Abstract
Recently, ice with stacking disorder structure, consisting of random sequences of cubic ice (Ic) and hexagonal ice (Ih) layers, was reported to be more stable than pure Ih/Ic. Due to a much lower free energy barrier of heterogeneous nucleation, in practice, the freezing process of water is controlled by heterogeneous nucleation triggered by an external medium. Therefore, we carry out molecular dynamic simulations to explore how ice polymorphism depends on the lattice structure of the crystalline substrates on which the ice is grown, focusing on the primary source of atmospheric aerosols, carbon materials. It turns out that, during the nucleation stage, the polymorph of ice nuclei is strongly affected by graphene substrates. For ice nucleation on graphene, we find Ih is the dominant polymorph. This can be attributed to structural similarities between graphene and basal face of Ih. Our results also suggest that the substrate only affects the polymorph of ice close to the graphene surface, with the preference for Ih diminishing as the ice layer grows.
1. Introduction
Ice nucleation is important in the physical environment and biological systems [1,2,3]. Thus, it is important to accurately estimate the nucleation rate of ice, which is highly related to polymorph of ice crystalline [4,5]. For a long time, hexagonal ice (Ih) was assumed to be the most stable ice phase at atmospheric pressure or below. However, this understanding has been questioned by a large number of reports by computer simulation [5,6,7,8] and experiment [9,10,11,12,13] in recent years. In the report of Lupi et al. [5] the stacking-disordered [14] critical ice crystallites are about 14 kJ/mol of crystallite more stable than hexagonal ice crystallites (at 230 K). In these simulations, homogeneous ice nucleation was considered, but in practice, it is almost impossible to eliminate the influence of impurities or external boundaries on ice nucleation [15,16]. Due to a much lower free energy barrier of heterogeneous nucleation, in practice, the freezing process of water is usually controlled by heterogeneous nucleation. This raises the question to what extent heterogeneous ice nucleation influences the recently discovered preference for stacking-disordered ice.
As the main component of atmospheric aerosols, carbon surfaces can greatly promote heterogeneous ice nucleation [17,18,19]. The crystallization temperature of ice on the graphite surface has been found to be about 12 K higher than the temperature for homogeneous ice nucleation [20,21]. This stimulated both experimental and molecular dynamics (MD) simulations investigation of heterogeneous ice nucleation on graphene/graphite and other carbon surfaces [20,22,23,24].
However, the issue of how nucleation on carbon surfaces influences the preference for stacking-disordered ice has so far not been addressed. To do so, we conducted MD simulations for a series of heterogeneous ice nucleation/growth processes on different carbon surfaces and found that the carbon surfaces have a strong influence on the local ice polymorphs.
2. Methods
Modeling. As shown in Figure 1, ice nucleation was studied on four types of atomic flat carbon surfaces with different lattice structures: Graphene (composed by six ring carbon atom), Oblique-Haeckelite (O-Haeckelite, composed by 5-6-7 ring carbon atom), Rectangular-Haeckelite (R-Haeckelite, composed by 5–7 ring carbon atom), and Random (all carbon atoms distributed randomly, refer to Figure S1) [25]. The reason behind these specific surfaces is that we aimed to clarify the effect of the hexagonal structure of graphene on the polymorph of ice, compared to the two non-hexagonal Haeckelite structures and an amorphous 2D carbon substrate. Analogous homogeneous ice nucleation (Homo) simulations without any substrate were also carried out as control. The size of the 3D periodic simulation boxes (which contain 15,029 water molecules) are 15.0 × 14.8 × 15.0 nm3 for the Graphene system, 16.3 × 15.3 × 15.0 nm3 for the O-Haeckelite system, 17.0 × 15.5 × 15.0 nm3 for the R-Haeckelite system, 15.0 × 14.9 × 15.0 nm3 for the Random system, and 15.0 ×15.0 × 15.0 nm3 for the Homo system, respectively.
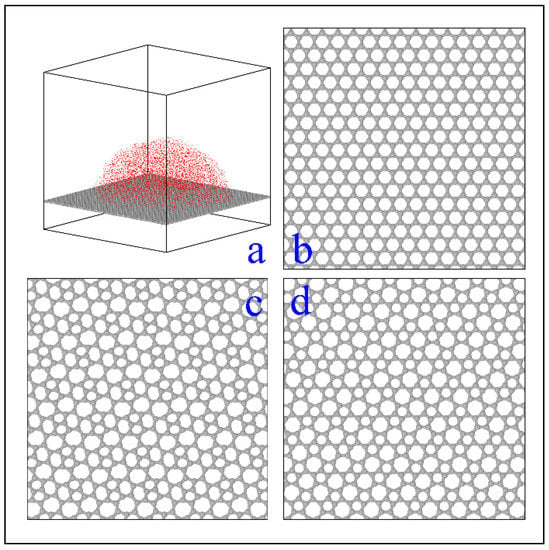
Figure 1.
(a) Example of a simulation box. (b–d) show top view (part) of substrates Graphene, O-Haeckelite, and R-Haeckelite, respectively. The top view (part) of substrates Random is shown in Figure S1. Carbon atoms in substrate are portrayed as gray spheres. The water molecules are showed in red dots.
Simulation Details. All MD simulations were performed using large-scale atomic/molecular massively parallel simulator (LAMMPS, Version 5Jun19) package [26]. The coarse-grained mono-atomic mW water model was applied in all simulations [27]. The interactions between mW water molecules consist of a sum of pairwise interactions, while the hydrogen bonding between water molecules is represented by three-body contributions, which can nucleate liquid water to ice homogeneously and heterogeneously at a certain supercooling without any advanced sampling method. The mW model correctly displays the anomalies and structures of liquid water, ices, and amorphous solid water and the transitions between them [28]. The equations of water motion were integrated with the velocity Verlet algorithm with a time step of 5 fs. All the simulations were conducted in the NVT ensemble. The temperature in the simulation systems was controlled by a Nosé-Hoover thermostat. The same as in previous reports [20], ice nucleation was studied through a cooling ramp of cooling rates of 1 K/ns, which was chosen after a systematic test of the cooling rates by the authors. To calculate the freezing efficiency [20], , 40 independent trajectories were performed for each system. The interactions between water molecules and carbon atoms are taken from the previous report by Lupi’ et al. [20], in which water–carbon interaction parameters are: = 0.32 nm and = 0.13 kcal/mol, to reproduce the experimental water contact angle of water on a graphene surface (namely 86°) [29,30]. All the carbon atoms in the substrates were fixed in all the MD simulations. Water molecules with Ih/Ic structure were identified using the Chill + algorithm proposed by Nguyen et al. [31], which is available in the OVITO package [32].
3. Results and Discussion
To investigate the effect of the substrate lattice structure on the polymorph of ice, MD simulations were employed to study the ice formation process on different substrates: Graphene, Oblique-Haeckelite (O-Haeckelite), Rectangular-Haeckelite (R-Haeckelite), and a Random carbonaceous substrate. As shown in Figure 2, consistent with previous reports [20,22,33,34], after an induction period (during which, the small water clusters with ice structure formed and then disappeared quickly, due to the thermal fluctuation of system), stable ice nuclei (larger than critical size Rc of ice) formed at the water-substrate interface for the systems of Graphene, O-Haeckelite, R-Haeckelite, and Random, which should be due to the much lower heterogeneous nucleation barrier. For these heterogeneous nucleation systems, especially for the nucleation stage, the ice crystals exhibit a single-crystal-like structure with barely any grain boundary, which is due to the 1-dimensional structure match (in the direction perpendicular to the substrate surface) between a flat substrate and a flat crystalline face of ice. While for the Homo system, the new-formed ice exhibits a polycrystalline structure.
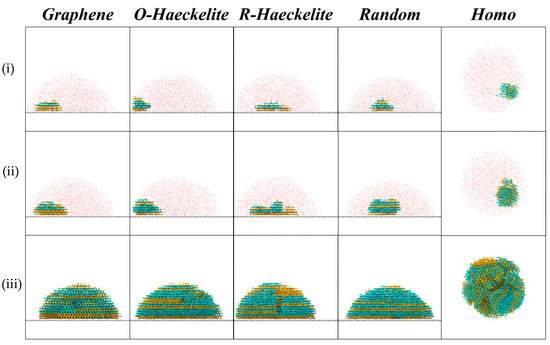
Figure 2.
Lateral view of the ice formation procedure from top to bottom: (i) ice nucleation; (ii) ice growth; (iii) water freezing completely. Liquid water is represented by red dots. Water molecules in ice crystallites are represented by colored ball-stick model (Ih: yellow; Ic, cyan). Carbon atoms in substrate are colored gray.
Intriguingly, for the system of Graphene, the Ih structure occupies an absolute majority in the ice nuclei during the stage of nucleation. With the growth of ice (after about 5–6 layers of Ih formed), the Ic turns up, which is consistent with the previous report that the Ic grows on Ih embryos to form a more stable stacking disorder structure [5]. When almost all the liquid water freezes to ice, the percentage of Ic is not much different from that of Ih. While for the systems of O-Haeckelite, R-Haeckelite, Random, and Homo, in all stages of ice formation, the number of water molecules in Ic is always comparable with the number of water molecules in Ih.
To reveal the phase change process of ice during heterogeneous nucleation processes, the molecular numbers of Ic and Ih as a function of system temperature (namely simulation time) were extracted from the ice formation MD trajectories (shown Figure 3). The snapshots in Figure 2 share the same trajectories with the results of Figure 3 for each system. As shown in Figure 3a (Graphene system), with the decrease in temperature, the nucleation process was observed in the Ih ice before the steady growth of Ic. In the growth stage, the Ic exhibited a lager growth rate than Ih, leading to an equivalent molecular number of Ih and Ic at the stage of water freezing completely. While for each of the systems in Figure 3b–e, the Ic and Ih grow simultaneously as the system temperature decreases, indicating that these systems have no selectivity to ice polymorph.
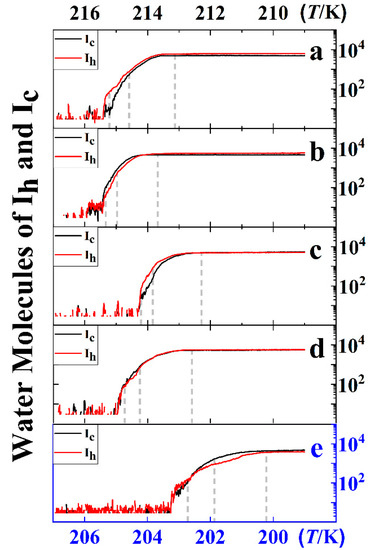
Figure 3.
Molecular numbers of water molecules in Ic and Ih during a cooling ramp for the systems of (a) Graphene, (b) O-Haeckelite, (c) R-Haeckelite, (d) Random, and (e) Homo. The dashed vertical lines indicate position of snapshots of the ice nucleation, ice growth, and water freezing completely in Figure 2. The blue borders are for panel (e).
To quantitatively reveal the polymorph of ice formed on different substrates, the cubicity [6] (i.e., the proportion of Ic) of the new-formed ices was calculated from the 40 independent MD trajectories for each system (refer to Table 1 and Table S1). As shown in Table 1, the cubicity of ice in the Homo system is 52.5 ± 1.3%, 53.9 ± 1.6%, and 58.2 ± 0.7% for the stage of ice nucleation, ice growth, and water freezing completely, respectively, which is consistent with previous reports (about 55%) [5,6,7,35]. For ice formed on Graphene, the cubicity is only 28.4 ± 2.5% at the nucleation stage, which is significantly lower than that of the Homo system. The results suggest that the graphene substrate has a preferential selectivity to Ih over Ic, which we attribute to the fact that Ih (basal face) and graphene share the same hexagonal structure and have a similar lattice structure. The distance between the center of two hexagon rings on graphene surface is 2.46 Å [24,36], and the distance of water molecules in the basal face of Ih is 2.76 Å [37,38]. According to previous reports [24,39,40], in the water–graphene system, the center of a hexagon formed by carbon atoms corresponds to the adsorption energy minima positions of water molecules. Due to the similarity of lattice structure between the basal plane of Ih and graphene, slight adjustment of the position of interfacial water molecules can match the lattice structure of basal plane of Ih. The calculated mismatch [41] between the substrate and the ice is 10.9%. The ice-nucleating protein, with a mismatch of 10% to ice, was found to be able to dramatically promote the nucleation of ice [42]. In the report of Bi et al. [24], it was found that the first ice layer on graphene substrate was mainly composed of Ih, while the first ice layer on an amorphous graphene substrate (similar to the O-Haeckelite substrate in this paper) was somewhat messy. The study of Bi et al. also suggests that the similarity between the graphene and the basal face of Ih results in the selective promotion of the formation of Ih over Ic. Moreover, in the study of ice formation on AgI [43,44], it is also found that the substrate lattice structure can alter the polymorph of ice: Ih mainly observed on the hexagonal β-AgI, while the Ic mainly found on cubic γ-AgI. These reports are in good agreement to our MD simulation results, confirming that the formation of Ih can be selectively promoted by graphene surface.

Table 1.
Average cubicity of each simulation system. From top to bottom are the stages of ice nucleation, ice growth, and water freezing completely, respectively.
To further investigate the effect of substrate lattice structure on heterogeneous ice nucleation, freezing efficiency of the substrates, based on the method of Lupi et al. [20], were calculated (shown in Figure 4). It follows that the calculated freezing efficiencies for Graphene, O-Haeckelite, R-Haeckelite, and Random substrates are 12.7 ± 0.6 K, 11.5 ± 1.2 K, 12.5 ± 1.0 K, and 11.1 ± 1.3 K, respectively, which are consistent with the previous reports that the crystallization temperature of ice on the graphite surface is 12 ± 3 K higher than the temperature of homogeneous ice nucleation [20,21]. The freezing efficiency of these different substrates has no significant difference, indicating that, although the substrates exhibit different polymorph selectivity of ice, the heterogeneous nucleation promotion effects of the substrates on ice are similar. Moreover, it should be pointed out that the heterogeneous nucleation promotion effect changes with the system temperature [24]. To calculate the freezing efficiency, the system temperature is steadily decreased. The freezing processes are driven by a very high supercooling, which can reduce the heterogeneous nucleation promotion effect. For instance, the heterogeneous ice nucleation rates on different substrates exhibit significant differences only when the system temperature increased to as high as 235 K [24]. Thus, for a systematic study, e.g., heterogeneous nucleation barrier and heterogeneous nucleation rate, it is necessary to gain more accurate results by taking into account the influence of temperature. Another thing that needs to be specified is that, in our results, the calculated homogeneous nucleation temperature of ice is 1.6 K higher than that in the previous report [20], which can be attributed to the difference in the number of water molecules between this work and the previous report (refer to Figure S2). According to classic nucleation theory (CNT), the greater the number of water molecules in the system, the greater the nucleation probability of ice formation is, which could lead to this 1.6 K discrepancy.
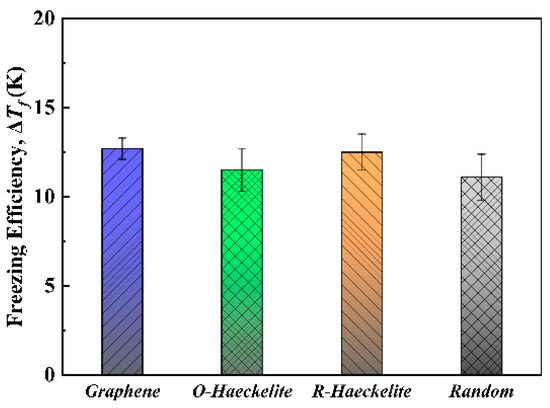
Figure 4.
Freezing efficiency of substrates in Graphene, O-Haeckelite, R-Haeckelite, and Random systems.
4. Conclusions
MD simulations of heterogeneous ice formation on a series of carbon surfaces show the significant impact of the substrate lattice structure on the polymorph of ice that is formed, but only during the nucleation stage. Specifically, graphene substrates have a preferential selectivity toward the formation of Ih over Ic during the nucleation stage, due to the similarity between the lattice structure of graphene and Ih. After the nucleation stage the cubicity of new-formed ice increases up to about 53%, due to the higher stability of stacking disordered ice. When subsequently the water freezes completely, the cubicity of ice ranges from 53% to 58%, in good agreement with homogeneous ice formation and previously reported results. This study enhances our understanding of the surface selectivity mechanism on certain ice polymorphs and provides a unique perspective in the field of crystal growth.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/cryst11091134/s1. Table S1: Cubicity of the nucleation stage in each simulation trajectory. Figure S1: Surface morphology of 2-dimensional carbon substrate with all the carbon atoms randomly distributed, the Random system. Figure S2: Freezing temperature in Homo-5241 (5241 water molecules) and Homo-15029 (15029 water molecules) systems.
Author Contributions
Conceptualization, C.Z., J.W. and Z.Z.; methodology, C.Z., T.L. and Z.Z.; software, C.Z. and Q.X.; investigation, C.Z. and H.L.; data curation, C.Z., H.L., A.P. and Z.Z.; writing—original draft preparation, C.Z., H.L. and Z.Z.; writing—review and editing, C.Z., H.L., R.d.V., H.Z. and Z.Z.; visualization, C.Z., H.L. and Z.Z.; supervision, Z.Z.; project administration, Z.Z.; funding acquisition, J.W. and Z.Z. C.Z. and H.L. contributed equally to this manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (Grant Nos. 11904300, 11772278 and 11502221), the Jiangxi Provincial Outstanding Young Talents Program (Grant No. 20192BCBL23029), the Fundamental Research Funds for the Central Universities (Xiamen University: Grant Nos. 20720180014, 20720180018 and 20720180066).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data generated or analyzed during this study are included in this published article and its additional files.
Acknowledgments
Y. Yu and Z. Xu from Information and Network Center of Xiamen University are acknowledged for the help with the high-performance computer clusters.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Hudait, A.; Moberg, D.R.; Qiu, Y.; Odendahl, N.; Paesani, F.; Molinero, V. Preordering of water is not needed for ice recognition by hyperactive antifreeze proteins. Proc. Natl. Acad. Sci. USA 2018, 115, 8266–8271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, J.Y.; Patey, G.N. Heterogeneous Ice Nucleation Induced by Electric Fields. J. Phys. Chem. Lett. 2011, 2, 2555–2559. [Google Scholar] [CrossRef]
- Herbert, R.J.; Murray, B.J.; Dobbie, S.J.; Koop, T. Sensitivity of liquid clouds to homogenous freezing parameterizations. Geophys. Res. Lett. 2015, 42, 1599–1605. [Google Scholar] [CrossRef] [PubMed]
- Hudait, A.; Molinero, V. What Determines the Ice Polymorph in Clouds? J. Am. Chem. Soc. 2016, 138, 8958–8967. [Google Scholar] [CrossRef] [PubMed]
- Lupi, L.; Hudait, A.; Peters, B.; Grünwald, M.; Mullen, R.G.; Nguyen, A.H.; Molinero, V. Role of stacking disorder in ice nucleation. Nature 2017, 551, 218–222. [Google Scholar] [CrossRef] [PubMed]
- Malkin, T.L.; Murray, B.J.; Salzmann, C.G.; Molinero, V.; Pickering, S.J.; Whale, T.F. Stacking disorder in ice I. Phys. Chem. Chem. Phys. 2015, 17, 60–76. [Google Scholar] [CrossRef] [Green Version]
- Moore, E.B.; Molinero, V. Is it cubic? Ice crystallization from deeply supercooled water. Phys. Chem. Chem. Phys. 2011, 13, 20008–20016. [Google Scholar] [CrossRef]
- Sosso, G.C.; Tribello, G.A.; Zen, A.; Pedevilla, P.; Michaelides, A. Ice formation on kaolinite: Insights from molecular dynamics simulations. J. Chem. Phys. 2016, 145, 211927. [Google Scholar] [CrossRef] [Green Version]
- Thurmer, K.; Nie, S. Formation of hexagonal and cubic ice during low-temperature growth. Proc. Natl. Acad. Sci. USA 2013, 110, 11757–11762. [Google Scholar] [CrossRef] [Green Version]
- Kuhs, W.F.; Sippel, C.; Falenty, A.; Hansen, T.C. Extent and relevance of stacking disorder in “ice I(c)”. Proc. Natl. Acad. Sci. USA 2012, 109, 21259–21264. [Google Scholar] [CrossRef] [Green Version]
- Shilling, J.E.; Tolbert, M.A.; Toon, O.B.; Jensen, E.J.; Murray, B.J.; Bertram, A.K. Measurements of the vapor pressure of cubic ice and their implications for atmospheric ice clouds. Geophys. Res. Lett. 2006, 33, 17. [Google Scholar] [CrossRef] [Green Version]
- Hansen, T.C.; Koza, M.M.; Lindner, P.; Kuhs, W.F. Formation and annealing of cubic ice: II. Kinetic study. J. Phys. Cond. Matt. 2008, 20, 285105. [Google Scholar] [CrossRef]
- Morishige, K.; Uematsu, H. The proper structure of cubic ice confined in mesopores. J. Chem. Phys. 2005, 122, 44711. [Google Scholar] [CrossRef] [PubMed]
- Malkin, T.L.; Murray, B.J.; Brukhno, A.V.; Anwar, J.; Salzmann, C.G. Structure of ice crystallized from supercooled water. Proc. Natl. Acad. Sci. USA 2012, 109, 1041–1045. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, X.Y.; Du, N. Zero-sized effect of nano-particles and inverse homogeneous nucleation. Principles of freezing and antifreeze. J. Biol. Chem. 2004, 279, 6124–6131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Z.; Liu, X.Y. Control of ice nucleation: Freezing and antifreeze strategies. Chem. Soc. Rev. 2018, 47, 7116–7139. [Google Scholar] [CrossRef]
- Lary, D.; Shallcross, D.; Toumi, R. Carbonaceous aerosols and their potential role in atmospheric chemistry. J. Geophys. Res. 1999, 104, 15929–15940. [Google Scholar] [CrossRef] [Green Version]
- Lupi, L.; Kastelowitz, N.; Molinero, V. Vapor deposition of water on graphitic surfaces: Formation of amorphous ice, bilayer ice, ice I, and liquid water. J. Chem. Phys. 2014, 141, 18C508. [Google Scholar] [CrossRef]
- Penner, J.; Eddleman, H.; Novakov, T. Towards the development of a global inventory for black carbon emissions. Atmos. Environ. Part A Gen. Top. 1993, 27, 1277–1295. [Google Scholar] [CrossRef]
- Lupi, L.; Hudait, A.; Molinero, V. Heterogeneous nucleation of ice on carbon surfaces. J. Am. Chem. Soc. 2014, 136, 3156–3164. [Google Scholar] [CrossRef]
- Lupi, L.; Molinero, V. Does hydrophilicity of carbon particles improve their ice nucleation ability? J. Phys. Chem. A 2014, 118, 7330–7337. [Google Scholar] [CrossRef]
- Cabriolu, R.; Li, T. Ice nucleation on carbon surface supports the classical theory for heterogeneous nucleation. Phys. Rev. E 2015, 91, 052402. [Google Scholar] [CrossRef] [Green Version]
- Whale, T.F.; Rosillo-Lopez, M.; Murray, B.J.; Salzmann, C.G. Ice nucleation properties of oxidized carbon nanomaterials. J. Phys. Chem. Lett. 2015, 6, 3012–3016. [Google Scholar] [CrossRef]
- Bi, Y.; Cabriolu, R.; Li, T. Heterogeneous ice nucleation controlled by the coupling of surface crystallinity and surface hydrophilicity. J. Phys. Chem. C 2016, 120, 1507–1514. [Google Scholar] [CrossRef] [Green Version]
- Terrones, H.; Terrones, M.; Hernández, E. New metallic allotropes of planar and tubular carbon. Phys. Rev. Lett. 2000, 84, 1716. [Google Scholar] [CrossRef] [Green Version]
- Plimpton, S. Fast parallel algorithms for short-range molecular dynamics. J. Comput. Phys. 1995, 117, 1–19. [Google Scholar] [CrossRef] [Green Version]
- Molinero, V.; Moore, E.B. Water Modeled As an Intermediate Element between Carbon and Silicon. J. Phys. Chem. B 2009, 113, 4008–4016. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnston, J.C.; Molinero, V. Crystallization, melting, and structure of water nanoparticles at atmospherically relevant temperatures. J. Am. Chem. Soc. 2012, 134, 6650–6659. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zeng, X.C. Wetting and interfacial properties of water nanodroplets in contact with graphene and monolayer boron–nitride sheets. ACS Nano 2012, 6, 2401–2409. [Google Scholar] [CrossRef]
- Adamson, A.W.; Gast, A.P. Physical Chemistry of Surfaces; Interscience: New York, NY, USA, 1967; Volume 15. [Google Scholar]
- Nguyen, A.H.; Molinero, V. Identification of Clathrate Hydrates, Hexagonal Ice, Cubic Ice, and Liquid Water in Simulations: The CHILL+ Algorithm. J. Phys. Chem. B 2015, 119, 9369–9376. [Google Scholar] [CrossRef] [Green Version]
- Stukowski, A. Visualization and analysis of atomistic simulation data with OVITO–the Open Visualization Tool. Mod. Simul. Mater. Sci. Eng. 2010, 18, 015012. [Google Scholar] [CrossRef]
- Cox, S.J.; Raza, Z.; Kathmann, S.M.; Slater, B.; Michaelides, A. The microscopic features of heterogeneous ice nucleation may affect the macroscopic morphology of atmospheric ice crystals. Faraday Disc. 2013, 167, 389–403. [Google Scholar] [CrossRef] [Green Version]
- Xue, H.; Lu, Y.; Geng, H.; Dong, B.; Wu, S.; Fan, Q.; Zhang, Z.; Li, X.; Zhou, X.; Wang, J. Hydroxyl Groups on the Graphene Surfaces Facilitate Ice Nucleation. J. Phys. Chem. Lett. 2019, 10, 2458–2462. [Google Scholar] [CrossRef]
- Li, T.; Donadio, D.; Russo, G.; Galli, G. Homogeneous ice nucleation from supercooled water. Phys. Chem. Chem. Phys. 2011, 13, 19807–19813. [Google Scholar] [CrossRef] [PubMed]
- Pozzo, M.; Alfe, D.; Lacovig, P.; Hofmann, P.; Lizzit, S.; Baraldi, A. Thermal expansion of supported and freestanding graphene: Lattice constant versus interatomic distance. Phys. Rev. Lett. 2011, 106, 135501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, J.; Zhu, C.; Liu, K.; Jiang, Y.; Song, Y.; Francisco, J.S.; Zeng, X.C.; Wang, J. Distinct ice patterns on solid surfaces with various wettabilities. Proc. Natl. Acad. Sci. USA 2017, 114, 11285–11290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nguyen, A.H.; Koc, M.A.; Shepherd, T.D.; Molinero, V. Structure of the Ice–Clathrate Interface. J. Phys. Chem. C 2015, 119, 4104–4117. [Google Scholar] [CrossRef]
- Zhang, Z.S.; Kang, Y.; Liang, L.J.; Liu, Y.C.; Wu, T.; Wang, Q. Peptide encapsulation regulated by the geometry of carbon nanotubes. Biomaterials 2014, 35, 1771–1778. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-C.; Shen, J.-W.; Gubbins, K.E.; Moore, J.D.; Wu, T.; Wang, Q. Diffusion dynamics of water controlled by topology of potential energy surface inside carbon nanotubes. Phys. Rev. B 2008, 77, 125438. [Google Scholar] [CrossRef]
- Cox, S.J.; Kathmann, S.M.; Purton, J.A.; Gillan, M.J.; Michaelides, A. Non-hexagonal ice at hexagonal surfaces: The role of lattice mismatch. Phys. Chem. Chem. Phys. 2012, 14, 7944–7949. [Google Scholar] [CrossRef]
- Qiu, Y.; Hudait, A.; Molinero, V. How Size and Aggregation of Ice-Binding Proteins Control Their Ice Nucleation Efficiency. J. Am. Chem. Soc. 2019, 141, 7439–7452. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roudsari, G.; Reischl, B.; Pakarinen, O.H.; Vehkamäki, H. Atomistic Simulation of Ice Nucleation on Silver Iodide (0001) Surfaces with Defects. J. Phys. Chem. C 2019, 124, 436–445. [Google Scholar] [CrossRef]
- Zielke, S.A.; Bertram, A.K.; Patey, G.N. A Molecular Mechanism of Ice Nucleation on Model AgI Surfaces. J. Phys. Chem. B 2015, 119, 9049–9055. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).