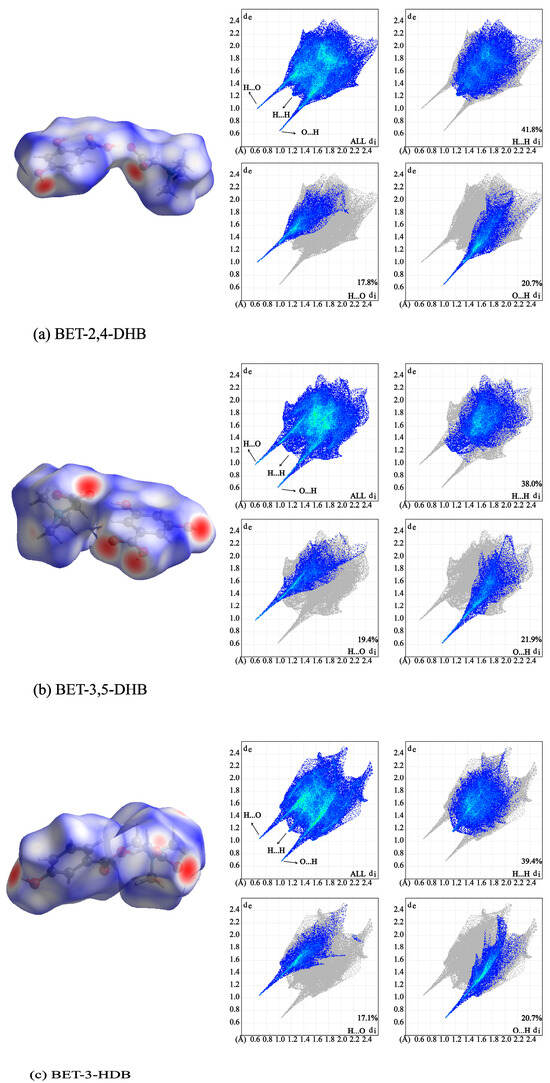
Figure 5.
Hirshfeld surface and 2D fingerprint plots of the three co-crystals.
In 2D fingerprint maps, de is the distance from the point to the nearest nucleus external to the surface, and di is the distance to the nearest nucleus internal to the surface [54]. Figure 6 illustrates the top three contribution contacts, H…H, H…O, and O…H. The maximum contribution of H...H contacts is found in BET-2,4-DHB, BET-3,5-DHB, and BET-3-HDB for 41.8%, 38.0%, and 39.4%, respectively. Due to the presence of carboxyl groups in all CCFs, the hydrogen bonds formed by H…O and O…H have an important role to play, exhibited as elongated sharp peaks. The contributions of O…H and H…O contacts accounted for 20.7% and 17.8% in BET-2,4-DHB, 21.9% and 19.4% in BET-3,5-DHB, and 20.7% and 17.1% in BET-3-HDB, respectively.
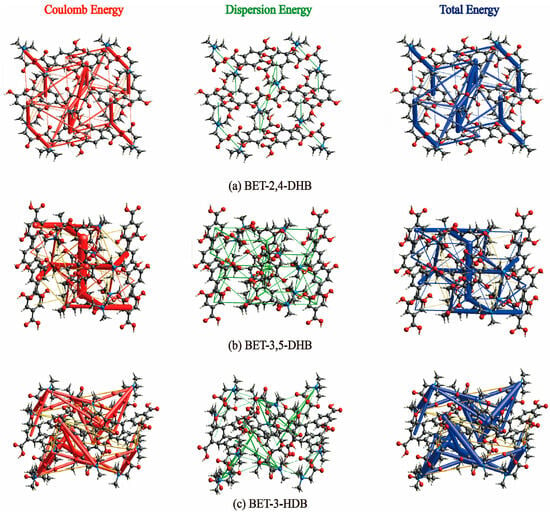
Figure 6.
Energy frameworks of the three co-crystals.
Figure 6 illustrates the 3D energy frameworks of the three co-crystals calculated by the CrystalExplorer 21.3 software, which is utilized to analyze the interaction of energy between molecules. Red is the Coulomb energy, green is the dispersion energy, and purple is the total energy. The cylinder diameter changes according to the magnitude of the interaction force. Due to betaine’s status as a zwitterion, we speculated that Coulomb energy would be the main energy involved in the formation of co-crystals of BET, and the results have been consistent with our speculations. The calculations demonstrate that the red cylinder in the three co-crystals is thicker than the other two colors, implying that Coulomb energy dominates in the three co-crystals.
3.6. Hygroscopicity Measurement
DVS is a gravimetric technique that measures substance weight loss/gain as a function of humidity (RH). The curves of sorption (red) and desorption (blue) for BET, BET-2,4-DHB, 3,5-DHB, and BET-3-HDB are presented in Figure 7.
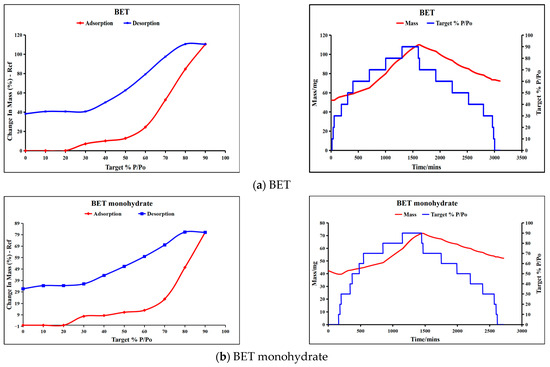
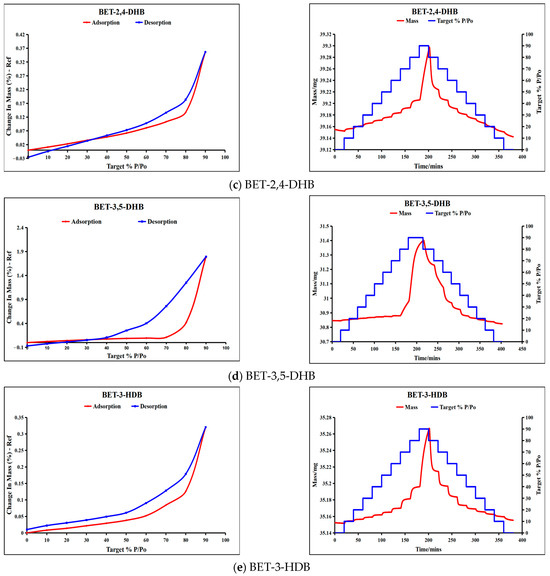
Figure 7.
DVS results of BET, BET monohydrate and the three co-crystals.
The weight change in the raw material BET increases to 110.5% when the relative humidity (RH) hits 90%. Conversely, when the RH falls to 0%, the BET is in a solution condition and its weight change decreases to 38.3%, indicating that the BET has a significantly stronger hygroscopicity. The hygroscopicity of BET monohydrate is inferior to that of BET; however, it maintains significant hygroscopicity. The weight change in BET monohydrate attains 81.07% at 90% RH, and when the humidity decreases to 0%, BET monohydrate exists as a solid powder. The formation of co-crystals can significantly improve the hygroscopicity of BET. At 90% RH, the weight changes in BET-2,4-DHB (0.36%), BET-3,5-DHB (1.79%), and BET-3-HDB (0.32%) are 306.9, 61.7, and 345.3 times more than those of the raw material BET, respectively. All three co-crystals are in solid powder when RH drops to 0%. The results of DVS reveal that the three co-crystals markedly improve the hygroscopicity of BET, especially BET-3-HDB.
As shown in Figure 1 and Figure S1, variations in hygroscopicity can be explained by the crystal structure and intermolecular interactions. Figure S1 illustrates the structure of the BET (WEMWEQ) and BET monohydrate (KICCOO) through the CIF file obtained at the Cambridge Crystallographic Data Centre (CCDC). Due to its carboxylate ions, BET readily forms hydrogen bonding interaction forces with water molecules, resulting in hygroscopicity. Nevertheless, BET-2,4-DHB and BET-3-HDB co-crystals, wherein both oxygen atoms of the carboxylate participate in hydrogen bond formation, exhibit an inability to interact with water molecules, leading to a markedly reduced weight change at 90% RH. Conversely, the BET-3,5-DHB co-crystal exhibits greater hygroscopicity than the other two co-crystals, as only one oxygen atom of the carboxylate participates in hydrogen bond formation.
Figure 8 illustrates that the low hygroscopicity of 3-HDB can be explained by MEPS, where red regions denote electron-rich areas and blue regions represent electron-deficient areas. 3-HDB exhibits two electron-rich and two electron-deficient regions, with the total of these regions being the lowest among the CCFs. This is followed by 2,4-DHB, while 3,5-DHB has the highest total. This sequence aligns with the established order of hygroscopicity. The ortho-hydroxyl group in 2,4-DHB lacks an electron-rich region and possesses only an electron-deficient region, which is associated with the formation of intramolecular hydrogen bonds. Therefore, the choice of benzoic acid-like structures as CCFs for BET, which primarily engages in hydrogen bonding with meta-hydroxyl and para-hydroxyl groups, represents a strategy to reduce hygroscopicity.
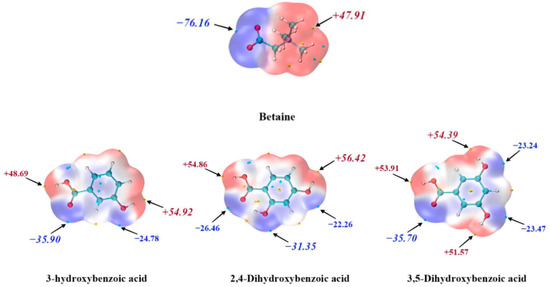
Figure 8.
MEPS of BET, 3-HDB, 2,4-DHB and 3,5-DHB.
4. Conclusions
This study successfully synthesized three co-crystals of BET using solvent-assisted grinding methods: BET-2,4-DHB, BET-3,5-DHB, and BET-3-HDB. The formation of co-crystals was confirmed through SCXRD, PXRD, DSC, TGA, and IR analyses. Additionally, the hygroscopicity of the co-crystals and BET was evaluated using DVS. The results of DVS reveal that the three co-crystals markedly reduce the hygroscopicity of BET, especially BET-3-HDB. Due to its carboxylate ions, BET readily forms hydrogen bonding interaction forces with water molecules, resulting in hygroscopicity. In co-crystals, carboxylate ions establish hydrogen bonds with CCFs, thereby minimizing interactions with water molecules and reducing hygroscopicity. In addition, the choice of benzoic acid-like structures as CCFs for BET, which primarily engages in hydrogen bonding with meta-hydroxyl and para-hydroxyl groups, represents a strategy to reduce hygroscopicity. The Hirschfeld surface illustrates the contribution of forces in co-crystals: H-H > O-H > H-O. Coulomb forces are predominant in the co-crystals, as indicated by 3D energy frameworks.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/cryst14110917/s1, Figure S1: Crystal packing patterns of (a) BET (viewed along the c axis) and (b) BET monohydrate (viewed along the b axis); Table S1: Parameters (Å, Degree) of the Main Hydrogen Bonds for the Co-crystals.
Author Contributions
Conceptualization, L.Z. and Y.L.; methodology, Q.L. software, D.Y. and S.L.; validation, D.Y. and S.L.; formal analysis, Q.L.; investigation, Y.X., S.L. and Q.L.; resources, Q.L. and Z.W.; data curation, Z.W. and Q.L.; writing—original draft preparation, Q.L.; writing—review and editing, D.Y. and L.Z.; visualization, S.Y.; supervision, L.Z., D.Y. and Y.L.; project administration, Y.L.; funding acquisition, Y.L., L.Z. and D.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (Grant No. 22278443), CAMS Innovation Fund for Medical Sciences (Grant No. 2022-I2M-1-015), the Key R&D Program of Shan Dong Province (Grant No. 2021ZDSYS26), Chinese Pharmacopoeia Commission Drug Standard Promoting Fund (Grant No. 2023Y11), Independent Innovation and Achievement Transformation Plan Project of Zaozhuang City (Grant No. 2022GH15), 2021 Tengzhou Talent Project, and the Xinjiang Uygur Autonomous Region Innovation Environment Construction Special Fund and Technology Innovation Base Construction Key Laboratory Open Project (Grant No. 2022D04016).
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Acknowledgments
The authors are thankful to the National Natural Science Foundation of China, CAMS Innovation Fund for Medical Sciences, the Key R&D Program of Shan Dong Province, Chinese Pharmacopoeia Commission Drug Standard Promoting Fund, Independent Innovation and Achievement Transformation Plan Project of Zaozhuang City, and the Xinjiang Uygur Autonomous Region Innovation Environment Construction Special Fund and Technology Innovation Base Construction Key Laboratory Open Project for financial support.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Arumugam, M.K.; Paal, M.C.; Donohue, T.M.J.; Ganesan, M.; Osna, N.A.; Kharbanda, K.K. Beneficial effects of betaine: A comprehensive review. Biology 2021, 10, 456. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, J.R.; Ratamess, N.A.; Kang, J.; Rashti, S.L.; Faigenbaum, A.D. Effect of betaine supplementation on power performance and fatigue. Int. Soc. Sports Nutr. 2009, 6, 7–10. [Google Scholar] [CrossRef] [PubMed]
- Willingham, B.D.; Ragland, T.J.; Ormsbee, M.J. Betaine Supplementation May Improve Heat Tolerance: Potential Mechanisms in Humans. Nutrients 2020, 12, 2939. [Google Scholar] [CrossRef] [PubMed]
- Pukale, D.D.; Lazarenko, D.; Aryal, S.R.; Khabaz, F.; Shriver, L.P.; Leipzig, N.D. Osmotic contribution of synthesized betaine by choline dehydrogenase using in vivo and in vitro models of post-traumatic syringomyelia. Cell. Mol. Bioeng. 2023, 16, 41–54. [Google Scholar] [CrossRef] [PubMed]
- Pukale, D.D.; Farrag, M.; Gudneppanavar, R.; Baumann, H.J.; Konopka, M.; Shriver, L.P.; Leipzig, N.D. Osmoregulatory role of Betaine and Betaine/γ-Aminobutyric acid transporter 1 in post-traumatic Syringomyelia. ACS Chem. Neurosci. 2021, 12, 3567–3578. [Google Scholar] [CrossRef]
- Lawson-Yuen, A. and Levy H L. The use of betaine in the treatment of elevated homocysteine. Mol. Genet. Metab. 2006, 88, 201–207. [Google Scholar] [CrossRef]
- Truitt, C.; Hoff, W.D.; Deole, R. Health functionalities of betaine in patients with homocystinuria. Front. Nutr. 2021, 8, 690359. [Google Scholar] [CrossRef]
- Kumar, T.; Sharma, G.S.; Singh, L.R. Homocystinuria: Therapeutic approach. Clin. Chim. Acta 2016, 458, 55–62. [Google Scholar] [CrossRef]
- Al Mutairi, F. Hyperhomocysteinemia: Clinical insights. J. Cent. Nerv. Syst. Dis. 2020, 12, 1179573520962230. [Google Scholar] [CrossRef]
- Imbard, A.; Toumazi, A.; Magréault, S.; Garcia-Segarra, N.; Schlemmer, D.; Kaguelidou, F.; Perronneau, I.; Haignere, J.; de Baulny, H.O.; Kuster, A.; et al. Efficacy and pharmacokinetics of betaine in CBS and cblC deficiencies: A cross-over randomized controlled trial. Orphanet J. Rare Dis. 2022, 17, 417. [Google Scholar] [CrossRef]
- Hossain, M.A.; Boily, S.; Beauregard, N.; Forest, J.M.; Leclair, G. Stability of Betaine Capsules. Int. Sch. Res. Not. 2013, 2013, 458625. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S. Role of betaine in liver disease-worth revisiting or has the die been cast. World J. Gastroenterol. 2020, 26, 5745. [Google Scholar] [CrossRef] [PubMed]
- Kharbanda, K.K. Role of transmethylation reactions in alcoholic liver disease. World J. Gastroenterol. 2007, 13, 4947–4954. [Google Scholar] [CrossRef] [PubMed]
- Singhal, N.K.; Sternbach, S.; Fleming, S.; Alkhayer, K.; Shelestak, J.; Popescu, D.; Weaver, A.; Clements, R.; Wasek, B.; Bottiglieri, T.; et al. Betaine restores epigenetic control and supports neuronal mitochondria in the cuprizone mouse model of multiple sclerosis. Epigenetics 2020, 15, 871–886. [Google Scholar] [CrossRef]
- Bhatt, M.; Di Iacovo, A.; Romanazzi, T.; Roseti, C.; Bossi, E. Betaine—The dark knight of the brain. Basic Clin. Pharmacol. Toxicol. 2023, 133, 485–495. [Google Scholar] [CrossRef]
- Golzarand, M.; Mirmiran, P.; Azizi, F. Association between dietary choline and betaine intake and 10.6-year cardiovascular disease in adults. Nutr. J. 2022, 21, 1. [Google Scholar] [CrossRef]
- Ashtary-Larky, D.; Bagheri, R.; Ghanavati, M.; Asbaghi, O.; Tinsley, G.M.; Mombaini, D.; Kooti, W.; Kashkooli, S.; Wong, A. Effects of betaine supplementation on cardiovascular markers: A systematic review and Meta-analysis. Crit. Rev. Food Sci. Nutr. 2022, 62, 6516–6533. [Google Scholar] [CrossRef]
- Alvarenga, L.; Ferreira, M.S.; Kemp, J.A.; Mafra, D. The role of betaine in patients with chronic kidney disease: A narrative review. Curr. Nutr. Rep. 2022, 11, 395–406. [Google Scholar] [CrossRef]
- Zawieja, E.E.; Zawieja, B.; Chmurzynska, A. Betaine supplementation moderately increases total cholesterol levels: A systematic review and meta-analysis. J. Diet. Suppl. 2021, 18, 105–117. [Google Scholar] [CrossRef]
- Du, J.; Shen, L.; Tan, Z.; Zhang, P.; Zhao, X.; Xu, Y.; Gan, M.; Yang, Q.; Ma, J.; Jiang, A.; et al. Betaine Supplementation Enhances Lipid Metabolism and Improves Insulin Resistance in Mice Fed a High-Fat Diet. Nutrients 2018, 10, 131. [Google Scholar] [CrossRef]
- Olli, K.; Lahtinen, S.; Rautonen, N.; Tiihonen, K. Betaine reduces the expression of inflammatory adipokines caused by hypoxia in human adipocytes. Br. J. Nutr. 2013, 109, 43–49. [Google Scholar] [CrossRef] [PubMed]
- O’Neil, M.J.; Heckelman, P.E.; Koch, C.B.; Roman, K.J. (Eds.) The Merck Index: An Encyclopedia of Chemicals, Drugs, and Biologicals; Merck & Co.: Rahway, NJ, USA, 2006. [Google Scholar]
- Tereshchenko, A.G. Deliquescence: Hygroscopicity of water-soluble crystalline solids. J. Pharm. Sci. 2015, 104, 3639–3652. [Google Scholar] [CrossRef] [PubMed]
- Reutzel-Edens, S.M.; Braun, D.E.; Newman, A.W. Hygroscopicity and Hydrates in Pharmaceutical Solids. In Polymorphism in the Pharmaceutical Industry: Solid Form and Drug Development; Wiley: Hoboken, NJ, USA, 2018; pp. 159–188. [Google Scholar]
- Thakur, T.S.; Thakuria, R. Crystalline multicomponent solids: An alternative for addressing the hygroscopicity issue in pharmaceutical materials. Cryst. Growth Des. 2020, 20, 6245–6265. [Google Scholar] [CrossRef]
- Anbarasan, A.; Nataraj, J.; Shanmukhan, N.; Radhakrishnan, A. Effect of hygroscopicity on pharmaceutical ingredients, methods to determine and overcome: An overview. J. Chem. Pharm. Res. 2018, 10, 61–67. [Google Scholar]
- Chang, S.Y.; Sun, C.C. Superior Plasticity and Tabletability of Theophylline Monohydrate. Mol. Pharm. 2017, 14, 2047–2055. [Google Scholar] [CrossRef]
- Newman, A.W.; Reutzel-Edens, S.M.; Zografi, G. Characterization of the "hygroscopic" properties of active pharmaceutical ingredients. J. Pharm. Sci. 2008, 97, 1047–1059. [Google Scholar] [CrossRef]
- Liu, F.; Hooks, D.E.; Li, N.; Mara, N.A.; Swift, J.A. Mechanical Properties of Anhydrous and Hydrated Uric Acid Crystals. Chem. Mater. 2018, 30, 3798–3805. [Google Scholar] [CrossRef]
- Duggirala, N.K.; Vyas, A.; Krzyzaniak, J.F.; Arora, K.K.; Suryanarayanan, R. Mechanistic Insight into Caffeine–Oxalic Co-crystal Dissociation in Formulations: Role of Excipients. Mol. Pharm. 2017, 14, 3879–3887. [Google Scholar] [CrossRef]
- Koranne, S.; Sahoo, A.; Krzyzaniak, J.F.; Luthra, S.; Arora, K.K.; Suryanarayanan, R. Challenges in Transitioning Co-crystals from Bench to Bedside: Dissociation in Prototype Drug Product Environment. Mol. Pharm. 2018, 15, 3297–3307. [Google Scholar] [CrossRef]
- Kaur, N.; Duggirala, N.K.; Thakral, S.; Suryanarayanan, R. Role of Lattice Disorder in Water-Mediated Dissociation of Pharmaceutical Co-crystal Systems. Mol. Pharm. 2019, 16, 3167–3177. [Google Scholar] [CrossRef]
- Pudipeddi, M.; Serajuddin, A.T.M. Trends in Solubility of Polymorphs. J. Pharm. Sci. 2005, 94, 929–939. [Google Scholar] [CrossRef] [PubMed]
- Rohrs, B.R.; Thamann, T.J.; Gao, P.; Stelzer, D.J.; Bergren, M.S.; Chao, R.S. Tablet Dissolution Affected by a Moisture Mediated Solid-State Interaction Between Drug and Disintegrant. Pharm. Res. 1999, 16, 1850–1856. [Google Scholar] [CrossRef] [PubMed]
- Balbach, S.; Korn, C. Pharmaceutical evaluation of early development candidates “the 100 mgapproach”. Int. J. Pharm. 2004, 275, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Kale, D.P.; Ugale, B.; Nagaraja, C.M.; Dubey, G.; Bharatam, P.V.; Bansal, A.K. Molecular Basis of Water Sorption Behavior of Rivaroxaban-Malonic Acid Co-crystal. Mol. Pharm. 2019, 16, 2980–2991. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.; Sun, X.; Chen, J.; Cai, T. Pharmaceutical co-crystals: A review of preparations, physicochemical properties and applications. Acta Pharm. Sin. B 2021, 11, 2537–2564. [Google Scholar] [CrossRef]
- Kuminek, G.; Cao, F.; de Oliveira da Rocha, A.B.; Cardoso, S.G.; Rodríguez-Hornedo, N. Co-crystals to Facilitate Delivery of Poorly Soluble Compounds Beyond-Rule-of-5. Adv. Drug Deliv. Rev. 2016, 101, 143–166. [Google Scholar] [CrossRef]
- Berry, D.J.; Steed, J.W. Pharmaceutical Co-crystals, Salts and Multicomponent Systems; Intermolecular Interactions and Property Based Design. Adv. Drug Deliv. Rev. 2017, 117, 3–24. [Google Scholar] [CrossRef]
- Watanabe, T.; Ito, M.; Suzuki, H.; Terada, K.; Noguchi, S. Reduced deliquescency of isosorbide by co-crystallization and mechanisms for hygroscopicity. Int. J. Pharm. 2021, 607, 120959. [Google Scholar] [CrossRef]
- Sun, J.; Jia, L.; Wang, M.; Liu, Y.; Li, M.; Han, D.; Gong, J. Novel drug-drug multicomponent crystals of epalrestat–metformin: Improved solubility and photostability of epalrestat and reduced hygroscopicity of metformin. Crystal Growth and Design. 2022, 22, 1005–1016. [Google Scholar] [CrossRef]
- Puschmann, H.; Bourhis, L.J.; Dolomanov, O.V.; Gildea, R.J.; Howard, J.A. OLEX2-a complete package for molecular crystallography. Acta Crystallogr. A 2011, 67, C593. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2, Teaching New Software Old And New Tricks. J. Appl. Crystallogr. 2019, 42, 339–341. [Google Scholar] [CrossRef]
- Lübben, J.; Wandtke, C.M.; Hübschle, C.B.; Ruf, M.; Sheldrick, G.M.; Dittrich, B. Aspherical scattering factors for SHELXL–model, implementation and application. Acta Crystallogr. Sect. A Found. Crystallogr. 2019, 75, 50–62. [Google Scholar] [CrossRef] [PubMed]
- Macrae, C.F.; Sovago, I.; Cottrell, S.J.; Galek, P.T.; McCabe, P.; Pidcock, E.; Platings, M.; Shields, G.P.; Stevens, J.S.; Towlera, M.; et al. Mercury: Visualization and analysis of crystal structures. J. Appl. Crystallogr. 2006, 39, 453–457. [Google Scholar] [CrossRef]
- An, Q.; Xing, C.; Wang, Z.P.; Li, S.; Wang, W.; Yang, S.; Kong, L.L.; Yang, D.Z.; Zhang, L.; Du, G.H.; et al. Metformin-Mediated Improvement in Solubility, Stability, and Permeability of Nonsteroidal Anti-Inffammatory Drugs. Pharmaceutics 2024, 29, 2208. [Google Scholar]
- Wang, Z.P.; Li, S.; Li, Q.; Wang, W.W.; Liu, M.J.; Yang, S.Y.; Zhang, L.; Yang, D.Z.; Du, G.H.; Lu, Y. A Novel Co-crystal of Daidzein with Piperazine to Optimize the Solubility, Permeability and Bioavailability of Daidzein. Molecules 2024, 29, 1710. [Google Scholar] [CrossRef]
- Allen, F.H.; Bruno, I.J. Bond lengths in organic and metal-organic compounds revisited: X—H bond lengths from neutron diffraction data. Acta Crystallogr. Sect. B Struct. Sci. 2010, 66, 380–386. [Google Scholar] [CrossRef]
- Li, J.; Sun, J. Application of X-ray Diffraction and Electron Crystallography for Solving Complex Structure Problems. Acc. Chem. Res. 2017, 50, 2737–2745. [Google Scholar] [CrossRef]
- Munjal, B.; Suryanarayanan, R. Applications of synchrotron powder X-ray diffractometry in drug substance and drug product characterization. TrAC Trends Anal. Chem. 2021, 136, 116181. [Google Scholar] [CrossRef]
- Bunaciu, A.A.; Udriştioiu, E.G.; Aboul-Enein, H.Y. X-ray diffraction: Instrumentation and applications. Crit. Rev. Anal Chem. 2015, 45, 289–299. [Google Scholar] [CrossRef]
- Garbacz, P.; Paukszta, D.; Sikorski, A.; Wesolowski, M. Structural characterization of co-crystals of chlordiazepoxide with p-aminobenzoic acid and lorazepam with nicotinamide by dsc, X-ray diffraction, ftir and raman spectroscopy. Pharmaceutics 2020, 12, 648. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, F.; Zhao, X.; Wang, S.; Yang, Q.; Zhang, X. Crystal structure, solubility, and pharmacokinetic study on a hesperetin co-crystal with piperine as coformer. Pharmaceutics 2022, 14, 94. [Google Scholar] [CrossRef] [PubMed]
- McKinnon, J.J.; Fabbiani, F.P.A.; Spackman, M.A. Comparison of Polymorphic Molecular Crystal Structures through Hirshfeld Surface Analysis. Cryst. Growth Des. 2007, 7, 755–769. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).