Abstract
Solar energy applications rely heavily on p-block elements and transition metals. Silicon is, by far, the most commonly used material in photovoltaic cells and accounts for about 85% of modules sold presently. Of late, thin film photovoltaic cells have gained momentum because of their higher efficiencies. Most of these thin film devices are made out of just five elements, namely, cadmium, tellurium, selenium, indium, gallium and copper. The present manuscript describes an elegant and inexpensive molten salt-based electrolytic process for fabricating a tellurium-coated metallic substrate. A three-electrode set up was employed to coat iridium with tellurium from a molten bath containing lithium chloride, lithium oxide and tellurium tetrachloride (LiCl-Li2O-TeCl4) at 650 °C for a duration ranging from 30 to 120 min under a galvanostatic mode. The tellurium coating was observed to be thick, uniform, smooth and homogeneous. Additionally, the deposited tellurium did not chemically react with the iridium substrate to form intermetallic compounds, which is a good feature from the standpoint of the device’s performance characteristics. The present process, being generic in nature, shows the potential for the manufacture of both the coated substates and high-purity elements not just for tellurium but also for other p-block elements.
1. Introduction
P-block elements, ranging from group 13 through 18 except helium from group 18 in the periodic table, consist of metals, nonmetals and semimetals/metalloids. Some of these elements, such as silicon, selenium, tellurium, bismuth, indium, gallium, germanium and their compounds/alloys, are extensively used in many key technologies including the clean energy sector. Due to an increased demand for photovoltaic cells, some of these elements have become the most sought-after commodities for the manufacture of these devices/modules to capture solar energy in a cost-effective manner. At present, silicon (both in crystalline and amorphous forms) dominates the photovoltaic market. Other forms of solar modules (thin film/perovskite/organic/quantum dots/multijunction/concentration photovoltaics) are gradually emerging in the scene because of their inexpensiveness, versality and relatively better efficiencies. In order to cater to the increasing demand, the recycling of waste photovoltaic modules to recover precious metals, such as germanium, selenium, tellurium and remove toxic elements (cadmium, lead) has, in recent years, gained global momentum [1,2,3].
From a device-fabrication standpoint, these elements are mostly preferred as coatings on a suitable substrate, such as metals, glass or plastics, and are routinely used as semiconductors, photovoltaics, phase change memory materials, high performance thermoelectric materials and alloy-additive components/films [4,5]. Tellurium, being one of the p-block elements, is widely used in photovoltaic modules [6]. Coating of tellurium has been traditionally carried out by multiple techniques, such as physical vapor deposition (PVD), chemical vapor deposition (CVD), ion implantation, atomic layer deposition (ALD) and electrodeposition [7,8,9,10]. Unlike the vacuum-assisted manufacturing methods (which are essentially top-down processes with several flow control/adjustment attachments), electrodeposition processes offer a one-pot and bottom-up synthesis approach whereby all the precursor components are present in one single solution. As a result of the inherent advantages associated with the electrodeposition techniques, these processes have been widely used in the fabrication of advanced engineering materials including metals, alloys, compounds, semimetals, non-metals and thin films.
A number of studies on the electrodeposition of p-block elements, their alloys and compounds including tellurium, from aqueous-based baths have shown limited success. Some of these drawbacks include (i) the low solubility of the functional electrolytes (compounds containing silicon, tellurium, selenium) in water/organic electrolytes (ii) non-adherence of the films/coatings on the substrate materials (iii) solubility of the electrodeposited material/coating in the electrolyte and (iv) formation of relatively thin deposits/coatings. For example, tellurium coatings prepared from aqueous solutions including ionic liquids, were reported to be of inferior quality [11,12,13]. These coatings, when prepared from a ternary chloride melt (AlCl3-NaCl-KCl, using TeCl4 as the functional electrolyte), have also been observed to suffer from similar inadequacies. The electrochemical behavior of tellurium in the ternary melt has been reported to be complex in nature [12]. Moreover, the electrodeposited layers were observed to be non-adherent and dissolved into the melt immediately after formation [12]. Another example is aluminum-coated steels, prepared from low temperature chloride melts in the temperature range of 160–180 °C. These coatings were also observed to be of non-adherent type besides not being able to show good corrosion-resistance abilities. [13,14]. It appears that a relatively elevated temperature may remove some of the limitations that are inherent to low-temperature coating/deposition processes. A recent study from our laboratory, has indeed shown the superior features of the coated components in terms of good adhesion and excellent corrosion-resistance properties in a simulated marine environment [15]. Several published literatures also support such a viewpoint wherein elevated temperatures have been shown to promote formation of smooth, adherent, thick and relatively uniform deposits/coatings [16,17]. One of the key factors, among others, that contribute to the formation of relatively thicker electrodeposits/coatings can be ascribed to the increased solubility of the functional electrolyte. Unlike aqueous electrolytes and ionic liquids, molten salts are endowed with this property as these can operate at elevated temperatures.
The present study describes results from one of our recent experimental research efforts whereby the focus was to examine the suitability of a platinum group metal (PGM) for its deployment as an oxygen-evolving electrode during the electrochemical reduction of used uranium oxide. The specific objective of the research was to study the chemical interactions of a group of reactive gaseous elements (oxygen, selenium, tellurium and iodine) that were made to generate electrochemically, on the performance characteristics as well as mechanical integrity of the PGM. Platinum, as an inert anode, is known to undergo degradation during the electrochemical reduction of uranium oxide [18]. The specific objective of the present experiment was to replace platinum with another PGM, namely iridium, to examine its interaction with tellurium. In situ generated tellurium, via the electrochemical reduction of tellurium tetrachloride (TeCl4), was deposited on the iridium to examine its chemical interaction with iridium.
2. Experimental
The electrochemical runs were carried out in an argon atmosphere (containing < 0.1 ppm of moisture and oxygen) by placing the electrochemical cell (Figure 1) in the glove box. High purity and a combination of ultra-dry LiCl-1.0 wt.% Li2O was used as the supporting electrolyte. Anhydrous TeCl4 (equivalent to 0.25 wt.% tellurium) was used as the source of tellurium (functional electrolytes). A three-electrode setup, consisting of an iridium wire (1 mm dia., 99.8% pure), molybdenum coil (made from 1 mm dia., 99.9% pure Mo wire) and glassy carbon (3 mm dia. and 100 mm long) as working, counter and reference electrodes, respectively (Figure 2), was used to carry out the electrodeposition test runs. The electrodes were sheathed with high purity alumina tubes to insulate them (from establishing possible electrical contacts with the electrochemical cell). A magnesia crucible was used to contain the molten electrolyte.
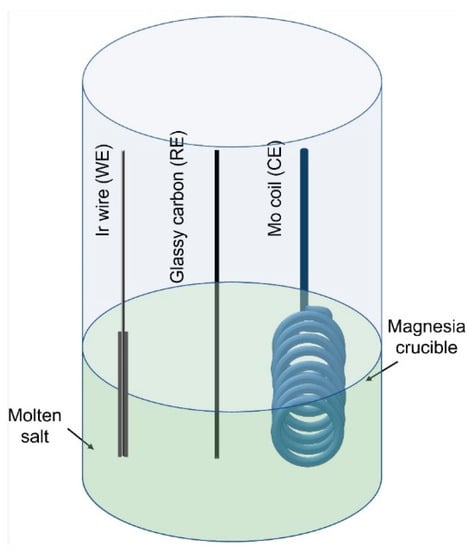
Figure 1.
Schematic diagram of the electrochemical cell assembly.

Figure 2.
Three-electrode set up (iridium wire as the working electrode; glassy carbon as the pseudo reference electrode and molybdenum coil as the counter electrode).
The electrochemical cell was assembled, electrodes were placed above the crucible (in a vertical position) and the furnace was switched on to the heating mode in a controlled manner. The temperature was set at 650 °C and the measured temperature, during electrodeposition tests, was observed to be 650 ± 2 °C. The melt was allowed to homogenize for about an hour prior to the electrochemical measurements. The electrodes were lowered into the melt and electrical connections were made via the Biologic potentiostat-galvanostat to record the experimental data. The electrodeposition runs were carried out, at constant current mode (0.5–2.0 A) and at 650 °C, using a suite of transient electrochemical techniques. The coated samples were evaluated and characterized by XRD and ICP-MS measurements.
3. Results and Discussion
3.1. Cyclic Voltammetric Measurements
A series of cyclic voltammetric (CV) measurements, at different scan rates (0.025–0.15 V s−1), were performed to determine the decomposition voltage of the TeCl4. The CV data showed consistency (in the measured values of the decomposition voltage), both during the cathodic deposition of tellurium (on iridium) and anodic stripping of tellurium (back to the electrolyte). Figure 3 shows a typical CV indicating the deposition of tellurium (during cathodic sweep) and its dissolution (in the electrolyte) during the reverse (also known as anodic) sweep. The appearance of a single redox peak indicated that the deposition of tellurium and its subsequent stripping occurred in one step. The nature of the CV plot indicated the electrode kinetics to be reversible and diffusion controlled.
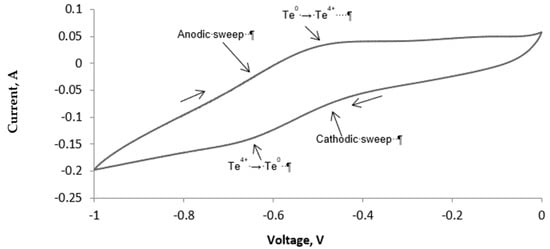
Figure 3.
Cyclic voltammogram (CV) of TeCl4 in LiCl-Li2O-TeCl4 electrolyte, [Te4+] –0.25 wt.%, S = 0.5 cm2, T −650 °C.
The appearance of a single peak, during the square wave (SQV) measurement, followed by the CV measurements, further confirmed the fact that the tellurium deposition and dissolution occurred in just one step (Figure 4).
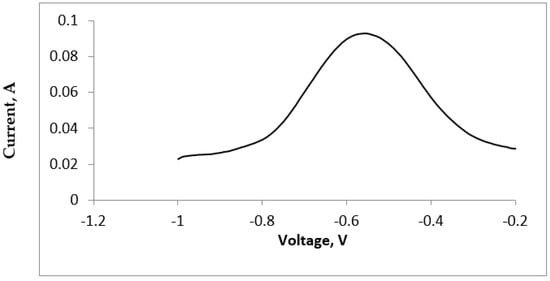
Figure 4.
Square Wave Voltammogram of the TeCl4: Pulse height −25 mV, potential step −10 mV, Frequency −8 Hz.
3.2. Galvanostatic Experiment
After experimentally determining the decomposition voltage of TeCl4, both by CV and SQV electrolysis experiments, were performed at constant current to deposit tellurium onto the iridium cathode. The galvanostatic experiments were carried out both at 0.1 A and then at 0.2 A for a total duration ranging between 30–120 min. A steady voltage (after an initial instability) indicated the deposition of tellurium into iridium (Figure 5).
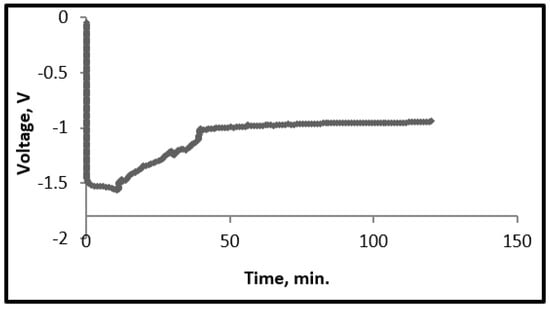
Figure 5.
Voltage vs. time profile: current −0.2 A and duration −120 min.
The deposition run was stopped after 120 min to allow the furnace to cool to room temperature. The iridium wire was subsequently taken out of the electrochemical cell for further evaluation and characterization. The iridium wire, after the removal from the electrochemical cell, was observed to have been coated with a smooth, adherent, uniform and thick tellurium (surface) coating (Figure 6).

Figure 6.
Tellurium-coated iridium wire (before washing/cleaning).
3.3. Inductively Coupled Plasma–Mass Spectrometric (ICP-MS) Analysis of the Deposit
The deposit (Figure 6) was scraped with a knife to analyze its chemical composition (without washing). As expected, the analysis indicated the presence of two major elements (tellurium and lithium) (Table 1).

Table 1.
ICP-MS analysis of the scraped coating.
3.4. XRD Patterns of the Deposits
A small portion of the unwashed deposit was cut and subjected to the XRD to determine the phase compositions. The XRD pattern indicated the formation of four distinct phases, as follows: elemental tellurium, two lithium telluride phases, LiTe3, Li2Te, respectively and elemental iridium. The XRD did not reveal the formation of any iridium telluride phase(s) (IrxTey). The coated wire was subsequently washed with distilled water to remove the salt and analyze the integrity of surface tellurium on iridium. The XRD (Figure 7) indicated the presence of tellurium as the major phase with traces of lithium and iridium as the minor phase.
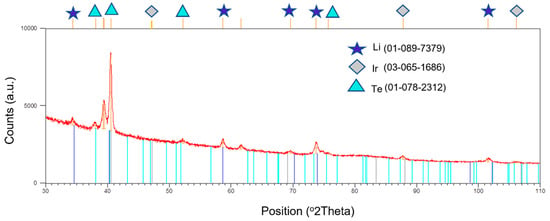
Figure 7.
XRD pattern of the washed tellurium-coated iridium substrate/wire.
Although thermodynamics favors the formation of two iridium tellurides (IrTe2 and IrTe2.67, respectively) [19], the XRD pattern did not indicate their formation. Perhaps their formation was inhibited due to kinetic considerations. These results have led to the following conclusions:
- Unlike platinum, the mechanical integrity of iridium was not observed to have been adversely impacted. Iridium and tellurium did not form any brittle telluride phases. These studies have clearly indicated the superior features of iridium over platinum.
- The tellurium deposit was observed to be highly pure.
- The tellurium coating on iridium was observed to be smooth, thick and adherent. The coating did not undergo any spallation and/or form cracks even after repeated washing.
- Iridium can be used as a metal substrate to form tellurium-coated components for solar energy devices.
4. Conclusions
Present experimental studies have conclusively established the feasibility of preparing p-block elements, such as tellurium, via an inexpensive molten-salt electrochemical process. Besides being cost-effective in nature, the electrochemical process shows five tangible benefits: (i) preparation of pure elements (ii) possibility of the fabrication of in situ coated components for direct use in a device (iii) easy control of deposition parameters to control the tellurium thickness on the metallic substrates (iv) generic nature of the molten salt electrochemical process for the fabrication of other p-block elements/coatings and (v) superior features of iridium as a potential metallic substrate material.
Author Contributions
Conceptualization, P.K.T.; methodology, K.M. All authors have read and agreed to the published version of the manuscript.
Funding
No external funding was received.
Institutional Review Board Statement
The institutional approval number is: INL/JOU-21-64989.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The work was supported by the Idaho National Laboratory Directed Research and Development Program under DOE Idaho Operations Office. The manuscript was authorized by Battelle Energy Alliances under the contract No. DE-AC07-05ID14517, with the US Department of Energy, for publication. The US government retains and the publisher, by accepting the manuscript for publication, acknowledges that the US government retains a non-exclusive, paid up irrevocable worldwide license to publish or reproduce the published form of this manuscript or allow others to do so for United States Government purposes.
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Smith, Y.R.; Nagel, J.R.; Rajamani, R.K. Electrodynamic Eddy Current Separation of End-of-Life PV Materials. In Energy Technology 2017; The Minerals, Metals & Materials Series; Zhang, L., Ed.; Springer: Berlin/Heidelberg, Germany, 2017; pp. 379–386. [Google Scholar]
- Bogust, P.; Smith, Y.R. Physical separation and beneficiation of end-of-life photovoltaic panel materials: Utilizing tem-perature swings and particle shape. JOM 2020, 72, 2615–2623. [Google Scholar] [CrossRef]
- Bruckman, L.S. Transformative Opportunities from Data Science and Big Data Analytics: Applied to Photovoltaics. Electrochem. Soc. Interface 2019, 28, 57–61. [Google Scholar] [CrossRef]
- Bartlett, P.N.; Cook, D.; de Groot, C.H.; Hector, A.L.; Huang, R.; Jolleys, A.; Kissling, G.P.; Levason, W.; Pearce, S.J.; Reid, G. Non-aqueous electrodeposition of p-block metals and metalloids from halometallate salts. RSC Adv. 2013, 3, 15645–15654. [Google Scholar] [CrossRef]
- Kowalik, R.; Kutyła, D.; Mech, K.; Tokarski, T.; Zabinski, P. Electrowinning Of Tellurium From Acidic Solutions. Arch. Met. Mater. 2015, 60, 591–596. [Google Scholar] [CrossRef]
- Redlinger, M.; Eggert, R.; Woodhouse, M. Evaluating the availability of gallium, indium, and tellurium from recy-cled photovoltaic modules. Sol. Energy Mater. Sol. Cells 2015, 138, 58–71. [Google Scholar] [CrossRef]
- Sen, S.; Bhatta, U.M.; Kumar, V.; Muthe, K.P.; Bhattacharya, S.; Gupta, S.K.; Yakhmi, J.V. Synthesis of Tellurium Nanostructures by Physical Vapor Deposition and Their Growth Mechanism. Cryst. Growth Des. 2008, 8, 238–242. [Google Scholar] [CrossRef]
- Ma, Y.-T.; Gong, Z.-Q.; Xu, W.-H.; Huang, J. Structural and optical properties of tellurium films obtained by chemical vapor deposition(CVD). Tran. Nonferr. Met. Soc. China 2006, 16, 693–699. [Google Scholar] [CrossRef]
- Kowalik, R.; Kutyła, D.; Mech, K.; Żabiński, P. Analysis of tellurium thin films electrodeposition from acidic citric bath. Appl. Surf. Sci. 2016, 388, 817–824. [Google Scholar] [CrossRef]
- Johnson, R.W.; Hultqvist, A.; Bent, S.F. A brief review of atomic layer deposition: From fundamentals to applications. Mater. Today 2014, 17, 236–246. [Google Scholar] [CrossRef]
- Ito, S.; Kitagawa, N.; Shibahara, T.; Nishino, H. Electrochemical Deposition of Te and Se on Flat TiO2for Solar Cell Application. Int. J. Photoenergy 2014, 2014, 5. [Google Scholar] [CrossRef]
- Ebe, H.; Ueda, M.; Ohtsuka, T. Electrodeposition of Sb, Bi, Te, and their Alloys in AlCl3–NaCl–KCl Molten Salt. Electrochim. Acta 2007, 53, 100–105. [Google Scholar] [CrossRef]
- Fellner, P.; Chrenkova-Paucirova, M.; Matiasovsky, K. Electrolytic Aluminum Plating in Molten Salt Mixtures Based on A1C13 I: Influence of the Addition of Tetra methyl ammonium chloride. Surf. Technol. 1981, 14, 101–108. [Google Scholar] [CrossRef]
- Paucirova, M.; Matiasovsky, K. Electrolytic Aluminum-Plating in Fused Salts Based on Chlorides. Electrodepos. Surf. Treat. 1975, 3, 121–128. [Google Scholar] [CrossRef]
- Tripathy, P.K.; Wurth, L.A.; Dufek, E.J.; Gutknecht, T.Y.; Gese, N.J.; Hahn, P.A.; Frank, S.M.; Fredrickson, G.L.; Herring, J.S. Aluminum electroplating on steel from a fused bromide electrolyte. Surf. Coat. Technol. 2014, 258, 652–663. [Google Scholar] [CrossRef][Green Version]
- Wu, G.; Li, N.; Zhou, D.; Mitsuo, K. Electrodeposited Co–Ni–Al2O3 composite coatings. Surf. Coat. Technol. 2004, 176, 157–164. [Google Scholar] [CrossRef]
- Gu, Y.; Liu, J.; Qu, S.; Deng, Y.; Han, X.; Hu, W.; Zhong, C. Electrodeposition of alloys and compounds from high-temperature molten salts. J. Alloys Compd. 2017, 690, 228–238. [Google Scholar] [CrossRef]
- Herrmann, S.D.; Tripathy, P.K.; Frank, S.M.; King, J.A. Comparative Study of Monolithic Platinum and Iridium as Oxy-gen-evolving Anodes during the Electrolytic Reduction of Uranium Oxide in a Molten LiCl-Li2O Electrolyte. J. Appl. Electrochem. 2019, 49, 379–388. [Google Scholar] [CrossRef]
- H.S.C. Chemistry, Ver. 7; Outotec: Pori, Finland, 2015.
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).