Abstract
In this study, nanostructured ZnO arrays were synthesized by an accessible thermal oxidation (TO) methodology. The Zn films were chemically etched with nitric acid (HNO3) and then oxidized in a furnace at 500 °C for 5 h. Two different morphologies were achieved by modifying the HNO3 concentration in the etching process: (a) ZnO grass-like nanostructures and (b) rod-like nanostructures, with an etching process in HNO3 solution at 2 and 8 M concentration, respectively. The physical and chemical properties of the samples were analyzed by X-ray diffraction (XRD), scanning (SEM) and transmission electron microscopy (TEM), energy dispersive X-ray spectroscopy (EDS), and Raman spectroscopy. Both morphologies were functionalized with hemoglobin, and a difference was found in the efficiency of functionalization, which was monitored by UV–Vis spectroscopy. The sample with the highest efficiency was the ZnO grass-like nanostructures. Afterward, the capture of carbon dioxide was evaluated by monitoring a sodium carbonate solution interacting with the as-functionalized samples. The evaluation was analyzed by UV–Vis spectroscopy and the results showed a CO2 capture of 98.3% and 54% in 180 min for the ZnO grass-like and rod-like nanostructures, respectively.
1. Introduction
Today, energy production is still dominated by fossil-fuel-based power plants such as thermoelectric stations, coal plants, or gas plants [1]. As a consequence, the energy industry produces around 30 gigatons of carbon dioxide (CO2) emissions every year [2]. This has led to resource depletion, an increase in costs, and a severe negative environmental impact as global temperatures increasingly rise, ice poles melt, and the number of forest fires increases [3]. CO2 is considered the main cause of the greenhouse effect. In just one century, it has managed to increase the planet’s temperature by 0.6 K. This temperature increase has contributed to the most significant changes in the last two decades [4]. As the Earth’s average temperature has increased, many consequences have appeared, such as the rising sea level, promotion of ocean acidification, imbalance of ecosystems, and threats to the life of the species that inhabit them [5]. In addition, great efforts have been made by the scientific community to reduce and control CO2 emissions. Among the recent proposals, CO2 capture has appeared as an alternative that takes advantage of the chemical nature of this molecule to trap it on the surface of an active material [6]. CO2 capture constitutes one step of the artificial photosynthesis (AP) process, which transforms the CO2 molecule into products such as alcohols and light hydrocarbons, which constitute the current fuels employed by humans in small systems such as gas stoves and water heaters [7]. The AP process comprises systems to convert solar energy into chemical energy [7]. This can be achieved through different nanostructured materials that allow the absorption of light, transportation of electrons, and capture and modification of CO2 molecules [8]. Thus, proposals for the development of each step that conforms to the process of AP (such as CO2 capture) are important, and each effort contributes to solving the problems that the surplus of CO2 in the atmosphere has brought.
Zinc oxide (ZnO) is a widely studied semiconductor material. The ZnO has been obtained as an array of micro- and nanostructures with modified chemical and physical properties. These characteristics make it useful for applications such as gas sensors [9], pressure sensors [10], photonic sensors [11], photodegradation of water pollutants [12,13], optical material for high-power laser applications [14], a highly efficient catalyst for various organic reactions [15], and for corrosion protection and self-cleaning for intelligent surface development [16] or CO2 capture [17]. These previously mentioned applications are related to the surface properties of ZnO. The electronic properties of ZnO allow the capture of molecules such as CO2 [17]. Among the methodologies for obtaining ZnO, thermal oxidation (TO) is an accessible technique that promotes modification of the surface due to defects in induction- and atomic-restructuration-related processes [18,19]. Recently, the chemical modification of the Zn surface before TO has become a useful tool to obtain different ZnO morphologies with variations in chemical and physical surface properties in a very accessible way [12,19].
Hemoglobin (Hb) is a molecule capable of transporting mainly O2 and CO2. This action depends on factors such as the hydrogen potential (pH), partial pressure of oxygen (pO2), and partial pressure of carbon dioxide (pCO2) [20]. At a high pH, Hb mainly transports oxygen; however, at a low pH, Hb can transport 15% to 20% of the undissociated CO2 in the blood. This transportation is achieved by binding the molecules to hemoglobin at the N terminals of the chains through a reaction [10,21]. Thus, the Hb molecule can be combined with semiconductor materials to promote CO2 capture. As ZnO has interesting surface properties, which drive the many applications mentioned before, it is a great candidate to serve as a base to be linked with Hb [22]. Even though ZnO has been functionalized with many molecules in other studies that focused on CO2 capture (such as benzimidazole-based ionic liquid [23], carbon nitride [24], and multiwalled carbon nanotubes and SiO2 [25]), finding accessible methodologies for achieving a commercial device is a current need [26]. Furthermore, understanding the relationship between the morphology in an inorganic material and the functionalization efficiency with an organic molecule is a topic of great interest for many applications [27].
This paper presents a contribution to the development of accessible CO2 capture materials by understanding the relationship between the surface properties of ZnO nanostructures and the efficiency of functionalization with Hb. In this study, two kinds of ZnO nanostructures were synthesized by etching treatment in nitric acid, followed by TO. Afterward, the ZnO nanostructures were functionalized with a Hb molecule. The efficiency of the Hb functionalization process was studied in each morphology by UV–Vis spectroscopy. The CO2 capture was evaluated by monitoring the spectroscopic changes in carbonate dilution across time. The influence of morphology on the surface properties of the ZnO nanostructures was determined. In addition, the influence of ZnO morphology on the CO2 capture effectiveness was discussed.
2. Materials and Methods
2.1. Chemicals and Reagents
In this study, zinc foil (Zn, SKU: GF00595391, SIGMA-ALDRICH, USA) was used as a substrate for the growth of ZnO. Nitric acid (HNO3, SKU: 225711-475ML, SIGMA-ALDRICH, USA), hydrogen chloride (HCl, SKU: 17935-250ML, SIGMA-ALDRICH, USA), sodium carbonate (Na2CO3, SKU: 223484-500G, SIGMA-ALDRICH, USA), ethanol (CH3CH2OH, SKU: 1070179026, SIGMA-ALDRICH, USA), deionized water (Resistivity of 18 MΩ), ammonia (NH3, SKU: 294993, SIGMA-ALDRICH, USA), tetraethyl orthosilicate (TEOS, SKU: 333859, SIGMA-ALDRICH, USA), and chromatographic nitrogen (N2) were commercially acquired and used in the experiments without further purification processes.
2.2. Synthesis of the Nanostructured ZnO Array
The nanostructured ZnO arrays were obtained by the known thermal oxidation (TO) methodology [12,19]. Two morphologies were selected from our past studies [19,28]: (a) ZnO grass-like nanostructures and (b) cane-like nanostructures. To create the ZnO grass-like nanostructures, the Zn film was cleaned in a HCl solution for 10 min to remove organic pollutants attached to the surface of the Zn foil. Then, the Zn substrate was etched into a 2 M HNO3 solution for 1 min. To create the ZnO rod-like nanostructures, the Zn foil was cleaned in an HCl solution for 10 min, and was subsequently etched into an 8 M HNO3 solution for 1 min. In both cases, the samples were cleaned in deionized water after each step and were immediately afterward dried by a N2 flux of 20 L/min. Afterward, the samples were oxidized under a 20% of oxygen (weight) in the atmosphere at 500 °C. The oxidation process was continued for 5 h by an isothermal process in a horizontal furnace. After the oxidation process, the samples were rinsed using deionized water and dried under a N2 flux.
2.3. Characterization of Physic and Chemical Properties
Once the samples were synthesized, the chemical and physical properties of the samples were compared. The structural features were analyzed by X-ray diffraction (XRD) by a Bruker D8 Eco Advance diffractometer. The excitation source was a copper tube with Kα radiation (λ = 1.5418 Å). The achieved morphologies in the ZnO arrays were studied by field-emission scanning electron microscopy (FESEM). The electron microscopy was a JEOL 7401F model equipped with an energy dispersive spectroscopy (EDS) system, which was employed to determine the chemical composition of samples. The evolution of atoms in the individual nanostructures of the ZnO arrays was analyzed across the TO process by transmission electron microscopy (TEM) in high-resolution (HRTEM) mode, employing a transmission electron microscope (JEOL ARM200F model). The vibrational modes exhibited by the samples were analyzed by Raman spectroscopy in INTEGRA Spectra equipment with a green laser as the excitation source (wavelength of 532 nm).
2.4. Functionalization of Nanostructured ZnO Array with Hemoglobin
For Hb functionalization, Hb was diluted in deionized water to obtain a 2 mM aqueous solution. The ZnO structures were preconditioned for interacting with the Hb molecule. Thus, 500 mL of ethanol, 500 mL of deionized water, and 400 mL of ammonia were mixed and placed in an ultrasonic bath for 10 min. Afterward, the ZnO nanostructures and 500 mL of the TEOS were added to the previously mentioned mixture. The mixture was agitated for 8 h at room temperature (RT). Then, the ZnO structures were removed from the reaction and immersed in deionized water to remove the remanent molecules. The preconditioned ZnO structures were immersed in the aqueous dispersion of Hb at a temperature of 80 °C for 1 h and then suddenly cooled down at 0 °C. Afterward, the samples were held at this temperature for 180 min. The remanent liquid was analyzed every 30 min by measuring the absorbance spectra with a UV–Vis spectrometer (i3 UV-VIS SPECTROPHOTOMETER, Hanon Instruments) to determine the percentage of Hb functionalized with the ZnO structure.
2.5. Evaluation of CO2 Capture
The capability of the samples (Hb-functionalized ZnO structures) for CO2 capture was studied. The samples were exposed to CO2 (gas) coming from the following reaction:
The samples’ interaction with CO2 was performed in a hermetic quartz cell to determine the functionality of the immobilized Hb for CO2 capture. Thus, a Na2CO3 solution (186 mM concentration) was employed for the test. The CO2 adsorption was indirectly measured from the absorbance signal measured from the remanent solution across time with a UV–Vis spectrometer (i3 UV-VIS SPECTROPHOTOMETER, Hanon Instruments). Absorbance spectra were recorded at room temperature every 15 min for 180 min.
3. Results and Discussion
3.1. Morphological and Structural Characterization of ZnO Arrays
A comparison between the XRD pattern of the Zn foil after the HNO3 etching process under a solution with 2 and 8 M concentrations is shown in Figure 1. The XRD pattern was measured before and after the TO process for both etching conditions. When the Zn foil was etched with the 2 M HNO3 solution, three peaks at 43.22, 70.09, and 70.29 degrees were found to be in agreement with the diffraction exhibited by the (101), (103), and (110) planes in the hexagonal Zn phase (PDF # 04–0831). For the Zn foil etched with the 8 M HNO3 solution, three peaks were located at 43.38, 54.40, and 70.08 degrees. These peaks were in agreement with the (101), (102), and (103) planes of the hexagonal Zn phase (PDF # 04–0831). In the Zn foil etched with the lower concentration, a predominant intensity of the plane (103) was observed, while the sample etched with the higher concentration promoted the prevalence of (102) plane exposition. These observations suggest that the concentration of HNO3 has an important effect on the plane exposition in a similar way that was reported in the grain observation process for metallurgical practices [29]. After the oxidation process was completed, the peaks located at 47.67, 56.60, 62.91, 68.06, and 69.19 degrees were found in the sample treated with 2 M HNO3 solution, which is labeled as ZnO-g in Figure 1. The peaks located at 47.59, 56.64, 62.91, 67.98, and 69.04 were found in the sample etched with 8 M HNO3. In both cases, the peaks corresponded with the diffraction of the (102), (110), (103), (112), and (201) planes for the wurtzite hexagonal structure of ZnO (PDF # 036–1451). Both samples exhibited the diffraction of the (101) and (103) planes for Zn, which indicated that the substrate was not totally oxidized during the TO process.
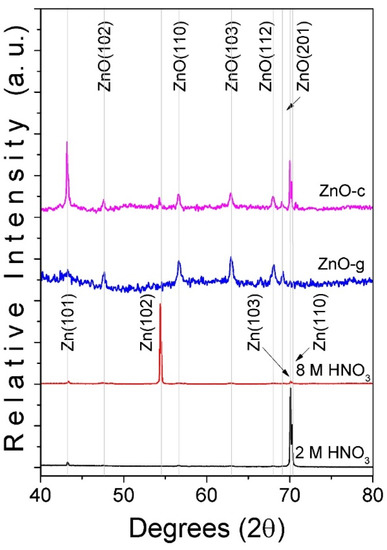
Figure 1.
Comparison between XRD patterns of Zn foil etched with HNO3 (2 and 8 M concentration) before and after TO at 500 °C. Note that samples obtained after TO for 2 and 8 M were the grass-like (ZnO-g) and cane-like (ZnO-c) ZnO nanostructures.
Figure 2 shows a comparison between the obtained morphology from the Zn foil after the TO process across the explored etching conditions. As can be seen, both conditions led to nanostructures growing on the entire surface of the substrate. The sample etched with the 2 M HNO3 solution was obtained with grass-like morphology (see Figure 2a), while the sample etched with the 2 M HNO3 solution exhibited cane-like morphology (see Figure 2b). A close examination of both kinds of structures showed that ZnO grass-like structures (ZnO-g) were formed by flat structures with an average area of 1 ± 0.01 µm2 and a thickness of 10 ± 0.2 nm (see Figure 2c). The ZnO cane-like structures (ZnO-c) were 100 ± 0 0.5 nm in length and had a diameter of 10 nm ± 0.1 nm. In addition, EDS was performed on each sample after the TO process, finding Zn and O as unique elements. This suggested that this methodology offers a great level of clean control for obtaining ZnO. For the ZnO-g (see Figure 2e), the amount of Zn was less than that found in the ZnO-c (see Figure 2f). This suggested that the etching with a higher concentration of HNO3 promotes a reduction in oxidation in the Zn substrates, showing the signal of Zn that was not fully oxidized.
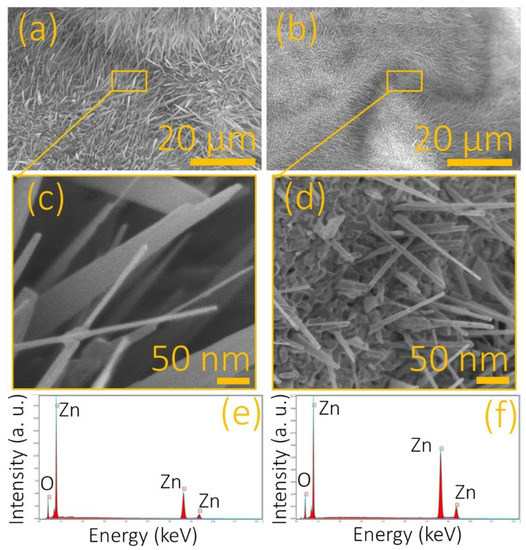
Figure 2.
Zn foils etched with HNO3 at (a) 2 M and (b) 8 M imaged by FESEM after the TO processing. (c,d) Close views of the samples in (a,b), respectively. Recorded EDS spectra from the (e) ZnO grass-like and (f) ZnO cane-like nanostructures.
3.2. Growing Path of ZnO Arrays
The individual structures were analyzed by electron microscopy to identify the differences in the interfaces of ZnO grass-like structures (see Figure 3a) and ZnO cane-like structures (see Figure 3c). Once the samples were imaged by HRTEM, the interplanar distance was measured, and we an average distance of 2.85 Å for the ZnO-g and 1.95 Å for the ZnO-c structures. Thus, according to the measured data, the prevalence of plane (010) in the ZnO grass-like structures was revealed (see Figure 3b), while the plane (012) was found in high density on the ZnO cane-like structures (see Figure 3d). The difference in the exposed planes in each kind of structure explained the resulting morphology for each substrate, as was seen in other studies [12].
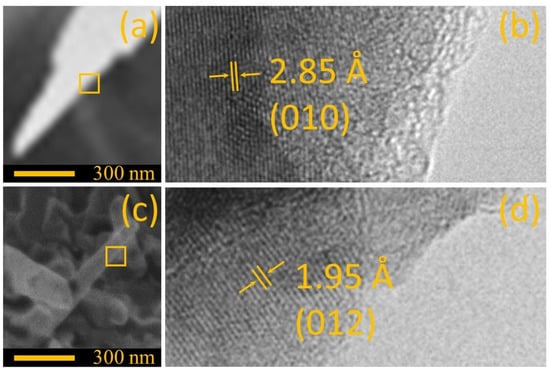
Figure 3.
Comparison between (a) a single ZnO grass-like nanostructure and (b) ZnO cane-like nanostructure imaged by SEM. (c,d) Close view of the interface imaged by HRTEM of samples (a,b), respectively.
According to the results, the Zn etching made with HNO3 is an important factor in producing morphological changes on the surface of the substrates that influence the final characteristics of the ZnO samples. It was reported [30] that oxide preferential growth occurs in Zn zones affected by etching along grain boundaries and scratches on nano-scaled dimensions. These nanostructured oxide regions [31] act as nuclei for further ZnO surface array deposition, as shown in Figure 2. Yu et al. [31] explained that ZnO nanowire deposition occurs at temperatures between the Zn melting and boiling point by means of two mechanisms: first, the development of ZnO nuclei sites formed by vapor-solid action; second, the nanowire formation controlled by the vapor–liquid mechanism. The oxidation temperature (T) in the experiments carried out in this study was 600 °C, which is a temperature between the Zn melting and boiling points (420 °C < T < 907 °C) [31]. The ZnO nanofeatures’ growth can be explained by the liquid–solid growth mechanism with preferential nucleation on the oxidized Zn nuclei first produced by acid etching [30] and then by the initial thermal process [30,31]. A scheme showing the influence of HNO3 etching on the growth mechanism is depicted in Figure 4. The process starts with the formation of surface features by acid etching; according to the results, a nitric acid concentration of 2 M induces scratches on the surface, and the 8 M concentration promotes spherical craters; scratches and craters are marked in red in the scheme in Figure 4a. On the etched surface, nanostructured oxide formation takes place (Figure 4b), mainly on the features promoted by etching (preferential sites for oxidized nuclei formation). Finally, when the temperature reaches the isothermal point, at 500 °C, which is a temperature between Zn melting and boiling points [31], the known liquid–solid growth mechanism controls the arrays’ growth, with surface features acting as nucleation sites (Figure 4c), allowing the formation of ZnO surface nanoarrays on Zn foils.
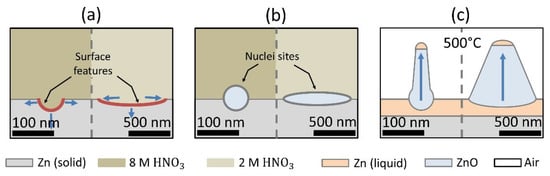
Figure 4.
Schematic representation for the growth path of nanostructured ZnO arrays: (a) the pattern on Zn foil promoted by the acid etching process, (b) the phase transition given by the nucleation mechanism, and (c) growth of nanostructured ZnO arrays by the solid–liquid mechanism on nucleation sites into the TO process.
The Raman spectra were recorded to understand the structural differences between the ZnO grass-like and ZnO cane-like nanostructures. Figure 5 shows a comparison between the spectra recorded from the Raman spectroscopy from the Zn foils etched with the 2 and 8 M HNO3 solutions before and after TO. As shown, the Raman shift was completely modified after the TO process. A Lorentzian fit was applied to the spectra recorded from the ZnO grass-like (see Figure 5b) and ZnO cane-like (see Figure 5c) nanostructures. In both samples, six vibrational modes were identified (see Table 1). The E2 (H)–E2 (L), A1 (TO), E2 (H), and E1 (LO) vibrational modes corresponded to the ZnO phase and were localized in all samples [19,32,33,34,35]. Although the same vibrational modes were found in both kinds of structures, the relationship between the area under the curve of the E2 (H) mode with respect to the others was different in the ZnO-g and ZnO-c. These results suggested a difference in the defect concentration from one sample to another, which could have a significant impact on the functionalization efficiency of the Hb molecule.
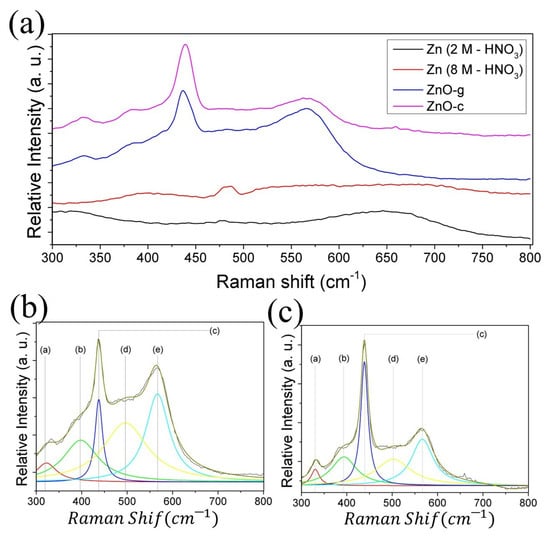
Figure 5.
(a) Comparison of the recorded Raman shift between the Zn foil etched with HNO3 2 M (Black line) and 8 M (red line), before and after TO (blue and magenta line, respectively). Lorentzian fit applied on the Raman shift recorded from (b) ZnO grass-like and (c) ZnO cane-like structures.

Table 1.
Comparison of Raman shift values for vibrational modes between the as-oxidized ZnO structures previously etched with 2 M (ZnO-g) and 8 M (ZnO-c) HNO3 solutions. Note that * point a vibrational mode not reported for ZnO.
3.3. Hb Functionalization of ZnO Nanostructures and CO2 Capture
The supernatant from the Hb-functionalization reaction was analyzed over time with UV–Vis spectroscopy (see Section 2 for details). Figure 6a shows the remnant from the interaction of Hb with the ZnO grass-like structures, while Figure 6b shows the results from the interaction with the ZnO cane-like structures. By comparing these figures, it is clear that the ZnO grass-like structures exhibited a high efficiency for attaching to Hb molecules on the surface. This is clearest in Figure 6c, where the percentage of remnant Hb molecule over the reaction time is depicted. The cane-like structures presented the slowest functionalization rate and achieved a 32.1% Hb-functionalization efficiency. In comparison, the grass-like structures exhibited the fastest rate with an efficiency of 99.2%. This difference in Hb-functionalization efficiency could be attributed to the predominance of the (010) plane in the grass structures, along with the high density of defects in ZnO. This difference in functionalization efficiency due to morphology was seen in other systems [27]. The insert in Figure 6c shows the Hb-functionalized ZnO grass-like structures after the whole process, where a complete surface modification can be seen, possibly due to the incorporation of the Hb molecule at the surface of the sample.
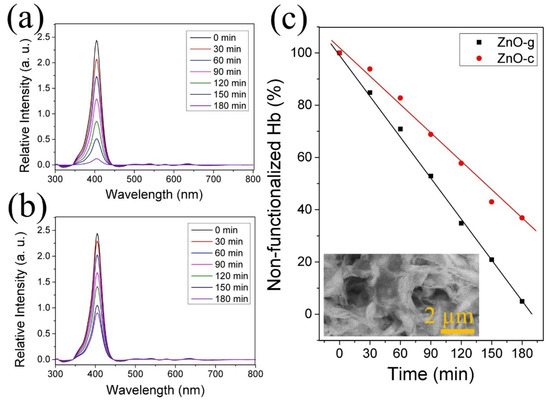
Figure 6.
Measured absorbance by UV–Vis of the remanent hemoglobin in the functionalization process over time with (a) grass-like and (b) cane-like structures. (c) Relationship between absorbance signal over time of supernatant in the Hb-functionalization process. Insert in (c) shows a Hb-functionalized sample imaged by SEM.
The CO2 capture due to the Hb-functionalized ZnO structures was indirectly measured by the absorption spectra of sodium carbonate (see details in Section 2). The reaction for obtaining CO2 presented an absorption peak below 250 nm. Thus, the decrease in this intensity was indicative of CO2 capture. Note that the shape of the UV–Vis spectrum did not change in the entire process. This could be explained because the molecules in the sample not changing their chemical structure by the interaction with the ZnO structures. The intensity of the UV–Vis spectrum decreased over time because some molecules were trapped by the ZnO; here, this was the CO2 molecule. As Figure 7a shows, the ZnO grass-like structures allowed significant CO2 capture after 180 min, with a 98.3% of reduction in the initial intensity. The ZnO cane-like structures exhibited a 54% of reduction in intensity after 180 min of exposure to the Na2CO3 solution (see Figure 6b). To compare the rate of CO2 adsorption, the kinetics of the reaction was computed according to the following equation:
where CO is the concentration when time is equal to zero, C is the concentration at time (t), and k is the reaction rate constant [12]. For the sample ZnO-g, the k at 180 min was computed as 0.0118, while that for sample ZnO-c was 0.0040. This provided evidence of a the great difference in CO2 capture promoted by the morphology in the ZnO arrays. The ZnO can capture CO2 by itself [17]. According to our results, the morphology of ZnO nanostructures influences the functionalization efficiency with Hb because of the charge distribution on the surface (which is directly related to the structural properties). These observations are in agreement with the theoretical results reported by Usseinov et al. [17]. After the Hb-functionalization process, both kinds of ZnO nanostructures adsorbed the CO2 molecule by the known interaction between the hemoglobin and CO2 molecule [36]. Thus, the CO2 capture difference depends on the Hb efficiency achieved by each ZnO nanostructure.
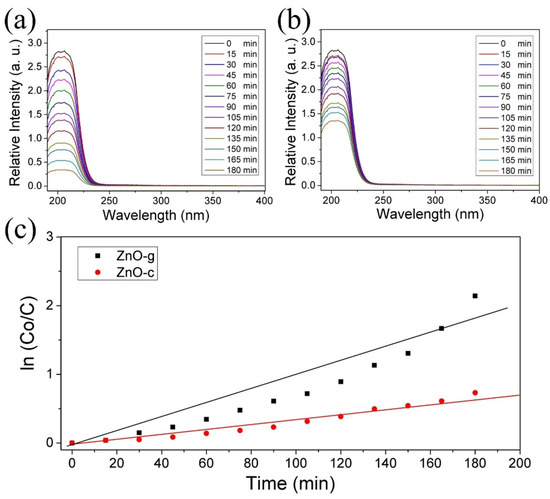
Figure 7.
Absorbance spectra of Na2CO3 dissolution over time of interaction with (a) ZnO grass-like (ZnO-g) and (b) ZnO cane-like (ZnO-c) structures. (c) The comparison of kinetics in CO2 adsorption between ZnO-g and ZnO-c.
4. Conclusions
In this study, ZnO grass-like (ZnO-g) and ZnO cane-like (ZnO-c) nanostructures were obtained as an array with an accessible thermal oxidation technique. The ZnO-g structures were mainly constituted by the (010) plane, while the ZnO-c structures were mainly constituted by the (012) plane. The growth and formation of both kinds of ZnO structures explored in this study can be explained by the vapor–liquid mechanism influenced by the HNO3 etching treatment. The ZnO structures were functionalized with hemoglobin, and we found that the ZnO grass-like structures exhibited the best efficiency, possibly due to the predominance of the (010) plane at the interface. This kind of structure also presented the best efficiency for CO2 capture, with a 98.3% of initial concentration after 180 min.
Author Contributions
Conceptualization, A.M.-S.; methodology, M.H.-R. and O.C.-M.; software, F.J.C.; validation, M.H.-R. and O.C.-M.; formal analysis, A.M.-S.; investigation, A.M.-S.; resources, M.H.-R. and O.C.-M.; data curation, F.J.C.; writing—original draft preparation, A.M.-S. and F.J.C.; writing—review and editing, M.H.-R. and O.C.-M.; visualization, F.J.C.; supervision, O.C.-M.; project administration, M.H.-R.; funding acquisition, O.C.-M.; All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Secretaría de Investigación y Posgrado of the Instituto Politécnico Nacional, grant number SIP 20220370 and SIP 20221569.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data included in this study are available upon request by contact with the corresponding author.
Acknowledgments
The authors acknowledge the Secrtaría de Investigación y Posgrado of the Instituto Politécnico Nacional for the economic support for this research. The authors also thank the Centro de Nanociencias y Micro y Nanotecnologias of the IPN for the sample characterization facilities.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Vigoya, M.F.; Mendoza, J.G.; Abril, S.O. International Energy Transition: A Review of its Status on Several Continents. Int. J. Energy Econ. Policy 2020, 10, 216–224. [Google Scholar] [CrossRef]
- Castells-Quintana, D.; Dienesch, E.; Krause, M. Air pollution in an urban world: A global view on density, cities and emissions. Ecol. Econ. 2021, 189, 107153. [Google Scholar] [CrossRef]
- Kim, J.S.; Kug, J.S.; Jeong, S.; Zeng, N.; Hong, J.; Jeong, J.-H.; Zhao, Y.; Chen, X.; Williams, M.; Ichii, K.; et al. Arctic warming-induced cold damage to East Asian terrestrial ecosystems. Commun. Earth Environ. 2022, 3, 16. [Google Scholar] [CrossRef]
- Beusch, L.; Nauels, A.; Gudmundsson, L.; Gütschow, J.; Schleussner, C.-F.; Seneviratne, S.I. Responsibility of major emitters for country-level warming and extreme hot years. Commun. Earth Environ. 2022, 3, 7. [Google Scholar] [CrossRef]
- Lyam, P.T.; Duque-Lazo, J.; Hauenschild, F.; Schnitzler, J.; Muellner-Riehl, A.N.; Greve, M.; Ndangalasi, H.; Myburgh, A.; Durka, W. Climate change will disproportionally affect the most genetically diverse lineages of a widespread African tree species. Sci. Rep. 2022, 12, 7035. [Google Scholar] [CrossRef]
- Alivand, M.S.; Mazaheri, O.; Wu, Y.; Stevens, G.W.; Scholes, C.A.; Mumford, K.A. Catalytic Solvent Regeneration for Energy-Efficient CO2 Capture. ACS Sustain. Chem. Eng. 2020, 8, 18755–18788. [Google Scholar] [CrossRef]
- Nguyen, V.-H.; Nguyen, B.-S.; Jin, Z.; Shokouhimehr, M.; Jang, H.W.; Hu, C.; Singh, P.; Raizada, P.; Peng, W.; Lam, S.S.; et al. Towards artificial photosynthesis: Sustainable hydrogen utilization for photocatalytic reduction of CO2 to high-value renewable fuels. Chem. Eng. J. 2020, 402, 126184. [Google Scholar] [CrossRef]
- Liu, Y.; Deng, L.; Sheng, J.; Tang, F.; Zeng, K.; Wang, L.; Liang, K.; Hu, H.; Liu, Y.-N. Photostable core-shell CdS/ZIF-8 composite for enhanced photocatalytic reduction of CO2. Appl. Surf. Sci. 2019, 498, 143899. [Google Scholar] [CrossRef]
- Zheng, C.; Zhang, C.; He, L.; Zhang, K.; Zhang, J.; Jin, L.; Asiri, A.M.; Alamry, K.A.; Chu, X. ZnFe2O4/ZnO nanosheets assembled microspheres for high performance trimethylamine gas sensing. J. Appl. Phys. 2020, 849, 156461. [Google Scholar] [CrossRef]
- Park, J.; Ghosh, R.; Song, M.S.; Hwang, Y.; Tchoe, Y.; Saroj, R.K.; Ali, A.; Guha, P.; Kim, B.; Kim, S.-W.; et al. Individually addressable and flexible pressure sensor matrixes with ZnO nanotube arrays on graphene. NPG Asia Mater. 2022, 14, 40. [Google Scholar] [CrossRef]
- Oh, H.; Tchoe, Y.; Kim, H.; Yun, J.; Park, M.; Kim, S.; Lim, Y.-S.; Kim, H.; Jang, W.; Hwang, J.; et al. Large-scale, single-oriented ZnO nanostructure on h-BN films for flexible inorganic UV sensors. J. Appl. Phys. 2021, 130, 223105. [Google Scholar] [CrossRef]
- Rojas-Chávez, H.; Miralrio, A.; Hernández-Rodríguez, Y.M.; Cruz-Martínez, H.; Pérez-Pérez, R.; Cigarroa-Mayorga, O.E. Needle- and cross-linked ZnO microstructures and their photocatalytic activity using experimental and DFT approach. Mater. Lett. 2021, 291, 129474. [Google Scholar] [CrossRef]
- Lin, Y.-P.; Polyakov, B.; Butanovs, E.; Popov, A.A.; Sokolov, M.; Bocharov, D.; Piskunov, S. Excited States Calculations of MoS2@ZnO and WS2@ZnO Two-Dimensional Nanocomposites for Water-Splitting Applications. Energies 2022, 15, 150. [Google Scholar] [CrossRef]
- Uklein, A.V.; Multian, V.V.; Kuz’micheva, G.M.; Linnik, R.P.; Lisnyak, V.V.; Popov, A.I.; Gayvoronsky, V.Y. Nonlinear optical response of bulk ZnO crystals with different content of intrinsic defects. Opt. Mater. 2018, 84, 738–747. [Google Scholar] [CrossRef]
- Khaliullin, S.M.; Zhuravlev, V.D.; Ermakova, L.V.; Buldakova, L.Y.; Yanchenko, M.Y.; Porotnikova, N.M. Solution Combustion Synthesis of ZnO Using Binary Fuel (Glycine + Citric Acid). Int. J. Self-Propag. High-Temp. Synth. 2019, 28, 226–232. [Google Scholar] [CrossRef]
- Zheng, H.; Liu, W.; He, S.; Wang, R.; Zhu, J.; Guo, X.; Liu, N.; Guo, R.; Mo, Z. A superhydrophobic polyphenylene sulfide composite coating with anti-corrosion and self-cleaning properties for metal protection. Colloids Surf. A: Physicochem. Eng. Asp. 2022, 648, 129152. [Google Scholar] [CrossRef]
- Usseinov, B.; Akilbekov, A.T.; Kotomin, E.A.; Popov, A.I.; Seitov, D.D.; Nekrasov, K.A.; Giniyatova, S.G.; Karipbayev, Z.T. The first principles calculations of CO2 adsorption on (10⎯10) ZnO surface. AIP Conf. Proc. 2019, 2174, 020181. [Google Scholar] [CrossRef]
- Tang, C.; Sun, F.; Chen, Z.; Chen, D.; Liu, Z. Improved thermal oxidation growth of non-flaking CuO nanorod arrays on Si substrate from Cu film and their nanoscale electrical properties for electronic devices. Ceram. Int. 2019, 45, 14562–14567. [Google Scholar] [CrossRef]
- Rojas-Chávez, H.; Cruz-Martínez, H.; Montejo-Alvaro, F.; Farías, R.; Hernández-Rodríguez, Y.M.; Guillen-Cervantes, A.; Ávila-García, A.; Cayetano-Castro, N.; Medina, D.I.; Cigarroa-Mayorga, O.E. The formation of ZnO structures using thermal oxidation: How a previous chemical etching favors either needle-like or cross-linked structures. Mater. Sci. Semicond. Process. 2020, 108, 104888. [Google Scholar] [CrossRef]
- Gibson, J.S.; Cossins, A.R.; Ellory, J.C. Oxygen-sensitive membrane transporters in vertebrate red cells. J. Exp. Biol. 2000, 203 Pt 9, 1395–1407. [Google Scholar] [CrossRef]
- Chu, H.; McKenna, M.M.; Krump, N.A.; Zheng, S.; Mendelsohn, L.; Thein, S.L.; Garrett, L.J.; Bodine, D.M.; Low, P.S. Reversible binding of hemoglobin to band 3 constitutes the molecular switch that mediates O2 regulation of erythrocyte properties. Blood 2016, 128, 2708–2716. [Google Scholar] [CrossRef]
- Gibson, J.S. Mechanism of O2-sensitive red cell properties. Blood 2016, 128, 2593–2595. [Google Scholar] [CrossRef] [Green Version]
- Rojas, L.M.G.; Huerta-Aguilar, C.A.; Orta-Ledesma, M.T.; Sosa-Echeverria, R.; Thangarasu, P. Zinc oxide nanoparticles coated with benzimidazole based ionic liquid performing as an efficient CO2 capture: Experimental and Theoretical studies. J. Mol. Struct. 2022, 1265, 133466. [Google Scholar] [CrossRef]
- Anuar, S.A.; Ahmad, K.N.; Al-Amiery, A.; Masdar, M.S.; Isahak, W.N.R.W. Facile Preparation of Carbon Nitride-ZnO Hybrid Adsorbent for CO2 Capture: The Significant Role of Amine Source to Metal Oxide Ratio. Catalysts 2021, 11, 1253. [Google Scholar] [CrossRef]
- Jena, K.K.; Panda, A.P.; Verma, S.; Mani, G.K.; Swain, S.K.; Alhassan, S.M. MWCNTs-ZnO-SiO2 mesoporous nano-hybrid materials for CO2 capture. J. Alloys Compd. 2019, 800, 279–285. [Google Scholar] [CrossRef]
- Barzagli, F.; Giorgi, C.; Mani, F.; Peruzzini, M. Screening Study of Different Amine-Based Solutions as Sorbents for Direct CO2 Capture from Air. ACS Sustain. Chem. Eng. 2020, 8, 14013–14021. [Google Scholar] [CrossRef]
- Cigarroa-Mayorga, O.E. Tuning the size stability of MnFe2O4 nanoparticles: Controlling the morphology and tailoring of surface properties under the hydrothermal synthesis for functionalization with myricetin. Ceram. Int. 2021, 47, 32397–32406. [Google Scholar] [CrossRef]
- Mendoza-Sánchez, R.; Hernández-Rodríguez, Y.M.; Cigarroa-Mayorga, O.E. Degradation of ZnO Nanostructures Interface by Effect of Photocatalytic Activity in Methylene Blue. In Proceedings of the 2021 IEEE International Summer Power Meeting/International Meeting on Communications and Computing (RVP-AI/ROC&C), Acapulco, Mexico, 14–18 November 2021; pp. 1–5. [Google Scholar] [CrossRef]
- Tan, W.K.; Razak, K.A.; Ibrahim, K.; Lockman, Z. Oxidation of etched Zn Foil for the formation of ZnO nanostructure. J. Alloys Compd. 2011, 509, 6806. [Google Scholar] [CrossRef]
- Yuan, L.; Wang, C.; Cai, R.; Wang, Y.; Zhou, G. Temperature-dependent growth mechanism and microstructure of ZnO nanostructures grown from the thermal oxidation of zinc. J. Crystal Growth. 2014, 390, 101. [Google Scholar] [CrossRef]
- Xiang, K.; Chai, L.; Wang, Y.; Wang, H.; Guo, N.; Ma, Y.; Murty, K.L. Microstructural characteristics and hardness of CoNiTi medium-entropy alloy coating on pure Ti substrate prepared by pulsed laser cladding. J. Alloys Compd. 2020, 849, 156704. [Google Scholar] [CrossRef]
- Xing, Y.J.; Xi, Z.H.; Xue, Z.Q.; Zhang, X.D.; Song, J.H.; Wang, R.M.; Xu, J.; Song, Y.; Zhang, S.L.; Yu, D.P. Optical properties of the ZnO nanotubes synthesized via vapor phase growth. Appl. Phys. Lett. 2003, 83, 1689–1691. [Google Scholar] [CrossRef]
- Wang, M.; Luo, Q.; Hussain, S.; Liu, G.; Qiao, G.; Kim, E.J. Sharply-precipitated spherical assembly of ZnO nanosheets for low temperature H2S gas sensing performances. Mater. Sci. Semicond. Process. 2019, 100, 283–289. [Google Scholar] [CrossRef]
- Souissi, A.; Sartel, C.; Sayari, A.; Meftah, A.; Lusson, A.; Galtier, P.; Sallet, V.; Oueslati, M. Zn- and O-polar surface effects on Raman mode activation in homoepitaxial ZnO thin films. Solid State Commun. 2012, 152, 794–797. [Google Scholar] [CrossRef]
- Souissi, A.; Sartel, C.; Amiri, G.; Meftah, A.; Lusson, A.; Galtier, P.; Sallet, V.; Oueslati, M. Raman study of activated quasi-modes due to misorientation of ZnO nanowires. Solid State Commun. 2012, 152, 1729–1733. [Google Scholar] [CrossRef]
- Christiansen, J.; Douglas, C.G.; Haldane, J.S. The absorption and dissociation of carbon dioxide by human blood. J. Physiol. 1914, 48, 244–271. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).