Structure and Anharmonicity of α- and β-Sb2O3 at Low Temperature
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| ADP | atomic displacement parameter |
| BVS | bond valence sum |
| NIS | nuclear inelastic scattering |
| TOF | time-of-flight |
| SEM | scanning electron microscopy |
| SE | secondary electron |
| EDS | energy dispersive spectroscopy |
| SNS | Spallation Neutron Source |
| ORNL | Oak Ridge National Laboratory |
References
- Bozorth, R.M. The Crystal Structures Of The Cubic Forms Of Arsenious And Antimonious Oxides. J. Am. Chem. Soc. 1923, 45, 1621–1627. [Google Scholar] [CrossRef]
- Buerger, M.J.; Hendricks, S.B. The Crystal Structure of Valentinite (Orthorhombic Sb2O3). Z. Kristallogr. Cryst. Mater. 1938, 98, 1–30. [Google Scholar] [CrossRef]
- Orosel, D.; Dinnebier, R.E.; Blatov, V.A.; Jansen, M. Structure of a new high-pressure–high-temperature modification of antimony(III) oxide, γ-Sb2O3, from high-resolution synchrotron powder diffraction data. Acta. Crystallogr. B 2012, 68, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Kokkalas, D.E.; Bikiaris, D.N.; Karayannidis, G.P. Effect of the Sb2O3 catalyst on the solid-state postpolycondensation of poly(ethylene terephthalate). J. Appl. Polym. Sci. 1995, 55, 787–791. [Google Scholar] [CrossRef]
- Venkateshwarlu, G.; Rajanna, K.C.; Saiprakash, P.K. Antimony Trioxide as an Efficient Lewis Acid Catalyst for the Synthesis of 5-Substituted 1H-Tetrazoles. Synth. Commun. 2009, 39, 426–432. [Google Scholar] [CrossRef]
- Shaw, J.B. Antimony oxide as an opacifier in cast iron enamels. J. Am. Chem. Soc. 1918, 1, 502–513. [Google Scholar] [CrossRef]
- Pavlish, A.E.; O’Bannon, L.S. An Investigation of Antimony Oxide as an Opacifier for Porcelain Enamels and Glass. Science 1952, 116, 196–200. [Google Scholar] [CrossRef]
- Naidu, B.; Pandey, M.; Sudarsan, V.; Vatsa, R.; Tewari, R. Photoluminescence and Raman spectroscopic investigations of morphology assisted effects in Sb2O3. Chem. Phys. Lett. 2009, 474, 180–184. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, H.; Yang, K.; Yang, Y.; Ma, J.; Pan, K.; Wang, G.; Ren, F.; Pang, H. Enhanced Electrochemical Performance of Sb2O3 as an Anode for Lithium-Ion Batteries by a Stable Cross-Linked Binder. Appl. Sci. 2019, 9, 2677. [Google Scholar] [CrossRef]
- Hall, D.W.; Newhouse, M.A.; Borrelli, N.F.; Dumbaugh, W.H.; Weidman, D.L. Nonlinear optical susceptibilities of high-index glasses. Appl. Phys. Lett. 1989, 54, 1293–1295. [Google Scholar] [CrossRef]
- Chagraoui, A.; Yakine, I.; Tairi, A.; Moussaoui, A.; Talbi, M.; Naji, M. Glasses formation, characterization, and crystal-structure determination in the Bi2O3–Sb2O3–TeO2 system prepared in an air. J. Mater. Sci. 2011, 46, 5439–5446. [Google Scholar] [CrossRef]
- Petkova, P.; Soltani, M.T.; Petkov, S.; Tacheva, J.; Nedkov, V. Visible and far-infrared spectroscopic studies of Co-doped (80–x)Sb2O3–20Na2O–xWO3 glasses. Phys. Scr. 2012, T149, 014057. [Google Scholar] [CrossRef]
- Tolborg, K.; Gatti, C.; Iversen, B.B. Expression and interactions of stereochemically active lone pairs and their relation to structural distortions and thermal conductivity. IUCrJ 2020, 7, 480–489. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, M.D.; Ozolins, V.; Heremans, J.P. Lone pair electrons minimize lattice thermal conductivity. Energy Environ. Sci. 2013, 6, 570–578. [Google Scholar] [CrossRef]
- Walsh, A.; Payne, D.J.; Egdell, R.G.; Watson, G.W. Stereochemistry of post-transition metal oxides: Revision of the classical lone pair model. Chem. Soc. Rev. 2011, 40, 4455–4463. [Google Scholar] [CrossRef] [PubMed]
- Sans, J.A.; Manjón, F.J.; Pereira, A.L.d.J.; Ruiz-Fuertes, J.; Popescu, C.; Muñoz, A.; Rodríguez-Hernández, P.; Pellicer-Porres, J.; Cuenca-Gotor, V.P.; Contreras-García, J.; et al. Unveiling the role of the lone electron pair in sesquioxides at high pressure: Compressibility of β-Sb2O3. Dalton Trans. 2021, 50, 5493–5505. [Google Scholar] [CrossRef] [PubMed]
- Orman, R.G. Phase Transitions in Antimony Oxides and Related Glasses. Ph.D. Thesis, University of Warwick, Coventry, UK, 2005. [Google Scholar]
- Orman, R.; Holland, D. Thermal phase transitions in antimony (III) oxides. J. Solid State Chem. 2007, 180, 2587–2596. [Google Scholar] [CrossRef]
- Roberts, E.J.; Fenwick, F. The antimony-antimony trioxide electrode and its use as a measure of acidity. J. Am. Chem. Soc. 1928, 50, 2125–2147. [Google Scholar] [CrossRef]
- Golunski, S.; Nevell, T.; Pope, M. Thermal stability and phase transitions of the oxides of antimony. Thermochim. Acta 1981, 51, 153–168. [Google Scholar] [CrossRef]
- Trofimov, V.; Sheinkman, A.; Kleshchev, G. Antimony pentoxide in the crystalline state. Izv. Vyssh. Uchebn. Zaved. Fiz. 1973, 3, 135–137. [Google Scholar] [CrossRef]
- White, W.; Dachille, F.; Roy, R. High-pressure polymorphism of As2O3 and Sb2O3. Z. Kristallogr. 1967, 125, 450–458. [Google Scholar] [CrossRef]
- Cody, C.A.; DiCarlo, L.; Darlington, R.K. Vibrational and thermal study of antimony oxides. Inorg. Chem. 1979, 18, 1572–1576. [Google Scholar] [CrossRef]
- Agrawal, Y.; Shashimohan, A.; Biswas, A. Studies on antimony oxides: Part I thermal analysis of Sb2O3 in air, nitrogen and argon. J. Therm. Anal. 1975, 7, 635–641. [Google Scholar] [CrossRef]
- Jones, S.; Fenerty, J.; Pearce, J. The enantiotropic phase transition of antimony(III) oxide. Thermochim. Acta 1987, 114, 61–66. [Google Scholar] [CrossRef]
- Centers, P.W. Sublimation-controlled oxidation of antimony trioxide. J. Solid State Chem. 1988, 72, 303–308. [Google Scholar] [CrossRef]
- Han, W.; Huang, P.; Li, L.; Wang, F.; Luo, P.; Liu, K.; Zhou, X.; Li, H.; Zhang, X.; Cui, Y.; et al. Two-dimensional inorganic molecular crystals. Nat. Commun. 2019, 10, 4728. [Google Scholar] [CrossRef]
- Gopalakrishnan, P.; Manohar, H. Kinetics and mechanism of the transformation in antimony trioxide from orthorhombic valentinite to cubic senarmontite. J. Solid State Chem. 1975, 15, 61–67. [Google Scholar] [CrossRef]
- Jafari, A.; Klobes, B.; Sergueev, I.; Moseley, D.H.; Manley, M.E.; Dronskowski, R.; Deringer, V.L.; Stoffel, R.P.; Bessas, D.; Chumakov, A.I.; et al. Phonon Spectroscopy in Antimony and Tellurium Oxides. J. Phys. Chem. A 2020, 124, 7869–7880. [Google Scholar] [CrossRef]
- Coughlin, J.P. Contributions to the data on theoretical metallurgy. XII. heats and free energies of formation of inorganic oxides. US Bur. Mines Bull. 1954, 542. [Google Scholar]
- Vuković, M.; Branković, Z.; Poleti, D.; Rečnik, A.; Branković, G. Novel simple methods for the synthesis of single-phase valentinite Sb2O3. J. Solgel Sci. Technol. 2014, 72, 527–533. [Google Scholar] [CrossRef]
- Huq, A.; Hodges, J.P.; Gourdon, O.; Heroux, L. Powgen: A third-generation highresolution high-throughput powder diffraction instrument at the Spallation Neutron Source. In Proceedings of the European Powder Diffraction Conference, Darmstadt, Germany, 27–30 August 2010; Oldenbourg Wissenschaftsverlag: München, Germany, 2011; pp. 127–136. [Google Scholar] [CrossRef]
- Huq, A.; Kirkham, M.; Peterson, P.F.; Hodges, J.P.; Whitfield, P.S.; Page, K.; Hugle, T.; Iverson, E.B.; Parizzi, A.; Rennich, G. POWGEN: Rebuild of a third-generation powder diffractometer at the Spallation Neutron Source. J. Appl. Crystallogr. 2019, 52, 1189–1201. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Carvajal, J. Recent advances in magnetic-structure determination by neutron powder diffraction. Phys. B 1993, 192, 55–69. [Google Scholar] [CrossRef]
- Whitten, A.E.; Dittrich, B.; Spackman, M.A.; Turner, P.; Brown, T.C. Charge density analysis of two polymorphs of antimony(iii) oxide. Dalton Trans. 2004, 1, 23–29. [Google Scholar] [CrossRef]
- Pertlik, F. Strukturverfeinerung von Kubischem As2O3 (Arsenolith) mit Einkristalldaten. Czech. J. Phys. B 1978, 28, 170–176. [Google Scholar] [CrossRef]
- Sidey, V. On the accurate bond-valence parameters for the Sb3+/O2− ion pair. Acta Cryst. B 2010, 66, 307–314. [Google Scholar] [CrossRef] [PubMed]
- Bernert, A.; Chatterji, T.; Thalmeier, P.; Fulde, P. Structure determination, valence, and superexchange in the dimerized low temperature phase of α-NaV2O5. Eur. Phys. J. B 2001, 21, 535–546. [Google Scholar] [CrossRef]
- Locock, A.J.; Burns, P.C. Revised Tl(I)–O bond valence parameters and the structures of thallous dichromate and thallous uranyl phosphate hydrate. Z. Kristallogr. Cryst. Mater. 2004, 219, 259–266. [Google Scholar] [CrossRef]
- Mills, S.J.; Christy, A.G.; Chen, E.C.C.; Raudsepp, M. Revised values of the bond valence parameters for Sb(V)–O and Sb(III)–O. Z. Kristallog. 2009, 224, 423–431. [Google Scholar] [CrossRef]
- Ballirano, P.; Maras, A. Refinement of the crystal structure of arsenolite, As2O3. Z. Krist. 2002, 217, 177–178. [Google Scholar] [CrossRef]
- Cornei, N.; Tancret, N.; Abraham, F.; Mentré, O. New ϵ-Bi2O3 Metastable Polymorph. Inorg. Chem. 2006, 45, 4886–4888. [Google Scholar] [CrossRef]
- Kumada, N.; Kinomura, N. A New Allotropic Form of Bi2O3. Mat. Res. Soc. Symp. Proc. 1998, 547, 227–232. [Google Scholar] [CrossRef]
- Jansen, M.; Moebs, M. Structural investigations on solid tetraphosphorus hexaoxide. Inorg. Chem. 1984, 23, 4486–4488. [Google Scholar] [CrossRef]
- Zav’yalova, A.A.; Imamov, R.M. Special features of the crystal structure of bismuth oxides. J. Struct. Chem. 1973, 13, 811–814. [Google Scholar] [CrossRef]
- Guńka, P.A.; Dranka, M.; Hanfland, M.; Dziubek, K.F.; Katrusiak, A.; Zachara, J. Cascade of High-Pressure Transitions of Claudetite II and the First Polar Phase of Arsenic(III) Oxide. Cryst. Growth Des. 2015, 15, 3950–3954. [Google Scholar] [CrossRef]
- Guńka, P.A.; Hanfland, M.; Chen, Y.S.; Zachara, J. High-pressure and low-temperature structural study of claudetite I, a monoclinic layered As2O3 polymorph. CrystEngComm 2021, 23, 638–644. [Google Scholar] [CrossRef]

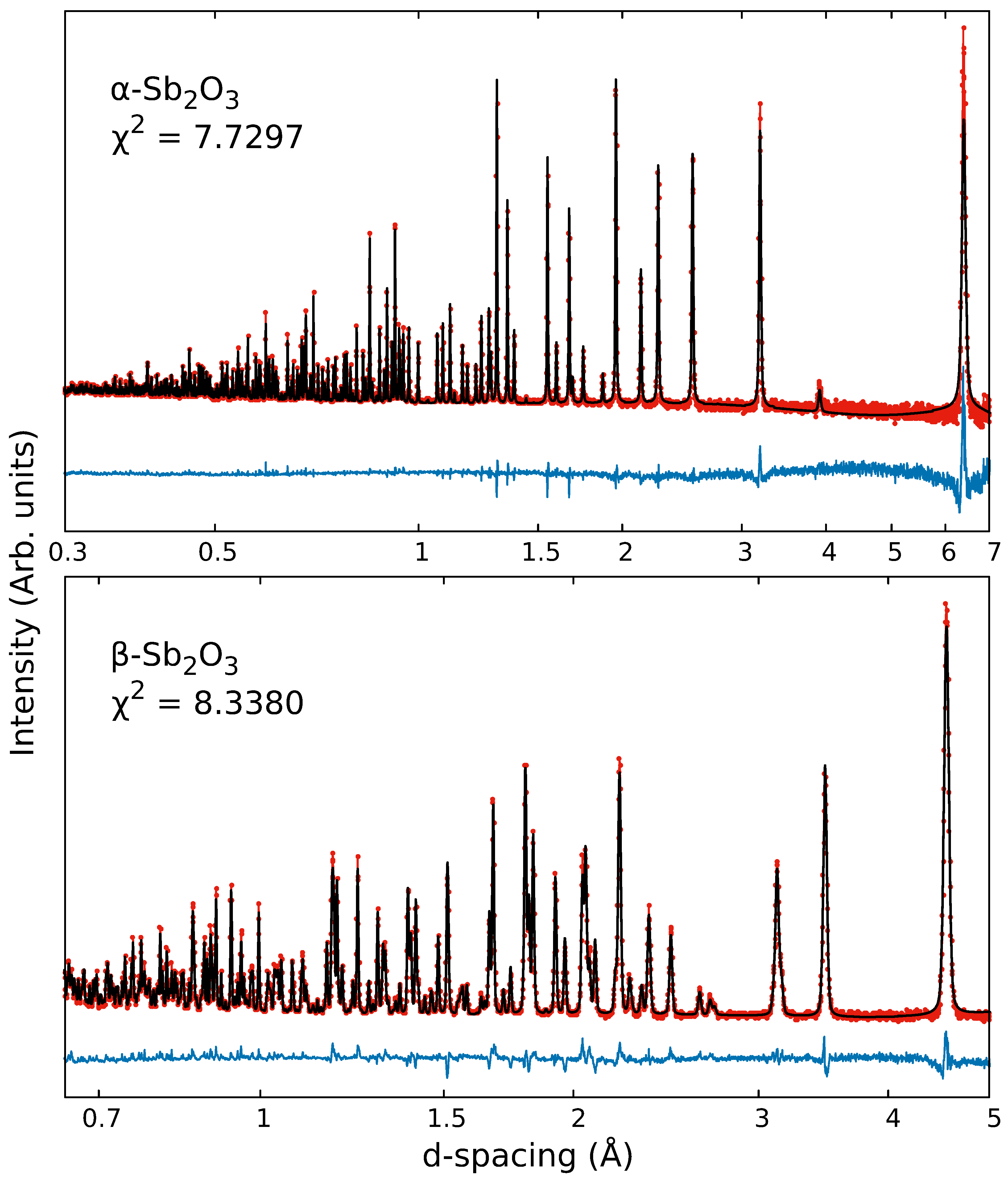
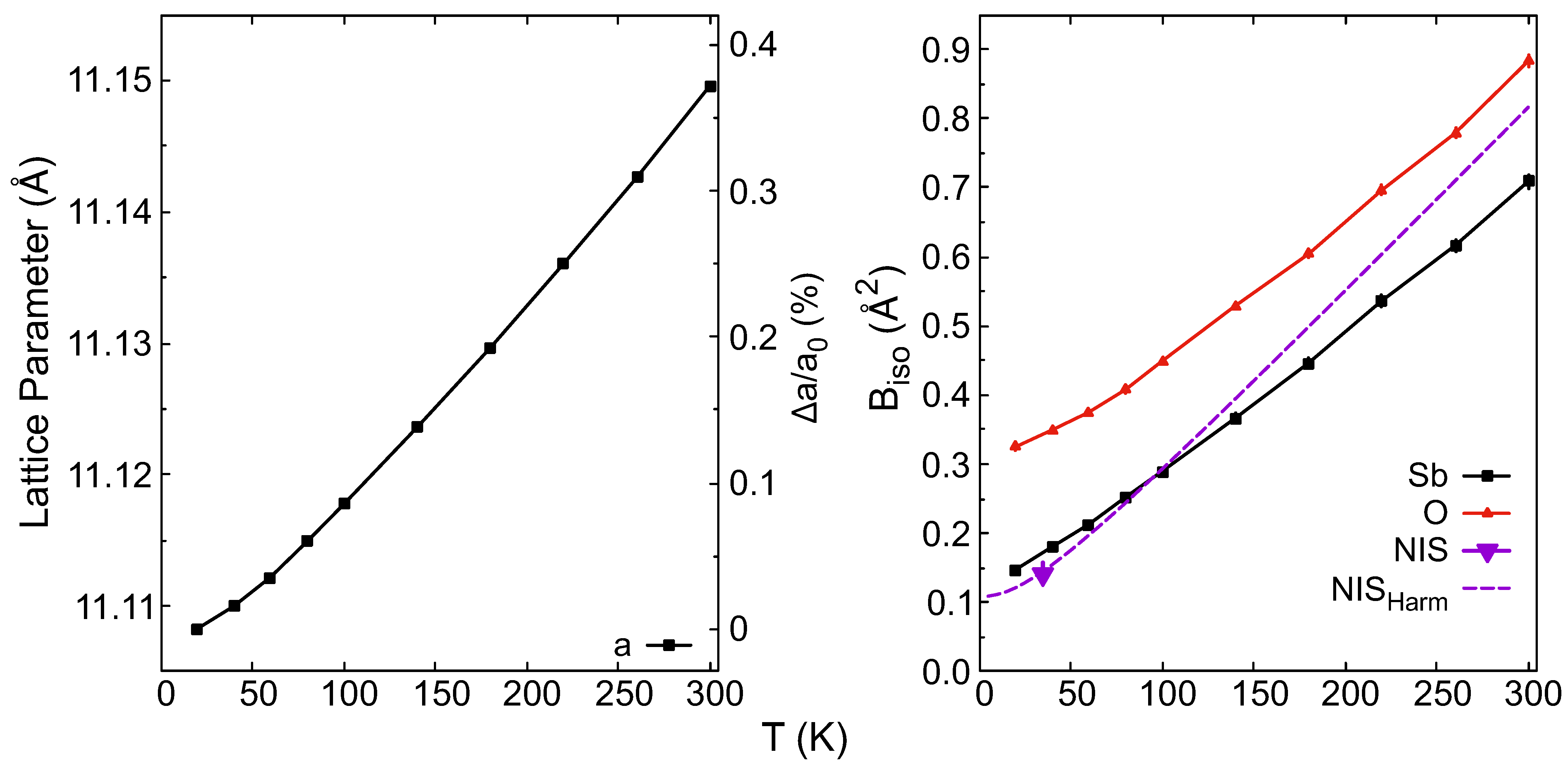
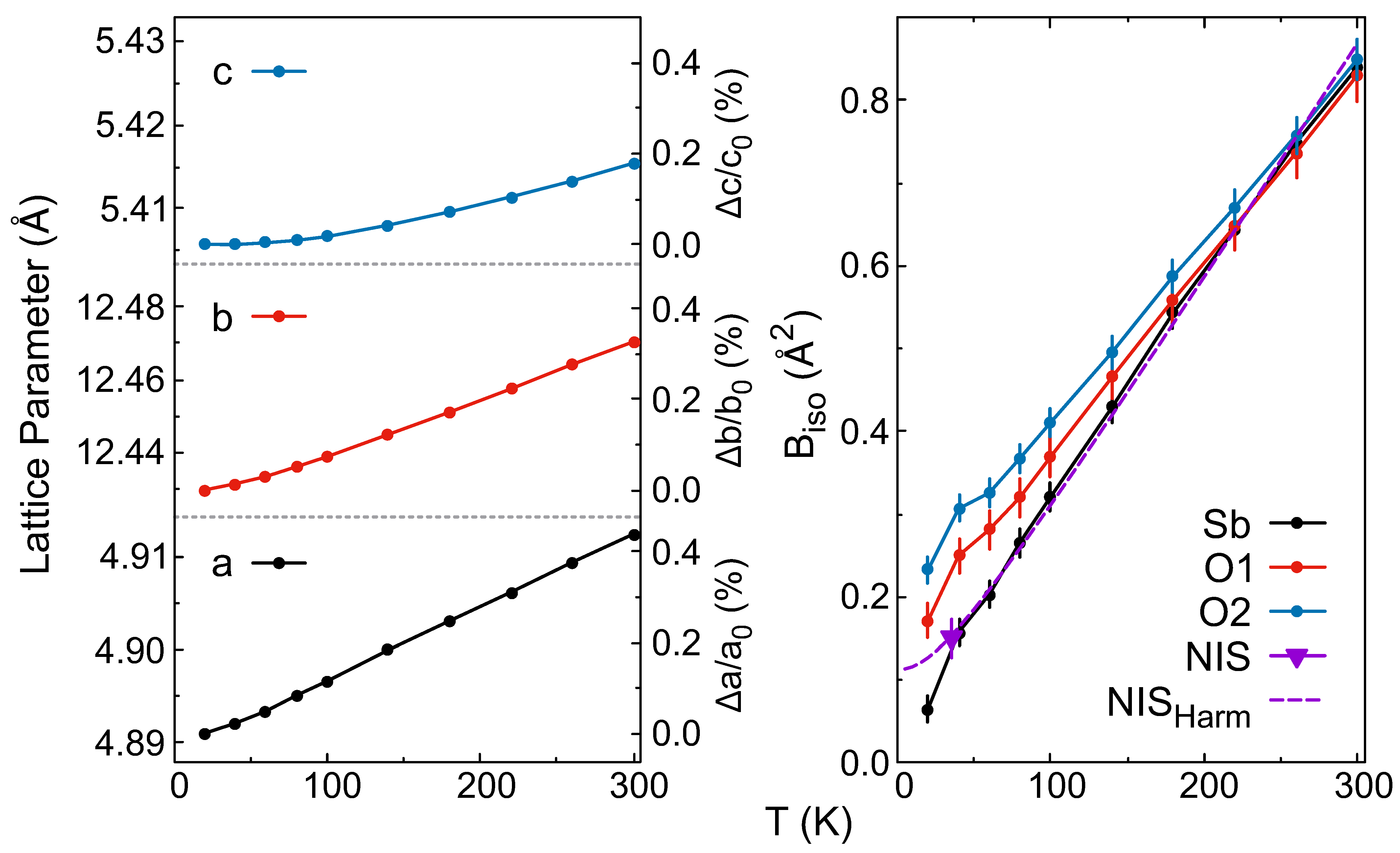
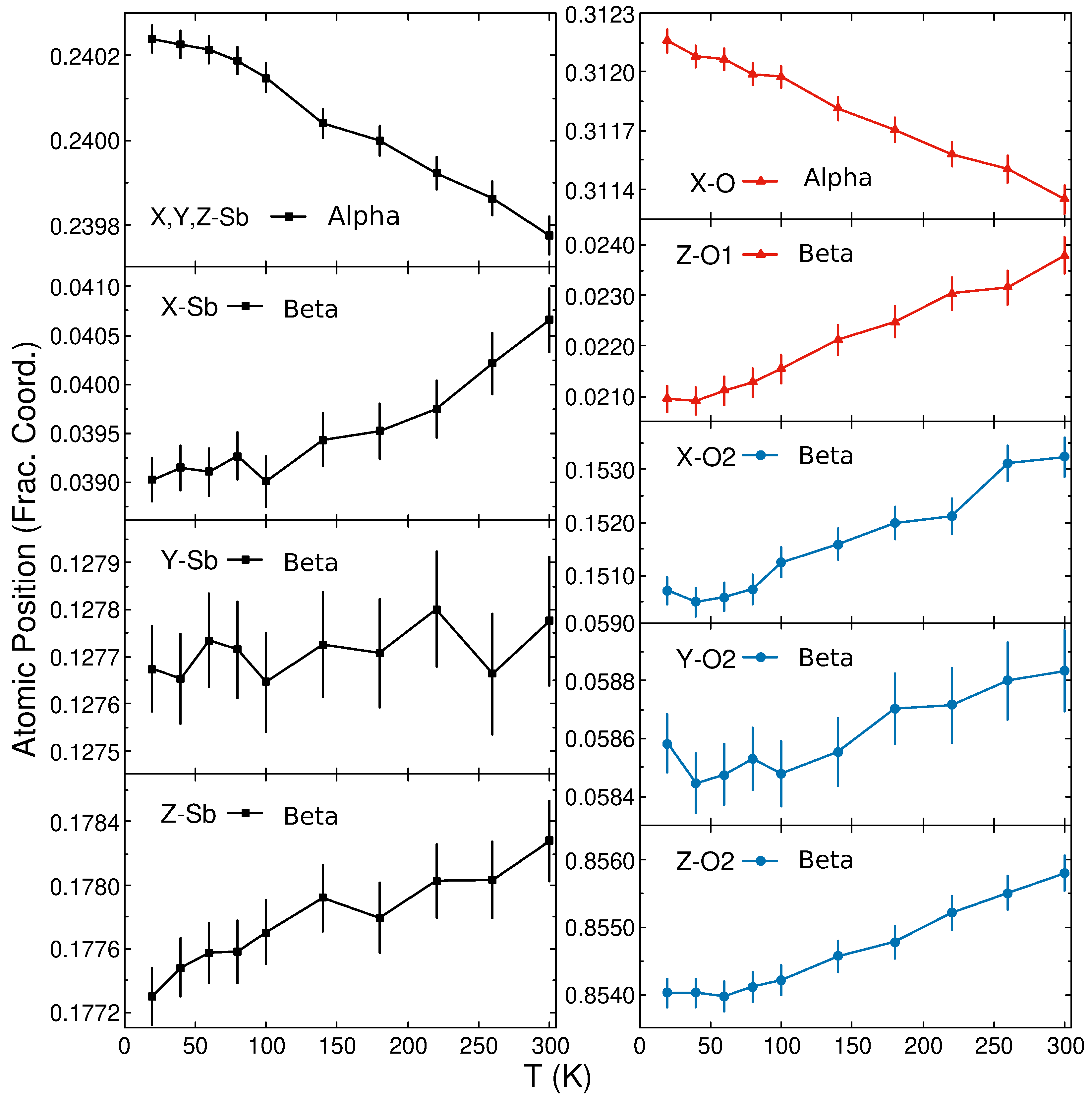
| 20 K | a (Å) | b (Å) | c (Å) | V (Å3) | Wyck. | X | Y | Z | (Å2) | |
| 11.108 (1) | 11.108 (1) | 11.108 (1) | 1370.685 (8) | Sb | 32e | 0.2402 (1) | 0.5098 (1) | 0.0098 (1) | 0.159 (5) | |
| O | 48f | 0.31216 (6) | 0.625 | 0.125 | 0.325 (4) | |||||
| 4.8910 (1) | 12.430 (1) | 5.4058 (1) | 328.637 (8) | Sb | 8e | 0.0390 (2) | 0.1277 (1) | 0.1773 (2) | 0.064 (15) | |
| O1 | 4c | 0.25 | 0.25 | 0.0210 (3) | 0.17 (2) | |||||
| O2 | 8e | 0.1507 (3) | 0.0586 (1) | 0.8540 (2) | 0.232 (15) | |||||
| 300 K | (Å) | (Å) | (Å) | (Å3) | Wyck. | (Å2) | ||||
| 11.1496 (1) | 11.1496 (1) | 11.1496 (1) | 1386.039 (10) | Sb | 32e | 0.2398 (1) | 0.5102 (1) | 0.01021 (1) | 0.709 (9) | |
| O | 48f | 0.3114 (1) | 0.625 | 0.125 | 0.883 (8) | |||||
| 4.9125 (1) | 12.470 (1) | 5.4154 (1) | 331.75 (1) | Sb | 8e | 0.0407 (3) | 0.1278 (1) | 0.1783 (3) | 0.839 (2) | |
| O1 | 4c | 0.25 | 0.25 | 0.0238 (4) | 0.830 (3) | |||||
| O2 | 8e | 0.1532 (4) | 0.0588 (1) | 0.8558 (3) | 0.849 (2) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moseley, D.H.; Bridges, C.A.; Daemen, L.L.; Zhang, Q.; McGuire, M.A.; Cakmak, E.; Hermann, R.P. Structure and Anharmonicity of α- and β-Sb2O3 at Low Temperature. Crystals 2023, 13, 752. https://doi.org/10.3390/cryst13050752
Moseley DH, Bridges CA, Daemen LL, Zhang Q, McGuire MA, Cakmak E, Hermann RP. Structure and Anharmonicity of α- and β-Sb2O3 at Low Temperature. Crystals. 2023; 13(5):752. https://doi.org/10.3390/cryst13050752
Chicago/Turabian StyleMoseley, Duncan H., Craig A. Bridges, Luke L. Daemen, Qiang Zhang, Michael A. McGuire, Ercan Cakmak, and Raphaël P. Hermann. 2023. "Structure and Anharmonicity of α- and β-Sb2O3 at Low Temperature" Crystals 13, no. 5: 752. https://doi.org/10.3390/cryst13050752
APA StyleMoseley, D. H., Bridges, C. A., Daemen, L. L., Zhang, Q., McGuire, M. A., Cakmak, E., & Hermann, R. P. (2023). Structure and Anharmonicity of α- and β-Sb2O3 at Low Temperature. Crystals, 13(5), 752. https://doi.org/10.3390/cryst13050752






