Abstract
Polymeric terbium(III) squarate hydrate [{Tb2(C4O4)3(H2O)8}n] was prepared from TbCl3 or Tb2O3 and squaric acid. The crystal structure was determined in a monoclinic Pc space group, and the whole molecular arrangement gives a sandwiched two-dimensional structure. The coordination polyhedra are described as a square antiprism. The solid complex emits green light under UV irradiation at room temperature with the quantum yield of 25%. Although Tb3+ is a non-Kramers ion, the alternating-current magnetic susceptibility showed frequency dependence in a 2000-Oe DC field, and the effective energy barrier for magnetization reorientation was 33(2) K. Thus, [{Tb2(C4O4)3(H2O)8}n] displayed functions of a potential luminescent magnet.
1. Introduction
Squarate, a dianion of 3,4-dihydroxycyclobut-3-ene-1,2-dione (C4O42–, abbreviated as sq hereafter; Scheme 1), has two strongly polarized carbonyl groups to gain the stabilization energy of 2π aromaticity [1]. The negative charges are delocalized among four oxygen atoms which can serve as good O donors in coordination chemistry [2,3,4]. Metal sq salts are known to afford polymeric polynuclear complexes due to μ2- to μ4-modes of sq ligands. Owing to this bridging ability, sq has recently been used as a building block in metal–organic frameworks with various functionalities [5,6,7,8,9].
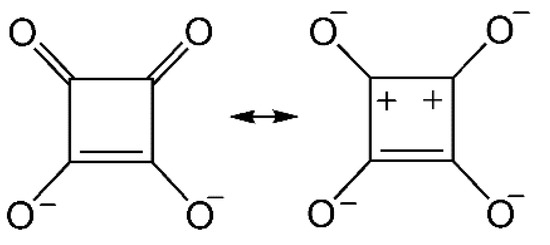
Scheme 1.
Canonical structures of squarate (sq).
Transition metal sq salts are often subjected to studies on exchange-coupled magnetic materials, as exemplified with Ni2+ and Cu2+ salts [10]. Lanthanide (Ln) sq complexes have also been well-known and studied since the 1970s [11,12], thanks to the Ln-O affinity. Even neutral carbonyl compounds can ligate Ln ions [13]. In contrast to transition metal sq salts, Ln sq salts give a scarce chance to investigate 4f-4f superexchange interactions, because magnetic 4f orbitals are shielded with 5s, 5p, and 6s orbitals. The Weiss temperature of Gd3+ squarate was reported to be as small as –0.12 K [14]. Instead, single-ion magnets (SIMs) emerge as a next-generation material, especially when exchange coupling is negligible [15]. SIM behavior is regulated with a relatively slow magnetization reorientation, as a result of a large magnetic moment and strong magnetic anisotropy, which is found in heavy Ln ions, typically 65Tb3+, 66Dy3+, 67Ho3+, and 68Er3+ [16].
Meanwhile, Ln ions are available for various luminescent materials [17,18], owing to sharp and strong emission, and their coordination compounds have been applied to light-emitting diodes in lighting devices [19,20] and luminescent probes in biology [21]. The green-light emission from Tb3+ ion is popular, and the red to NIR luminescence from Eu3+, Nd3+, Sm3+, Ho3+, Er3+, Yb3+, etc. is of interest as well [22,23]. We aimed at materials showing multifunctionalities by using a Tb3+ ion. In this Communication, the crystal structure of terbium(III) squarate hydrate [{Ln2(sq)3(H2O)8}n] (Ln = Tb; abbreviated as Tb-sq hereafter) has been determined for the first time. The photoluminescent and magnetic properties will be reported.
2. Materials and Methods
2.1. Preparation
Method A. An aqueous solution (10 mL) containing TbCl3·6H2O (Aldrich, 150 mg; 0.40 mmol) and an aqueous solution (10 mL) containing squaric acid (H2sq) (Junsei Chemical, 69 mg; 0.60 mmol) were combined at room temperature and allowed to stand for a week. Very thin plates of Tb-sq were precipitated, and they were collected on a filter, washed with water, and air-dried. The yield was 94.3 mg (59%). Mp. > 300 °C. Anal (PerkinElmer 2400 II). Calcd.: C, 18.06; H, 2.02% for C12H16O20Tb2. Found: C, 18.11; H, 1.81%. IR (neat, attenuated total reflection (Nicolet FT-IR 6700)) 640, 664, 1093, 1463, 1602, 3089, 3404 cm−1.
Method B. A hydrothermal synthesis technique was applied with a Parr Teflon autoclave in an As-one ON-300S thermostat dryer. A brown suspension containing TbIII2O3 (Angene Chemical, 37 mg; 0.10 mmol), H2sq (46 mg; 0.40 mmol), and water (10 mL) was heated at 150 °C for 72 h and then slowly cooled down to room temperature for 48 h. Off-white prismatic and powdery products were collected on a filter, washed freely with water, and air-dried. The yield was 69.0 mg (85%). The product was identical to Tb-sq, as confirmed by means of a powder X-ray diffraction (PXRD) study.
2.2. Structural Analysis
X-ray diffraction data of Tb-sq were collected on a Rigaku Saturn70 hybrid pixel array detector with graphite monochromated Mo Kα radiation (λ = 0.71073 Å). The structures were directly solved by a heavy-atom method and expanded using Fourier techniques in the Olex2 1.3 program [24]. The parameters were refined on Shelxl [25]. Hydrogen atoms were located at calculated positions and treated as “riding”. Selected data are as follows: C12H16O20Tb2, monoclinic Pc, a = 11.9331(8), b = 8.1820(6), c = 10.0563(7) Å, β = 96.103(6)°, V = 976.29(11) Å3, Z = 2, d = 2.715 g cm–3, μ(Mo Kα) = 7.292 mm−1, R(F) (I > 2σ(I)) = 0.0247, wR(F2) (all reflections) = 0.0603, goodness-of-fit parameter = 1.020, T = 296(1) K for 4200 reflections. CCDC (Supplementary Materials) reference number 2108278.
The PXRD patterns were recorded on a Rigaku Ultima III diffractometer using Cu Kα radiation (λ = 1.54178 Å) at room temperature.
2.3. Photoluminescent Measurements
The emission spectrum and the absolute quantum yield of polycrystalline Tb-sq were measured on a Hamamatsu Photonics Quantaurus-QY C11347 absolute PL quantum yield measurement system. The holder blank data were measured separately and subtracted from the raw sample data. The emission spectrum was recorded after the excitation wavelength was set to the excitation maximum.
2.4. Magnetic Measurements
Direct-current (DC) magnetic susceptibilities of Tb-sq were measured on a Quantum Design MPMS-XL7 SQUID magnetometer with a static field of 500 Oe. The magnetic responses were corrected with diamagnetic blank data of the sample holder measured separately. The diamagnetic contribution of the sample itself was estimated from Pascal’s constants [26]. The alternating-current (AC) magnetic susceptibilities on Tb-sq were recorded on a Quantum Design PPMS apparatus equipped with an AC/DC magnetic susceptibility option.
3. Results and Discussion
In the previous work [11,12,27,28], several pseudo-polymorphs are separated depending on the bridging mode of sq and the number of solvent (water) molecules ligating to metal ions and incorporated as a guest: [{Ln2(sq)3(H2O)4}n], [{Ln2(sq)3(H2O)11}n]· (H2O)2, [Ln(Hsq)(sq)(H2O)6], [Ln(Hsq)(sq)(H2O)6]·(H2O), [{Ln2(sq)2(ox)(H2O)4}n] (ox = oxalate), and Ln-sq. In the present study, such diversity was actually observed in the reactions from Tb and other Ln ions. However, we found that a dilute aqueous solution with a stoichiometric molar ratio according to Reaction Equation (1), namely TbCl3 (0.2 mmol) and H2sq (0.3 mmol) in water (10 mL), is preferable to afford Tb-sq (Method A). As Figure 1a shows, the purity as a bulk solid was confirmed by means of PXRD.
2 TbCl3·6H2O + 3 H2sq ⟶ Tb2(sq)3(H2O)8 + 6 HCl + 4 H2O
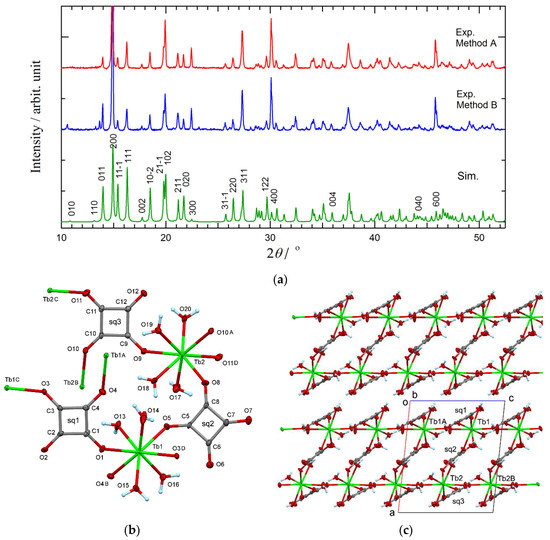
Figure 1.
(a) Powder X-ray diffraction patterns of the data on Tb-sq prepared by Method A (top), the data on Tb-sq prepared by Method B (middle), and simulation from the single-crystal structure result (bottom). (b) X-ray crystal structure of a repeating unit Tb2(sq)3(H2O)8 in Tb-sq. (c) Molecular arrangement viewed along the b axis. The thermal ellipsoids are drawn at the 50% probability level. The symmetry operation codes: A: x, 1−y, −1/2+z; B: x, 1−y, 1/2+z; C: x, −1+y, z; D: x, 1+y, z.
Since Ln oxides are basic solids, organic acids such as H2sq (pKa = 2.2) can dissolve them [29]. To avoid the formation of undesired compounds, protonated Hsq− and partially decomposed ox2−, possibly caused by an acidic by-product, we utilized Tb2O3 as a starting material in hydrothermal synthesis (Method B), as described with Reaction Equation (2). A slightly excess amount of H2sq led to complete consumption of Tb2O3, and thus the following molar ratio is recommended; Tb2O3 (0.1 mmol) and H2sq (0.4 mmol) in water (10 mL). The PXRD patterns (Figure 1a) clarify that both products from Methods A and B are identical. Polycrystalline Tb-sq obtained from Method B was satisfactorily pure, but a sample from Method A after being checked with elemental analysis was subjected to detailed physical measurements.
Tb2O3 + 3 H2sq + 5 H2O ⟶ Tb2(sq)3(H2O)8
The crystallographic analysis of Tb-sq was successful in a monoclinic Pc space group. Surprisingly, this is the first structural report on Tb-sq, despite the simple composition. However, we have to cite and compare practically the same structure with very close cell parameters for the Eu, Gd, Dy, and Er analogues. The report by Petit et al. [11] can be regarded as a pioneering work, and the space group of Eu-sq was determined to be Pc, while the possibility of centrosymmetric P2/c was rejected. Since then, the space groups reported were controversial; P21/c was chosen for Gd-sq [14] with one sq missing, presumably owing to disordered weak electron density. For Dy- and Er-sq [28], P21/c was assumed, but a severe overlap of the atomic coordinates remained. We encountered a similar centrosymmetry/non-centrosymmetry problem, and finally concluded that the most plausible space group is Pc based on the rational crystal structure (Figure 1b,c).
The asymmetric unit contains two Tb ions, three sq ions, and eight water ligands. The Tb-O distances vary from 2.331(8) to 2.449(7) Å. The polyhedra around the Tb ions are best described as an approximate square antiprism from the SHAPE analysis [30]; the CShM values for the Tb1 and Tb2 ions were 0.367 and 0.292, respectively, with reference to SAPR-8. Figure 1c illustrates that the local molecular axis of each Tb3+ polyhedron is aligned almost parallel in a crystal.
The crystal of Tb-sq consists of a bilayer structure parallel to the crystallographic bc plane (Figure 1c). Every sq bridges Tb ions. Squarate C1-C2-C3-C4 (abbreviated as sq1) binds three Tb1 ions with the O1, O3, and O4 coordination bonds, while three sq1 ions are coordinated to a Tb1 ion to form a Tb1/sq1-sheet parallel to the bc plane. Quite similarly, three Tb2 ions and three μ3-sq3 anions (C9-C10-C11-C12 squarate) construct a Tb2/sq3-sheet with the O9, O10, and O11 coordination bonds. Between the two sheets, another sq (sq2) interconnects Tb1 and Tb2 ions with the O5 and O8 donor atoms. Consequently, a sandwiched two-dimensional polynuclear polymer appears. The shortest intra-sheet Tb1•••Tb1 and Tb2•••Tb2 distances are 6.4246(5) and 6.4671(5) Å, respectively, which are comparable to the shortest inter-sheet Tb1•••Tb2 distance 6.5479(5) Å. They are linked across O-C-C-O atoms. The shortest Tb1•••Tb1 and Tb2•••Tb2 distances across O-C-C-C-O atoms are somewhat longer (8.1820(6) Å, corresponding to the b-axis translation).
The sq rings are stacked along the a axis (Figure 2a). The presence of π-π interaction has already been discussed in Eu-sq [11]. In addition to this, dipolar interaction between the polarized carbonyl groups (Scheme 1) should be pointed out, because the shortest interplane distances are found between the C and O atoms in a slipped π-π overlap. Furthermore, the dense hydrogen-bonding network also contributes to stabilizing the crystal. Relatively short O(w)•••O(sq) distances are depicted in Figure 2b-d. Seven-membered rings comprising O-C-C-O(sq)-Tb-O-H(w) atoms are an important motif. The oxygen atoms of H-donating water molecules are located nearly on the H-accepting sq molecular plane, indicating the sp2-hybridization of the sq oxygen atoms and the substantial O•••H interaction.
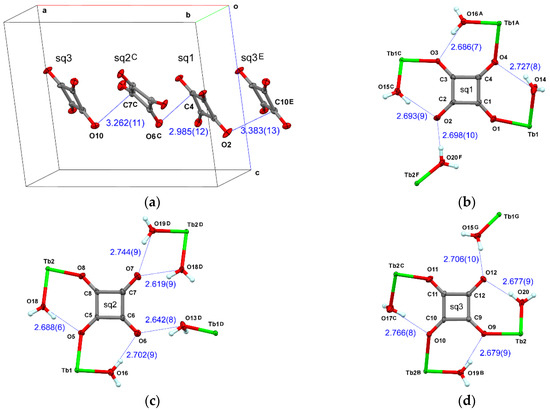
Figure 2.
(a) Stacking motif of sq planes. The shortest interplane distances are indicated with dotted lines. (b) Hydrogen bonds around sq1, (c) sq2, and (d) sq3. The interatomic O•••O distances are indicated. The symmetry operation codes: E: x, 1+y, z; F: −1+x, 1−y, 1/2+z; G: 1+x, 1−y, −1/2+z.
Figure 3a shows the intense photoluminescence of a solid sample of Tb-sq under ambient conditions with irradiation from a handheld UV light (375 nm). The emission spectrum of Tb-sq at room temperature was recorded under irradiation at λex = 320 nm (Figure 3b). Major luminescence peaks appeared at 490, 544, 583–588, and 620–621 nm, which are assigned to the f–f transitions of 5D4 – 7FJ with J = 6, 5, 4, and 3, respectively [31]. The excitation spectrum of Tb-sq is superimposed in Figure 3b. A broad band at 300–360 nm corresponds to the absorption of the organic ligand. The solution absorption spectrum of Tb3+ squarate was reported to show a peak at 270 nm and a shoulder around 300 nm [32]. The polymeric solid form of Tb-sq seems to lead to a red shift in absorption. The quantum yield of the photoluminescence (Φ) was recorded with the emission monitored at 450 – 700 nm at room temperature (Figure 3c). Almost constant Φ = 0.25 – 0.24 was found in the range λex = 310 – 350 nm. The efficient photoluminescence can be accounted for with an antenna effect [33,34], which involves a sensitizing organic chromophore allowing indirect excitation of the Ln ion. The absorption from Ln ions themselves is usually weak, so that the organic UV-vis absorbents are necessary adjacent to the Ln ion. The luminescent bands were accompanied by fine structures, and further investigation would clarify the sublevel regulated with Jz [35,36,37,38].
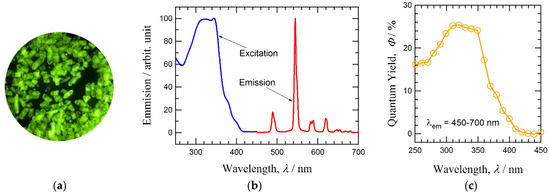
Figure 3.
(a) A photo of a solid sample of Tb-sq under irradiation of UV light (375 nm). (b) Emission spectrum (λex = 320 nm) and excitation spectrum (λem = 450–700 nm) of polycrystalline Tb-sq recorded at room temperature. (c) Absolute photoluminescence quantum yield as a function of excitation wavelength.
Figure 4a displays the DC magnetic susceptibility result on a formula {Tb2(C4O4)3(H2O)8} basis of Tb-sq. At 300 K, the χmT value was 22.9 cm3 K mol−1, in good agreement with the theoretical value 23.6 cm3 K mol−1 expected for magnetically isolated Tb3+ ion from the total angular moment J = 6 and the Landé g factor gJ = 3/2. On cooling, the χmT value gradually decreased and reached 16.4 cm3 K mol−1 at 1.8 K, and this finding can be explained in terms of the depopulation effect with respect to the Jz sublevels. The M–H curves (Figure 4a, inset) display a nearly saturated curve, but the apparent saturation magnetization (11.6 NAμB at 7 T and 1.8 K when the sample was unfixed) was lower than the maximal value, 18 NAμB from Jz = 6, mainly because of the strong magnetic anisotropy of Tb3+.
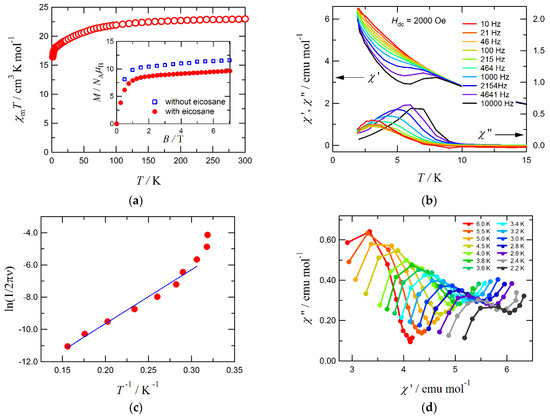
Figure 4.
(a) Temperature dependence of χmT for Tb-sq, measured at 500 Oe. The sample was fixed with a small amount of eicosane for removing the field-align effect due to torqueing. Inset: M–H curves for Tb-sq, measured at 1.8 K with and without eicosane. (b) AC magnetic susceptibilities for Tb-sq at an applied DC field of 2000 Oe. Solid lines are drawn as a guide for the eye. (c) Arrhenius plot for Tb-sq with the data taken from (b). For a solid line, see the text. (d) Cole–Cole plots for Tb-sq. Solid lines are drawn as a guide for the eye.
Figure 4b shows the in-phase and out-of-phase portions of the AC magnetic susceptibilities (χ’m and χ”m, respectively) for Tb-sq, measured at an applied DC field of 2000 Oe. No appreciable χ”m was recorded without any applied field. Possible quantum tunneling of the magnetization is suppressed with a field bias [39]. The Arrhenius plot on Tb-sq is drawn in Figure 4c, using the frequency dependence of the χ”m peak data. The effective activation energy (Ueff) for the magnetization reversal and pre-exponential factor (τ0) were determined according to the equation, ln(1/2πν) = ln(τ0) + Ueff/kBT [40], giving Ueff/kB = 33(2) K and τ0 = 8.1(40) × 10–8 s. A straight line suggests that the magnetization would undergo Orbach-type relaxation [41]. In a lowest temperature region around 2 K, the χ”m peaking temperature seems to become insensitive to frequency. The Cole–Cole plot [42] did not trace a simple semicircle; in fact, a second relaxation process may be operative. The origin of the additional behavior is unclear at present, and further investigation on Tb3+-sq-Tb3+ superexchange magnetic coupling, together with the energy potential surface regarding Jz, is now underway.
We have already reported another system, the Tb3+ complexes carrying “mer”- [tris(N-[(imidazol-4-yl)-methylidene]-dl-phenylalaninato) and related “fac”-dl-alaninato ligands [43]. These complexes exhibited luminescence and SIM behavior, but no blocking was recorded above 2 K in the same AC-field frequency window as that of the present study. Interestingly and ironically, a much simpler Tb-sq presents better SIM characteristics. This may be because the D4d square-antiprism crystal field in Tb-sq is just suitable for SIM behavior, similarly to the Tb3+ phthalocyanino complex [15]. The oblate electron density around Tb3+ minimizes the energies at the large-|Jz| sublevels to give a double-well potential, although Tb3+ is a non-Kramers ion, when the square-antiprism crystal field is axially compressed [44].
4. Conclusions
We prepared Tb-sq in two ways and found that a hydrothermal synthesis from Tb2O3 would be an excellent method for specific synthesis. The crystal structure of Tb-sq was determined in a monoclinic Pc space group. A sandwiched two-dimensional structure was characterized. The solid complex emits green light under UV irradiation at room temperature with the quantum yield of 25%. The AC magnetic susceptibility measurement showed frequency dependence, as an indication for a SIM with Ueff/kB = 33(2) K in a 2000-Oe DC field. It seems to be related with a D4d crystal field from the square-antiprism coordination structure around Tb3+. Compound Tb-sq is an easily obtainable and prototypical luminescent magnet. More sophisticated compounds [45] will be designed and synthesized from the viewpoint of Jz energy surface engineering to explore magnet-based multi-functional Ln coordination materials.
Supplementary Materials
CCDC deposition number 2108278 contains the supplementary crystallographic data for this paper. These data are provided free of charge by the Cambridge Crystallographic Data Centre at www.ccdc.cam.ac.uk/structures.
Author Contributions
R.T. participated in all the experiments. T.I. designed and supervised the study. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported in part from Izumi Science and Technology Foundation (Grant number 2019-J-033) and JSPS KAKENHI (Challenging Exploratory Research JP20K21170 and Scientific Research on Innovative Areas “Soft Crystals” JP17H06371).
Conflicts of Interest
The authors declare no conflict of interest.
References
- West, R. Oxocarbons; Academic Press: New York, NY, USA, 1980. [Google Scholar]
- Brenner, N.; Sperling, J.M.; Poe, T.N.; Celis-Barros, C.; Brittain, K.; Villa, E.M.; Albrecht-Schmitt, T.E.; Polinski, M.J. Trivalent f-element squarates, squarate-oxalates, and cationic materials, and the determination of the nine-coordinate ionic radius of Cf(III). Inorg. Chem. 2020, 59, 9384–9395. [Google Scholar] [CrossRef]
- Lin, X.Y.; Zhao, L.M.; Wang, D.H.; Wang, Y.K.; Li, M.; Li, H.H.; Chen, Z.R. Structural diversities of squarate-based complexes: Photocurrent responses and thermochromic behaviours enchanced by viologens. Inorg. Chem. Front. 2018, 5, 189–199. [Google Scholar] [CrossRef]
- Goswami, S.; Saha, R.; Steele, I.M.; Dasgupta, P.; Poddar, A.; Kumar, S. An unusual μ-1,2,3-squarato- bridged two dimensional coordination polymer: Crystal structure, thermal, photoluminescence and magnetic studies. Inorg. Chim. Acta 2014, 410, 111–117. [Google Scholar] [CrossRef]
- Thorarinsdottir, A.E.; Harris, T.D. Metal–organic framework magnets. Chem. Rev. 2020, 120, 8716–8789. [Google Scholar] [CrossRef]
- Tu, R.; Zhang, W.; Zhang, J.; Wang, M.; Zhang, F.; Yang, K.; Li, J.; Pan, H.; Bernards, M.T.; Xie, P.; et al. Squarate-calcium metal–organic framework for molecular sieving of CO2 from flue gas with high water vapor resistance. Energy Fuel. 2021, 35, 13900–13907. [Google Scholar] [CrossRef]
- Babaryk, A.A.; Contreras Almengor, O.R.; Cabrero-Antonino, M.; Navalón, S.; García, H.; Horcajada, P. A semiconducting Bi2O2(C4O4) coordination polymer showing a photoelectric response. Inorg. Chem. 2020, 59, 3406–3416. [Google Scholar] [CrossRef]
- Goswami, S.; Jena, H.S.; Konar, S. Study of Heterogeneous Catalysis by iron-squarate based 3D metal organic framework for the transformation of tetrazines to oxadiazole derivatives. Inorg. Chem. 2014, 53, 7071–7073. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Guo, L.; Zhang, Z.; Yang, Q.; Yang, Y.; Bao, Z.; Ren, Q.; Li, J. A robust squarate-based metal–organic framework demonstrates record-high affinity and selectivity for xenon over krypton. J. Am. Chem. Soc. 2019, 141, 9358–9364. [Google Scholar] [CrossRef]
- Massoud, S.S.; Louka, F.R.; Mautner, F.A. Polynuclear and polymeric squarato-bridged coordination compounds. CrystEngComm 2015, 17, 7604–7617. [Google Scholar] [CrossRef]
- Petit, J.F.; Gleizes, A.; Trombe, J.C. Lanthanide(III) squarates 1. Five families of compounds obtained from aqueous solutions in an open system. Crystal structure and thermal behaviour. Inorg. Chim. Acta 1990, 167, 51–68. [Google Scholar] [CrossRef]
- Huskowska, E.; Glowiak, T.; Legendziewicz, J.; Oremek, G. Luminescence and crystal structure of neodymium and europium squarate hydrates. J. Alloys Compd. 1992, 179, 13–25. [Google Scholar] [CrossRef]
- Kanetomo, T.; Ishida, T. Luminescent single-ion magnets from lanthanoid(III) complexes with monodentate ketone ligands. AIP Conf. Proc. 2016, 1709, 020015. [Google Scholar]
- Biswas, S.; Adhikary, A.; Goswami, S.; Konar, S. Observation of a large magnetocaloric effect in a 2D Gd(III)-based coordination polymer. Dalton Trans. 2013, 42, 13331–13334. [Google Scholar] [CrossRef]
- Ishikawa, N.; Sugita, M.; Ishikawa, T.; Koshihara, S.Y.; Kaizu, Y. Lanthanide double-decker complexes functioning as magnets at the single-molecular level. J. Am. Chem. Soc. 2003, 125, 8694–8695. [Google Scholar] [CrossRef] [PubMed]
- Bernot, K.; Bogani, L.; Caneschi, A.; Gatteschi, D.; Sessoli, R. A family of rare-earth-based single chain magnets: Playing with anisotropy. J. Am. Chem. Soc. 2006, 128, 7947–7956. [Google Scholar] [CrossRef] [PubMed]
- Armelao, L.; Quici, S.; Barigelletti, F.; Accorsi, G.; Bottaro, G.; Cavazzini, M.; Tondello, E. Design of luminescent lanthanide complexes: From molecules to highly efficient photo-emitting materials. Coord. Chem. Rev. 2010, 254, 487–505. [Google Scholar] [CrossRef]
- Yan, B. Lanthanide-functionalized metal–organic framework hybrid systems to create multiple luminescent centers for chemical sensing. Acc. Chem. Res. 2017, 50, 2789–2798. [Google Scholar] [CrossRef]
- Kido, J.; Okamoto, Y. Organo lanthanide metal complexes for electroluminescent materials. Chem. Rev. 2002, 102, 2357–2368. [Google Scholar] [CrossRef] [PubMed]
- Bünzli, J.C.G.; Piguet, C. Taking advantage of luminescent lanthanide ions. Chem. Soc. Rev. 2005, 34, 1048–1077. [Google Scholar] [CrossRef]
- Xu, J.; Gulzar, A.; Yang, P.; Bi, H.; Yang, D.; Gai, S.; He, F.; Lin, J.; Xing, B.; Jin, D. Recent advances in near-infrared emitting lanthanide-doped nanoconstructs: Mechanism, design and application for bioimaging. Coord. Chem. Rev. 2019, 381, 104–134. [Google Scholar] [CrossRef]
- Quici, S.; Cavazzini, M.; Marzanni, G.; Accorsi, G.; Armaroli, N.; Ventura, B.; Barigelletti, F. Visible and near-infrared intense luminescence from water-soluble lanthanide [Tb(III), Eu(III), Sm(III), Dy(III), Pr(III), Ho(III), Yb(III), Nd(III), Er(III)] complexes. Inorg. Chem. 2005, 44, 529–537. [Google Scholar] [CrossRef]
- Sahadevan, S.A.; Monni, N.; Oggianu, M.; Abhervé, A.; Marongiu, D.; Saba, M.; Mura, A.; Bongiovanni, G.; Mameli, V.; Cannas, C.; et al. Heteroleptic NIR-Emitting YbIII/Anilate-based neutral coordination polymer nanosheets for solvent sensing. ACS Appl. Nano. Mater. 2020, 3, 94–104. [Google Scholar] [CrossRef] [Green Version]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Bain, G.A.; Berry, J.F. Diamagnetic corrections and Pascal’s constants. J. Chem. Educ. 2008, 85, 532–536. [Google Scholar] [CrossRef]
- Trombe, J.C.; Petit, J.F.; Gleizes, A. Lanthanide(III) squarates 2. High diversity of rare coordination modes of the squarate anion in a series of weakly hydrated cerium(III) squarates prepared by pseudo-hydrothermal methods. Inorg. Chim. Acta 1990, 167, 69–81. [Google Scholar] [CrossRef]
- Wang, L.; Gu, W.; Deng, X.J.; Zeng, L.F.; Liao, S.Y.; Zhang, M.; Yang, L.-Y.; Liu, X. A Series of 2D and 3D Novel Lanthanide Complexes Constructed from Squarate C4O42–: Syntheses, Structures, Magnetic Properties, and Near-infrared Emission Properties. Aust. J. Chem. 2011, 64, 1373–1382. [Google Scholar] [CrossRef]
- Wang, X.; Zhai, Q.G.; Li, S.N.; Jiang, Y.C.; Hu, M.C. Synthesis, crystal structures, and solid-state luminescent properties of diverse Ln–Pyridine-3,5-Dicarboxylate coordination polymers modulated by the ancillary ligand. Cryst. Growth Des. 2014, 14, 177–188. [Google Scholar] [CrossRef]
- Lluncll, M.; Casanova, D.; Circra, J.; Bofill, J.M.; Alcmany, P.; Alvarez, S.; Pinsky, M.; Avnir, D. SHAPE, version 2.1; University of Barcelona: Barcelona, Spain; Hebrew University of Jerusalem: Jerusalem, Israel, 2005. [Google Scholar]
- Leonard, J.P.; Gunnlaugsson, T. Luminescent Eu(III) and Tb(III) complexes: Developing lanthanide luminescent-based devices. J. Fluoresc. 2005, 15, 585–595. [Google Scholar] [CrossRef]
- Ribeiro, S.J.; Gonçalves, R.R.; de Oliveira, L.F.; Santos, P.S. Spectroscopic study of lanthanide squarate hydrates. J. Alloys Compd. 1994, 216, 61–66. [Google Scholar] [CrossRef]
- Moore, E.G.; Samuel, A.P.; Raymond, K.N. From antenna to assay: Lessons learned in lanthanide luminescence. Acc. Chem. Res. 2009, 42, 542–552. [Google Scholar] [CrossRef] [Green Version]
- Marin, R.; Brunet, G.; Murugesu, M. Shining New Light on Multifunctional Lanthanide Single-Molecule Magnets. Angew. Chem. Int. Ed. 2021, 60, 1728–1746. [Google Scholar] [CrossRef]
- Karbowiak, M.; Rudowicz, C.; Ishida, T. Determination of crystal-field energy levels and temperature dependence of magnetic susceptibility for Dy3+ in [Dy2Pd] heterometallic complex. Inorg. Chem. 2013, 52, 13199–13206. [Google Scholar] [CrossRef]
- Ehama, K.; Ohmichi, Y.; Sakamoto, S.; Fujinami, T.; Matsumoto, N.; Mochida, N.; Ishida, T.; Sunatsuki, Y.; Tsuchimoto, M.; Re, N. Synthesis, structure, luminescent, and magnetic properties of carbonato-bridged ZnII2LnIII2 complexes [(μ4-CO3)2{ZnIILnLnIII(NO3)}2] (LnIII = GdIII, TbIII, DyIII.; L1 = N,N′-Bis(3-methoxy-2-oxybenzylidene)-1,3-propanediaminato, L2 = N,N′-Bis(3-ethoxy-2-oxybenzylidene)-1,3-propanediaminato). Inorg. Chem. 2013, 52, 12828–12841. [Google Scholar]
- Yamashita, K.; Miyazaki, R.; Kataoka, Y.; Nakanishi, T.; Hasegawa, Y.; Nakano, M.; Yamamura, T.; Kajiwara, T. A luminescent single-molecule magnet: Observation of magnetic anisotropy using emission as a probe. Dalton Trans. 2013, 42, 1987–1990. [Google Scholar] [CrossRef]
- Karbowiak, M.; Rudowicz, C.; Nakamura, T.; Murakami, R.; Ishida, T. Spectroscopic and magnetic studies of erbium(III)-TEMPO complex as a potential single-molecule magnet: Interplay of the crystal-field and exchange coupling effects. Chem. Phys. Lett. 2016, 662, 163–168. [Google Scholar] [CrossRef]
- Castro, S.L.; Sun, Z.; Grant, C.M.; Bollinger, J.C.; Hendrickson, D.N.; Christou, G. Single-molecule magnets: Tetranuclear vanadium(III) complexes with a butterfly structure and an S = 3 ground state. J. Am. Chem. Soc. 1998, 120, 2365–2375. [Google Scholar] [CrossRef]
- Gatteschi, D.; Sessoli, R.; Villain, J. Molecular Nanomagnets; Oxford University Press: Oxford, UK, 2006. [Google Scholar]
- Giansiracusa, M.; Gransbury, G.; Chilton, N.; Mills, D. Single Molecule Magnets. In Encyclopedia of Inorganic and Bioinorganic Chemistry; John Wiley & Sons: New York, NY, USA, 2021. [Google Scholar]
- Sessoli, R.; Powell, A.K. Strategies towards single molecule magnets based on lanthanide ions. Coord. Chem. Rev. 2009, 253, 2328–2341. [Google Scholar] [CrossRef]
- Yamauchi, S.; Fujinami, T.; Matsumoto, N.; Mochida, N.; Ishida, T.; Sunatsuki, Y.; Watanabe, M.; Tsuchimoto, M.; Coletti, C.; Re, N. Synthesis, Structure, Luminescence, and Magnetic Properties of a Single-Ion Magnet “mer”-[Tris(N-[(imidazol-4-yl)- methylidene]-dl-phenylalaninato)terbium(III) and Related “fac”-dl-Alaninato Derivative. Inorg. Chem. 2014, 53, 5961–5971. [Google Scholar] [CrossRef]
- Rinehart, J.D.; Long, J.R. Exploiting single-ion anisotropy in the design of f-element single-molecule magnets. Chem. Sci. 2011, 2, 2078–2085. [Google Scholar] [CrossRef]
- Long, J.; Guari, Y.; Ferreira, R.A.; Carlos, L.D.; Larionova, J. Recent advances in luminescent lanthanide based Single-Molecule Magnets. Coord. Chem. Rev. 2018, 363, 57–70. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).