Abstract
The synthesis and characterization of Co(II) and Ni(II) mixed ligand complexes are derived from isoniazid, 9-fluorenoneandoxalate. The metal complexes were characterized on the basis of elemental analysis, IR, UV-visible, CV, PXRD, and molar conductance analytical data, viz., all the metal complexes were suggested in an octahedral geometry, respectively. The mixed ligand complexes are formed in the 1:1:2:1 (M:L1:L2:L3) ratios, as found from the elemental analyses, and originate to have the formula [M(L1)(L2)2(L3)]. Where M = Co(II), Ni(II), L1 = isoniazid, L2 = 9-fluorenone, and L3 = oxalate. The molar conductance data reveals that the complexes are non-electrolytes. The cyclic voltammogram of the Co(II) complex revealed that the quasi-reversible single electron transfer process and Ni(II) complex corresponding to a one-electron transfer process were observed during controlled potential electrolysis. IR spectra show that the ligands are coordinated to the metal ions through N and O donor sites of isoniazid-N, 9-fluorenone-O and oxalate-O. Magnetic moment values and UV-visible spectra were used to infer the coordinating of the geometrics of these complexes found to be octahedral. The PXRD patterns suggest that all the complexes are crystalline phases. The metal chelates have been screened for antimicrobial, antioxidant and anti-inflammatory activities, and our findings have been reported, explained and compared with some known antibiotics.
1. Introduction
The mixed ligand–metal complexes are important fields of bioinorganics, medicine, chemistry, photochemistry, molecular biology, electromagnetism chemistry, etc. [1]. The coordination behavior of Co and Ni has been explored as an interesting topic in inorganic biochemistry [2]. Typically, the pharmacological importance of such compounds’ magnetic properties and diverse catalytic performance is being explored. Recently, a few studies on the antimicrobial effects of Co(II) and Ni(II) complexes have been reviewed [3]. Co(II) and Ni(II) complexes exhibit higher activity than their source donors and are therefore favorable to human health. The antibacterial and antifungal properties of Co(II) and Ni(II) complexes were exceptional [4]. Numerous inorganic researchers are working to improve pharmacological therapeutic effects by synthesizing powerful medicines with improved therapeutic effects on microorganisms that have developed resistance to prior drugs. Researchers are more interested in metal-drug complexes because of their various medical applications in the treatment of ailments [5]. Transition metal compounds play such an important role in the creation of Co(II) and Ni(II) metal complexes as medicines for the treatment of a wide range of diseases in one of the most active fields of research. One of the most essential aspects of medical bioinorganic chemistry is the ability to understand and cure diseases caused by poor metal-ion function at the molecular level. In biology, metal ions are needed as medicines and diagnostic agents [6]. The researchers selected the metal and its oxidation state, as well as the amount and types of coordinated ligands and the complexes coordination geometry. The ligands are important in defining the type of secondary coordination sphere interactions involved in the identification of biological targets, as well as controlling the metal’s reactivity [7].
Isonicotinylhydrazide, also known as isoniazid, was first used in the treatment of tuberculosis in 1952. Isoniazid complexes have been found to be relatively stable and to have interesting biological properties. As a result, more research into the synthesis and structural elucidation of metformin and isoniazid complexes has been conducted [8]. The INH contains donor atoms, such as nitrogen, oxygen, and carbonyl, and when they combine with metal ions, they form complexes with various geometries that are biologically active [9]. INH metal complexes have been identified as models for biologically significant species. As a consequence, the study of metal complexes of INH has exploded in popularity in the recent decade. Most of the compounds have anti-inflammatory, analgesic, anticonvulsant, antituberculous, anticancer, anti-HIV, and antibacterial effects, according to reports [10]. The biocompatibility of 9-fluorenone has been discovered to be quite interesting. The arylsulfonylspiro[fluorene-9,5′-imidazolidine]-2′,4′-diones, which are found in mitochondrial enzyme moieties, have been shown to be effective [11]. In vitro, 9-fluorene possesses cytotoxicities that are identical to cisplatin, a common chemotherapy drug.
The current research focuses on the synthesis, characterization, antibacterial, antioxidant, and anti-inflammatory properties ofisoniazid, oxalate, and 9-fluorenone and their Co(II) and Ni(II) mixed ligand complexes.
2. Materials and Methods
2.1. Materials
The reagents and solvents listed below were utilized in all the preparations and characterizations. Without additional purification, all the reagents and solvents were applied. Isoniazid, 9-fluorenone purchased from (Sigma-Aldrich, St. Louis, MO, USA) at Mumbai, India; solvents: DMSO, DMF, methanol, ethanol, and acetonitrile (AnalaR grade), cobalt nitrate, nickel nitrate, sodium oxalate (AnalaR grade) purchased from Merck at Mumbai, India.
2.2. Characterization
The bonding and structure of the complexes generated were explained using various physical methods. While elemental analyses with an Elementar Vario EL III CHN(O) analyzer at SITC, Cochin were used to describe the complexes, they were also computerized. Finally, the elemental percentage, metal estimate, and electrical conductance analysis were performed at 25 °C with a dip-type standardized platinum cell using a digital conductivity bridge (Equiptronics EQ 660). The researchers utilized complicated acetonitrile solutions to the order of 10−3 M. Cyclic voltammograms of a complexes in DMF solution at room temperature were recorded at Versa STAT (Princeton Applied Research-model) electrochemical analyzer and MC. FT-IR spectra were recorded using Agilent spectrophotometer with the range of 4000–400 cm−1 based on KBr pellet technique. The UV-visible spectra of the complexes were recorded in the absorption range of 200 to 800 nm and wavelength accuracy, which was about ± 0.1 nm, by using the Perkin-Elmer spectrophotometer. The magnetic moments of the complexes at room temperature were determined by using a Lakeshore VSM-7410 model vibrating sample magnetometer. The powder X-ray diffraction meter (XRD-600, Shimadzu, model—PW3050/60) using CuKα1 = 1.5406 Å and Kα2 1.5444.
2.3. Metal Estimation
With dil. HCl or dil. H2SO4, a known weight of metal complexes, was decomposed and made up to the mark in a standard measuring flask. Metal ions in the complexes formed metal sulphate or chloride, which was used to estimate metal ions in the complexes using volumetric, colorimetric, and gravimetric estimations [12].
2.4. Preparation of Cobalt Complex
Cobalt nitrate 1.00 g (3 mM) diluted in 10 mL of methanol, isoniazid (3 mM) (L1) 0.471 g in 20 mL of ethanol was added in drops with steady stirring and then 9-fluorenone (6 mM) (L2) 1.238 g in 20 mL of ethanol. The mixture was stirred and sodium oxalate (3 mM) (L3) 0.460 g in 10 mL of water was added and heated on a magnetic stirrer for about 10–15 min. The dark pink colored precipitated colored complex was filtered, washed with ethanol and dried and kept in an airtight glass container (Schematic, Figure 1).
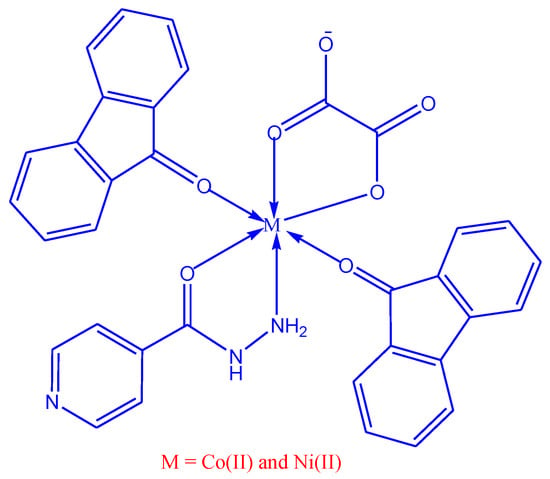
Figure 1.
Schematic representation of Co(II) and Ni(II) mixed ligand complexes.
2.5. Preparation of Nickel Complex
Nickel nitrate 1.00 g (3 mM) dissolved in 10 mL of methanol, and isoniazid 0.472 g (3 mM) in 20 mL of ethanol was added in drops with constant stirring and then 9-fluorenone 1.239 g (6 mM) in 20 mL of ethanol. The mixture was stirred and sodium oxalate 0.461 g (3 mM) in 10 mL water was added and heated on a magnetic stirrer for about 10–15 min. The green-colored precipitated complex was filtered, washed with ethanol and dried and kept in an airtight glass container (Schematic, Figure 1).
2.6. Antimicrobial Activities
2.6.1. Determination of Antimicrobial Activity
Microorganisms
The microbial strains employed in the biological assays were Escherichia coli (MTCC 732), Staphylococcus aureus (MTCC 3160) and Bacillus subtilis (MTCC 441) for bacteria and Aspergillus niger (MTCC 10180) and Candida albicans (MTCC 183) for fungal strains. Obtained from the microbial type culture collection (MTCC) at the Institute of Microbial Technology (IMTECH), Chandigarh, India.
Preparation of Medium
In 1000 mL of distilled water, we dissolved 28.0 g of nutritional agar, brought it to a boil, and then thoroughly dissolved the medium. We sterilized for 15 min in an autoclave at 15 Ibs pressure (121 °C). We then filled the sterilized Petri dishes with the mixture. In 1000 mL of distilled water, 39.0 g of PDA was suspended. We brought it to a boil, and then thoroughly dissolved the medium. We sterilized for 15 min using an autoclave at 15 Ibs pressure (121 °C). When a pH of 3.5 was necessary, we thoroughly mixed the medium before dispensing in a specified flask, acidified the medium with sterile 10% tartaric acid.
For 100 mL of sterile cooled medium, approximately 1 mL of acid was required. After adding the acid, do not heat the medium. In a Roux bottle, a loop containing each of the microorganisms was suspended in about 10 mL of physiological saline. Except for fungal, which was incubated at 25 °C for 48 h, each of these was streaked onto the appropriate culture slants and incubated at 37 °C for 24 h. When growth was observed after the incubation period, the tubes were stored at 2–8 °C until used.
Preparation of sample solutions and dried filter paper discs. Samples were taken, 10 mg dissolved in 10 mL of DMSO and standard solution as chloramphenicol for bacteria (10 mg/mL distilled water), fluconazole (10 mg/mL distilled) for fungi. They were kept under refrigerated conditions unless they were used for the experiment. Whatman filter paper (No:1) was used to prepare four discs approximately 6 mm in diameter, which were placed in hot air for sterilization. After sterilization, discs were loaded with 30μL of each sample. The standard solution was chloramphenicol and fluconazole 30μL and control 30 μL was used to compare the test solution, respectively. They were kept under refrigerated conditions unless they were used for the experiment.
Antimicrobial Assay
Antibiogram was done by the disc diffusion method using samples. Petri plates were prepared by pouring 30 mL of nutrient agar (NA) medium and potato dextrose agar (PDA) medium. The test organism was inoculated on a solidified agar plate with the help of a micropipette and spread and allowed to dry for 10 min. The surfaces of the media were inoculated with bacteria from a broth culture. A sterile cotton swab was dipped into a standardized microbes test suspension and used to evenly inoculate the entire surface of the nutrient agar/PDA plates. Briefly, inoculums containing microbial strains were spread on nutrient agar/PDA plates. Using sterile forceps, the sterile filter papers (6 mm diameter) containing discs were loaded with 30 μL of each sample, 30 μL of control, and 30μL of the standard solution were laid down on the surface of an inoculated agar plate. The plates were incubated at 37 °C for 24 h for the bacteria and 48 h for fungal strains. Each sample was tested in triplicate. The antimicrobial potential of test compounds was determined on the basis of a mean diameter of the zone of inhibition around the disc in millimeters. The zones of inhibition of the tested microorganisms by the samples were measured using a millimeter scale [13,14].
2.7. In Vitro Antioxidant Activity
DPPH Radical-Scavenging Activity Assay
In a nutshell, a 2 mL aliquot of DPPH methanol solution (25 µg/mL) was added to 0.5 mL of the sample solution at various concentrations (20, 40, 60 and 80 µg/mL). The mixture was vigorously shaken and set aside for 30 min at room temperature in the dark. The absorbance was then measured in a spectrophotometer at 517 nm. Higher free radical-scavenging activity was indicated by the reaction mixture’s lower absorbance [15].
where AC = control denotes the absorbance of the control and AS = sample denotes the absorbance of the reaction mixture (in the presence of sample).
Radical Scavenging activity (%) = (AC − AS)/AC × 100
2.8. In Vitro Anti-Inflammatory Activity
Inhibition of Albumin Denaturation
The reaction mixture (5 mL) consisted of 0.2 mL of egg albumin (from fresh hen’s egg), 2.8 mL of phosphate-buffered saline (PBS, pH 6.4) and 2 mL of varying concentrations of extracts (100, 200, 300, 400 and 500 µg/mL), respectively. A similar volume of double-distilled water served as the control. Then the mixtures were incubated at 37 ± 2 °C in an incubator for 15 min and then heated at 70 °C for 5 min. After cooling, their absorbance was measured at 660 nm by using a vehicle as blank. Diclofenacsodium at the final concentrations (100–500 µg/mL) was used as a reference drug and treated similarly for the determination of absorbance. The percentage inhibition of protein denaturation was calculated by using the following formula [16]:
where, Vt = absorbance of a test sample, Vc = absorbance of control.
Percentage of inhibition (%) = [1 − Vt/Vc] × 100
Tests were carried out in triplicate for three separate experiments. The amount of sample needed to inhibit free radicals concentration by 50%, IC50, was graphically determined by a linear regression method using Ms-Windows-based GraphPad Instate (version 3) software. Results were expressed as graphically/mean ± standard deviation [17].
3. Results and Discussion
3.1. Elemental Analysis and Molar Conductance
The experimental value of Co(II) and NI(II) mixed ligand complexes are in good agreement with the theoretically calculated value with complexes molar radioratio 1:1:2:1 between metal and ligands. The complexs molecular formulas were determined using the elemental analytical data. The percentage of each element contained in the complexes provided in Table 1 can be used to confirm the complex based on the analytical results. At 25 °C and a 1 × 10−3 M concentration of water, the molar conductance of metal complexes was measured.

Table 1.
Physical and analytical data for the mixed ligand complexes.
The low conductance range 06–20 Ω−1 cm2 mole−1 indicates Co(II) and Ni(II) complexes are of a non-electrolyte nature (1:0 types) as shown in Table 1 [18].
3.2. Cyclic Voltammogram
The electrochemical characteristics of cobalt complex were investigated using cyclic voltammetry (CV) in a DMSO solution at ambient temperature in the potential range –2.0 to 1.5 V versus an Ag/AgCl reference electrode under a nitrogen environment. Figure 2a shows the cyclic voltammogram of the cobalt complex. It reveals two cathodic reduction peaks at −0.64 and +0.9 during the forward scan, which is attributed to Co(II) → Co(I) and Co(I) → Co(0), respectively. The reverse scan shows two anodic oxidation peaks at +1.2 and −1 V are attributed to Co(0) → Co(I) and Co(I) → Co(II), respectively. The non-equivalent current intensity of cathodic and anodic peaks indicates that the cobalt complex has a quasi-reversible characteristic [19].
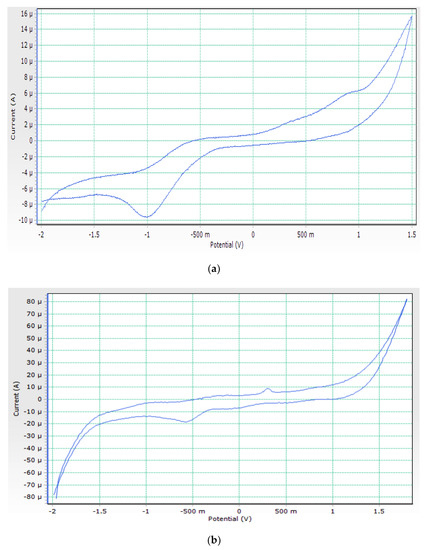
Figure 2.
(a). Cyclic voltammogram of Co(II) complex. (b). Cyclic voltammogram of Ni(II) complex.
Using cyclic voltammetry (CV) in a DMSO solution at ambient temperature in the potential range –2.0 to 2 V versus an Ag/AgCl reference electrode in a nitrogen atmosphere, the electrochemical properties of Ni(II) complex were examined. Figure 2b depicts the electrochemical properties of the Ni(II) complex having a reduction peak at 0.31 V that corresponds to the Ni(II) reduction to Ni(I), as well as an oxidation peak at 0.52 V that corresponds to Ni(I) oxidation to Ni(II) (II). During controlled potential electrolysis, a peak corresponding to a one-electron transfer process was observed [20].
3.3. UV-Visible Spectra and Magnetic Moments
In the DMSO medium, the electronic spectra of Co(II) and Ni(II) metal complexes were recorded, as shown in Figure 3. The Co(II) complex exhibited three bands at 608 nm, 502 nm and 362 nm, which are assigned to 4T2g(F) ← 4T1g, 4A2g(F) ← 4T1g (LMCT) and π − π* transitions respectively, and the lower bands are due to d-d transitions. These transitions suggest octahedral geometry for the complex. Furthermore, the Co(II) complex effective magnetic moment (µeff) is 4.30 BM, which is characteristic of octahedral geometry [21].
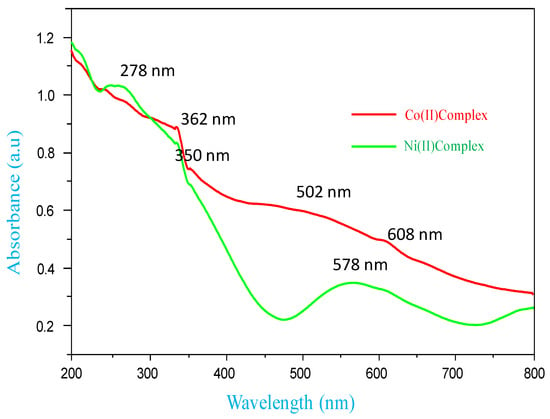
Figure 3.
UV-visible spectra of Co(II) and Ni(II) complexes.
The Ni(II) complex showed three prominent bands at 578 nm, 350 nm and 260 nm, which may be tentatively assigned to 3T2g(F) ← 3A2g, 3T1g(F) ← 3A2g(LMCT) and π − π* transitions, arising from the octahedral geometry. The effective magnetic moment value at 2.90 BM also indicated the octahedral arrangement [22].
3.4. IR-Spectrum of Metal Complexes
This section discusses the infrared spectra of INH, 9-flurorenone and oxalate ion Co(II) and Ni(II) complexes are shown in Figure 4 and Figure 5. In complexes, the major amide group band emerges at 3208–3264 cm−1, and NH stretching appears at 3431 cm−1 to 3381 cm−1. The main amine involvement in the coordination of the metal ions is confirmed by the band shifting to lower or higher wavenumbers.
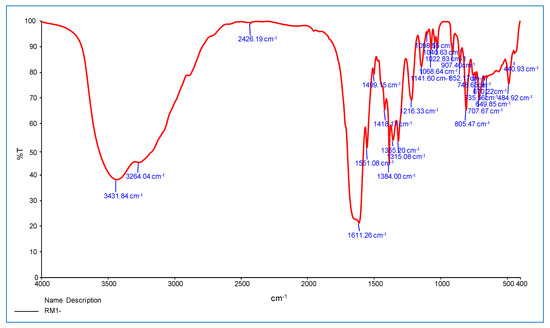
Figure 4.
FT-IR spectrum of Co(II) mixed ligands complex.
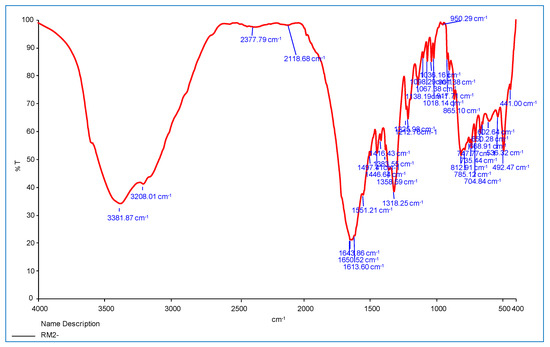
Figure 5.
FTIR spectrum of Ni(II) mixed ligands complex.
The v(C=O) stretching vibrations of INH reveal a band at 1643 cm−1 for Ni(II) complexes and 1640 cm−1 for Co(II) complexes, confirming the ligands entry into the coordination sphere through an oxygen atom in a carbonyl group. These changes also support INH role as a bidentate ligand that coordinates through the –NH2 and C=O groups. Other frequencies in the complexes are moved to substantial alterations at the lower or higher level, such as N–H (stretching), C–H (aromatic), and N–N (stretching) [23].
In addition, the oxalate ions extra ligand shows bands at 1551–1651 cm−1 and 1497–1499 cm−1 in the complexes, which correspond to vas(C=O) and sy(C=O), respectively. These two carbonyl frequencies show that the oxalate ion active as a bidentate Chelation that can coordinate to the metal ion via both oxygen atoms. The stretching frequency of 9-fluorenone C=O moved to 1611–1613 cm−1, verifying the ligand coordination through the monodentate model’s oxygen atom in these complexes.
3.5. Powder XRD Analysis
The investigation of powder X-ray diffraction of Co(II) and Ni(II) complexes is given in (Figure 6). The presence of prominent crystalline peaks in the PXRD pattern of metal complexes shows that they are crystalline. The lattice parameters of metal complexes were calculated using a computer tool called XPERT high score plus, version 3.0.
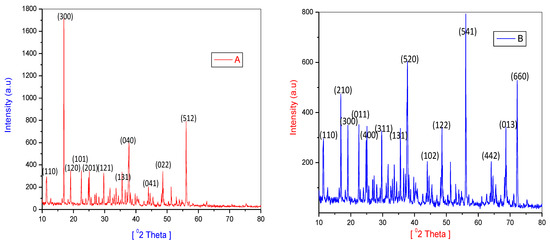
Figure 6.
PXRD patterns of (A) Co(II)complex and (B) Ni(II)complex.
The lattice constants for the Co(II) complex are a = 10.82, α = 90°, b = 9.24(4), β = 90°, c = 6.03(2), γ = 90°, V = 65.25 and the crystal system Orthorhombic, Bravais, Primitive (P) are the three types of polygons.
The complex lattice constants of Ni(II) have PXRD patterns of a = 11.14, α = 90°, b = 9.59(4), β = 90°, c = 4.79(2), γ = 90°, V = 511.05, respectively. Orthorhombic, Bravais, Primitive (P) crystal system.
Scherrer’s equation was used to determine the crystallite size [24].
where λ = wavelength of X-ray radiation (Cu Kα = 1.5406 A0), β = (FWHM) full width at half maximum, θ = diffraction angle, and constant as 0.9. The average crystallite size of Co(II), Ni(II) complexes were found to be 30.7 ± 14.7 and 53.1 ± 18.1, accordingly.
D = 0.9λ/β cosθ
3.6. Antibacterial and Antifungal Activities
The microbial activities of Co(II) and Ni(II) complexes were screened for gram-positive and gram-negative bacteria and fungi. All test sample solution was prepared in MIC 30 µL using DMSO solvent. Chloramphenicol and fluconazole were used as standards for antibacterial and antifungal activities [25]. The antimicrobial activities of metal complexes result are given in Figure 7 and Figure 8 and Table 2. The zone of inhibition results indicates that the complex is an effect of the microorganism under an identical experimental condition. The metal complexes show higher activity against E. coli and S. aureus at 30 µL concentration compared to other bacteria. In-vitro antifungal activity of the Co(II) and Ni(II) complexes shows good activity against C. albicans compared to A. niger. The increase shown of mixed ligand–metal complexes can be explained based on the Chelation theory. On Chelation, the polarity of the metal ion will be reduced to a greater extent due to the overlap of the ligand orbital and partial sharing of the positive charge of the metal ion with donor groups. Further, it increases the delocalization of π-electrons over the whole chelate ring and enhances the lipophilicity of the complexes. However, in comparison to the standard drugs, the antimicrobial activities of the metal complexes are relatively low [26,27].
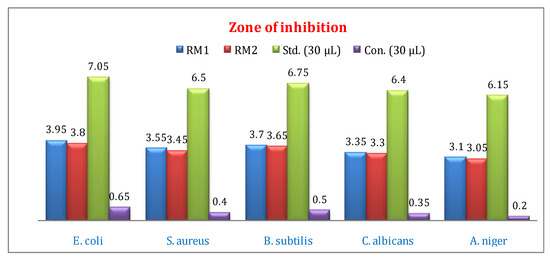
Figure 7.
Antibacterial activity of (RM1) Co(II) and (RM2) Ni(II) complexes zone of inhibition and MIC 30 µL.

Figure 8.
Antibacterial activity of Bacillus subtilis and Staphylococcus aureus.

Table 2.
Antimicrobial activity of Co(II) and Ni(II) complexes.
3.7. In Vitro Anti-Inflammatory Activities
The in-vitro anti-inflammatory activities of Co(II) and Ni(II) complexes were studied by the denaturation method using protein (bovine serum albumin) with different concentrations (100, 200, 300, 400 & 500 μg/mL), with diclofenacsodium used as a reference drug. The percentage inhibition of protein denaturation was calculated and compared with references shown in Table 3 and Figure 9. Three separate experiments were carried out and the inhibition concentration of IC50 was also found. The results indicated the comparing diclofenacsodium (96.05%) IC50 value as 230.75, the Ni(II) complex (85.52%) IC50 value as 265.09 with a very good anti-inflammatory agent compared to the Co(II) complexes (81.64%) IC50 value as 265.03. The presence of a donor site in the mixed ligand (‘N’ donor), and (‘O’ donor) present in the complex, shows the activity of the complexes [28].

Table 3.
In vitro anti-inflammatory (egg albumin) activity of our samples.
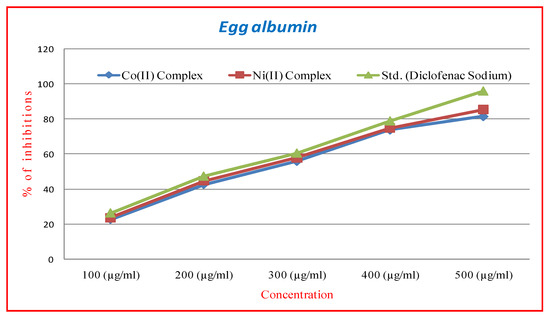
Figure 9.
In-vitro anti-inflammatory (egg albumin) activity of Co(II) and Ni(II) complexes.
3.8. In Vitro Antioxidant Activities
The antioxidant activities of the Co(II) and Ni(II) complex were evaluated based on the radical-scavenging method using a standard DPPH free radical. The IC50 value is the concentration of the complexes necessary to scavenge 50% of DPPH free radical. They assessed changes in the free radical-scavenging ability of the Co(II) and Ni(II) on the basis of the percentage of inhibition given in Figure 10 and Table 4, as well as the IC50 value of the tested complexes. When lowering the concentration of the reaction mixture, the free radical-scavenging activity is higher. The DPPH scavenging activity of the Co(II) complex is 85%,the IC50 value is 42.28, and the Ni(II) complex is 82%, IC50 value is 44.88, representing that the complex is stronger free radical scavengers conferred to the standard ascorbic acid [29,30].
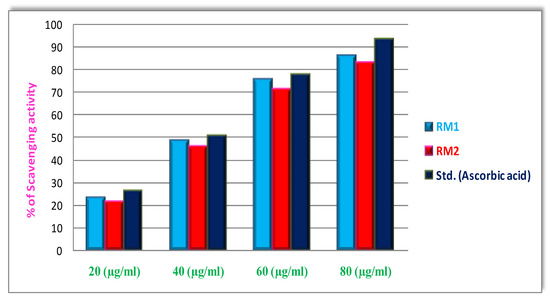
Figure 10.
In vitro antioxidant (DPPH) activity of Co(II) and Ni(II) complexes.

Table 4.
In vitro antioxidant (DPPH) activity of metal complexes.
4. Conclusions
A number of physical, chemical, and spectroscopic approaches have been used to develop and construct novel mixed ligands Co(II) and Ni(II) complexes. Based on FTIR, UV-visible, C.H.N.O. analysis, magnetic moment, and powder XRD analysis, an octahedral geometry was postulated for all the discovered compounds. The compounds typical average crystallite size of Co(II), Ni(II) complexes were found to be 30.7 ± 14.7 and 53.1 ± 18.1 nm, according to powder XRD examination. According to the molar conductance measurements of the complexes are non-electrolytes. The cyclic voltammogram of the compounds revealed a single electron transport route. When compared to a typical antibiotic, the antibacterial activity of the mixed ligand Co(II) and Ni(II) complexes is moderate, according to the findings. The outcomes of DPPH techniques showed that chemicals had the ability to grab electrons or hydrogen atoms and then interact with free radicals or stop chain processes in a dose-dependent way. In comparison to normal ascorbic acid, these complexes show a substantial scavenge efficacy. The complexes have good anti-inflammatory properties; when compared to the standard medicine diclofenac sodium, the Co(II) and Ni(II) complexes showed a good percentage inhibition.
Author Contributions
Conceptualization, M.M., A.A.O. and S.B.; methodology, S.M., P.M., G.M.; software, A.M. and M.N.A.; validation, M.P., H.S.A. and A.K.; formal analysis, A.M.A.; investigation, M.M.R.; resources, M.M., G.M., H.S.A., A.M.A. and M.M.R.; data curation, A.K.; writing—original draft preparation, A.M.; writing—review and editing, S.M.; visualization, M.M.; supervision, A.M.; project administration. All authors have read and agreed to the published version of the manuscript.
Funding
The Deanship of Scientific Research (DSR) at King Abdulaziz University, Jeddah, Saudi Arabia has funded this project, under grant number (KEP-18-130-42).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Singh, R.K.; Gupta, A.K.; Prakash, S.; Prakash, D. Mixed Ligand Complexes of Al(III) with Chelating Organic Acids and Ethylenediamine. Orient. J. Chem. 2020, 36, 1225–1228. [Google Scholar] [CrossRef]
- Dorkov, P.; Pantcheva, I.N.; Sheldrick, W.S.; Mayer-Figge, H.; Petrova, R. Synthesis, structure and antimicrobial activity of manganese (II) and cobalt (II) complexes of the polyether ionophore antibiotic Sodium Monensin A. J. Inorg. Biochem. 2008, 102, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Nishat, N.; Rahis-Ud-Din Dhyani, S. Synthesis, characterization and antimicrobial activity of a new macrocycle and its transition metal complexes. J. Coord. Chem. 2009, 62, 996–1004. [Google Scholar] [CrossRef]
- Akter, J.; Hanif, M.A.; Islam, M.S.; Haque, M.M.; Lee, S.H.; Banu, L.A. Synthesis, characterization and antimicrobial activity of Ni(II) and Zn(II) complexes with amino acids and heterocyclic amine. Der. Chem. Sin. 2016, 7, 75. [Google Scholar]
- Mercy, O.; Bamigboye, I.; Ejidike, P. Synthesis, Characterization, Antimalarial and Antimicrobial activities of Mixed Ibuprofen-Pyrimethamine M(II) Complexes [M=Cd, Co, Zn, Mn]. Nat. Appl. Sci. J. 2019, 2, 39. [Google Scholar] [CrossRef]
- Sodhi, R.K.; Paul, S. Metal Complexes in Medicine: An Overview and Update from Drug Design Perspective. Canc. Therapy Oncol. Int. J. 2019, 14, 555883. [Google Scholar] [CrossRef]
- Hossain, S.; Mannan, A.; Camellia, F.K.; Zaman, A.K.B.; Zakaria, C.M.; Kudrat-E-Zahan, M. Isoniazid Containing Metal Based Drugs as Potential Antimicrobial Agent: A Short Review. Sci. J. Chem. 2017, 5, 62–70. [Google Scholar] [CrossRef][Green Version]
- Akinyele, O.F.; Fakola, E.G.; Durosinmi, L.M.; Ajayeoba, T.A.; Ayeni, A.O. Synthesis and characterization of heteroleptic metal complexes of isoniazid and metformin. Ife J. Sci. 2019, 21, 184–192. [Google Scholar] [CrossRef]
- Nesa, S.; Hossain, S.; Nasira, S.; Uddin, N.; Ashrafuzzaman, M.; Habib, A.; Rashid, A.M.; Haque, M. Mixed ligand complexes: Synthesis, characterization and antibacterial activity investigation. Int. J. Chem. Stud. 2020, 8, 306–312. [Google Scholar] [CrossRef]
- Ogunniran, K.O.; Mesubi, M.A.; Adekoya, J.A.; Siyanbola, O.O.; Inegbinebor, A.I.; Ojo, O.O.; Adedapo, A.E.; Edobor-Osoh, A.; Narender, T. SYNTHESIS OF (E)-N’-(5-bromo-2-hydroxybenzylidene)nicotinohydrazide) AND ITS Pt(II), Zn(II), Mn(II), Ni(II) AND Mo(V) COMPLEXES AS POTENTIAL ANTI- TUBERCULAR AGENT. Can. J. Pure Appl. Sci. 2015, 9, 3519–3534. [Google Scholar]
- Kumbar, M.; Patil, S.A.; Toragalmath, S.S.; Kinnal, S.M.; Shettar, A.; Hosakeri, J.H. Anticancer activity studies of novel metal complexes of ligands derived from polycyclic aromatic compound via greener route. J. Organomet. Chem. 2020, 15, 121219. [Google Scholar] [CrossRef] [PubMed]
- Jeffery, G.H.; Bassett, J.; Mendham, J.; Denney, R.C. Vogel’s Textbook of Quantitative Chemical Analysis, 5th ed.; John Wiley and Sons Inc.: New York, NY, USA, 2005; p. 10158. ISBN 0-582-44693-7. [Google Scholar]
- Kuchárová, V.; Kuchár, J.; Zaric, M.; Canovic, P.; Arsenijevic, N.; Volarevic, V.; Misirkic, M.; Trajkovic, V.; Radojević, I.D.; Čomić, L.R.; et al. Low-dimensional compounds containing bioactive ligands. Part XI: Synthesis, structures, spectra, in vitro anti-tumor and antimicrobial activities of 3D metal complexes with 8-hydroxyquinoline-5-sulfonic acid. Inorg. Chim. Acta 2019, 497, 119062. [Google Scholar] [CrossRef]
- Tadavi, S.K.; Bendre, R.S.; Patil, S.V.; Gaguna, S.; Rajput, J.D. Synthesis, crystal structures and antimicrobial activity of palladium metal complexes of sulfonyl hydrazone ligands. Eur. J. Chem. 2020, 11, 377–384. [Google Scholar] [CrossRef]
- Aly, S.A.; Fathalla, S.K. Preparation, characterization of some transition metal complexes of hydrazone derivatives and their antibacterial and antioxidant activities. Arab. J. Chem. 2020, 13, 3735–3750. [Google Scholar] [CrossRef]
- Shukla, S.; Mishra, A.P. Metal complexes used as anti-inflammatory agents: Synthesis, characterization and anti-inflammatory action of VO(II)-complexes. Arab. J. Chem. 2019, 12, 1715–1721. [Google Scholar] [CrossRef]
- Mary, S.J.; Chithra, B.; Sivajiganesan, S. In vitro anti -inflammatory activity of the flowers of nerium oleander (white). Int. J. Res. Granthaalayah 2017, 5, 123–128. [Google Scholar] [CrossRef]
- Ali, I.; Wani, W.A.; Saleem, K. Empirical Formulae to Molecular Structures of Metal Complexes by Molar Conductance. Synth. React. Inorg. Met. Org. Nano-Met. Chem. 2013, 43, 1162–1170. [Google Scholar] [CrossRef]
- Kuate, M.; Conde, M.A.; Mainsah, E.N.; Paboudam, A.G.; Tchieno, F.M.M.; Ketchemen, K.I.Y.; Kenfack, I.T.; Ndifon, P.T. Synthesis, Characterization, Cyclic Voltammetry, and Biological Studies of Co(II), Ni(II), and Cu(II) Complexes of a Tridentate Schiff Base, 1-((E)-(2-Mercaptophenylimino) Methyl) Naphthalen-2-ol (H2L1). J. Chem. 2020, 2020, 5238501. [Google Scholar] [CrossRef]
- Shaju, K.; Joby, T.; Vinod, P.; Kuriakose, N. Spectral and cyclic voltammetric studies on Cu(II)-Schiff base complex derived from anthracene-9(10 H)-one. IOSR J. Appl. Chem. 2014, 7, 64–68. [Google Scholar]
- Singh, K.; Thakur, R.; Kumar, V. Co(II), Ni(II), Cu(II), and Zn(II) complexes derived from 4-[{3-(4-bromophenyl)-1-phenyl-1H-pyrazol-4-ylmethylene}-amino]-3-mercapto-6-methyl-5-oxo-1,2,4-triazine. Beni-Suef Univ. J. Basic Appl. Sci. 2016, 5, 21–30. [Google Scholar] [CrossRef]
- Geeta, B.; Shravankumar, K.; Reddy, P.M.; Ravikrishna, E.; Sarangapani, M.; Reddy, K.K.; Ravinder, V. Binuclear cobalt(II), nickel(II), copper(II) and palladium(II) complexes of a new Schiff-base as ligand: Synthesis, structural characterization, and antibacterial activity. Spectrochim. Acta Part. A Mol. Biomol. Spectrosc. 2010, 77, 911–915. [Google Scholar] [CrossRef] [PubMed]
- Ramadevi, P.; Singh, R.; Prajapati, A.; Gupta, S.; Chakraborty, D. Cu(II) Complexes of Isoniazid Schiff Bases: DNA/BSA Binding and Cytotoxicity Studies on A549 Cell Line. Hindawi Publ. Corp. Adv. Chem. 2014, 2014, 630575. [Google Scholar] [CrossRef]
- Adly, O.M.I.; Shebl, M.; Abdelrhman, E.M.; El-Shetary, B.A. Synthesis, spectroscopic, X-ray diffraction, antimicrobial and antitumor studies of Ni(II) and Co(II) complexes derived from 4- acetyl-5,6-diphenyl-3(2H)-pyridazinone and ethylenediamine. J. Mol. Struct. 2020, 1219, 128607. [Google Scholar] [CrossRef]
- Sovari, S.N.; Zobi, F. Recent Studies on the Antimicrobial Activity of Transition Metal Complexes of Groups 6–12. Chemistry 2020, 2, 418–452. [Google Scholar] [CrossRef]
- Manimaran, P.; Balasubramaniyan, S. Synthesis, Characterization and Biological Evaluation of Fe(III) and Cu(II) Complexes with 2,4-Dinitrophenyl hydrazine and Thiocyanate Ions. Asian J. Chem. 2019, 31, 780–784. [Google Scholar] [CrossRef]
- Hossain, S.; Zakaria, C.M.; Kudrat-E-Zahan, M. Metal Complexes as Potential Antimicrobial Agent: A Review. Am. J. Heterocycl. Chem. 2018, 4, 1–21. [Google Scholar] [CrossRef]
- Szczepaniak, A.; Fichna, J. Organometallic Compounds and Metal Complexes in Current and Future Treatments of Inflammatory Bowel Disease and Colorectal Cancer—A Critical Review. Biomolecules 2019, 9, 398. [Google Scholar] [CrossRef]
- Tadele, K.T. Antioxidant Activity of Schiff Bases and Their Metal Complexes. A Recent Rev. J. Pharm. Med. Res. 2017, 3, 73–77. [Google Scholar]
- Ejidike, I.P.; Ajibade, P.A. Synthesis, Characterization, Anticancer, and Antioxidant Studies of Ru(III) Complexes of Monobasic Tridentate Schiff Bases. Bioinorg. Chem. Apps. Hindawi Publ. Corp. 2015, 2015, 890734. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).