Abstract
A new spirooxindole hybrid engrafted imidazo[2,1-b]thiazole core structure was designed and achieved via [3+2] cycloaddition reaction approach. One multi-component reaction between the ethylene derivative based imidazo[2,1-b]thiazole scaffold with 6-Cl-isatin and the secondary amine under heat conditions afforded the desired compound in a stereoselective manner. The relative absolute configuration was assigned based on single-crystal X-ray diffraction analysis. Hirshfeld calculations for 4 revealed the importance of the H…H (36.8%), H…C (22.9%), Cl…H (10.4%) and S…H (6.6%), as well as the O…H (4.7%), N…H (5.3%), Cl…C (1.6%), Cl…O (1.0%) and N…O (0.5%) contacts in the crystal stability. DFT calculations showed excellent straight-line correlations (R2 = 0.9776–0.9962) between the calculated and experimental geometric parameters. The compound has polar nature (3.1664 Debye). TD-DFT and GIAO calculations were used to assign and correlate the experimental UV-Vis and NMR spectra, respectively.
1. Introduction
Imidazo [2,1-b]thiazole is an important bicyclic nitrogen and sulfur containing compound in some natural as well as synthetic pharmacologically active compounds and agrochemicals [1,2]. Many compounds reported in the literature incorporating imidazo[2,1-b]thiazole exhibited pharmaceutical targets such as antihelminthic [3], antifungal [4] and antibacterial [5], and also in cancer research development as anti-tumor agents [6,7,8]. Levamisolum is one of the representative pharmaceutically relevant molecules for immunomodulatory and antihelminthic agents which have partially hydrogenated imidazo[2,1-b]thiazole as a core constituent (Figure 1). Due to a drug-resistant bacterial infection and in the effort to develop a novel antimicrobial agent, Li et al. reported a new series of bicyclic incorporating dihydroimidazothiazole scaffold and exhibited high potency against methicillin-resistant S. aureus (MERSA) [9].
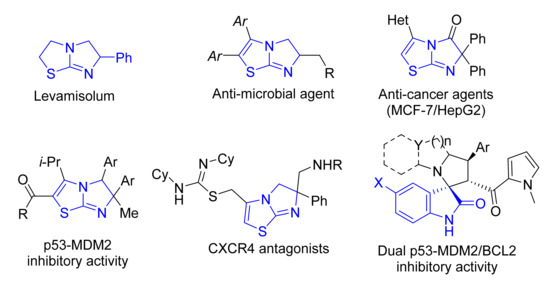
Figure 1.
Representative examples of biologically active compounds having imidazothiazole and spirooxindole derivatives.
Indeed, Miyazaki et al. reported a lead compound comprising imidazo[2,1-b]thiazole scaffold for cancer treatment, targeting p53–MDM2 protein–protein interaction inhibitors [10].
The Éric Marsault and Emanuel Escher research group designed, synthesized and studied the biological evaluation of the imidazo[2,1-b]thiazole system against CXCR4 antagonists [11]. The chemical and biological studies of this privileged structure have gained a lot of attention from the researchers.
Spirooxindole is an important pharmacophore exhibiting a lot of pharmaceutical targets including anti-tumor [12], and anti-inflammatory targets [13], but also for treating Alzheimer’s disease [14,15], and other pharmacological activity [16]. Recently, Barakat et al. reported a spirooxindole lead compound for cancer treatment targeting p53–MDM2 protein–protein interaction inhibitors [17,18]. The combination of these two spirooxindole and imidazo [2,1-b]thiazole pharmacophores may integrate the biological properties of both.
In the area of synthetic and medicinal chemistry research, multi-component reactions (MCRs) became important methodological arsenal as they were eco-friendly, had less reaction time, had a step-/atom economy and provided high-chemical yields. Additionally, for drug discovery and development, MCRs acted as an amenable tool for the generation of a library of new chemical entities. The [3+2] Cycloaddition (32CA) reaction [19,20,21,22] is a class of the multi-component reactions which afford a diversity of highly complex molecules efficiently and with a straightforward transformation.
In this text, we have reported the straightforward synthesis of a new compound having two pharmacophores based on the spirooxindole and imidazothiazole scaffolds via [3+2] cycloaddition (32CA) reaction. The crystal structure and the physical properties of the synthesized molecule were studied.
2. Materials and Methods
All technical instruments and chemicals used in this study are provided in the Supplementary Materials. The synthesis of imidazo[2,1-b]thiazole derivative 1 followed by the reported procedure [23].
(2S,7a’S)-6’’-Chloro-7’-(4-chlorophenyl)-5,6-diphenyl-7’,7a’-dihydro-1’H,3H,3’H-dispiro[imidazo[2,1-b]thiazole-2,6’-pyrrolo[1,2-c]thiazole-5’,3’’-indoline]-2’’,3-dione 4
A mixture of imidazo[2,1-b]thiazole derivative 1 (207 mg, 0.5 mmol), 6-Cl-isatin (90.5 mg, 0.5 mmol) and (R)-thiazolidine-4-carboxylic acid (66.5 mg, 0.5 mmol) in methanol (10 mL)/dichloromethane (DCM) (10 mL) was refluxed on an oil bath for the appropriate time of 5 h. After the completion of the reaction as evident from TLC, the reaction was kept at room temperature overnight and the solid precipitate was filtered off without any further purification in 92% chemical yield. Crystalline compound was obtained by slow evaporation in methanol.
1H NMR (400 MHz, CDCl3) δ 8.74 (s, 1H), 7.55–7.16 (m, 14H), 7.14 (d, J = 5.0 Hz, 1H), 6.98 (d, J = 8.0 Hz, 1H), 6.76 (s, 1H), 4.88 (q, J = 7.6 Hz, 1H), 4.15 (d, J = 9.0 Hz, 1H), 3.76 (d, J = 5.9 Hz, 1H), 3.58 (d, J = 5.9 Hz, 1H), 2.96 (dd, J = 9.8, 5.5 Hz, 1H), 2.84–2.73 (m, 1H); 13C NMR (101 MHz, CDCl3) δ 176.3, 168.7, 149.9, 146.1, 143.5, 137.3, 134.7, 134.6, 132.0, 131.5, 130.7, 129.5, 129.3, 128.7, 128.4, 128.4, 127.4, 127.3, 124.9, 123.1, 120.8, 111.7, 82.4, 75.7, 71.0, 56.2, 46.8, 32.9, 14.2; IR (KBr, cm−1): 3419, 3318, 2921, 2855, 1730, 1620, 1511, 1210; Chemical Formula: C35H24Cl2N4O2S2.
3. Results and Discussion
3.1. Chemistry
The target spirooxindole compound based imidazo[2,1-b]thiazole scaffold was designed and synthesized from the starting material named (Z)-2-(4-chlorobenzylidene)-5,6-diphenylimidazo[2,1-b]thiazol-3(2H)-one 1 with the 6-chloroisatin 2 and (R)-thiazolidine-4-carboxylic acid in MeOH/DCM (1:1) under reflux for 5 h (Scheme 1). The generated azomethine ylide was involved in the reaction as the intermediate which further moved to the [3+2] cycloaddition (32CA) reaction with the ethylene derivative based imidazo[2,1-b]thiazole to afford the new bis-spiro compound 4 (Scheme 2). The transition states were proposed to afford the only regioisomer and diastereoisomer based on the recent literature [19,20,21,22,24] (Scheme 2). The chemical feature of the bis-spiro compound 4 was assigned based on 1H-NMR, 13C-NMR, IR and single-crystal X-ray diffraction analysis. The data analysis revealed that the chemical structure fully agreed with the designed structure. The 1H-NMR spectrum showed the characteristic protons in the proposed structure as follows: NH proton assigned at δ 8.74 ppm as a singlet signal; in the aromatic region δ 7.55–7.16 ppm appeared for the overlapped 14 protons of the aromatic rings (two phenyl rings plus p-Cl-Ph); the three protons of the oxindole ring shown as two protons doublet and one proton singlet in the chemical shift at δ 7.14, 6.98 and 6.76 ppm, respectively. The protons of the fused cyclic ring appeared in the region between δ 4.88 until 2.73 ppm. 13C-NMR spectrum exhibited the assigned carbons of the synthesized compound in very good manner. IR spectrum showed most functional groups existing in the proposed structure, such as NH, C-H, C=O, C=C, C=N, C-S, C-N, stretching as well as the pending vibrational assignment.
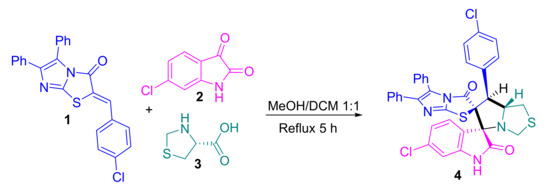
Scheme 1.
Synthesis of spirooxindole based imidazo[2,1-b]thiazole scaffold 4.
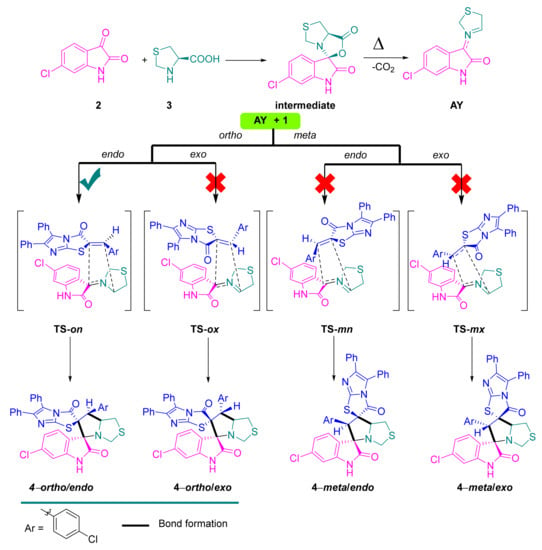
Scheme 2.
Proposed approach of AY to ethylene derivative 1, explaining the regio- and stereoselective synthesis of 4.
3.2. Crystal Structure Description of 4
The X-ray single-crystal structure analysis of 4 revealed the expected structure based on the spectral characterizations very well (Figure 2). The compound crystallized in a monoclinic crystal system and centrosymmetric P21/c space group with lattice parameters: a = 6.57040(10) Å, b = 29.6357(5)Å, c = 15.5008(4), β = 96.898(2)° (Table 1). The molecule comprised many ring systems, for more clarity these rings were designated as A to H as shown in Figure 2. The two fused rings A and B are nearly coplanar where both rings deviated from one another by 3.27°. Similarly, the two rings C and D deviated from one another by only 1.78°, indicating a coplanar fused-ring system. In contrast, the two fused five membered rings H and I are not perfectly coplanar where the perfectly planar parts of these rings are C8C7C19C11 and C8C9S1C10, respectively. It is clear that both rings have envelope conformation where the N atom is located out of the plane of each ring by distances of 0.700 and 0.644 Å, respectively. As expected, the three phenyl moieties are perfectly planar where the two rings E and F make angles of 22.78 and 64.07° with the mean plane of C8C7C19C11 atoms. The molecular structure of this compound is stabilized by the three intramolecular C8-H8...O1, C1-H1...O1 and C17-H17...O2 with donor–acceptor distances of 2.953(2), 3.423(3) and 3.129(2) Å (Table 2). For better clarity, this hydrogen bonding interaction is presented as the turquoise-dotted line in the left part of Figure 3.
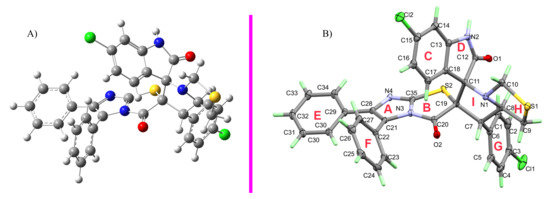
Figure 2.
(A) AM1 semiempirical optimization (4−ortho-endo); (B) X−ray structure of 4.

Table 1.
Crystal Data of compound 4.

Table 2.
Hydrogen bond parameters (Å and °) for 4.
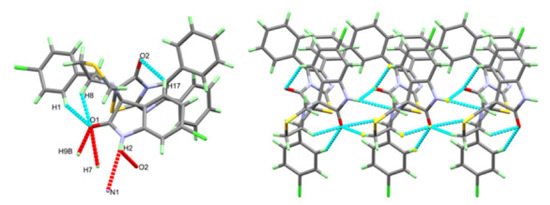
Figure 3.
Hydrogen bond contacts in 4.
The molecules of 4 are packed by three hydrogen bonds presented as red-dotted lines in Figure 3. The molecules are packed via strong N2-H2...O2 with donor–acceptor distances of 2.826(2) Å (Table 3). Additionally, the N2-H2...N1, C7-H7...O1 and C9-H9B...O1 have longer interactions distances of 3.470(3), 3.419(2) and 3.207(2) Å, respectively (Figure 3, right part).

Table 3.
Bond lengths (Å) and angles (°) for 4.
3.3. Analysis of Molecular Packing
The Hirshfeld surfaces of 4 are shown in Figure 4 while the intermolecular contacts are presented in Figure 5. The molecular packing is dominated by H…H (36.8%), H…C (22.9%), Cl…H (10.4%) and S…H (6.6%) contacts where the majority of these interactions have longer interaction distances than the vdWs radii sum of the interacting atoms except for the H…C contacts. In addition to the short H…C contacts, the packing is controlled by many other short contacts such as O…H (4.7%), N…H (5.3%), Cl…C (1.6%), Cl…O (1.0%) and N…O (0.5%) contacts. The shortest interactions along with the corresponding distances are listed in Table 4. Most of these interactions are short with characteristic sharp peaks in the fingerprint plot (Figure 6) and red regions in dnorm map indicating significant interactions (Figure 7).
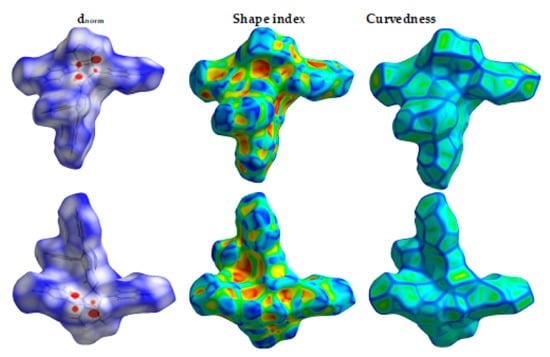
Figure 4.
Hirshfeld surfaces of 4.
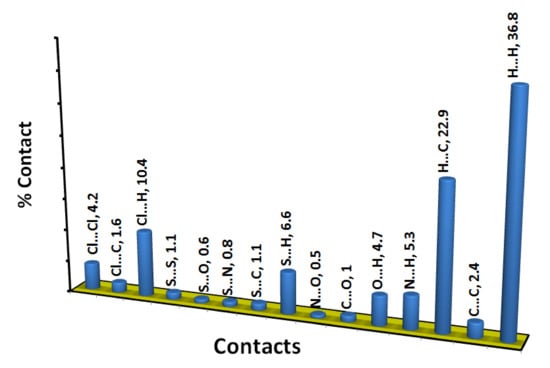
Figure 5.
Intermolecular interactions in 4.

Table 4.
Intermolecular interactions and their distances in 4 a.
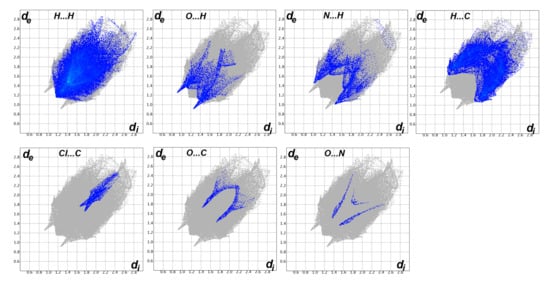
Figure 6.
Decomposed fingerprint plots for the important interactions in 4.
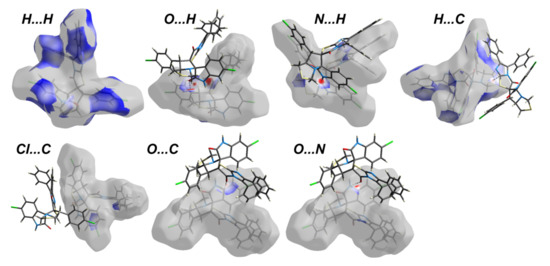
Figure 7.
Decomposed dnorm surfaces for the important interactions in 4.
3.4. DFT Studies
Using a B3LYP method, the structure of 4 was optimized (Figure 8). The computed structure of 4 showed good matching with the experimental geometry. The list of bond distances and angles depicted in Table S1 (Supplementary Data) reveal the good agreement between the calculations and the experiment. In this regard, the correlation graphs shown in Figure 9 indicated an excellent straight-line relation (R2 = 0.9776–0.9962) between the calculated and experimental values. The presence of intermolecular interactions in the solid state could be the main reason for such deviations.
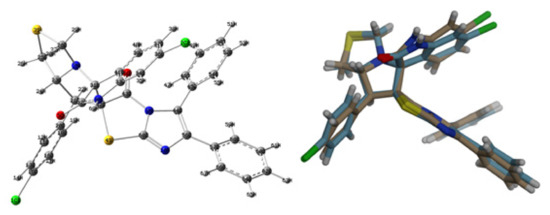
Figure 8.
The calculated geometry using a B3LYP method (left) and its overlay with an experimental one (right) for 4.
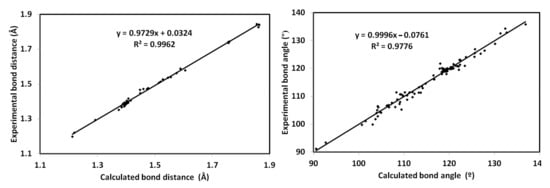
Figure 9.
Correlations between the calculated and the experimental geometric parameters.
The results of the charge calculations indicated the electropositive nature of both S-atoms (Table S2 (Supplementary Data)). It is clear that the S-atom (0.3759 e) located in the five membered ring which contain the carbonyl group, has a higher positive charge than the other S-site (0.1929 e). In addition, the two chlorine atoms have very small natural charges of −0.0040 and 0.0075 e. All nitrogen and oxygen atomic sites are electronegative where the carbonyl oxygen atom of the cyclic amide has the highest negative charge of −0.6032 e. In contrast, the hydrogen atoms have positive charge where the NH proton has the highest positive natural charge of 0.4481 e. The net dipole moment of 4 is calculated to be 3.1664 Debye. In MEP, the intense blue region close to the NH proton reveals its electropositive nature while the intense red regions (most electronegative) are close to the carbonyl oxygen atoms (Figure 10).
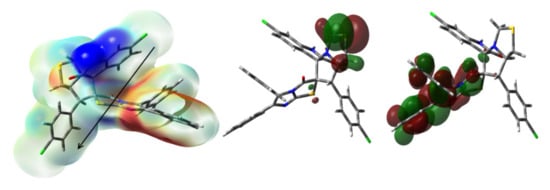
Figure 10.
The MEP, HOMO and LUMO of 4. Black arrow indicates the direction of the dipole moment vector.
On the other hand, the HOMO and LUMO presentations are explored in Figure 10. The HOMO level is mainly located over the S-atom of the thiazolidinyl ring while the LUMO one is localized over the π-system of one of the aryl groups which indicate n-π* excitation for the HOMO→LUMO intramolecular charge transfer. Based on the HOMO and LUMO energies, the ionization potential (I = −EHOMO), electron affinity (A = −ELUMO), chemical potential (μ = −(I + A)/2), hardness (η = (I − A)/2) as well as electrophilicity index (ω = μ2/2η) were calculated [25,26,27,28,29,30,31]. These reactivity parameters are calculated to be 5.6663, 1.8719, −3.7691, 3.7944 and 1.8720 eV, respectively.
Experimentally, three electronic transitions at 339, 246 and 221 nm in the electronic spectra of the studied molecule were detected as shown in Figure S1 (Supplementary Data). Obviously, the electronic spectra showed very little changes due to solvent effects. The longest wavelength band observed at 335 nm in ethanol was calculated using the TD-DFT calculations at 331.9 nm (f = 0.031) which was assigned to the HOMO-2→LUMO (63%)/HOMO-1→LUMO (23%) mixed excitations. Hence, this band could be assigned as mixed π-π* and n-π* transitions (Figure 11).
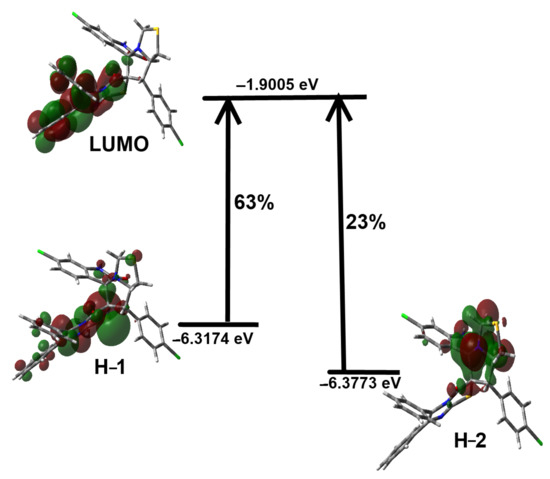
Figure 11.
MOs included in the longest wavelength electronic absorption band for the studied system.
On the other hand, NMR calculations were used to compute the 1H and 13C chemical shifts (Table S3 (Supplementary Data)). In Figure 12, the computed chemical shifts correlated well with the experimental values (R2 = 0.96–0.97).
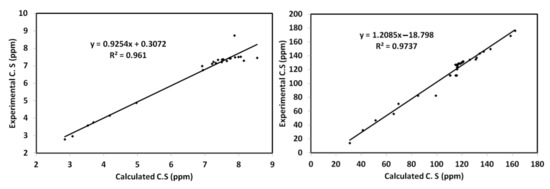
Figure 12.
1H and 13C NMR correlations between the calculated and experimental data.
4. Conclusions
The new spirooxindole hybrid incorporating the imidazo[2,1-b]thiazole derivative was designed, synthesized and elucidated its chemical structure successfully. Based on Hirshfeld calculations, many intermolecular contacts such as H…H, H…C, Cl…H and S…H, as well as O…H, N…H, Cl…C, Cl…O and N…O interactions affect the molecular pacing of 4. The studied compound has polar nature (3.1664 Debye). The NMR chemical shifts correlated well with the experimental results. The UV-Vis electronic spectral bands observed experimentally were assigned based on TD-DFT calculations. DFT calculations were used to compute the electronic and spectroscopic properties of the studied system.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/cryst12010005/s1, Figures S1–S4: 1HNMR, 13CNMR, IR and UV-Vis spectrum, Tables S1–S3: computational investigations.
Author Contributions
Conceptualization, A.B.; methodology, M.S.A. and A.A.A.; software, S.M.S. and M.H.; validation, M.S.A., N.H.A.-S., and A.A.A.; formal analysis, M.S.A., N.H.A.-S., M.H., and A.A.A.; investigation, M.S.A.; resources, M.S.A. and A.B.; data curation, A.B., and S.M.S.; writing—original draft preparation, A.B., and S.M.S.; writing—review and editing, A.B., and S.M.S.; visualization, A.B., M.S.A. and N.H.A.-S.; supervision, A.B., and M.S.A.; project administration, A.A.A.; funding acquisition, M.S.A. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the Deanship of Scientific Research at Princess Nourah bint Abdulrahman University, through the Research Groups Program Grant no. (RGP-1443-0040).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
This work was funded by the Deanship of Scientific Research at Princess Nourah bint Abdulrahman University, through the Research Groups Program Grant no. (RGP-1443-0040).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Li, J.J. Heterocyclic Chemistry in Drug Discovery; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2013. [Google Scholar]
- Shareef, M.A.; Khan, I.; Babu, B.N.; Kamal, A. A Comprehensive review on the therapeutic versatility of imidazo [2,1-b]thiazoles. Curr. Med. Chem. 2020, 27, 6864–6887. [Google Scholar] [CrossRef]
- Shetty, N.S.; Khazi, I.A.M.; Ahn, C.-J. Synthesis, anthelmintic and anti-inflammatory activities of some novel imidazothiazole sulfides and sulfones. Bull. Korean Chem. Soc. 2010, 31, 2337–2340. [Google Scholar] [CrossRef]
- Çapan, G.; Ulusoy, N.; Ergenç, N.; Kiraz, M. New 6-phenylimidazo[2,1-b]thiazole derivatives: Synthesis and antifungal activity. Monatsh. Chem. 1999, 130, 1399–1407. [Google Scholar] [CrossRef]
- Cascioferro, S.; Parrino, B.; Petri, G.L.; Cusimano, M.G.; Schillaci, D.; DI Sarno, V.; Musella, S.; Giovannetti, E.; Cirrincione, G.; Diana, P. 2,6-Disubstituted imidazo[2,1-b][1,3,4]thiadiazole derivatives as potent staphylococcal biofilm inhibitors. Eur. J. Med. Chem. 2019, 167, 200–210. [Google Scholar] [CrossRef]
- Sbenati, R.M.; Semreen, M.H.; Semreen, A.M.; Shehata, M.K.; Alsaghir, F.M.; El-Gamal, M.I. Evaluation of imidazo[2,1–b]thiazole-based anticancer agents in one decade (2011–2020): Current status and future prospects. Bioorg. Med. Chem. 2021, 29, 115897. [Google Scholar] [CrossRef] [PubMed]
- Başoğlu, F.; Ulusoy-Güzeldemirci, N.; Akalın-Çiftçi, G.; Çetinkaya, S.; Ece, A. Novel imidazo [2,1-b] thiazole-based anticancer agents as potential focal adhesion kinase inhibitors: Synthesis, in silico, and in vitro evaluation. Chem. Biol. Drug. Des. 2021, 98, 270–282. [Google Scholar] [CrossRef]
- Potikha, L.M.; Brovarets, V.S. Synthesis of imidazo [2,1-b][1,3] thiazoles–potential anticancer agents derived from γ-bromodipnones. Chem. Heterocycl. Compd. 2020, 56, 1073–1077. [Google Scholar] [CrossRef]
- Li, Y.; Bionda, N.; Fleeman, R.; Wang, H.; Ozawa, A.; Houghten, R.A.; Shaw, L. Identification of 5,6-dihydroimidazo[2,1- b ]thiazoles as a new class of antimicrobial agents. Bioorg. Med. Chem. 2016, 24, 5633–5638. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Miyazaki, M.; Naito, H.; Sugimoto, Y.; Kawato, H.; Okayama, T.; Shimizu, H.; Miyazaki, M.; Kitagawa, M.; Seki, T.; Fukutake, S.; et al. Lead optimization of novel p53-MDM2 interaction inhibitors possessing dihydroimidazothiazole scaffold. Bioorg. Med. Chem. Lett. 2013, 23, 728–732. [Google Scholar] [CrossRef] [PubMed]
- Mona, C.E.; Besserer-Offroy, É.; Cabana, J.; Leduc, R.; Lavigne, P.; Heveker, N.; Marsault, É.; Escher, E. Design, synthesis, and biological evaluation of CXCR4 ligands. Org. Biomol. Chem. 2016, 14, 10298–10311. [Google Scholar] [CrossRef] [PubMed]
- Barakat, A.; Islam, M.S.; Ghawas, H.M.; Al-Majid, A.M.; Elsenduny, F.; Badria, F.A.; Elshaier, Y.; Ghabbour, H.A. Design and synthesis of new substituted spirooxindoles as potential inhibitors of the MDM2–p53 interaction. Bioorg. Chem. 2019, 86, 598–608. [Google Scholar] [CrossRef]
- Altowyan, M.S.; Barakat, A.; Al-Majid, A.M.; Al-Ghulikah, H. Spiroindolone analogues bearing benzofuran moiety as a selective cyclooxygenase COX-1 with TNF-α and IL-6 inhibitors. Saudi J. Biol. Sci. 2020, 27, 1208–1216. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.S.; Al-Majid, A.M.; Azam, M.; Verma, V.P.; Barakat, A.; Haukka, M.; Elgazar, A.A.; Mira, A.; Badria, F.A. Construction of spirooxindole analogues engrafted with indole and pyrazole scaffolds as acetylcholinesterase inhibitors. ACS Omega 2021, 6, 31539–31556. [Google Scholar] [CrossRef]
- Barakat, A.; Alshahrani, S.; Al-Majid, A.M.; Ali, M.; Altowyan, M.S.; Islam, M.S.; Alamary, A.S.; Ashraf, S.; Ul-Haq, Z. Synthesis of a new class of spirooxindole–benzo[b]thiophene-based molecules as acetylcholinesterase inhibitors. Molecules 2020, 25, 4671. [Google Scholar] [CrossRef]
- Zhou, L.-M.; Qu, R.-Y.; Yang, G.-F. An overview of spirooxindole as a promising scaffold for novel drug discovery. Expert Opin. Drug Discov. 2020, 15, 603–625. [Google Scholar] [CrossRef] [PubMed]
- Lotfy, G.; Aziz, Y.M.A.; Said, M.M.; El Ashry, E.S.H.; El Tamany, E.S.H.; Abu-Serie, M.M.; Teleb, M.; Dömling, A.; Barakat, A. Molecular hybridization design and synthesis of novel spirooxindole-based MDM2 inhibitors endowed with BCL2 signaling attenuation; a step towards the next generation p53 activators. Bioorg. Chem. 2021, 117, 105427. [Google Scholar] [CrossRef]
- Aziz, Y.M.A.; Lotfy, G.; Said, M.M.; El Ashry, E.S.H.; El Tamany, E.S.H.; Soliman, S.M.; Abu-Serie, M.M.; Teleb, M.; Yousuf, S.; Dömling, A.; et al. Design, Synthesis, Chemical and biochemical insights into novel hybrid spirooxindole-based p53-MDM2 inhibitors with potential Bcl2 signaling attenuation. Front. Chem. 2021, 9, 735236. [Google Scholar] [CrossRef]
- Ríos-Gutiérrez, M.; Domingo, L.R. Unravelling the mysteries of the [3+2] cycloaddition reactions. Eur. J. Org. Chem. 2019, 2019, 267–282. [Google Scholar] [CrossRef]
- Domingo, L.R.; Chamorro, E.; Perez, P. Understanding the high reactivity of the azomethine ylides in [3 + 2] cycloaddition reactions. Lett. Org. Chem. 2010, 7, 432–439. [Google Scholar] [CrossRef]
- Domingo, L.R.; Kula, K.; Ríos-Gutiérrez, M. Unveiling the reactivity of cyclic azomethine ylides in [3+2] cycloaddition reactions within the molecular electron density theory. Eur. J. Org. Chem. 2020, 2020, 5938–5948. [Google Scholar] [CrossRef]
- Domingo, L.R.; Ríos-Gutiérrez, M.; Pérez, P. A Molecular electron density theory study of the role of the copper metalation of azomethine ylides in [3+2] cycloaddition reactions. J. Org. Chem. 2018, 83, 10959–10973. [Google Scholar] [CrossRef] [PubMed]
- Mustafa, A.; Ali, M.I.; Abou-State, M.A.; Hammam, A.-E.G. Reactions with 4,5-disubstituted 2-mercaptoimidazoles and their derivatives. J. Prakt. Chem. 1972, 314, 785–792. [Google Scholar] [CrossRef]
- Barakat, A.; Haukka, M.; Soliman, S.M.; Ali, M.; Al-Majid, A.M.; El-Faham, A.; Domingo, L.R. Straightforward regio- and diastereoselective synthesis, molecular structure, intermolecular interactions and mechanistic study of spirooxindole-engrafted rhodanine analogs. Molecules 2021, 26, 7276. [Google Scholar] [CrossRef]
- Tannor, D.J.; Marten, B.; Murphy, R.; Friesner, R.A.; Sitkoff, D.; Nicholls, A.; Honig, B.; Ringnalda, M.; Goddard, W.A., III. Accurate first principles calculation of molecular charge distributions and solvation energies from ab initio quantum mechanics and continuum dielectric theory. J. Am. Chem. Soc. 1994, 116, 11875–11882. [Google Scholar] [CrossRef]
- Cheeseman, J.R.; Trucks, G.W.; Keith, T.A.; Frisch, M.J. A comparison of models for calculating nuclear magnetic resonance shielding tensors. J. Chem. Phys. 1996, 104, 5497–5509. [Google Scholar] [CrossRef]
- Foresman, J.B.; Frisch, Æ. Exploring Chemistry with Electronic Structure Methods, 2nd ed.; Gaussian: Pittsburgh, PA, USA, 1996. [Google Scholar]
- Chang, R. Chemistry, 7th ed.; McGraw-Hill: New York, NY, USA, 2001. [Google Scholar]
- Kosar, B.; Albayrak, C. Spectroscopic investigations and quantum chemical computational study of (E)-4-methoxy-2-[(p-tolylimino)methyl]phenol. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2011, 78, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Koopmans, T.A. Ordering of wave functions and eigenenergies to the individual electrons of an atom. Physica 1933, 1, 104–113. [Google Scholar] [CrossRef]
- Parr, R.G.; Yang, W. Density-Functional Theory of Atoms and Molecules; Oxford University Press: New York, NY, USA, 1989. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).