Abstract
New radical-cation salts based on tetramethyltetrathiafulvalene (TMTTF) and tetramethyltetraselenefulvalene (TMsTSF) with metallacarborane anions (TMTTF)[3,3′-Cr(1,2-C2B9H11)2], (TMTTF)[3,3′-Fe(1,2-C2B9H11)2], and (TMTSF)2[3,3′-Cr(1,2-C2B9H11)2] were synthesized by electrocrystallization. Their crystal structures were determined by single crystal X-ray diffraction, and their electrophysical properties in a wide temperature range were studied. The first two salts are dielectrics, while the third one is a narrow-gap semiconductor: σRT = 5 × 10−3 Ohm−1cm−1; Ea ≈ 0.04 eV (aprox. 320 cm−1).
1. Introduction
Radical-cation salts and charge transfer complexes based on derivatives of tetrathiafulvalene (TTF) constitute a wide class of organic materials with transport properties ranging from insulating to superconducting [1,2,3,4]. This work is part of the systematic study of radical-cation salts of tetrathiafulvalene and its derivatives with metallacarborane anions, of which earlier results were summarized in works [5,6,7].
Transition metal bis(dicarbollide) complexes [3,3′-M(1,2-C2B9H11)2]− (M = Fe, Co, or Ni) are of great interest as counterions for the synthesis of TTF-based molecular conductors due to the unique high stability, possibility of tuning the charge and nature of the metal, and wide range of options for modification with dicarbollide ligands via hydrogen substitution by other atoms and functional groups [5,6]. Although most of the compounds studied were BEDT-TTF-based radical-cation salts, recently, we have synthesized radical-cation salts based on such unconventional and rather exotic donors as bis(1,3-propylenedithio)-tetrathiafulvalene [8,9], dibenzotetrathiafulvalene [10], and 4,5-ethylenedithio-4′,5′-(2-oxa-1,3-propylenedithio)-tetrathiafulvalene [9]. On the other hand, although compounds of the composition (TMTXF)2Y (X = T, S) are usually classical organic metals among which the first organic superconductors were discovered [4,7], and TMTTF and TMTSF radical-cation salts continue to attract the attention of researchers [11,12,13,14,15], very little attention has been paid to TMTTF and TMTSF radical-cation salts with metallacarborane anions [16,17,18,19]. This prompted us to prepare and investigate new TMTTF and TMTSF radical-cation salts with metallacarborane anions.
This contribution describes the synthesis, structure, and electrical conductivity of new salts with TMTTF and TMTSF radical-cations and metallacarborane anions: (TMTTF)[3,3′-Cr(1,2-C2B9H11)2] (1), (TMTTF)[3,3′-Fe(1,2-C2B9H11)2] (2), and (TMTSF)2[3,3′- Cr(1,2-C2B9H11)2] (3).
2. Results and Discussion
Single crystals of compounds 1–3 suitable for X-ray diffraction studies in the form of thin plates were obtained by electrochemical crystallization (See Supplementary Materials and Table 1). The crystal structure of 1 is formed by the TMTTF radical-cations and the [3,3′-Cr(1,2-C2B9H11)2]− anions occupying general positions in the unit cell (Figure 1). (TMTTF)[3,3′-Cr(1,2-C2B9H11)2] has a pseudo-layered structure, in which anionic layers alternate along the ac diagonal with layers formed by radical-cation dimers (Figure 2). The dimer formation corresponds to the stoichiometry of the salt: in this case due to the Peierls instability a phase transition should occur with doubling of the stacks period [7]. The distances between the averaged planes of the TMTTF donors in the dimers are 3.38 Å (the planes are drawn through all S atoms), and the dihedral angle between the planes is 0° by symmetry conditions. There are short intermolecular S…S interactions (3.426(1)–3.432(1) Å) of the “face-to-face” type between the TMTTF donors in the dimers.

Table 1.
Crystal data and structure refinement for (TMTTF)[3,3′-Cr(1,2-C2B9H11)2] (1), (TMTTF)[3,3′-Fe(1,2-C2B9H11)2] (2), and (TMTSF)2[3,3′-Cr(1,2-C2B9H11)2] (3).
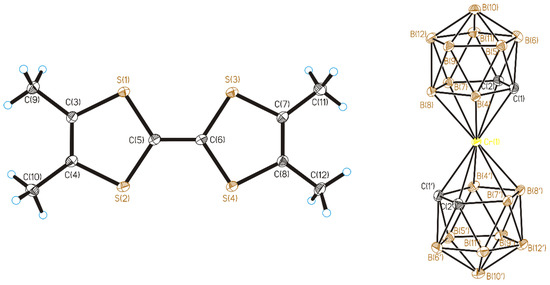
Figure 1.
TMTTF radical-cation and anion in (1). Thermal ellipsoids are given at 30% probability level. Cage H atoms omitted for clarity.
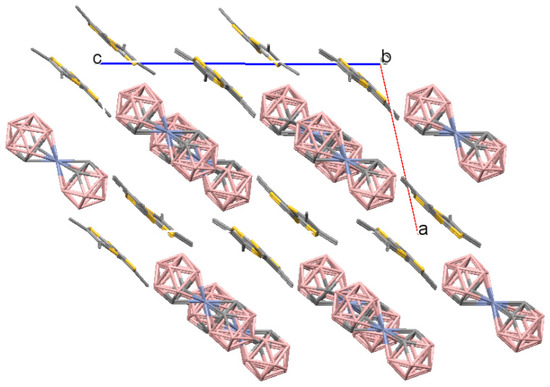
Figure 2.
Crystal packing fragment of (1). A view along the b axis. The unit cell is outlined. H atoms are omitted for clarity.
The TMTTF+ radical-cations are non-planar and have a “boat” conformation: the maximum deviations of terminal C(9), C(10), C(11), and C(12) atoms from the plane of the averaged molecule drawn through all sulfur atoms are 0.30–0.36 Å.
The Cr-C and Cr-B bond lengths are 2.173(2)–2.180(2) and 2.232(2)–2.279(2) Å, correspondingly. The distances from the chromium atom to the C2B3 faces of the dicarbollide ligands are equal to 1.68 Å, which is close to the corresponding distances found in the structures of Cs [3,3′-Cr(1,2-C2B9H11)2] [20], (TTF)[3,3′-Cr(1,2-C2B9H11)2] [21], and (BEDT-TTF)2[3,3′-Cr(1,2-C2B9H11)2] [22,23]. The dicarbollide ligands in the [3,3′-Cr(1,2-C2B9H11)2]− anion are turned relative to each other by 180°, forming the transoid conformation. The C2B3 faces deviate slightly from parallel, being inclined by 178.7° to each other.
The electrical conductivity measurements have shown that 1 is an insulator with σ293~10−11 Ohm−1cm−1. The low value of electrical conductivity is apparently connected with the absence of conducting layers and dimerization of the radical-cations stacks.
It should be noted that compound 1 is the first TMTTF radical-cation salt with an unsubstituted transition metal bis(dicarbollide), while the radical-cation salts (TMTTF)[8-HO-3,3′-Co(1,2-C2B9H10)(1′,2′-C2B9H11)] and (TMTTF)(8,8′-Cl2-3,3′-Co(1,2-C2B9H10)2]2 obtained earlier contained substituted bis(dicarbollide) anions [16,17].
The crystal structure of (TMTTF)[3,3′-Fe(1,2-C2B9H11)2] (2) is formed by a quarter of the TMTTF radical-cation in a special position placed on the m plane and a quarter of the [3,3′-Fe(1,2-C2B9H11)2]− anion in the 2/m special position of the unit cell (Figure 3). The compound 2 is characterized by a structure where the TMTTF cations and the metallacarborane anions form staggered stacks (Figure 4 and Figure 5). The distances between the averaged planes of the TMTTF donors in the dimers are 3.38 Å, and the dihedral angle between the planes is 0° by symmetry conditions.
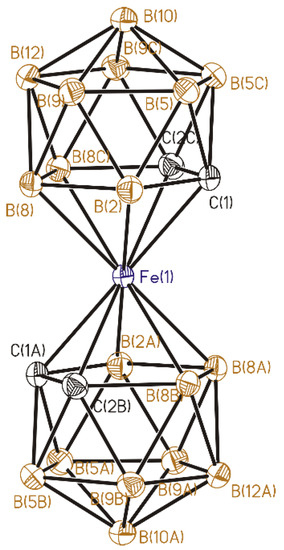
Figure 3.
Anion in (2). Thermal ellipsoids are given at 30% probability level. Cage H atoms omitted for clarity.
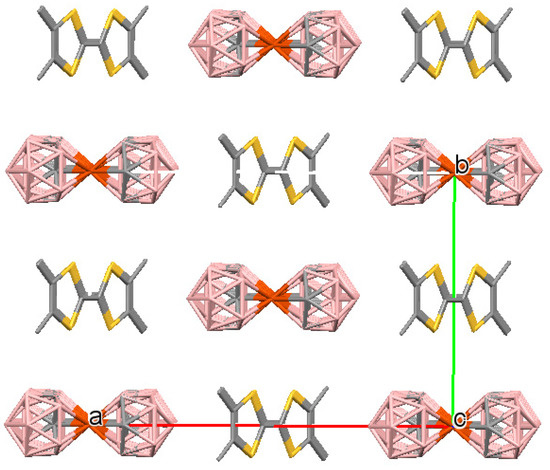
Figure 4.
Crystal packing fragment of (2). A view along the c axis. The unit cell is outlined. H atoms are omitted for clarity.
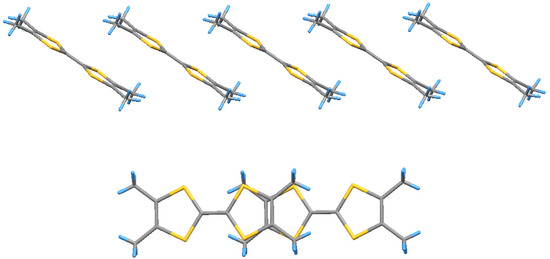
Figure 5.
A stack and radical-cations overlapping in (2).
The Fe-C and Fe-B bond lengths are 2.0790(9)–2.1001(8) and 2.1001(8)–2.1494(8) Å, correspondingly, and the overlapping values are due to the statistical disordering of carbon and boron atoms in the dicarbollide ligands. The distances from the iron atom to the C2B3 faces of the dicarbollide ligands are equal to 1.53 Å, which is close to the distances in analogous salts of the iron bis(dicarbollide) anion [19,24,25]. The dicarbollide ligands are turned relative to each by 180°, forming the transoid conformation. The C2B3 faces are parallel by symmetry conditions.
According to the electric conductivity measurements, compound 2 is an insulator with conductivity ~10−10 Ohm−1cm−1. The low value of electroconductivity is in an agreement with the 1:1 stoichiometry and non-layered structure of the salt, as well as with the inclination angle of the radical-cations in the stack, at which there is only slight overlap between neighboring radical-cations.
The (TMTSF)2[3,3′-Cr(1,2-C2B9H10)2] (3) crystals are isostructural to (TMTSF)2[3,3′-Co(1,2-C2B9H11)2] and (TMTSF)2[3,3′-Fe(1,2-C2B9H11)2] salts studied earlier, containing cobalt and iron bis(dicarbollide) anions [18,19]. The crystal structure of 3 is formed by the TMTSF cation in a general position and the [3,3′-Cr(1,2-C2B9H11)2]− anion in a special centrosymmetrical position (Figure 6). Compound 3 possesses a structure (Figure 7 and Figure 8) where the TMTSF+• radical-cations and anions form staggered stacks. The distances between the averaged planes of the TMTSF donors in the dimers are 3.70 and 3.73 Å, and the dihedral angle between the planes is 0° by symmetry conditions.
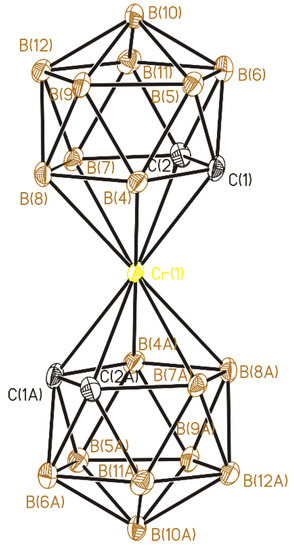
Figure 6.
Anion in (3). Thermal ellipsoids are given at 30% probability level.
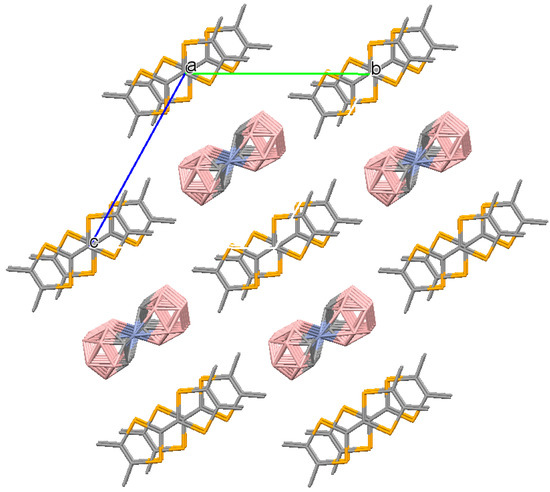
Figure 7.
Crystal packing fragment of (3). A view along the a axis. The unit cell is outlined. H atoms are omitted for clarity.
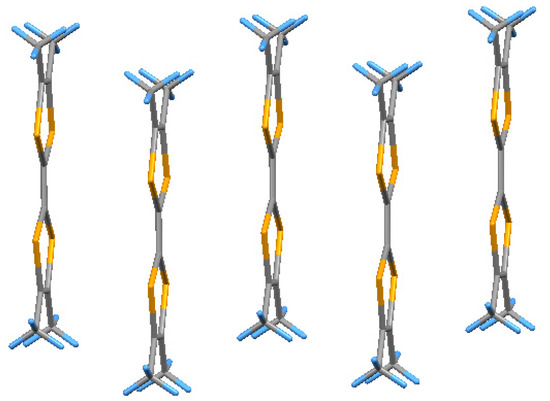
Figure 8.
A stack of radical-cations in (3).
The Cr-C and Cr-B bond lengths are 2.175(7)–2.176(7) and 2.226(8)–2.277(8) Å, correspondingly. The distances from the chromium atom to the C2B3 faces of the dicarbollide ligands are equal to 1.68 Å, and the dicarbollide ligands in the [3,3′-Cr(1,2-C2B9H10)2]− anion are turned relative to each other by 180°, forming the transoid conformation. The C2B3 faces are parallel to each other by the symmetry conditions.
The electroconductivity measurements have revealed that compound 3 in the range of 41–195 K behaves like a dielectric. However, above 195 K, the delocalization of the positive charge disappears due to the numerous intermolecular S…S contacts and an inconspicuous dielectric–semiconductor structural phase transition occurs, caused by charge ordering: stacks contain both TMTSF molecules and TMTSF radical-cations. The room temperature electric conductivity σ293 = 5·10−3 Ohm−1cm−1 and activation energy Ea ≅ 0.04 eV (Figure 9). It should be noted that analogous salts (TMTSF)2[3,3′-Co(1,2-C2B9H11)2] and (TMTSF)2[3,3′-Fe(1,2-C2B9H11)2] were characterized by electroconductivity values σ293 of 15 and 0.1 Ohm−1cm−1, correspondingly [18,19].
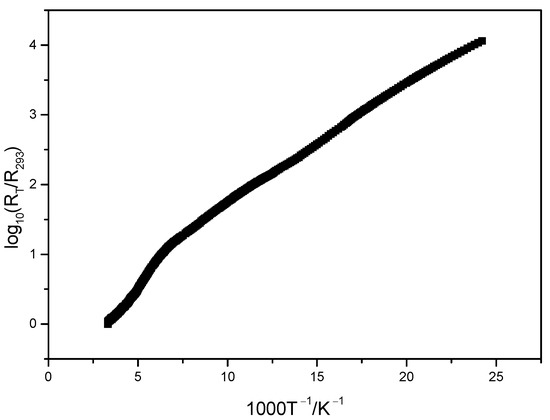
Figure 9.
Temperature dependence of electrical resistivity of (3).
In conclusion, new salts with the TMTTF and TMTSF radical-cations and metallacarborane anions (TMTTF)[3,3′-Cr(1,2-C2B9H11)2] (1), (TMTTF)[3,3′-Fe(1,2-C2B9H11)2] (2), and (TMTSF)2[3,3′-Cr(1,2-C2B9H11)2] (3) were electrochemically synthesized and investigated. Their crystal structures were determined by X-ray study and electroconductivities were measured. Salts (1) and (2) are insulators, which is explained by the 1:1 stoichiometry and the absence of an extended network of interdonor interactions, whereas (3) is a semiconductor at room temperature with electroconductivity σ293 = 5·10−3 Ohm−1cm−1, which is lower than in (TMTSF)2[3,3′-Fe(1,2-C2B9H11)2] and (TMTSF)2[3,3′-Co(1,2-C2B9H11)2] salts (electroconductivity values σ293 of 0.1 and 15 Ohm−1cm−1, correspondingly). The tendency of a rise in conductivity (5·10−3 < 0.1 < 15) is apparently connected with decreasing the cation size in the order Cr3+ > Fe3+ > Co3+ [26], which leads to decreasing the corresponding metallacarborane anion size and, in turn, to unit cell compression and a tighter radical-cation packing of the salts.
Supplementary Materials
Details of experimental data including synthesis of the title compounds, their X-ray diffraction studies, and electric resistivity measurements are available online at https://www.mdpi.com/article/10.3390/cryst11091118/s1.
Author Contributions
Synthesis, D.M.C., I.B.S., I.D.K., G.G.A., E.V.S.; measurements, L.I.B., D.N.P., T.N.S.; X-ray diffraction study, G.V.S.; data analysis and writing, O.N.K.; research conception, V.I.B., O.A.D. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The crystallographic data for this paper (the CCDC numbers 2091714, 2091713, 2091715 for (1), (2), (3), respectively) can be obtained free of charge via www.ccdc.cam.ac.uk/data_request/cif (accessed on 11 August 2021).
Acknowledgments
This work was partly performed in accordance with the state task of the Institute of Problems of Chemical Physics, Russian Academy of Sciences, State Registration No. AAAA-A19-119092390076-7. Synthesis and characterization of the starting metallacarboranes were performed at A.N. Nesmeyanov Institute of Organoelement Compounds, Russian Academy of Sciences with support of the Ministry of Science and Higher Education of the Russian Federation.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Williams, J.M.; Ferraro, J.R.; Thorn, R.J.; Carlson, K.D.; Geiser, U.; Wang, H.H.; Kini, A.M.; Whangbo, M.-H. Organic SuperConductors (Including Fullerenes: Synthesis, Structure, Properties, and Theory); Prentice Hall: Englewood Cliffs, NJ, USA, 1992. [Google Scholar]
- Ishiguro, T.; Yamaji, K.; Saito, G. Organic Superconductors, 2nd ed.; Springer Series in Solid-State Sciences; Springer: Berlin, Germany, 1998. [Google Scholar]
- Saito, G.; Yoshida, Y. Organic superconductors. Chem. Rec. 2011, 11, 124–145. [Google Scholar] [CrossRef]
- Naito, T. Modern history of organic conductors: An overview. Crystals 2021, 11, 838. [Google Scholar] [CrossRef]
- Bregadze, V.I.; Dyachenko, O.A.; Kazheva, O.N.; Kravchenko, A.V.; Sivaev, I.B.; Starodub, V.A. Tetrathiafulvalene-based radical-cation salts with transition metal bis(dicarbollide) anions. CrystEngComm 2015, 17, 4754–4767. [Google Scholar] [CrossRef]
- Bregadze, V.I.; Dyachenko, O.A.; Kazheva, O.N.; Kosenko, I.D.; Kravchenko, A.V.; Sivaev, I.B.; Starodub, V.A. Electroconducting radical-cation salts based on tetrathiafulvalene derivatives and transition metals bis(dicarbollides). Russ. J. Gen. Chem. 2019, 89, 971–987. [Google Scholar] [CrossRef]
- Starodub, V.A.; Starodub, T.N.; Kazheva, O.N.; Bregadze, V.I. Materialy Sovremennoi Electroniki i Spintroniki (Materials of Modern Electronics and Spintronics); Fizmatlit: Moscow, Russia, 2018; 424p. [Google Scholar]
- Kazheva, O.N.; Chudak, D.M.; Shilov, G.V.; Komissarova, E.A.; Kosenko, I.D.; Kravchenko, A.V.; Shilova, I.A.; Shklyaeva, E.V.; Abashev, G.G.; Sivaev, I.B.; et al. First molecular conductors of BPDT-TTF with metallacarborane anions: (BPDT-TTF)[3,3′-Cr(1,2-C2B9H11)2] and (BPDT-TTF)[3,3′-Co(1,2-C2B9H11)2]—Synthesis, structure, properties. J. Organomet. Chem. 2018, 867, 375–380. [Google Scholar] [CrossRef]
- Kazheva, O.N.; Chudak, D.M.; Shilov, G.V.; Kosenko, I.D.; Abashev, G.G.; Shklyaeva, E.V.; Kravchenko, A.V.; Starodub, V.A.; Buravov, L.I.; Dyachenko, O.A.; et al. First EOTT and BPDT-TTF based molecular conductors with [8,8′-Cl2-3,3′-Fe(1,2-C2B9H10)2]− anion—Synthesis, structure, properties. J. Organomet. Chem. 2021, 949, 121956. [Google Scholar] [CrossRef]
- Kazheva, O.N.; Chudak, D.M.; Shilov, G.V.; Kravchenko, A.V.; Kosenko, I.D.; Sivaev, I.B.; Abashev, G.G.; Shklyaeva, E.V.; Starodub, V.A.; Buravov, L.I.; et al. First radical-cation salts based on dibenzotetrathiafulvalene (DBTTF) with metallacarborane anions: Synthesis, structure, properties. J. Organomet. Chem. 2020, 930, 121592. [Google Scholar] [CrossRef]
- Mroweh, N.; Mézière, C.; Allain, M.; Auban-Senzier, P.; Canadell, E.; Avarvari, N. Conservation of structural arrangements and 3:1 stoichiometry in a series of crystalline conductors of TMTTF, TMTSF, BEDT-TTF, and chiral DM-EDT-TTF with the oxo-bis[pentafluorotantalate(V)] dianion. Chem. Sci. 2020, 11, 10078–10091. [Google Scholar] [CrossRef] [PubMed]
- Yoshino, H.; Iwasaki, Y.; Tanaka, R.; Tsujimoto, Y.; Matsuoka, C. Crystal structures and electrical resistivity of three exotic TMTSF salts with I3−: Determination of valence by DFT and MP2 calculations. Crystals 2020, 10, 1119. [Google Scholar] [CrossRef]
- Sahadevan, S.A.; Abhervé, A.; Monni, N.; Auban-Senzier, P.; Cui, H.; Kato, R.; Mercuri, M.L.; Avarvari, N. Radical-cation salts of tetramethyltetrathiafulvalene (TM-TTF) and tetramethyltetraselenafulvalene (TM-TSF) with chlorocyananilate-based anions. Cryst. Growth Des. 2020, 20, 6777–6786. [Google Scholar] [CrossRef]
- Frąckowiak, A.; Barszcz, B.; Olejniczak, I.; Tomasik, M.; Jarzyniak, N.; Świetlik, R.; Auban-Senzier, P.; Fourmigué, M.; Jeannin, O.; Camerel, F. Mixed valence trimers in cation radical salts of TMTTF with the planar bis(6-sulfo-8-quinolato) platinum complex [Pt(qS)2]2−. New J. Chem. 2020, 44, 15538–15548. [Google Scholar] [CrossRef]
- Allain, M.; Mézière, C.; Auban-Senzier, P.; Avarvari, N. Old donors for new molecular conductors: Combining TMTSF and BEDT-TTF with anionic (TaF6)1−x/(PF6)x alloys. Crystals 2021, 11, 386. [Google Scholar] [CrossRef]
- Kazheva, O.N.; Alexandrov, G.G.; Kravchenko, A.V.; Kosenko, I.D.; Lobanova, I.A.; Sivaev, I.B.; Filippov, O.A.; Shubina, E.S.; Bregadze, V.I.; Starodub, V.A.; et al. Molecular conductors with a 8-hydroxy cobalt bis(dicarbollide) anion. Inorg. Chem. 2011, 50, 444–450. [Google Scholar] [CrossRef] [PubMed]
- Kazheva, O.N.; Alexandrov, G.G.; Kravchenko, A.V.; Sivaev, I.B.; Starodub, V.A.; Kosenko, I.D.; Lobanova, I.A.; Bregadze, V.I.; Buravov, L.I.; Dyachenko, O.A. New organic conductors with halogen and phenyl substituted cobalt bis(dicarbollide) anions. J. Chem. Eng. Chem. Res. 2015, 2, 497–503. [Google Scholar]
- Kazheva, O.N.; Chekhlov, A.N.; Alexandrov, G.G.; Buravov, L.I.; Kravchenko, A.V.; Starodub, V.A.; Sivaev, I.B.; Bregadze, V.I.; Dyachenko, O.A. Synthesis, structure and electrical conductivity of fulvalenium salts of cobalt bis(dicarbollide) anion. J. Organomet. Chem. 2006, 691, 4225–4233. [Google Scholar] [CrossRef]
- Kazheva, O.N.; Alexandrov, G.G.; Kravchenko, A.V.; Starodub, V.A.; Sivaev, I.B.; Lobanova, I.A.; Bregadze, V.I.; Buravov, L.I.; Dyachenko, O.A. New fulvalenium salts of bis(dicarbollide) cobalt and iron: Synthesis, crystal structure and electrical conductivity. J. Organomet. Chem. 2007, 692, 5033–5043. [Google Scholar] [CrossRef]
- Bednarska-Szczepaniak, K.; Przelazły, E.; Kania, K.D.; Szwed, M.; Litecká, M.; Grüner, B.; Leśnikowski, Z.J. Interaction of adenosine, modified using carborane clusters, with ovarian cancer cells: A new anticancer approach against chemoresistance. Cancers 2021, 13, 3855. [Google Scholar] [CrossRef] [PubMed]
- Forward, J.M.; Mingos, D.M.P.; Müller, T.E.; Williams, D.J.; Yan, Y.-K. Synthesis and structural characterization of metallacarborane sandwich salts with tetrathiafulvalene (ttf) [M(C2B9H11)2][ttf] (M = Cr, Fe, Ni). J. Organomet. Chem. 1994, 467, 207–216. [Google Scholar] [CrossRef]
- Yan, Y.-K.; Mingos, D.M.P.; Williams, D.J.; Kurmoo, M. Synthesis, structures and physical properties of bis(ethylenedithio) tetrathiafulvalenium salts of paramagnetic metallacarborane anions. J. Chem. Soc. Dalton Trans. 1995, 19, 3221–3230. [Google Scholar] [CrossRef]
- Čižmár, E.; Šoltésová, D.; Kazheva, O.N.; Alexandrov, G.G.; Kravchenko, A.V.; Chekulaeva, L.A.; Kosenko, I.D.; Sivaev, I.B.; Bregadze, V.I.; Fedorchenko, A.V.; et al. Large magnetic anisotropy of chromium(III) ions in a bis(ethylenedithio)tetrathiafulvalenium salt of chromium bis(dicarbollide), (ET)2[3,3′-Cr(1,2-C2B9H11)2]. Trans. Met. Chem. 2018, 43, 647–655. [Google Scholar] [CrossRef]
- Sivaev, I.B.; Bregadze, V.I. Chemistry of nickel and iron bis(dicarbollides). J. Organomet. Chem. 2000, 614–615, 27–36. [Google Scholar] [CrossRef]
- Kazheva, O.N.; Alexandrov, G.G.; Kravchenko, A.V.; Sivaev, I.B.; Kosenko, I.D.; Lobanova, I.A.; Kajňakov, M.; Buravov, L.I.; Bregadze, V.I.; Feher, A.; et al. Synthesis, structure, electrical and magnetic properties of (BEDT-TTF)2[3,3-Fe(1,2-C2B9H11)2]. Inorg. Chem. Commun. 2012, 15, 106–108. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Cryst. C 1976, 32, 751–767. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).