Abstract
The crystal structure of the ferromagnetically-coupled CuII3−pyrazolato complex, (Bu4N)2[Cu3(μ3-Cl)2(μ-4-NO2-pz)3Cl3] (1a, pz = pyrazolato anion), was originally determined in the triclinic P-1 space group. By varying the recrystallization solvent and temperature, two additional true polymorphs were crystallized in the monoclinic P21/n (1b) and orthorhombic Pbca (1c) space groups. Comparison of the metric parameters of the three polymorphs revealed only minor variations in their bond lengths and angles but clearly distinguishable packing patterns. The DFT calculations showed that, in vacuum, 1a had the lowest energetic minimum (also the densest of three polymorphs), whereas 1b and 1c lay at 6.9 kcal/mol and 7.8 kcal/mol higher energies. The existence of isolable 1b and 1c is, therefore, attributed to the intermolecular interactions analyzed by the Hirshfeld methods.
1. Introduction
Polymorphism [1] is a well-established phenomenon in organic compounds, resulting in the manifestation of critical differences in the pharmaceutical properties of compounds crystallized as different polymorphs [2,3]. Polymorphism is also common among minerals—calcium carbonate and the 14 known polymorphs of silica are good examples—and binary or ternary solid-state materials with often strikingly different properties manifested by their various forms [4,5]. Metal organic frameworks (MOFs) are also commonly encountered [6], as are materials undergoing a phase transition to a new polymorph under pressure. In contrast, transition metal complexes are rarely encountered as true polymorphs at ambient conditions [7,8,9,10,11]; a few more examples were included in a review article [12].
Trinuclear copper pyrazolato complexes of the general formula, [Cu3(µ3-E)(µ-4-R-pz)3X3]z, where E = OH, O, (Cl)2, (Br)2, and OMe; R = H, Cl, Br, I, Me, NO2, -CHO, and -COOEt; X = Cl, Br, PhCOO-, py, SCN-, and MeCOO-, and z = 2−, 1−, and 2+, have been studied in our laboratory for over two decades for their interesting magnetic, structural, and electrochemical properties [13,14,15,16,17,18,19,20,21]. The capping ligand E forces the Cu3E motif into one of three geometries: planar (Cu3O), trigonal pyramidal (Cu3(OH)), or trigonal bipyramidal (Cu3X2).
We have previously published a ferromagnetically coupled CuII3-pyrazolato complex, (Bu4N)2[Cu3(μ3-Cl)2(μ-4-NO2-pz)3Cl3] (1a), which crystallized in the triclinic P-1 space group [22]. Herein, we report its two true polymorphs in the P21/n (1b) and Pbca (1c) space groups and discuss the analyses of their Hirshfeld surfaces and energies calculated by DFT methods.
2. Materials and Methods
2.1. Materials
All reagents, except 4-nitro-pyrazole were purchased from commercial sources and used as received. 4-Nitro-pyrazole was synthesized following procedures in the literature [23]. The solvents were purified and dried using standard techniques [24].
2.2. Synthesis
The synthetic method for (Bu4N)2[Cu3(μ3-Cl)2(μ-4-NO2-pz)3Cl3] has been previously published along with the crystal structure of 1a [22]. Whereas the trinuclear complex was synthesized in the manner reported, the crystallization of 1a–c differed as follows: saturated solutions of the complex in boiling n-propyl alcohol (1a), n-butyl alcohol (1b), and methanol (1c) were filtered while hot and allowed to cool down under ambient conditions, resulting in X-ray quality crystals. Except for the methanol, a small amount of acetonitrile was added to the alcoholic solution during crystallization to prevent the formation of oily products on cooling.
2.3. X-ray Crystallography and Data Collection
X-ray diffraction data were collected with a Bruker AXS SMART 1K CCD diffractometer [25] with graphite-monochromated Mo Kα radiation (λ = 0.71073 Å) at ambient temperature. The data were corrected for Lorentz and polarization effects [26]. The structures were solved using the SHELXTL-direct methods program, then refined by full-matrix least squares methods on F2 [27]. The crystal data and structure refinement parameters are listed in Table 1. CCDC 2215557 (1b) and 2215558 (1c) contain the supplementary crystallographic data for this paper and can be obtained free of charge from the Cambridge Crystallographic Data Center via www.ccdc.cam.ac.uk/data_request/cif.

Table 1.
Crystal data and structure refinement parameters for 1a–c.
2.4. DFT Calculations
DFT calculations were carried out, with geometry optimization, without symmetry restrictions using the B3LYP [28,29] hybrid density functional with the 6-31G* basis set for all atoms [30] using Gaussian-09 [31] software. Correctness of the calculated electronic states was ensured by checking the stability of the SCF solutions; the calculations confirmed that all the considered structures had the quartet ground electronic state. Geometry optimizations were carried out in the gas phase.
2.5. Software
Geometric calculations and visual representations of the molecules were obtained from both Olex2 [32] and Mercury 2020.3.0 [33]. Hirshfeld surfaces, fingerprint plots, and the associated images were calculated and obtained using CrystalExplorer17 [34]. Hirshfeld surface analysis and the capabilities of CrystalExplorer are well discussed in the literature [35,36,37,38].
3. Results and Discussion
3.1. Crystal Structure Descriptions
3.1.1. General Structure Description
A representative structure of the [Cu3(μ3-Cl)2(μ-4-NO2-pz)3Cl3]2− unit of 1a, 1b, and 1c is shown in Figure 1, and selected bond lengths of each polymorph are listed in Table 2. The charge in each dianionic complex is balanced by two crystallographically independent tetrabutylammonium cations. 1a was previously crystallized from boiling n-propyl alcohol and reported in the P-1 space group, while 1b was crystallized from boiling n-butyl alcohol in the P21/n space group, and 1c was crystallized from boiling methanol in the Pbca space group. As the symmetry of each polymorph increased from the triclinic to monoclinic to orthorhombic crystal systems, the crystal density (as reported in Table 1) decreased from 1.394 g cm−3 to 1.357 g cm−3 to 1.346 g cm−3.
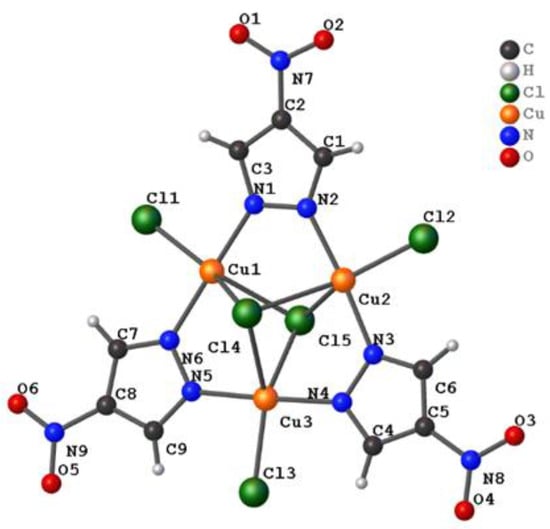
Figure 1.
Representative structure and atom numbering scheme of the [Cu3(μ3-Cl)2(μ-4-NO2-pz)3Cl3]2− unit for 1(a–c).

Table 2.
Selected bond lengths (Å) for 1a–1c.
3.1.2. Tau Parameter Determination
In all cases, the Cu centers are in a triangular arrangement and the 5-coordinate environment of each Cu atom is formed by two μ-4-NO2-pyrazolate bridges, two μ3-Cl caps, and one terminal Cl ligand. While the Cu-N bond lengths of each [Cu3(μ3-Cl)2(μ-4-NO2-pz)3Cl3]2− unit are the same within experimental error, each polymorph exhibits different ligand (chloride and pyrazolate) positions in relation to the Cu3-plane and different Cu-Cl bond lengths. The geometry of 5-coordinate metal centers can be ideally represented as either square–pyramidal (trans angles α = β = 180°) or trigonal bipyramidal (α = 120°; β = 180°) where β is the largest angle and α is the second largest. For complexes that deviate from ideal geometries, the τ parameter, defined as follows, provides a quantitative description of the coordination geometry [39]:
Therefore, τ = 0 for a perfectly square pyramidal complex, and τ = 1 for a perfectly trigonal bipyramidal complex. The tau parameters calculated for each one of the three Cu-centers of the polymorphs, along with the total deviation of the three Cu-centers of each polymorph, Στ, are listed in Table 3.

Table 3.
Selected bond angles (°) and calculated τ values for 1a–1c.
The tau parameters for complex 1b are most consistently between square pyramidal and trigonal bipyramidal. The highest tau value of 0.57 is encountered for the Cu3 of 1a, while the lowest one of 0.21 is for the Cu1 of 1c; thus, the geometries of these two metal centers better adheres to the trigonal bipyramidal and square pyramidal, respectively. However, the higher total deviation of the three Cu-centers is encountered in 1b. Figure 2 illustrates the differences in the geometries of these two extremes. For each case, the copper atom and immediate coordination sphere are shown in two different orientations: (1) Cl4 as the axial ligand (square pyramidal) and (2) the nitrogen atoms as the axial ligands (trigonal bipyramidal).
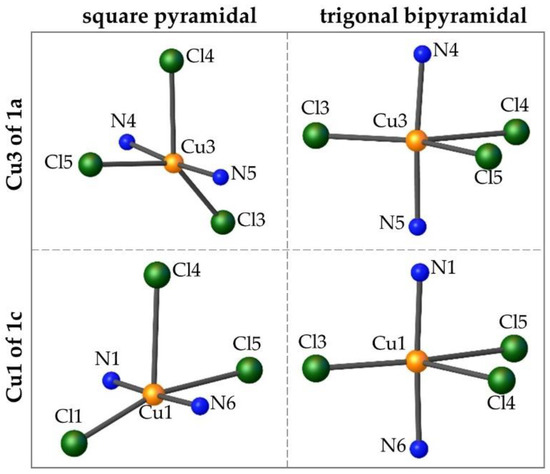
Figure 2.
Comparison of the square pyramidal and trigonal bipyramidal geometries for the Cu3 of 1a (τ = 0.57) and the Cu1 of 1c (τ = 0.21).
3.1.3. Ligand Position in Relation to the Cu3 Plane
Another illustrative parameter for describing the three polymorphs is the distance of the three terminal chloride atoms and the three 4-NO2-pyrazolate ligands from the Cu3-plane, as reported in Table 4. Here, a centroid was calculated for each pyrazolate ring: pz1 refers to the centroid of the plane that contains N1, N2, C1, C2, and C3; pz2 refers to the centroid of the plane that contains N3, N4, C4, C5, and C6; and pz3 refers to the centroid of the plane that contains N5, N6, C7, C8, and C9. A positive or negative sign is attributed to the ligand being either above or below the Cu-plane, respectively, with each molecule oriented so that Cl5 is above the plane and Cl4 is below the plane.

Table 4.
Distances (d, Å) of the terminal chloride ligands and pyrazolates from the Cu3-plane for 1a–c.
Due to the bridging nature of the pyrazolates, the distance between the terminal Cl ligands and the Cu3-plane is larger than that of the pyrazolate rings (as expected) except for 1b—in which and are larger by 0.054 and 0.044, respectively. 1c has the largest values for both and , therefore, exhibiting the most deviation either above or below the Cu3-plane. However, when the sum is calculated, allowing for the total effect to be taken into consideration— and —,1c has the smallest values of the three polymorphs.
3.1.4. Crystal Packing
As the name suggests, the three polymorphs give rise to vastly different crystal packing motifs. The types of interactions that dominate each polymorph are discussed more in depth in Section 3.3 (vide infra). For simplicity, the crystal packing of the complex is shown separately from that of the cations. The crystal packing viewed parallel to the Cu3 plane for each molecule is shown in Figure 3. The complexes of 1a are arranged in pairs that exhibit repulsions between a terminal chloride (Cl2) and the nitro group of a pyrazolate. The Cl2…N7 distance is 3.660 Å. These pairs of cations are eclipsed and extend down the a-axis. The complexes of 1b are arranged in a manner that allows for the nitro groups of pyrazolates to be stacked with a N7…N7 distance of 3.246 Å. Two other pyrazolates have reciprocal C-H…O interactions with a C6…O3 distance of 3.271 Å. These two types of pyrazolate interactions alternate parallel to the b-axis. The complexes of 1c are arranged in pairs with one nitro group (O1-N7-O2) having interactions with a terminal and capping chloride. The N7…Cl1 distance is 3.767 Å with the chloride ligand bending away from the nitro group. The O1…Cl5 distance is 3.432 Å. Unlike the pairs in 1a, the pairs in 1c do not extend into the plane; instead, they rotate ~90° down the plane shown in Figure 3.
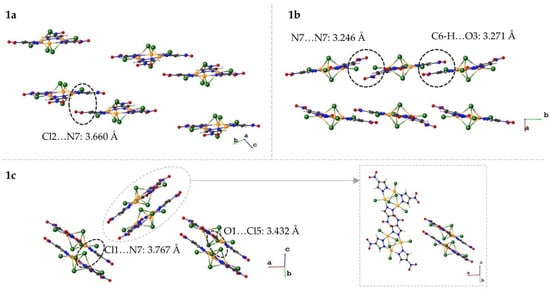
Figure 3.
Crystal packing of the dianionic complex viewed approximately parallel to the Cu3 plane for 1a (top left), 1b (top right), and 1c (bottom). The inset of 1c shows the rotation of the pair directly behind and in front of the specified pair. Significant interactions are circled and labeled with the identity and distance of the interaction.
The crystal packing of the tetrabutylammonium cations for each molecule are shown in Figure 4. In each polymorph, layers of chains are formed. In 1a, the chains are approximately linear, running alternatingly parallel to the b-axis and diagonally in the bc plane. The dianionic copper complexes occupy the spaces formed between the diagonal cation chains. The cations in 1b form sinusoidal waves that propagate parallel to the c-axis; the adjacent layers of cation waves are out of phase. The complex anions occupy the channels created by layers of waves. The cations in 1c form corrugated layers parallel to the a-axis. Between consecutive layers are pairs of crystallographically non-independent cations. Four complex molecules surround each pair of cations between the layers.
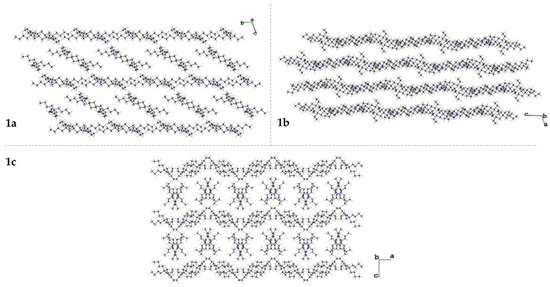
Figure 4.
Crystal packing of the two crystallographically independent tetrabutylammonium cations for 1a (top left), 1b (top right), and 1c (bottom).
3.2. DFT Calculations
The potential energies of the three polymorphs were calculated in the gas phase at the B3LYP/6-31G* level of theory. Since calculations of vibrational frequencies were not feasible for molecules of this size, zero-point vibrational energy corrections were not considered. However, this is not expected to affect the computed relative energies significantly. According to the calculations, all three structures correspond to the local minima at the potential energy surface, with 1a having the lowest energy, and 1b and 1c lying respectively 6.9 and 7.8 kcal/mol higher; these results should be interpreted qualitatively, because their differences are within the range of B3LYP accuracy. The main structural difference in the gas phase is the position of the tetrabutylammonium cations with respect to the central Cu3(μ3-Cl)2(μ-4-NO2-pz)3Cl3 anion, which is diagonal in 1a, horizontal in 1b, and vertical in 1c.
3.3. Hirshfeld Surface Analysis
Figure 5, Figure 6 and Figure 7 show the front and back of the dnorm Hirshfeld surface for 1a, 1b, and 1c, respectively. The “front” face of each molecule is the one that contains μ3-Cl4 while the “back” face is the one that contains μ3-Cl5. Although 1a is the densest polymorph, the Hirshfeld surface of 1b has the largest number of significant interactions. In total, the Cu complexes of 1a, 1b, and 1c are in contact with five, seven, and four different tetrabutylammonium cations, respectively. In addition, the surface of 1b has significant interactions with one dianionic complex, while 1a and 1c do not exhibit complex–complex interactions. The types of complex–cation interactions that dominate each polymorph differ as follows. 1a exhibits mainly C-H…Cl interactions and has two strong interactions with a capping Cl (C27-H…Cl5 (3.689(5) Å) and C30-H…Cl5 of 3.651(4) Å). 1b exhibits the strongest interactions involving the nitro group of the pyrazolate ligands (C24-H…O4 (3.356(9) Å), C26-H…O6 (3.375(8) Å), C10-H…O2 (3.404(8) Å, C38-H…O3 (3.520(6) Å), and C30-H…O3 (3.570(6) Å). 1b also has an interaction between the 4-NO2-pyrazolates of two separate Cu complexes: O3…H-C6′ and the reciprocal O3′…H-C6 of 3.271(7) Å. 1c also has its strongest interaction with the nitro group of a pyrazolate (C21-H…O2 (3.36(2) Å) and is the only polymorph to have an interaction with the ring of the 4-NO2-pyrazolate (C28-H…C7/C8 (3.72(3) Å). A more in-depth description of the Hirshfeld surface interactions can be found in the supplemental information.
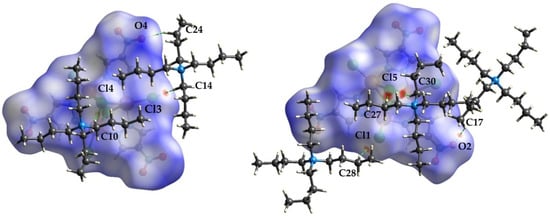
Figure 5.
Hirshfeld surface of the front (left) and back (right) of the [Cu3(μ3-Cl)2(μ-4-NO2-pz)3Cl3]2− unit of 1a showing the intermolecular interactions with the tetrabutylammonium cations.
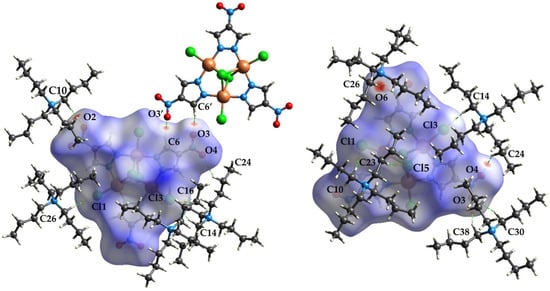
Figure 6.
Hirshfeld surface of the front (left) and back (right) of the [Cu3(μ3-Cl)2(μ-4-NO2-pz)3Cl3]2− unit of 1b showing the intermolecular interactions with the tetrabutylammonium cations and other Cu3 dianionic complexes.
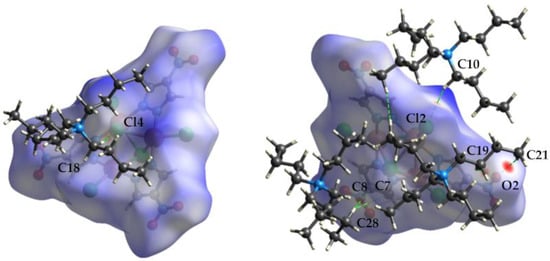
Figure 7.
Hirshfeld surface of the front (left) and back (right) of the [Cu3(μ3-Cl)2(μ-4-NO2-pz)3Cl3]2− unit of 1c showing the intermolecular interactions with the tetrabutylammonium cations.
From the dnorm Hirshfeld surfaces, 2D fingerprint plots were assembled as shown in Figure 8. All three polymorphs show the most interactions (dark blue = small number of interactions, while green = moderate number of interactions) around di ≈ 2 Å, de ≈ 1.4 Å. The main difference between the shape of each plot is the number of interactions at high di/de values. 1a is the densest polymorph, and thus its surface has fewer long-range interactions (those above di ≈ de ≈ 2.6 Å). The other main difference is the number of spikes of each fingerprint plot. 1a has two main spikes. The first is located roughly at di ≈ 1.4 Å, de ≈ 1.1 Å and continues diagonally upwards. This depicts the O…H interactions. The second spike is located roughly at di ≈ 1.6 Å, de ≈ 1.0 Å and continues diagonally upwards. This spike is attributed to the Cl…H interactions. 1b has the highest number of spikes (four) of the polymorphs: (1) di ≈ 1.1 Å, de ≈ 1.4 Å; (2) di ≈ de ≈ 1.2 Å; (3) di ≈ 1.3 Å, de ≈ 1.0 Å; and (4) di ≈ 1.7 Å, de ≈ 1.1 Å. The O…H interactions account for the first and third spikes. The second spike is attributed to the H…H interactions, and the fourth spike is attributed to the Cl…H interactions. The Cl…H interactions of 1b are not as strong as those of 1a; however, the O…H interactions of 1b are stronger than those of 1a. The plot of 1c shows three spikes: (1) di ≈ de ≈ 1.1 Å; (2) di ≈ 1.4 Å, de ≈ 1.0 Å; and (3) di ≈ 1.7 Å, de ≈ 1.1 Å. These are attributed to the H…H, O…H, and Cl…H interactions, respectively. Here, the H…H interactions are slightly stronger than in 1b, while the strength of the O…H interactions is similar to 1a, and the strength of the Cl…H interactions is similar to 1b. The breakdown of the 2D fingerprint plots by type of atomistic contribution is shown in Figures S1–S3 of the supplementary information.
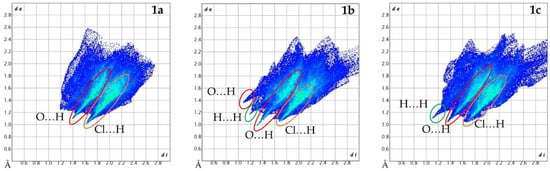
Figure 8.
2D fingerprint plots (di/de) of 1a (left), 1b (middle), and 1c (right) showing the types of shortest interactions for each Hirshfeld surface.
For all three polymorphs, approximately 93% of all interactions of the complex surface consist of five types: O…H, Cl…H, H…H, C…H, and N…H. There are small differences in the percentages of these interactions, as shown in Figure 9. The remaining interactions account for 2% of the interactions of each polymorph, and the same type of minor interactions do not occur for each polymorph. For example, 1b has no Cu…O interactions or Cl…N interactions and is the only polymorph that contains N…N interactions. Only 1a had Cl…C interactions. 1c had no O…C interactions.
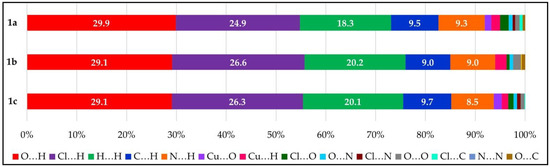
Figure 9.
Bar graph, compiling the Hirshfeld surface interactions for 1a–c analyzed by percent contributions of individual atomic contacts.
4. Conclusions
By varying the crystallization solvent and temperature of (Bu4N)2[Cu3(μ3-Cl)2(μ-4-NO2-pz)3Cl3], three true polymorphs of this complex, 1a–c, have been recognized so far. Aside from the numerous polymorphs of [M(acac)3] complexes (where M = Fe [40], Cr [41], or Mn [42] and acac = acetylacetonate), we are aware of only a few other examples of metal complexes known in three polymorphic structures under ambient conditions [7,43]. The X-ray crystal structures of the three polymorphic complexes revealed insignificant variations in their bond lengths and angles in contrast to their clearly differentiated 3D packing and, therefore, differences in intermolecular interactions. The DFT calculations showed that, in vacuum, the triclinic 1a was the ground state polymorph. However, in the solid state, the sum of the various intermolecular interactions (not quantified here) was significant enough to offset the polymorphic instabilities of 6.9 kcal/mol and 7.8 kcal/mol and allow phase transitions to the monoclinic and orthorhombic forms, 1b and 1c, respectively. The intermolecular interactions were qualitatively probed by Hirshfeld surface analysis. Interestingly, 1a was also the densest, therefore the one with the higher number of contacts involving the μ3-Cl, terminal Cl, and NO2 groups. However, 1b had the highest number of significant interactions with the Hirshfeld surface, whereas 1c had only two strong interactions—involving a terminal Cl and a NO2 group. It was not possible to assess here the role of the crystallization solvent polarity and boiling point.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/cryst12111611/s1, Figures S1–S3: Breakdown of 2D fingerprint plot by type of individual atomistic contribution for complexes 1a,b, respectively. Additional Hirshfeld surface analysis of Figure 5, Figure 6 and Figure 7 in the main text.
Author Contributions
K.L.R. data analysis and manuscript preparation; L.M. data analysis; G.M. research and data analysis; A.M.M. computational DFT data; R.G.R., supervision and manuscript preparation. All authors have read and agreed to the published version of the manuscript.
Funding
K.L.R. was supported by the U.S. Nuclear Regulatory Commission (NRC) fellowship grant No. 31310018M0012 awarded to FIU.
Data Availability Statement
Not applicable.
Acknowledgments
The authors would like to acknowledge Indranil Chakraborty for his crystallographic expertise.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Bernstein, J. Polymorphism in Molecular Crystals; International Union of Crystallography Monographs on Crystallography; Oxford University Press: Oxford, UK, 2007. [Google Scholar] [CrossRef]
- Stahly, G.P. Diversity in Single- and Multiple-Component Crystals. The Search for and Prevalence of Polymorphs and Cocrystals. Cryst. Grow. Des. 2007, 7, 1007–1026. [Google Scholar] [CrossRef]
- Hilfiker, R. Polymorphism: In the Pharmaceutical Industry; Wiley-VCH: Weinheim, Germany, 2006. [Google Scholar]
- Saha, B.K.; Nath, N.K.; Thakuria, R. Polymorphs with Remarkably Distinct Physical and/or Chemical Properties. Chem. Rec. 2002, e202200173. [Google Scholar] [CrossRef] [PubMed]
- Benito, Q.; Le Goff, X.F.; Maron, S.; Fargues, A.; Garcia, A.; Martineau, C.; Taulelle, F.; Kahlal, S.; Gacoin, T.; Boilot, J.-P.; et al. Polymorphic Copper Iodide Clusters: Insights into the Mechanochromic Luminescence Properties. J. Am. Chem. Soc. 2014, 136, 11311–11320. [Google Scholar] [CrossRef]
- Dave, S.; Sahu, R.; Tripathy, B.C. Electrochemical Applications of Metal-Organic Frameworks; Elsevier: Amsterdam, The Netherlands, 2022; pp. 17–35. [Google Scholar]
- Chai, W.; Hong, M.; Song, L.; Jia, G.; Shi, H.; Guo, J.; Shu, K.; Guo, B.; Zhang, Y.; You, W.; et al. Three Reversible Polymorphic Copper(I) Complexes Triggered by Ligand Conformation: Insights into Polymorphic Crystal Habit and Luminescent Properties. Inorg. Chem. 2015, 54, 4200–4207. [Google Scholar] [CrossRef]
- Muthukumar, P.; Pannipara, M.; Al-Sehemi, A.G.; Moon, D.; Anthony, S.P. Polymorphs of a Copper Coordination Compound: Interlinking Active Sites Enhance the Electrocatalytic Activity of the Coordination Polymer Compared to the Coordination Complex. CrystEngComm 2020, 22, 425–429. [Google Scholar] [CrossRef]
- Wöhlert, S.; Runčevski, T.; Dinnebier, R.E.; Ebbinghaus, S.G.; Näther, C. Synthesis, Structures, Polymorphism, and Magnetic Properties of Transition Metal Thiocyanato Coordination Compounds. Cryst. Grow. Des. 2014, 14, 1902–1913. [Google Scholar] [CrossRef]
- Fromm, K.M.; Doimeadios, J.L.S.; Robin, A.Y. Concomitant Crystallization of Two Polymorphs—A Ring and a Helix: Concentration Effect on Supramolecular Isomerism. Chem. Commun. 2005, 36, 4548–4550. [Google Scholar] [CrossRef]
- White-Morris, R.L.; Olmstead, M.M.; Attar, S.; Balch, A.L. Intermolecular Interactions in Polymorphs of Trinuclear Gold(I) Complexes: Insight into the Solvoluminescence of AuI3(MeNCOMe)3. Inorg. Chem. 2005, 44, 5021–5029. [Google Scholar] [CrossRef]
- Brog, J.-P.; Chanez, C.-L.; Crochet, A.; Fromm, K.M. Polymorphism, What It Is and How to Identify It: A Systematic Review. RSC Adv. 2013, 3, 16905. [Google Scholar] [CrossRef]
- Angaridis, P.A.; Baran, P.; Boča, R.; Cervantes-Lee, F.; Haase, W.; Mezei, G.; Raptis, R.G.; Werner, R. Synthesis and Structural Characterization of Trinuclear CuII −Pyrazolato Complexes Containing μ3 -OH, μ3 -O, and μ3 -Cl Ligands. Magnetic Susceptibility Study of [PPN]2[(μ3-O)Cu3(μ-pz)3Cl3]. Inorg. Chem. 2002, 41, 2219–2228. [Google Scholar] [CrossRef]
- Boudalis, A.K.; Rogez, G.; Heinrich, B.; Raptis, R.G.; Turek, P. Towards Ionic Liquids with Tailored Magnetic Properties: Bmim+ Salts of Ferro- and Antiferromagnetic CuII3 Triangles. Dalton Trans. 2017, 46, 12263–12273. [Google Scholar] [CrossRef]
- Kreiger, D.I.; Mathivathanan, L.; Raptis, R.G. Coordination Polymers Based on Pyrazole-4-Carboxaldehyde-Containing Cu3N6 Metallacycles as Building Units. CrystEngComm 2019, 21, 3047–3055. [Google Scholar] [CrossRef]
- Mathivathanan, L.; Boudalis, A.K.; Turek, P.; Pissas, M.; Sanakis, Y.; Raptis, R.G. Interactions between H-Bonded [CuII3(μ3-OH)] Triangles; a Combined Magnetic Susceptibility and EPR Study. Phys. Chem. Chem. Phys. 2018, 20, 17234–17244. [Google Scholar] [CrossRef] [PubMed]
- Mathivathanan, L.; Rogez, G.; Ben Amor, N.; Robert, V.; Raptis, R.G.; Boudalis, A.K. Origin of Ferromagnetism and Magnetic Anisotropy in a Family of Copper(II) Triangles. Chem. Eur. J. 2020, 26, 12769–12784. [Google Scholar] [CrossRef] [PubMed]
- Rivera-Carrillo, M.; Chakraborty, I.; Mezei, G.; Webster, R.D.; Raptis, R.G. Tuning of the [Cu3(μ-O)]4+/5+ Redox Couple: Spectroscopic Evidence of Charge Delocalization in the Mixed-Valent [Cu3(μ-O)]5+ Species. Inorg. Chem. 2008, 47, 7644–7650. [Google Scholar] [CrossRef] [PubMed]
- Mezei, G.; Raptis, R.G. Effect of Pyrazole-Substitution on the Structure and Nuclearity of Cu(II)-Pyrazolato Complexes. Inorg. Chim. Acta 2004, 357, 3279–3288. [Google Scholar] [CrossRef]
- Mezei, G.; Rivera-Carrillo, M.; Raptis, R.G. Effect of Copper-Substitution on the Structure and Nuclearity of Cu(II)-Pyrazolates: From Trinuclear to Tetra-, Hexa- and Polynuclear Complexes. Inorg. Chim. Acta 2004, 357, 3721–3732. [Google Scholar] [CrossRef]
- Boča, R.; Dlháň, L.; Mezei, G.; Ortiz-Pérez, T.; Raptis, R.G.; Telser, J. Triangular, Ferromagnetically-Coupled CuII3−Pyrazolato Complexes as Possible Models of Particulate Methane Monooxygenase (PMMO). Inorg. Chem. 2003, 42, 5801–5803. [Google Scholar] [CrossRef]
- Mezei, G.; Raptis, R.G.; Telser, J. Trinuclear, Antiferromagnetically Coupled CuII Complex with an EPR Spectrum of Mononuclear CuII: Effect of Alcoholic Solvents. Inorg. Chem. 2006, 45, 8841–8843. [Google Scholar] [CrossRef]
- Maresca, K.P.; Rose, D.J.; Zubieta, J. Synthesis and Characterization of a Binuclear Rhenium Nitropyrazole Complex [Re2O3Cl2(PPh3)2(C3H2N3O2)2]. Inorg. Chim. Acta 1997, 260, 83–88. [Google Scholar] [CrossRef]
- Perrin, D.D.; Armarego, W.L.F.; Perrin, D.R. Purification of Laboratory Chemicals; Pergamon Press: Oxford, UK, 1980. [Google Scholar]
- Data Collection: SMART-NT Software Reference Manual; Version 5.0; Bruker AXS, Inc.: Madison, WI, USA, 1998.
- Data Reduction: SAINT-NT Software Reference Manual; Version 4.0; Bruker AXS, Inc.: Madison, WI, USA, 1996.
- Sheldrick, G.M. SHELXTL-NT; Version 5.1; Bruker AXS, Inc.: Madison, WI, USA, 1999. [Google Scholar]
- Becke, A.D. Density-functional Thermochemistry. III. The Role of Exact Exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti Correlation-Energy Formula into a Functional of the Electron Density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef] [PubMed]
- Hariharan, P.C.; Pople, J.A. The Influence of Polarization Functions on Molecular Orbital Hydrogenation Energies. Theoret. Chim. Acta 1973, 28, 213–222. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09; Revision D.01; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A Complete Structure Solution, Refinement and Analysis Program. J. Appl. Cryst. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Macrae, C.F.; Sovago, I.; Cottrell, S.J.; Galek, P.T.A.; McCabe, P.; Pidcock, E.; Platings, M.; Shields, G.P.; Stevens, J.S.; Towler, M.; et al. Mercury 4.0: From Visualization to Analysis, Design and Prediction. J. Appl. Cryst. 2020, 53, 226–235. [Google Scholar] [CrossRef] [PubMed]
- Spackman, P.R.; Turner, M.J.; McKinnon, J.J.; Wolff, S.K.; Grimwood, D.J.; Jayatilaka, D.; Spackman, M.A. CrystalExplorer: A Program for Hirshfeld Surface Analysis, Visualization and Quantitative Analysis of Molecular Crystals. J. Appl. Cryst. 2021, 54, 1006–1011. [Google Scholar] [CrossRef]
- Spackman, M.A.; Jayatilaka, D. Hirshfeld Surface Analysis. CrystEngComm 2009, 11, 19–32. [Google Scholar] [CrossRef]
- Sundareswaran, S.; Karuppannan, S. Hirshfeld Surface Analysis of Stable and Metastable Polymorphs of Vanillin. Crys. Res. Technol. 2020, 55, 2000083. [Google Scholar] [CrossRef]
- McKinnon, J.J.; Fabbiani, F.P.A.; Spackman, M.A. Comparison of Polymorphic Molecular Crystal Structures through Hirshfeld Surface Analysis. Cryst. Grow. Des. 2007, 7, 755–769. [Google Scholar] [CrossRef]
- Spackman, M.A.; McKinnon, J.J. Fingerprinting Intermolecular Interactions in Molecular Crystals. CrystEngComm 2002, 4, 378–392. [Google Scholar] [CrossRef]
- Addison, A.W.; Rao, T.N.; Reedijk, J.; van Rijn, J.; Verschoor, G.C. Synthesis, Structure, and Spectroscopic Properties of Copper(II) Compounds Containing Nitrogen–Sulphur Donor Ligands; the Crystal and Molecular Structure of Aqua[1,7-bis(N-methylbenzimidazol-2′-yl)-2,6-dithiaheptane]copper(II) perchlorate. J. Chem. Soc. Dalton Trans. 1984, 7, 1349–1356. [Google Scholar] [CrossRef]
- Baker, T.M.; Howard, K.M.; Brennessel, W.W.; Neidig, M.L. Crystal Structure of a Third Polymorph of Tris (acetyl acetonato-κ2O,O′)iron(III). Acta Cryst. 2015, E71, m228–m229. [Google Scholar] [CrossRef]
- Morosin, B. The Crystal Structure of Trisacetylacetonatochromium(III). Acta Cryst. 1965, 19, 131–137. [Google Scholar] [CrossRef]
- Geremia, S.; Demitri, N. Crystallographic Study of Manganese(III) Acetylacetonate: An Advanced Undergraduate Project with Unexpected Challenges. J. Chem. Educ. 2005, 82, 460. [Google Scholar] [CrossRef]
- Vrdoljak, V.; Prugovečki, B.; Matković-Čalogović, D.; Hrenar, T.; Dreos, R.; Siega, P. Three Polymorphic Forms of a Monomeric Mo(VI) Complex: Building Blocks for Two Metal–Organic Supramolecular Isomers. Intermolecular Interactions and Ligand Substituent Effects. Cryst. Grow. Des. 2013, 13, 3773–3784. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).