Abstract
Spectroscopic and thermoactivation methods were used to study the processes of accumulation of electron and hole trapping centers and energy transfer of electronic excitations to impurities in and . It is shown that electronic trapping centers are created during the excitation of an anionic complex as a result of charge transfer from to closely spaced anionic complexes in and . In and , energy transfer from the host to impurities occurs at the moment of charge transfer from the excited anionic complex to the combined radiative electronic state at 2.95–3.1 eV. This combined state is formed from electronic trapping centers and . It was found that the emerging combined radiative states at 2.95–3.1 eV of sulfates, which are formed as a result of charge transfer from the excited anionic complexes to the excited state of impurities, , occupy the same energy levels as the intrinsic electronic trapping center of the host at 2.95–3.17 eV. Experimental results show that during UV photon irradiation, anionic complexes are excited mainly near impurities in sulfates.
1. Introduction
The practical use of these materials as phosphors, dosimeters, detectors, etc. is connected to the research of the mechanism of the formation of electron and hole trapping centers in irradiated sulfates of alkaline earth metals [1,2,3]. In irradiated sulfates of alkaline earth metals, the electronic excitations produced at trapping sites [4,5,6] relax as intrinsic and recombination emissions.
The creation of electron and hole trapping centers is related to the practical use of these crystals as dosimeters and detectors. The concentration of accumulated electron and hole trapping centers in TL (Thermo-luminescent) dosimeters is used to quantify the absorbed dosage in crystals [7,8,9,10]. Stability is a key issue for dosimeters. Stability may be enhanced by implementation difference impurities. Stability of ionic compounds [11,12] and perovskites [13] doped with impurities studied in term of continuous illumination with UV light. Local levels below the conduction band and above the top of the valence band correspond to intrinsic trapping centers in the host transparency region. Special impurities are added to concentrate accumulated defects and radiate the energy of recombination processes [14,15,16].
Experimental evidence has demonstrated that accumulated defects in practically all sulfates are associated with long-wavelength recombination emission bands at 3.0–3.1 eV, 2.6–2.7 eV, and 2.3–2.4 eV. At photon energies between 6 and 12.4 eV, free electron-hole pairs are formed, which results in the formation of these recombination emissions. It has been experimentally shown that, upon excitation in the recombination emission bands at 3.0–3.1 eV and 2.6–2.7 eV, excitations appear corresponding to 3.9–4.0 eV and 4.45–4.5 eV [17,18,19,20] in the transparency region of the host. These excitation energies must correspond to the local levels of electron and hole trapping centers.
When impurities capture electrons in irradiated K2SO4-Tl+ and Na2SO4-Cu+ crystals, it leads to the formation of electron trapping centers such as Tl0 [21] and Cu0 [18]. These centers are associated with and as a result create a hole trapping centers located under the conduction band. Within the 2.9–3.0 eV spectral range, the recombination emission bands that correspond to the impurity trapping centers are located below the conduction band. They are closely situated to the recombination emission of the host which is observed at 3.0–3.17 eV. In contrast to the emission band of the electronic impurity trapping centers, the emission centers of these impurities in sulfates, Tl+ (4.2 eV), and Cu+ (2.6–2.7 eV), are in distinct spectral ranges.
The formation of electron trapping centers complementary to hole trapping centers has been investigated in other similar ionic–molecular compounds. In these works, possible mechanisms for the creation of electronic trapping centers during the excitation of anionic complexes are discussed. For example, in the work [22] in the compounds , the formation of electronic trapping centers is assumed because of charge transfer from the anionic complex impurities . As a result, electronic trapping centers are formed. It is possible that a similar mechanism for the formation of impurity electron trapping centers is realized in activated sulfates of alkali and alkaline earth metals.
The main objective of this work is to study the mechanisms of creation of electronic Mn+ and hole trapping centers, as well as their sensitizing role in energy transfer to emitters. According to the intensity of TSL (thermostimulated luminescence), the dose absorptions are estimated.
2. Materials and Methods
Natural calcium sulfate crystals and extra pure barium sulfate 99.99% (Sigma Aldrich, St. Louis, MO, USA) were used as investigated samples. and samples were prepared by mechanical friction method. Powder samples were pressed in a form of tablet to convenience of measurements. As raw reagents , , and powders with 99.99% (Sigma Aldrich) purity were used.
Thermo activation and vacuum ultraviolet spectroscopic techniques were used. An irradiation was performed by X-ray source based on BSV-23 X-ray tube with a copper anode. During the experiments the tube’s current was 10 mA, voltage was 40 kV, and its photon energy was 10–15 keV. Photoluminescence measurements were performed on XBO 150 W xenon lamp (OSRAM, Munich, Germany) with a photon energy of 1.5–6.2 eV. Measurements in VUV area were performed by vacuum monochromator with a photon energy of 6.2–12 eV based on hydrogen lamp. Vacuum monochromator is assembled according to the Seya–Namioka scheme. The Solar M 266 with photomultiplier Hamamatsu H 11,890–110 was used as a recorded monochromator. All measurements were carried out in a wide temperature range from 15 to 300 K. The excitation spectrum in VUV area was corrected for the spectral distribution of the excitation emission intensity. XRD analysis is performed on X-ray Diffraction (XRD) System—SmartLab (Rigaku, Akishima, Tokyo, Japan).
3. Results
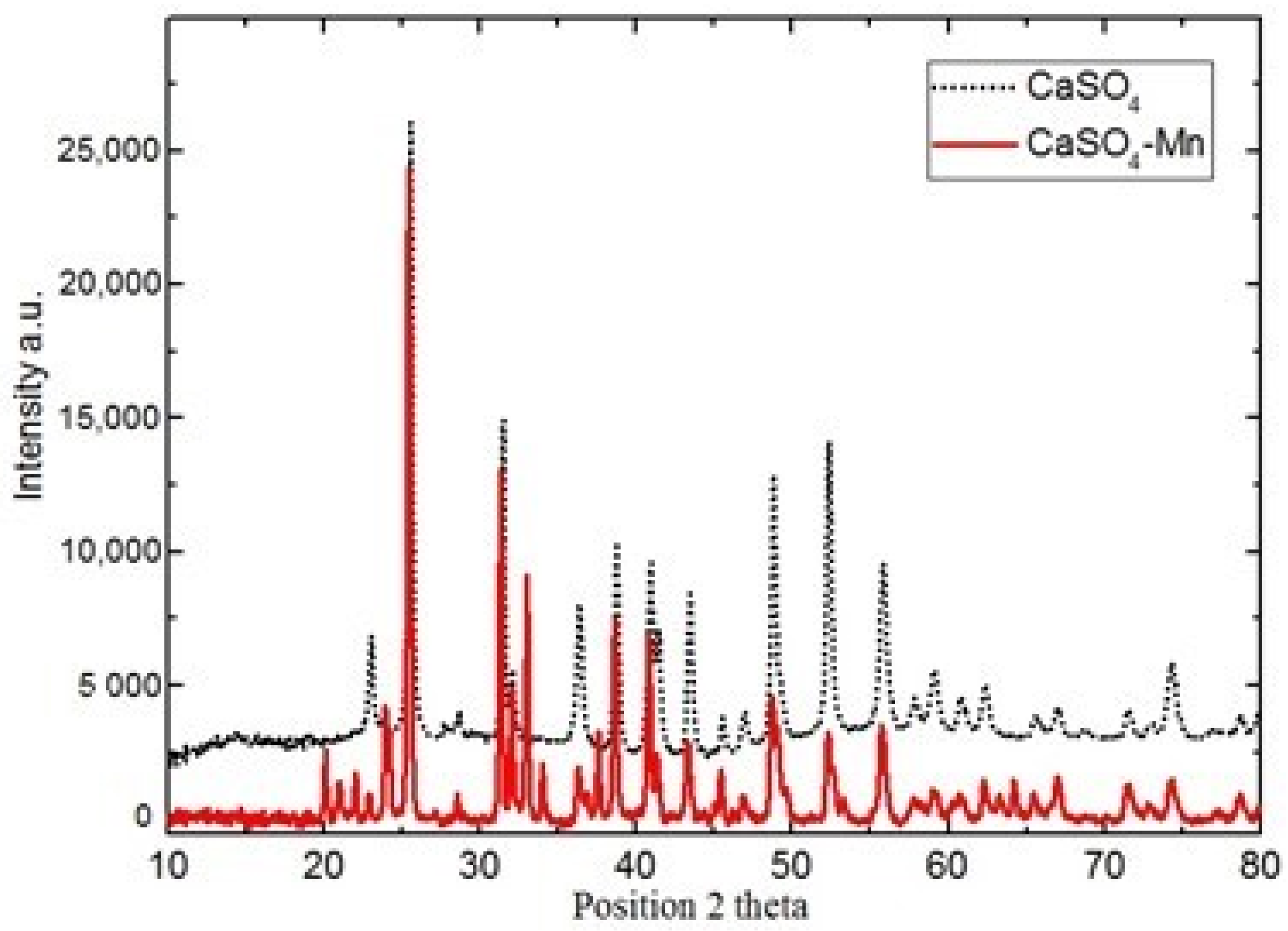
The sample’s XRD pattern is illustrated in Figure 1. The spectrum data shows that the sample of has orthorhombic structure and corresponds to JCPDS card no. 06-0226. Obtained results confirm the purity and existence of Mn in investigated samples. Similar results were also obtained for sample.
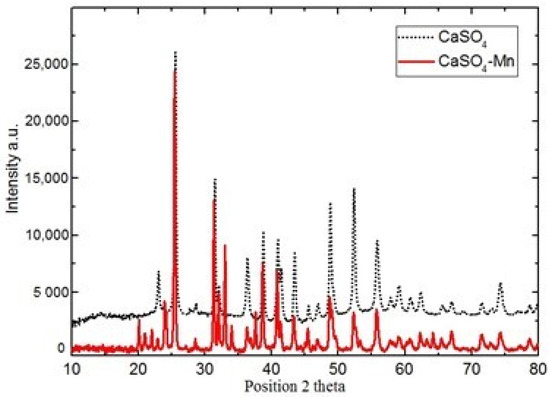
Figure 1.
XRD spectra of and at room temperature.
In this work the mechanism of generation of electron and hole trapping centers as well as the creation of electron and hole trapping centers in and are investigated.
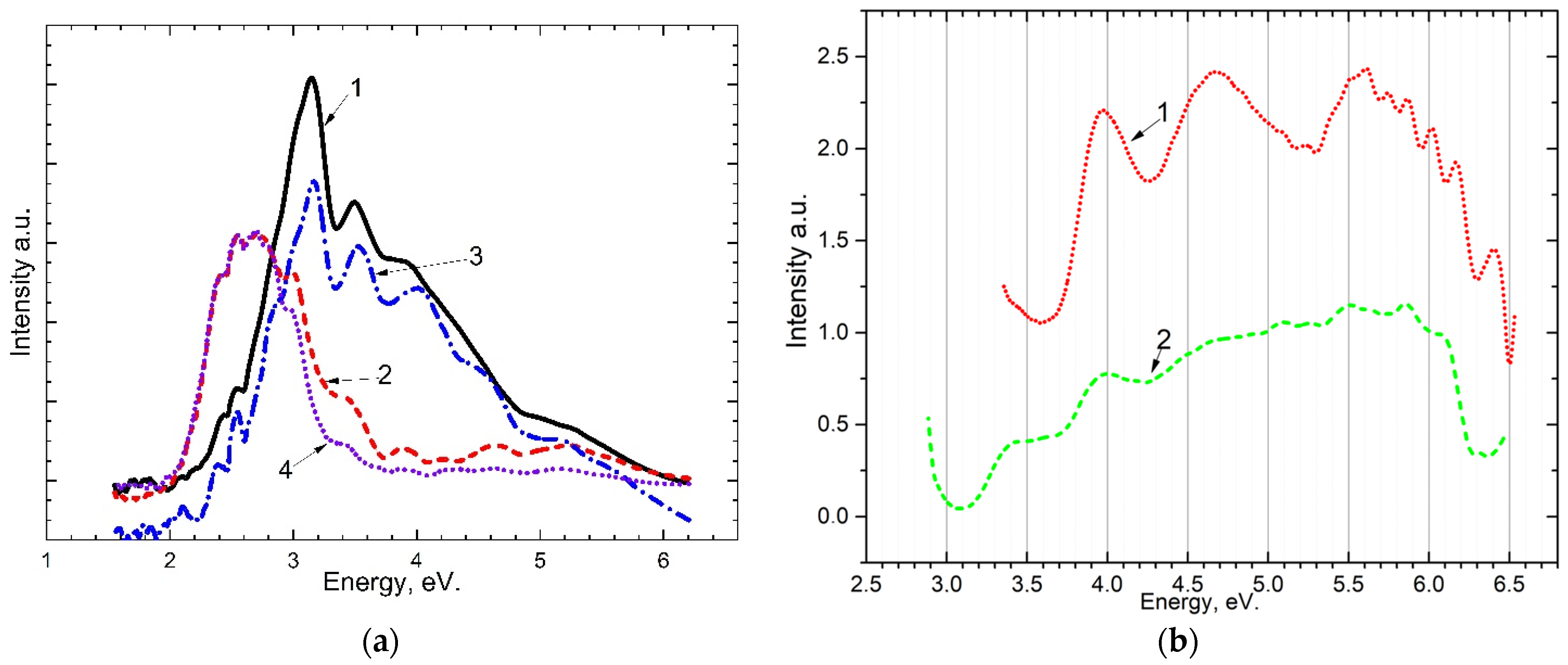
The emission Figure 2a and excitation Figure 2b spectra for the crystal are shown in Figure 2. When excited by photons with energies from 5.4 eV to 7.75 eV, long-wavelength emission bands at 2.3–2.4 eV, 2.6–2.7 eV, 3.0–3.1 eV, and short-wavelength at 3.45–3.8 eV, 4.5–5.0 eV appear. As a result of measuring the excitation spectrum of long-wavelength emission bands of 3.0–3.1 eV and 2.6–2.7 eV, it was shown that these bands of recombination emission are excited at photon energies of ~4.0 eV and ~4.5 eV in the region of transparency CaSO4.
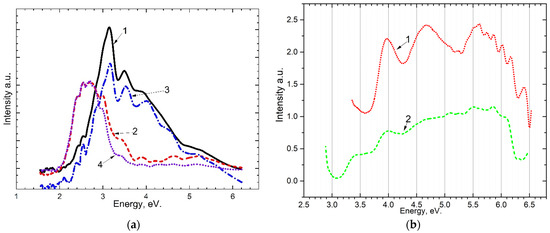
Figure 2.
(a) emission spectra of the crystal upon excitation by: 1—7.75 eV at 15 K; 2—7.75 eV at 300 K; 3—7.3 eV at 15 K; 4—7.3 eV at 300 K; (b) excitation spectrum of the crystal at 80 K after irradiation of 6.2 eV for 10 min: for the band ~3.1 (curve 1) and the band 2.7 (curve 2).
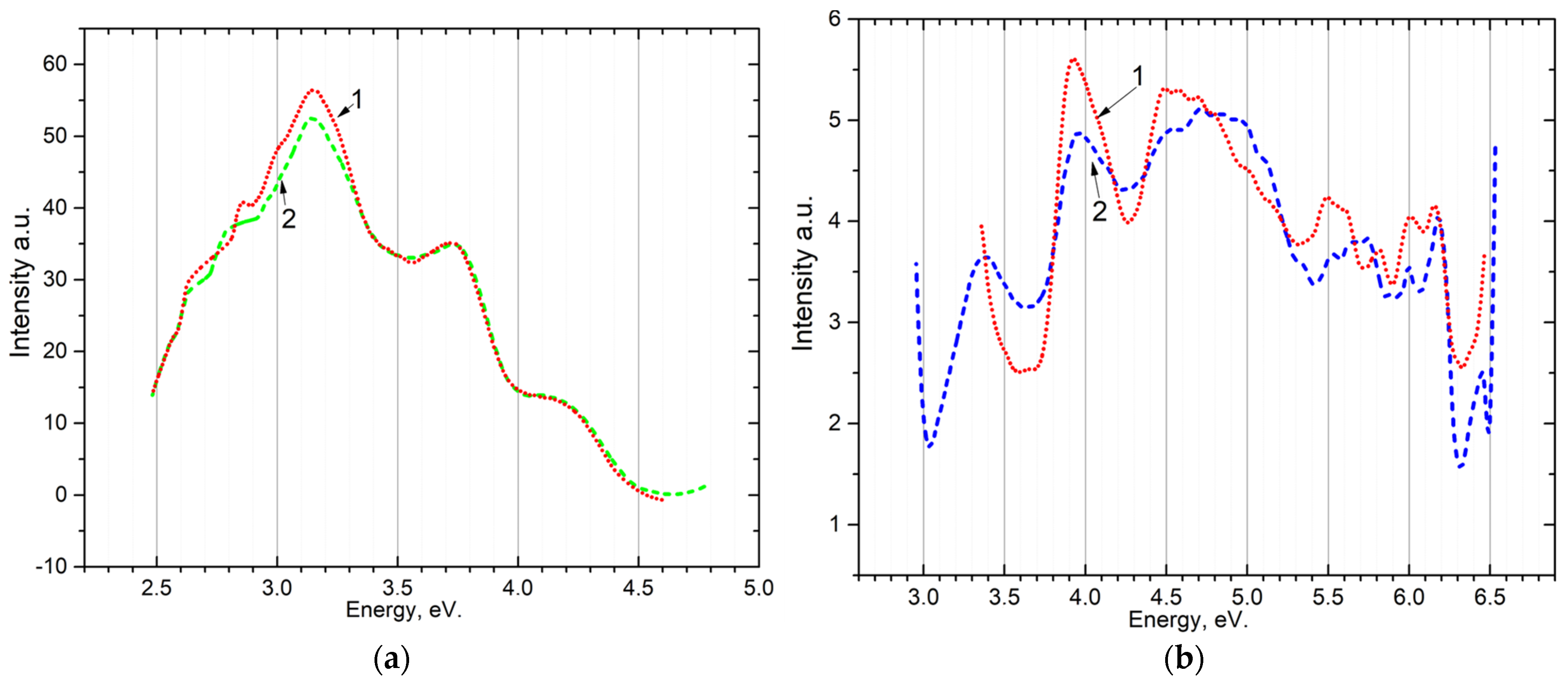
The emission Figure 3a and excitation Figure 3b spectra for the powder sample are shown in Figure 3. When excited by photons with energies from 5.4 eV to 7.75 eV, long-wavelength emission bands appear at 2.3–2.4 eV, 2.6–2.7 eV, 3.0–3.1 eV, and short-wavelength at 3.45–3.8 eV, 4.0–4.5 eV. The excitation spectrum of recombination emission 2.6–2.7 eV and 3.0–3.1 eV was measured. It can be seen that the bands of recombination emission are excited at photon energies of 4.0 eV and 4.5 eV in the transparency region.
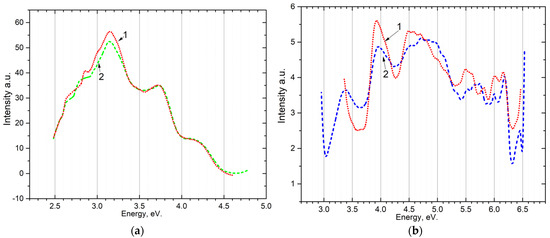
Figure 3.
(a) emission spectrum of powder at 80 K upon excitation by: 1—5.64 eV; 2—5.4 eV; (b) the excitation spectrum of the powder after irradiation with 6.2 eV for 10 min: for the band ~3.1 (curve 1—80 K) and the band 2.7 (curve 2—300 K).
In order to clarify the correspondence between the values of the excitation spectra at 4.0 eV and 4.5 eV for recombination emissions of 3.0–3.1 eV and 2.6–2.7 eV, the samples were excited by photons with an energy of 4.0 eV and 4.5 eV.
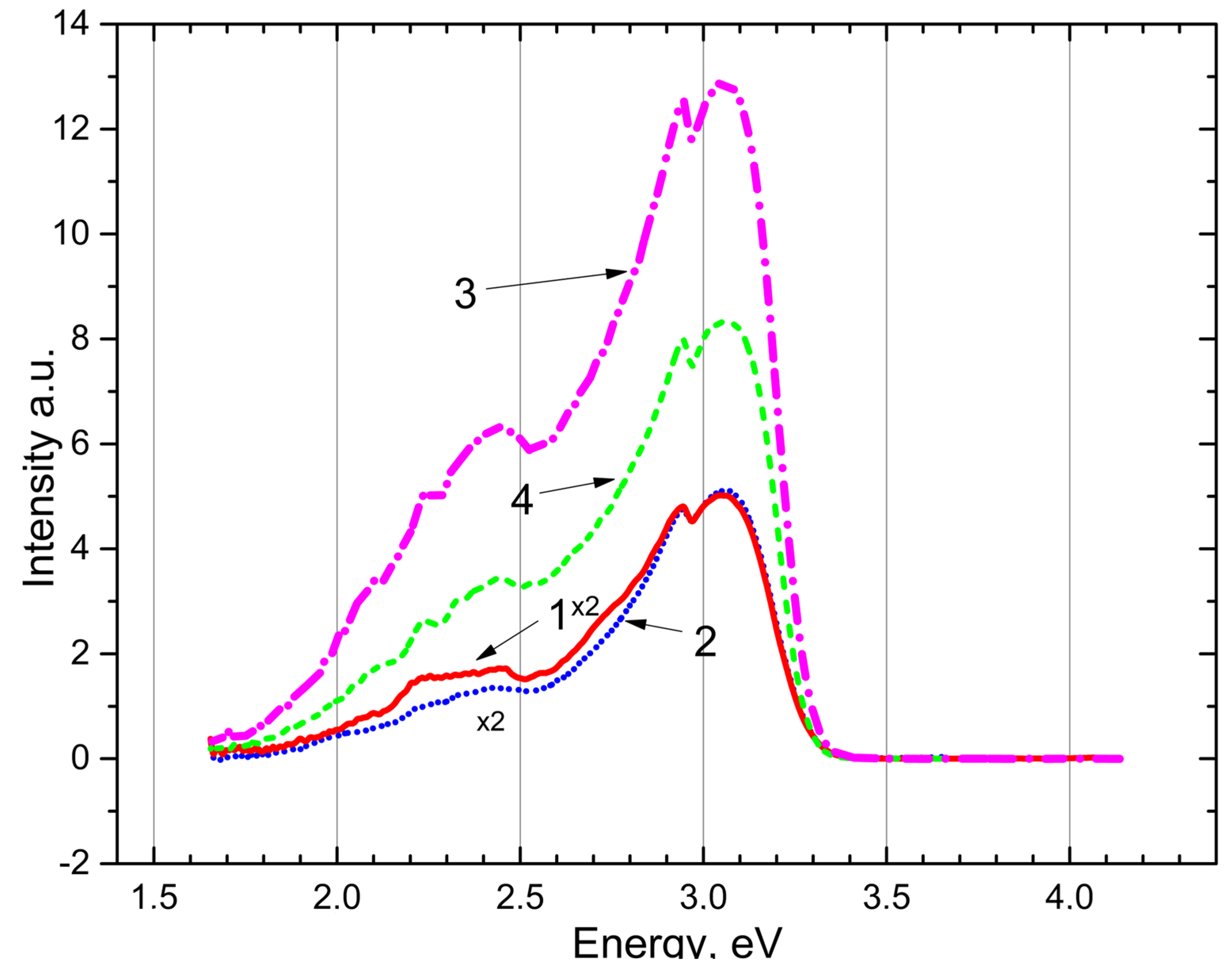
The emission spectrum of an irradiated
crystal and powder is shown in Figure 4. Under excitation by photons with the energies of 4.0 eV and 4.5 eV the recombination emissions reappear at 3.0–3.1 eV and 2.6–2.7 eV in the crystal (curves 1, 2) and (curves 3, 4). The experimental fact proves that recombination emission at 3.0–3.1 eV and 2.6–2.7 eV in and is associated with tunneling electronic transitions between local electronic levels at trapping centers.
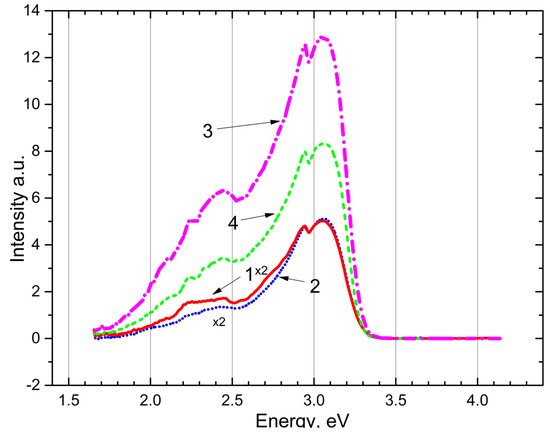
Figure 4.
The emission spectrum after irradiation by photons of 7.3–7.75 eV for 20 min at 80 K: CaSO4 crystal (curves 1, 2) and BaSO4 powder (curves 3, 4) excited photons 4.5 eV and 4.0 eV, respectively.
Thus, according to emission and excitation spectra the mechanisms of accumulation of electron-hole trapping centers in pure and are studied. It is shown that in irradiated crystals, electrons are trapped by the anionic complex , and holes are localized in the form of the radical . Electronic trapping centers are created by the reaction: + e− →. When electron-hole trapping centers and in irradiated crystals are excited with an energy of 5.5–6.2 eV, tunneling recombination emission ~3.1 eV and ~2.6–2.7 eV occurs. These bands are excited in the transparency region of the host ~4.0 eV and ~4.5 eV. These bands are bands of the absorption and excitation spectra of electron and hole trapping centers.
At the next stage, the mechanisms of accumulation of trapping centers and their recombination decay in and were studied.
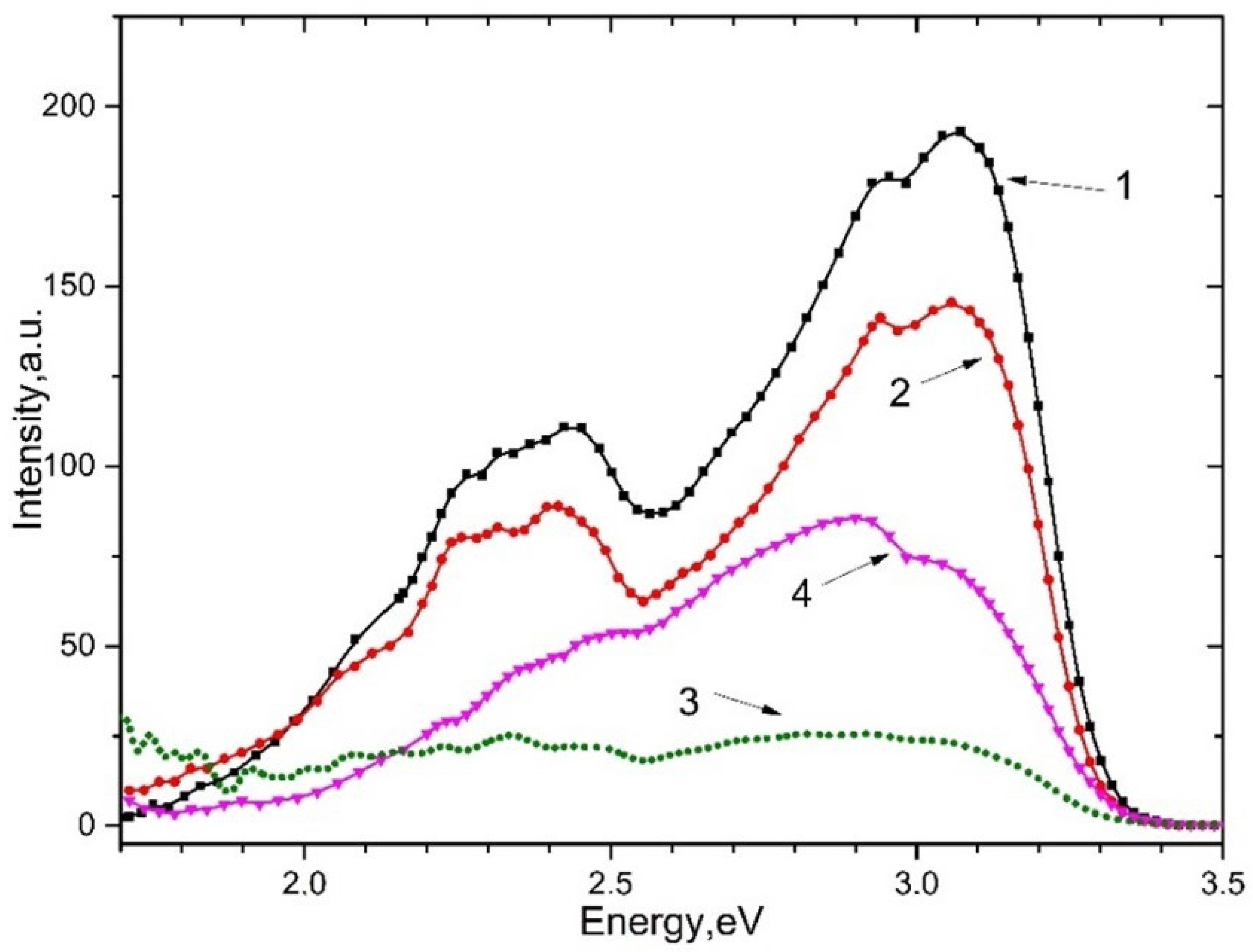
The photoluminescence of and irradiated (curves 1 and 4) and unirradiated (curves 2 and 3) with x-rays upon excitation by 5.6 eV photons at a temperature of 80 K is shown in Figure 5. It can be seen that emission associated with with an impurity of at ~2.3–2.4 eV and emission bands at ~2.95 eV and ~3.1 eV are arisen. The emission bands at 2.95 eV and 3.1 eV refer to intrinsic and impurity electron-hole trapping centers.
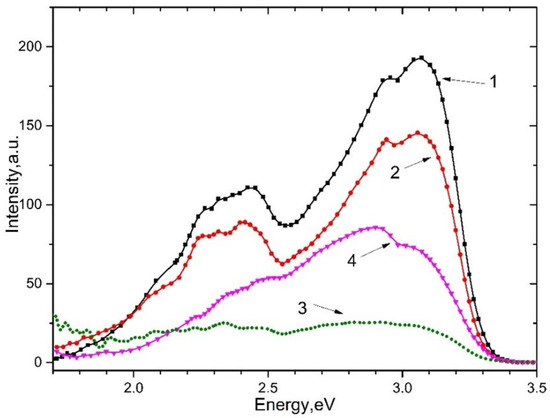
Figure 5.
The emission spectrum of crystals upon excitation of 5.6 eV, 80 K. For (curve 2) and (curve 3) and pre-irradiated with X-rays for 10 min (curve 1 and 4, respectively).
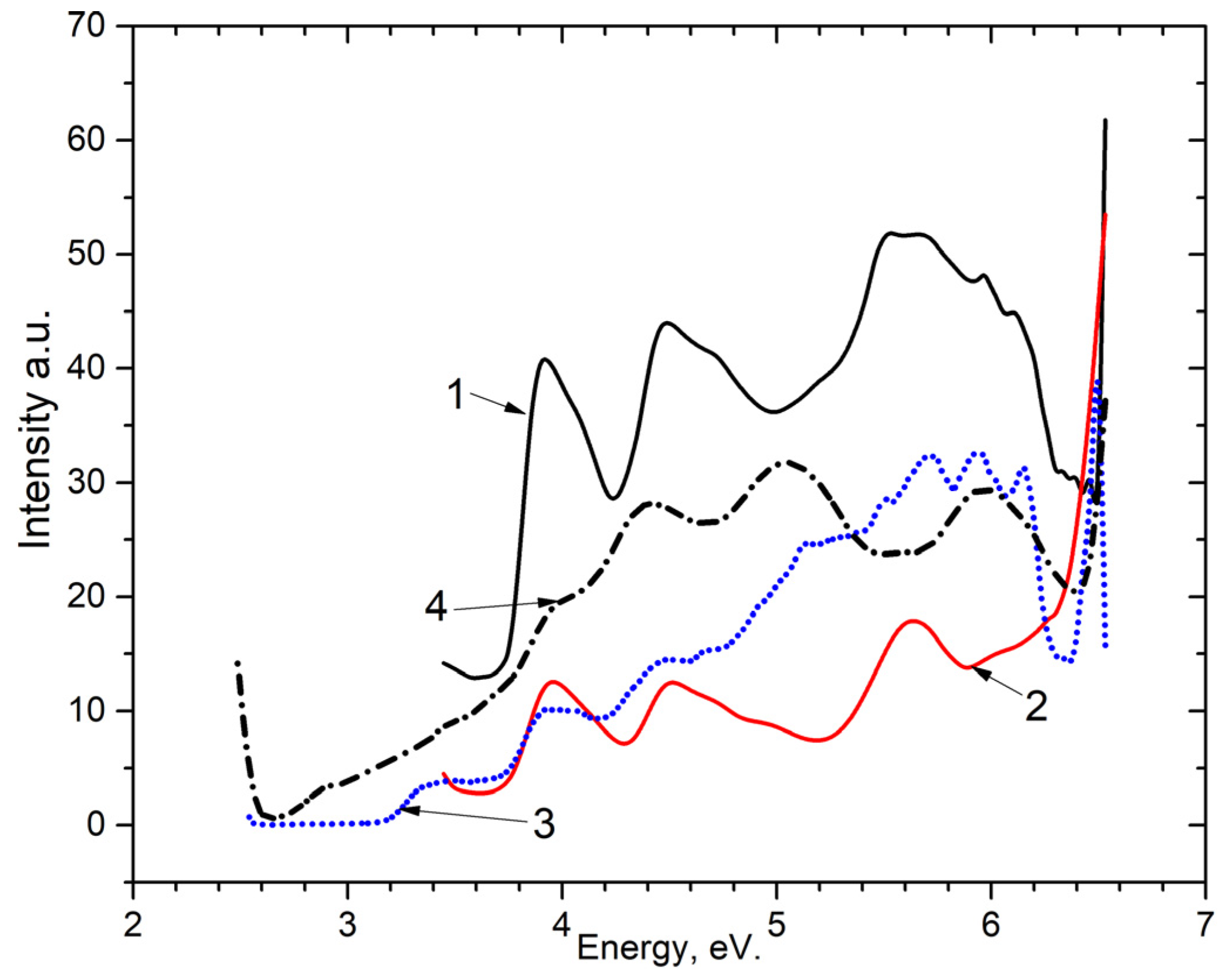
Following this, an analysis on the excitation spectra (as shown in Figure 6) of the emission center of Mn2+ impurity for the 2.3–2.4 eV band at 80 K, for (curve 2) and (curve 1) was conducted. As can be observed, excitation occurs in three spectral ranges: near 3.35 eV, 4.0 eV, 4.5 eV and the spectral interval 5.0–6.2 eV. The fundamental spectral area of the host is defined as 5.0–6.2 eV. New electron-hole trapping centers are formed in this region.
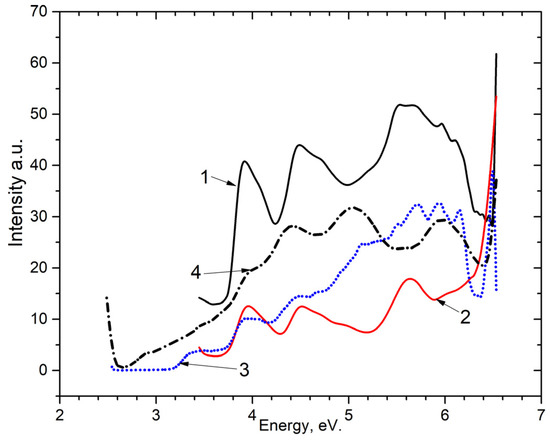
Figure 6.
Excitation spectra in 1—for the emission band of 2.3–2.4 eV at 80 K in BaSO4-Mn; 2—for the emission band of 2.3–2.4 eV at 80 K in CaSO4-Mn; 3—for the emission band 3.1 eV at 80 K in BaSO4-Mn; 4—for the emission band 2.7 eV at 80 K in BaSO4-Mn.
Figure 6 also displays the powder’s recombination emission excitation spectra at 3.1 eV (curve 3) and 2.7 eV (curve 4). The excitation bands observed in the ~4.0 eV and ~4.5 eV was found to be analogous to pure samples (as shown in Figure 2 and Figure 3).
It was experimentally shown that the excitation spectra of 4.0 eV and 4.5 of the recombination emission of an electron-hole trapping center in and coincide with the excitation energies of the Mn2+ impurity in these hosts.
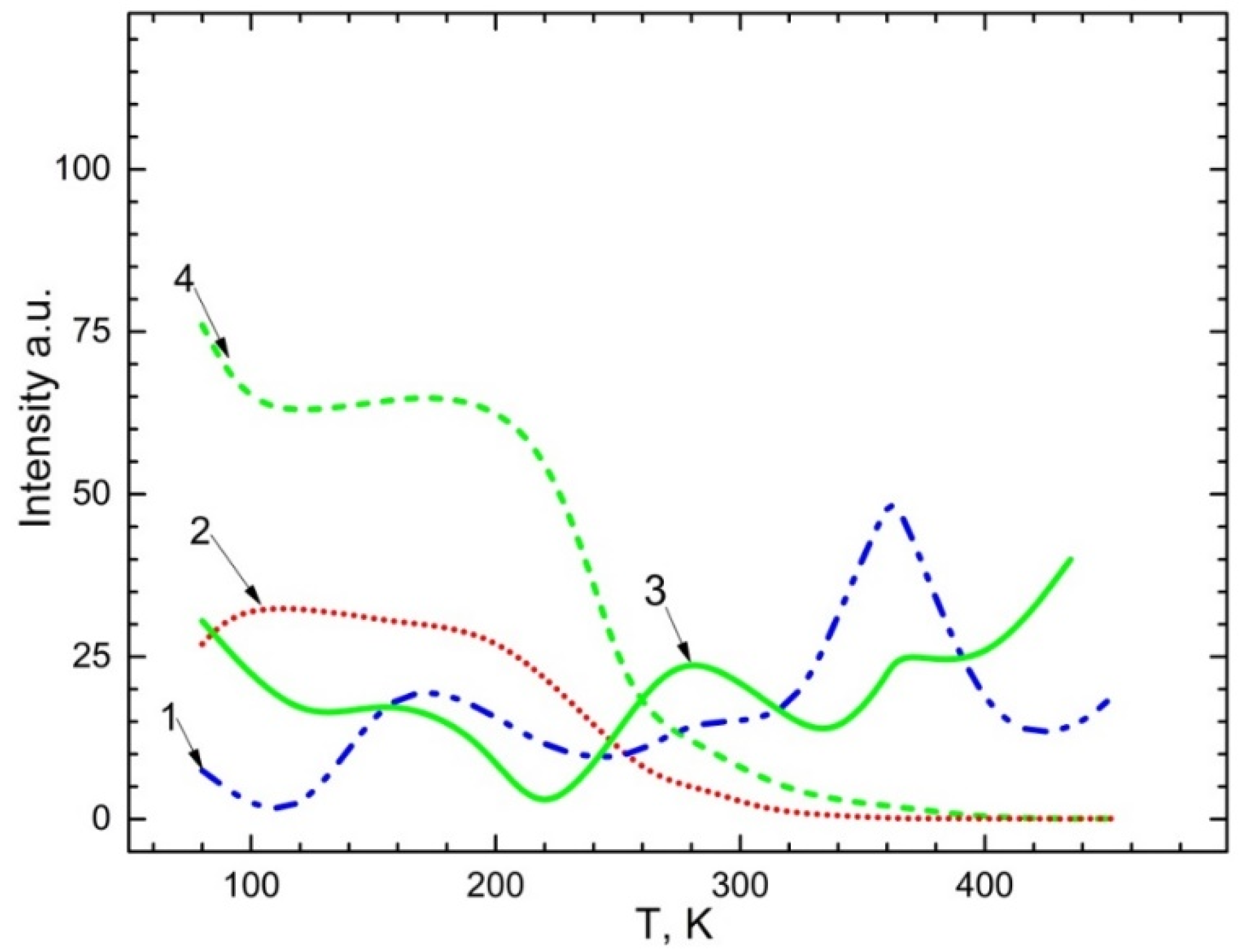
The temperature dependency of the emission spectra of 2.3–2.4 eV Mn2+ impurities as well as 2.95 eV and 3.1 eV recombination emissions are illustrated in Figure 7. The exciting energy of bands was 4.5 eV and 5.6–5.9 eV for CaSO4-Mn and BaSO4-Mn. From Figure 7, one could observe that:
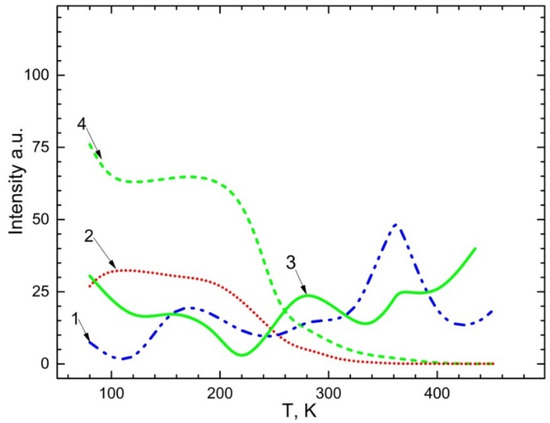
Figure 7.
Temperature dependence for emission bands: 1—2.3–2.4 eV upon excitation of 5.9 eV ; 2—2.95–3.1 eV upon excitation of 4.5 eV in ; 3—2.95–3.1 eV upon excitation of 5.9 eV ; 4—2.3–2.4 eV upon excitation of 4.5 eV .
(a) In CaSO4- Mn and BaSO4-Mn crystals, emission ~2.95 eV and 3.1 eV are steady up to 200–220 K (curve 4, 2). The band’s intensity starts to decline at a temperature of 200–220 K. It is presumable that after the electron delocalizes from the Mn+ trapping centers at this temperature. The intensity of the recombination emission band gradually diminishes until it reaches a minimum value.
(b) the impurity is ionized from the trapping centers in accordance with the following reaction: , i.e., the Mn2+ impurity is restored (curves 1, 3); the intensity of the emission band ~2.3–2.4 eV corresponding to the emission of Mn2+ increases. The delocalization of holes from centers, which occurs in the temperature range of 350–360 K, is linked to an increase in the Mn2+ impurity’s emission intensity.
4. Discussion
The excitation spectrum of recombination emission at 2.95–3.1 eV and 2.6–2.7 eV was measured. It is shown that emission is excited at photon energies of 4.5 eV and 4.0 eV. Under reverse excitation of CaSO4 and BaSO4 samples with induced trapping centers, with photon energy of 4.5 eV and 4.0 eV recombination emission 2.95–3.1 eV and 2.6–2.7 eV are detected. Based on the obtained results, a mechanism of the formation of trapping centers is proposed. We propose a band scheme for the arrangement of local states of trapping centers. Electron trapping centers are produced in accordance with the reaction when electrons are trapped by anionic complexes or during charge transfer during excitation of the anionic complex . The hole excitation component is localized in the form of the radical . The formation of the radical in irradiation sulfates was established by the authors of [23] using the EPR technique. This is how electron and hole trapping centers are formed in the form . The trapping centers correspond to recombination emission.
Based on theoretical calculations by the authors of [24], it was predicted that holes exist in various local states from the top of the valence band. These calculations revealed that the ground state of the unpaired electron in the radical will differ in each of the three crystallographic directions. Additionally, experimental evidence shows that the thermal decollation of a hole of two types in CaSO4 occurs at various temperatures [25]. All these data indicate the existence of three local states from the top of the valence band, corresponding to localized holes —differ—crystallographic directions in the transparency region of the crystal. As a result, the produced holes are localized at distinct distances of 3.35 eV, 4.0 eV, and 4.5 eV from the local level of electronic trapping centers.
The authors of [18,19,20] studied the mechanisms of energy transfer to impurities in alkali metal sulfates, in activated and crystals. The excitation spectra of impurities and intrinsic recombination emissions of the host were measured. In these and our previous works, the relation between the excitation spectra of the recombination emission of the host and impurities was not specified.
It is assumeI that in the irradiated crystals and powders of CaSO4-Mn and BaSO4-Mn in the spectral region of 2.95–3.1 eV, corresponding to the recombination emission of the host, a combined band appears, including the emission of its intrinsic recombination emission and the emission arising on impurity electron-hole trapping centers. The combined emission band 2.95–3.1 eV is excited in the same way at photon energies of 3.9–4.0 eV and 4.5–4.6 eV as shown on Figure 6. It is assumed that in the CaSO4-Mn and BaSO4-Mn powders irradiated with UV photons, upon excitation of the anionic complex, a combined radiative state of 2.95–3.1 eV is created by two mechanisms:
- -
- during charge transfer from oxygen to impurities ();
- -
- when electron-hole pairs are trapped by impurities.
In both cases, an impurity electron-hole state is created.
Parallel in the host:
- -
- when charge is transferred from oxygen () to the next to anionic complex , intrinsic electron-hole trapping centers are created near the impurity;
- -
- when an electron is captured by an anionic complex and a hole is localized in the form of , similar capture centers can be created.
Recombination decays of emerging trapping centers occur:
during the decay of , emission of 2.95–3.1 eV occurs;
during the decay of , an electron recombines with a hole located near the impurity and the energy of the recombination process excite impurities, emission of impurities is observed at 2.3–2.4 eV.
The formation of combined states 2.95–3.1 eV appear during the measurement of the temperature dependence of the recombination emission band and the intracenter emission of . At a temperature of 220–250 K, where electron delocalization from centers occurs, an increase in the intensity of the intra-center emission band corresponding to ions () is observed.
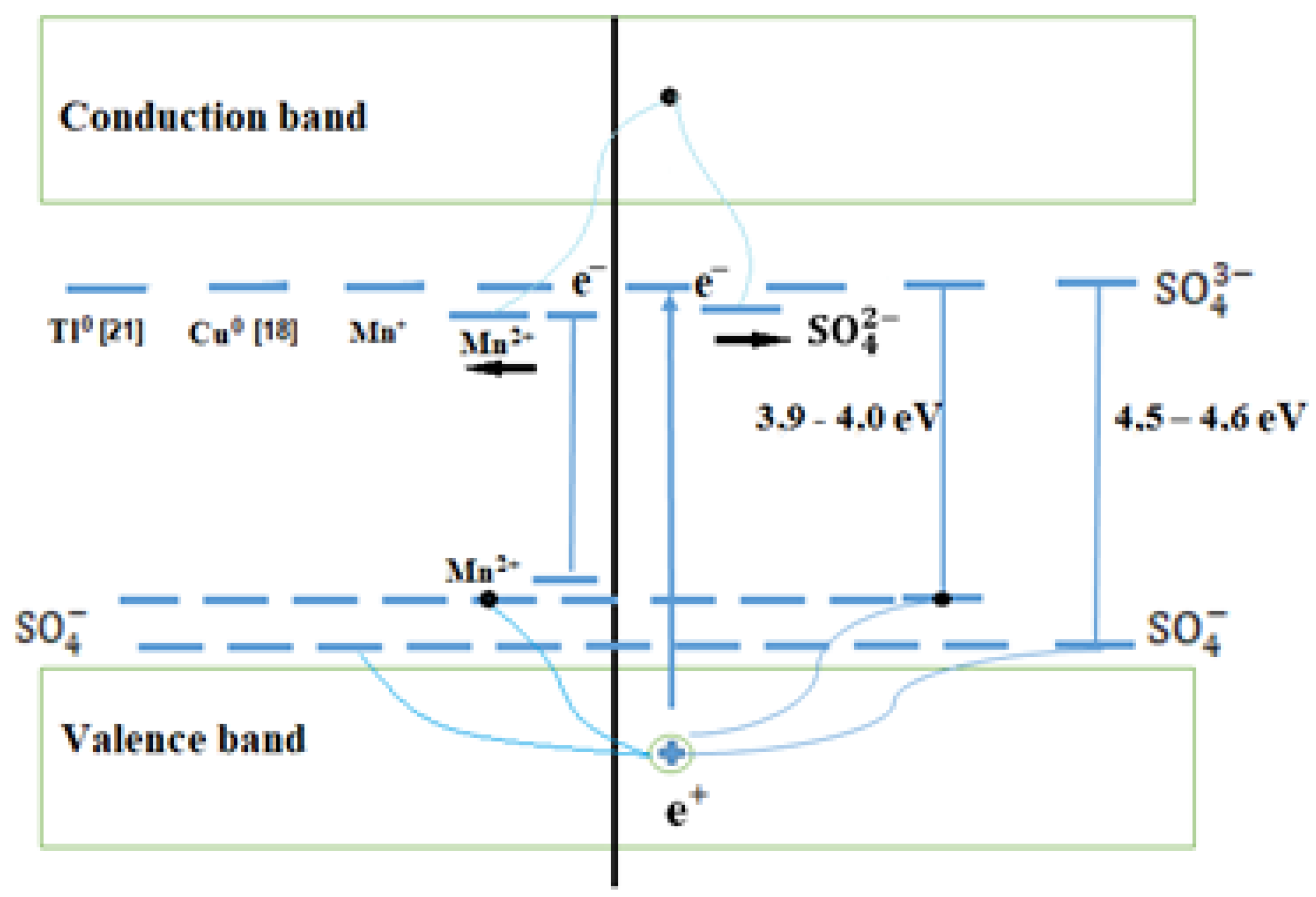
The exhibition of combined states is also characteristic of other alkali metal sulfates activated by Cu+ and Tl+ impurities. We have shown the formation of such states in the band diagram in Figure 8.
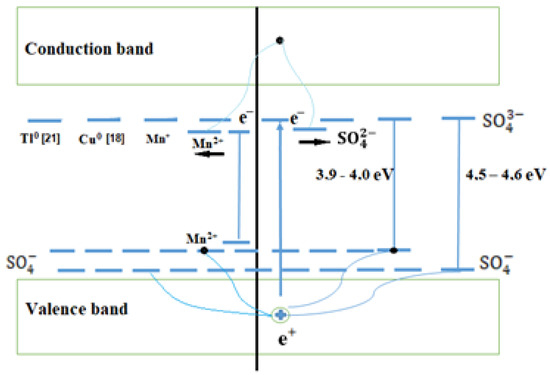
Figure 8.
Band scheme of impurity ) and intrinsic electron and hole trapping centers.
We have experimentally shown that impurity emission at 2.3–2.4 eV and recombination emission of 2.95–3.1 eV are excited at the same energies 4.0 eV and 4.5 eV. The pattern of formation of electron and hole trapping centers with different energy states in the transparency region of the host should be a characteristic feature of sulfates of alkali and alkaline earth metals. A distinctive characteristic of these hosts is the creation of Tl0, Cu0, , and Mn+ electronic trapping centers in both pure and doped sulfates, which possess local radiative energy states of approximately 2.95–3.17 eV.
5. Conclusions
- 1.
- The charge transfer by anionic complexes occurs during the excitation of . Trap centers are created as a result of charge transfer from . The alternative mechanism of formation occurs as trapping free electrons on .
- 2.
- Energy transfer to impurities occurs at the time of charge transfer . A common combined electronic state of 2.95–3.1 eV is created. The combined radiative state 2.95–3.1 eV consists of the radiative levels of and trapping centers.
- 3.
- In sulfates with Tl+, Cu+, and Mn2+ impurities, the combined radiative state 2.95–3.17 eV is formed during charge transfer (M-metall).
- 4.
- In sulfates, anionic complexes are excited mainly near impurities.
Author Contributions
Conceptualization, T.N.N. and A.M.Z.; methodology, A.Z.K.; software, T.T.A.; validation, D.H.D., A.Z.K. and B.M.S.; formal analysis, D.A.T.; investigation, R.K.S.; resources, K.B.Z.; data curation, D.H.D.; writing—original draft preparation, T.T.A.; writing—review and editing, T.T.A.; visualization, B.M.S.; supervision, T.T.A.; project administration, A.M.Z.; funding acquisition, D.H.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Science Committee of Ministry of Education and Science Republic of Kazakhstan, grant number AP09259303.
Data Availability Statement
The data that support the findings of this study are available upon reasonable request from the authors.
Acknowledgments
This work was supported by the Science Committee of Ministry of Education and Science Republic of Kazakhstan grants IRN No. AP09259303.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Jamkhaneh, K.B.; Saraee, K.R.E. Thermoluminescence characterization of nanocrystalline powder of SrSO4: Sm exposed to gamma radiation for dosimetric applications. Appl. Radiat. Isot. 2020, 160, 109128. [Google Scholar] [CrossRef]
- Bahl, S.; Kumar, V.; Bihari, R.R.; Kumar, P. Investigations of OSL properties of CaSO4: Mn phosphor exposed to gamma and beta radiations. J. Lumin. 2017, 181, 36. [Google Scholar] [CrossRef]
- Tang, Q.; Tang, H.; Luo, D.; Zhang, C.; Guo, J.; Wu, H. Thermoluminescence spectra and dose responses of SrSO4 phosphors doped with rare earths (Eu, Dy, Tm) and phosphorus. Rad. Protec. Dos. 2019, 187, 164. [Google Scholar] [CrossRef] [PubMed]
- Plekhanov, V.G.; Os’minin, V.S. Issledovaniye spektrov otrazheniya i lyuminestsentsii kristallov sul’fata kaliya pri nizkoy temperature. OiS 1975, 38, 120–123. [Google Scholar]
- Zhai, B.G.; Xu, H.; Zhang, Q.; Huang, Y.M. Blue Photoluminescence and Cyan-Colored Afterglow of Undoped SrSO4 Nanoplates. ACS Omega 2021, 6, 10129. [Google Scholar] [CrossRef] [PubMed]
- Boroznovskaya, N.N.; Zyryanova, L.A.; Pekov, I.V. Luminescent properties of natural barite: Evidence for its genesis. Dokl. Earth Sci. 2016, 471, 1171. [Google Scholar]
- Rao, T.G.; Bhatt, B.C.; Srivastava, J.K.; Nambi KS, V. On the sulphoxy radicals in CaSO4: Dy, Na thermoluminescent phosphor: Electron paramagnetic resonance studies. J. Phys. Condens. Matter 1993, 5, 1791. [Google Scholar] [CrossRef]
- Petö, Á.; Kelemen, A.; Ötvös, N. Radioluminescence characteristics of CaSO4:Dy, Cu. J. Lumin. 1997, 72, 778. [Google Scholar] [CrossRef]
- Morgan, M.D.; Stoebe, T.G. Optical absorption and luminescent processes in thermoluminescent CaSO4:Dy. J. Phys. Condens. Matter 1989, 1, 5773. [Google Scholar] [CrossRef]
- Okada, G.; Hirasawa, K.; Kusano, E.; Yanagida, T.; Nanto, H. Radio-photoluminescence properties of samarium-doped alkaline earth sulfates. NIMP B 2020, 466, 56. [Google Scholar] [CrossRef]
- Ávila, O.; Ramírez-Barbosa, E.; Gamboa-deBuen, I. Energy dependence of TLD-900 dosimeters exposed to low energy X-rays. Rad. Meas. 2014, 71, 127–132. [Google Scholar] [CrossRef]
- Niroomand-Rad, A.; DeWerd, L.A. The application of CaSO4:Dy (TLD-900) to diagnostic x-ray exposures. Med. Phys. 1983, 10, 691–694. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.; Wang, M.; Yang, Z.; Wang, H.; Padhiar, M.A.; Qiu, H.; Dang, J.; Miao, Y.; Zhou, Y.; Bhatti, A.S. Strong violet emission from ultra-stable strontium-doped CsPbCl3 superlattices. Nanoscale 2022, 14, 2359–2366. [Google Scholar] [CrossRef]
- Omanwar, S.K.; Palan, C.B. Synthesis and preliminary OSL studies of Ce3+ activated calcium sulfate (CaSO4) for radiation dosimetry. J. Mater. Sci. Mater. Electron. 2018, 29, 7388. [Google Scholar] [CrossRef]
- Yüksel, M.; Dogan, T.; Balci-Yegen, S.; Akca, S.; Portakal, Z.G.; Kucuk, N.; Topaksu, M. Heating rate properties and kinetic parameters of thermoluminescence glow curves of La-doped zinc borate. Radiat. Phys. Chem. 2019, 148, 197. [Google Scholar] [CrossRef]
- Beaugnon, F.; Quiligotti, S.; Chevreux, S.; Wallez, G. On the monoclinic distortion of β-anhydrite CaSO4. Solid State Sci. 2020, 108, 106399. [Google Scholar] [CrossRef]
- Nurakhmetov, T.N.; Salikhodzha, Z.M.; Zhunusbekov, A.M.; Kainarbay, A.Z.; Daurenbekov, D.H.; Alibay, T.T.; Sadykova, B.M.; Zhangylyssov, K.B.; Yussupbekova, B.; Tolekov, D.A. Intrinsic emission and electron-hole trapping centers in irradiated Na2SO4. Optik 2021, 242, 167081. [Google Scholar] [CrossRef]
- Yussupbekova, B.N.; Nurakhmetov, T.N.; Salikhodzha, Z.M.; Zhunusbekov, A.M.; Kainarbay, A.Z.; Daurenbekov, D.H.; Sadykova, B.M.; Zhangylyssov, K.B. Intrinsic and impurity emission and formation mechanism of trapping centers in LiKSO4-Cu crystals. NIMP B. 2020, 481, 19–23. [Google Scholar] [CrossRef]
- Nurakhmetov, T.N.; Salikhodzha, Z.M.; Zhunusbekov, A.M.; Kainarbay, A.Z.; Daurenbekov, D.H.; Alibay, T.T.; Sadykova, B.M.; Zhangylyssov, K.B.; Yussupbekova, B.N.; Tolekov D., A. Mechanisms formation of electron hole trap centers in LiKSO4 crystall. Eur. J. Ph. Fun. Mat. 2021, 5, 24–30. [Google Scholar] [CrossRef]
- Nurakhmetov, T.N.; Alibay, T.T.; Pazylbek, S.; Zhunusbekov, A.M.; Sadykova, B.M.; Tolekov, D.A.; Shamieva, R.K.; Nurpeissov, A.S. Electron-hole trapping centers in Na2SO4 with a transition metal impurity Mn. Eur. J. Ph. Fun. Mat. 2023, 7, 38–44. [Google Scholar] [CrossRef]
- Osminin, V.S.; Plekhanov, V.G.; Silkin, N.I. Recombination processes in potassium sulfate with thallium as an impurity. J. Appl. Spectrosc. 1974, 21, 908. [Google Scholar] [CrossRef]
- Zhou, R.; Lin, L.; Liu, C.; Dorenbos, P.; Tao, Y.; Huang, Y.; Liang, H. Insight into Eu redox and Pr3+ 5d emission in KSrPO4 by VRBE scheme construction. Dalton Trans. 2018, 47, 306–313. [Google Scholar] [CrossRef]
- Byberg, J.R. O—Detected by ESR as a primary electron-excess defect in x-irradiated K2SO4. J. Chem. Phys. 1986, 84, 6083–6085. [Google Scholar] [CrossRef]
- Nair, S.R.; Kondawar, V.K.; Upadeo, S.V.; Moharil, S.V.; Gundurao, T.K. Redox reactions, radio-photoluminescence and thermoluminescence in CaSO4-Eu. J. Ph. Con. Mat. 1997, 9, 8307. [Google Scholar] [CrossRef]
- Danby, R.J.; Boas, J.F.; Calvert, R.L.; Pilbrow, J.R. ESR of thermoluminescent centres in CaSO4 single crystals. J. Ph. C Sol. St. Ph. 1982, 15, 2483. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).