Abstract
Nowotny chimney ladder (NCL) phases are intermetallic compounds formed by transition metals and metals of groups 13 and 14. This family can be expanded by combining two p-elements from different groups with those transition metals, for which the corresponding binary NCL phases are unknown. In this paper, we present three new compounds in the V-Al-Ge, Nb-Al-Ge, and Nb-Ga-Ge systems related to the TiSi2 structure type (Sp. Gr. Fddd) obtained with the standard ampule technique. The crystal structures of the new compounds were determined using synchrotron powder X-ray diffraction data. A transition to the CrSi2 structure type was detected upon changing the composition from VAl0.72(2)Ge1.28(2) to VAl1.534(3)Ge0.466(3). According to the 18–n rule, all the compounds are metallic conductors, which was supported by the electronic structure calculations. It was shown that the expected energy gap located above the Fermi level in the vanadium-based NCL compound collapsed into a pseudogap upon the replacement of V by Nb.
1. Introduction
Intermetallics are a large class of compounds with a variety of crystal structures. Most of the metals in the Periodic Table allow for diverse combinations, which lead to a wealth of crystal and electronic structures [1,2,3]. Their diversity ensures a plethora of properties; as a result, intermetallic compounds are widely used in technology and are also an object of basic scientific interest [4,5,6]. For intermetallics, there is still no general theory that links their composition, structure, and properties [6]. This is not surprising; indeed, depending on the nature of the constituent chemical elements and details of the electronic structures, intermetallic compounds can be classified into three major groups. The extreme cases are represented by, on one side, ionic compounds such as Zintl phases with an almost complete charge transfer between cationic and anionic substructures and, on the opposite side, so-called electronic compounds such as Hume–Rothery phases with almost no charge transfer. For both groups, elaborate approaches have been devised to relate their crystal and electronic structures with properties [7,8]. Polar intermetallics are an intermediate case, which itself represents a heterogeneous field of multifarious structural and bonding patterns. It includes intermetallic compounds formed by metals from different blocks of the Periodic Table: transition metals on one side and metals or semimetals of the p-block on the other side. The so-called Nowotny chimney ladder (NCL) phases belong to this subclass of polar intermetallic compounds.
NCL phases are widely known and crystallize in more than 10 structure types [9,10,11,12,13,14,15,16]. Despite the variety of the crystal structures, they all have common dominant features. Transition metal atoms form a four-step helix that opens up in the form of a channel with a square base (slightly distorted in the case of TiSi2), inside which atoms of a p-metal/semimetal form spirals of different repeat intervals, depending on the stoichiometry of a phase. Channels of transition metal atoms resemble a “chimney”, inside of which there are spiral-shaped “ladders” made of p-element atoms (see Figure 1a,b).
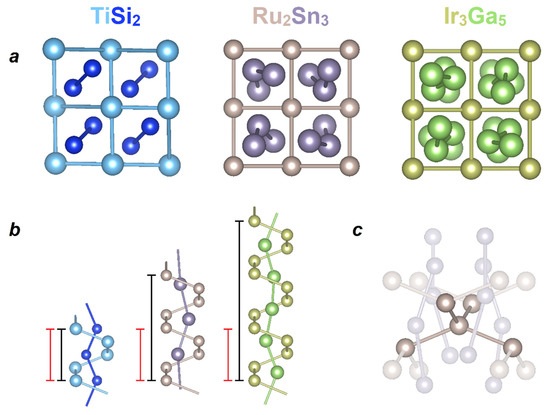
Figure 1.
Crystal structures of Nowotny chimney ladder (NCL) phases exemplified by TiSi2, Ru2Sn3, and Ir3Ga5 structure types. (a) View of the structures perpendicular to the d- and p-helices. (b) p-element helical “ladder” inside a transition metal “chimney” with periodicity (repeating periods of d-metal and p-element are shown by red and black lines, respectively). (c) Four shortest metal–metal interactions between the transition metal atoms in the NCL phases of the Ru2Sn3 crystal structure.
Another common feature of the NCL phases is the deviation from the stoichiometric composition, which leads to the formation of incommensurate structures due to the mismatch of the repeating periods of d- and p-helices. For many such compounds, the corresponding approximant structures have been proposed. Thus, single crystals of Fe2Ge3 possess a precise composition and commensurate structure, while a polycrystalline sample is characterized by a complex incommensurate crystal structure [10,11,12]. In addition, helices can be formed by more than one element. It should be noted that employing a combination of two p-elements allows one to obtain new NCL phases for some of the transition metals that do not form binary NCL phases. According to this approach, phases with the TiSi2 structure type have been obtained in the Cr-Al-Ge, Mo-Ga-Ge, Mn-Al-Si, Nb-Al-Si, Mo-Al-Ge, and Mo-Al-Si systems, despite the absence of binary compounds for those d-metals involved [17,18,19,20,21,22]. One more important feature of the NCL phases is the valence electron count (VEC), which varies within the range of 12 to 14 per transition element atom, with possible minor deviations to slightly higher values, such as for Rh17Ge22 (VEC = 14.18) [23]. Noteworthy is a unique case of hp-Co2Si3 (VEC = 15); however, this compound can be prepared only at a high pressure of 7 GPa [24]. Based on the crystal and electronic structures of NCL phases, the 14-electron rule has been formulated [9,15,16], which was later transformed into the 18–n rule [25,26]. According to it, in polar intermetallic compounds based on transition metals, the VEC should adhere to 18–n, where n is the number of electron pairs shared between transition metal atoms, which corresponds to the number of d-M–d-M bonds. This number is 4 for all NCL phases according to the features of their crystal structure (see Figure 1c). In addition, the formation of a gap or a pseudogap at the Fermi level is frequently observed at VEC = 14. However, a sizable number of phases are electron-poor but nonetheless stable [26].
To date, only two NCL phases with group 5 metals are known; they are binary V17Ge31 [27] and ternary NbAl0.6Si1.4 [19]; the latter compound is found to crystallize in the TiSi2 structure type.
The major goal of this work was the targeted synthesis of NCL phases with the TiSi2-type in systems where vanadium or niobium is employed as a transition metal forming a “chimney”, and a combination of two elements of groups 13 and 14 is used as a p-element-building “ladder”: in this case, aluminum and germanium or gallium and germanium. The compositional range of the phases’ existence and their crystal structures were investigated experimentally, and the electronic structure of the new compounds was calculated.
2. Materials and Methods
2.1. Synthesis
Chips of metallic vanadium (99.9%), powder of niobium (99.98%), geranium chips (99.999%), and gallium ingots (99.9999%) were used as starting materials. Before application, vanadium was treated with a diluted (2%) solution of hydrochloric acid to remove an oxide film from the metal surface. A stoichiometric mixture of the elements was loaded into a quartz ampule, which was evacuated (residual pressure of 1 × 10−3 Torr) and sealed. Ampules were annealed at 750 °C for one week, after which the samples were ground, pressed into pellets, and annealed under the same conditions for another week. For the synthesis, the following stoichiometric ratios were used: VAl2−xGex (x = 0.8, 0.9, 1, 1.1, 1.2, 1.3, 1.4, 1.5), VGa2−xGex (x = 0.5, 0.7, 0.8, 0.9, 1, 1.1, 1.2, 1.3, 1.5), NbAl2−xGex (x = 0.5, 0.7, 0.8, 0.9, 1, 1.1, 1.2, 1.3, 1.5), and NbGa2−xGex (x = 0.8, 0.9, 1, 1.1, 1.2, 1.3, 1.4).
Single crystals of VAl2−xGex were grown using chemical transport reactions with several crystals of I2 as a transport agent. A pre-synthesized phase-pure polycrystalline sample of VAl2−xGex was annealed in an evacuated quartz ampule in a two-zone horizontal furnace for 14 days. The load temperature was 450 °C, while the crystallization zone was kept at 400 °C.
2.2. Powder X-ray Diffraction
The phase composition of all the products was determined by powder X-ray diffraction (PXRD) analysis using a Huber G670 Guinier Camera (Cu Kα1 radiation, Ge monochromator, λ = 1.5406 Å). The data were collected by scanning the image plate 4 times after an exposure time of 2400 s at room temperature.
2.3. Energy Dispersive X-ray Analysis
A scanning electron microscope, JSM JEOL 6490-LV, equipped with an energy dispersive X-ray (EDX) analysis system, INCA x-Sight, was used for the chemical analysis. The accelerating voltage was 30 kV. The uncertainty of the measurements for each element was 1 at.%. For the measurements, the samples were finely ground and pressed into pellets. The detailed EDX analysis results as well as EDX mapping are shown in the Table S1 and Figure S1 of the Supplementary Materials.
2.4. Crystal Structure Investigation
The crystal structures of the phase-pure samples with the nominal composition VAl0.7Ge1.3 and NbGa0.8Ge1.2 were investigated using high-resolution PXRD data collected at the ID22 beamline of the European Synchrotron Radiation Facility (ESRF, Grenoble, France) (λ = 0.35451 Å, 2θ range of 1–37.914°, and a scan step of 0.002°). Room temperature measurements were performed on the samples enclosed in sealed quartz capillaries with an inner diameter of 0.5 or 0.2 mm. The capillaries were spun during the measurements. The Jana 2006 software was used for the structure refinement [28]. The PXRD patterns are presented in Figure S2 of the Supplementary Materials.
Single crystals of VAl2−xGex grown from a single-phase VAl0.7Ge1.3 polycrystalline sample were investigated at 100 K on a Bruker D8 VENTURE single-crystal X-ray diffractometer equipped with a PHOTON 100 CMOS detector, graphite monochromator, and Mo-target X-ray tube (λ = 0.73071 Å). A frame width of 0.50° and exposure time of 15 s/frame were employed for data collection. Data reduction and integration were performed with the Bruker software package SAINT (Version 8.38A) [29]. The absorption correction was performed using the multiscan routine as implemented in SADABS (Version 2014/5) [30,31]. The crystal structure was solved by the charge-flipping algorithm using the Superflip program [32]. Furthermore, the data were refined in the full-matrix anisotropic approximation against |F2| using the SHELXTL (Version 2017/1) program package [33]. The crystal structures were visualized using the VESTA 3 package [34].
Crystallographic data as well as structure solution and refinement details are presented in Table 1 and Table 2. CCDC/CSD 2017189, 2017190, and 2017191 contain the supplementary crystallographic data for this paper. These data can be obtained free of charge at www.ccdc.cam.ac.uk/data_request/cif, by emailing data_request@ccdc.cam.ac.uk, or by contacting The Cambridge Crystallographic Data Centre, 12 Union Road, Cambridge CB2 1EZ, UK; fax: +44 1223 336033.

Table 1.
Crystallographic data and structure refinement parameters for VAl2−xGex and NbGa2−xGex powder samples.

Table 2.
Crystallographic data and refinement parameters for VAl2−xGex single-crystal X-ray diffraction.
2.5. Electronic Strcuture Calculations
The atomic coordinates and unit cell parameters obtained from the crystal structure refinement were used for electronic structure calculations within the density functional theory (DFT) approach using a Full-Potential Local-Orbital minimum basis band-structure (FPLO) code (version 14.00-47) [35]. The local density approximation (LDA) functional was used to treat the exchange-correlation energy in the fully relativistic regime [36]. Integrations in the k-space were performed with an improved tetrahedron method [37] on a grid of 16 × 16 × 16 k-points.
Since, according to the crystal structure solution data, the formation of a superstructure or ordering of aluminum/gallium and germanium in a single p-element position was not found for all phases, we proposed several ordered models for the electronic structure calculations. A schematic representation of all the models is shown in Figure 2a,b: three models for the TiSi2-structure type and two models for the CrSi2-sctructure type. Despite the fact that the refined compositions of the new phases are different from the M:E1:E2 = 1:1:1 (M = V, Nb; E1 = Al, Ga; E2 = Ge) ratio, we used this integer stoichiometry in order to simplify the band structure calculations and to perform a qualitative comparison of the results for the different models against each other.
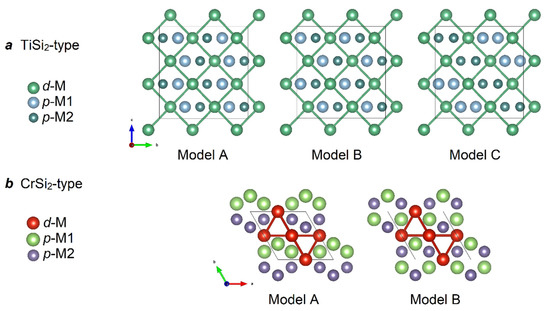
Figure 2.
Schematic representation of ordered models for band structure calculations. The black frame shows the unit cell. (a) TiSi2-type; in the case of VAlGe, d-M = V, p-M1 = Al, p-M2 = Ge; for NbGaGe, d-M = Nb, p-M1 = Ga, p-M2 = Ge. (b) CrSi2-type; for VAlGe, d-M = V, p-M1 = Al, p-M2 = Ge.
In addition, a virtual crystal approximation (VCA) was used for the NbGa2−xGex phase. In this case, we could freely vary the nuclear charge of the p-element from the ideal composition of the ordered models (NbGaGe, p-element nuclear charge of 31.5) to the real one (NbGa0.8Ge1.2, p-element nuclear charge of 31.6).
3. Results and Discussion
3.1. VAl2−xGex: Phase Equilibria, and Crystal and Electronic Structure
All VAl2−xGex (x = 0.8, 0.9, 1, 1.1, 1.2, 1.3, 1.4, 1.5) samples were investigated by the PXRD analysis, according to which we observed the formation of a new compound in the region of x between 0.9 and 1.5. A phase-pure sample was obtained for the VAl0.7Ge1.3 nominal composition, for which all reflections on the diffraction pattern were indexed in the orthorhombic crystal system (Sp. Gr. Fddd), and the composition V1.02(3)Al0.72(3)Ge1.26(3) was confirmed by the EDX analysis (for details, see Figure S1). An increase in the germanium content (x ≥ 1.4) in the samples leads to the formation of a second phase—VGe2 (CrSi2-type, Sp. Gr. P6222 [38])—as an impurity. At the same time, the amount of the target phase decreases. With a decrease in the germanium content (1 ≤ x ≤ 1.2), a decrease in the amount of the target compound and the formation of impurity phases V5Ge3 (W5Si3-type, Sp. Gr. I4/mcm [39]) and VAl3 (TiAl3-type, Sp. Gr. I4/mmm [40]) are observed. Finally, reflections of the new compound are not detected at the lowest investigated germanium content (x ≤ 0.9). An illustration of the phase composition change for the VAl2−xGex series is shown in Figure 3.
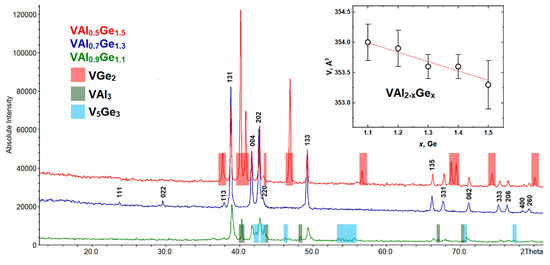
Figure 3.
Powder X-ray diffraction (PXRD) patterns of VAl2−xGex (x = 1.1, 1.3, and 1.5). Inset: the unit cell volume of VAl2−xGex (Fddd) vs. nominal Ge content.
It should be noted that the calculated unit cell parameters change only slightly with the nominal composition (see the inset of Figure 3), pointing at a narrow homogeneity range of the new compound, whose width could not be detected with reasonable accuracy.
A single-phase powder sample VAl0.7Ge1.3 was investigated using synchrotron PXRD, and the crystal structure was refined (Table 1). As expected, the new compound crystallizes in the TiSi2 structure type (Sp. Gr. Fddd) and belongs to the family of NCL phases. There are two unique positions in the crystal structure—one for vanadium and another for p-elements. No ordering of aluminum and germanium was observed; thus, there is a statistical population of the p-M1 site by Al and Ge with a refined occupancy equal to 0.36(2)Al + 0.64(2)Ge, which yields the VAl0.72(2)Ge1.28(2) composition, in perfect agreement with the EDX data. The mixed population of the 16f site by two p-elements is typical for the NCL phases with the TiSi2 crystal structure, even when p-elements with different atomic radii are combined [17,20]. The atomic parameters for the crystal structure of VAl0.72(2)Ge1.28(2) are presented in Table 3.

Table 3.
Atomic coordinates and thermal displacement parameters of the VAl0.72(2)Ge1.28(2).
The obtained phase-pure powder of VAl0.7Ge1.3 was used for growing crystals exploiting chemical transport reactions in a temperature gradient of 450–400 °C, with iodine serving as a transport agent. Surprisingly, VAl1.534(3)Ge0.466(3) crystals with a CrSi2 structure were obtained, and, as can be seen, the refined composition of the single crystal differs greatly from the composition of the initial sample (for details, see Table 2). This is actually an unexpected result. In the V–Al and V–Ge binary systems, there is only one CrSi2-type phase—VGe2, which was obtained under high pressure conditions [38]. Apparently, in our case, phase stabilization occurs due to the substitution of part of the germanium atoms by aluminum atoms, and then we could expect that the “VGe2” phase that we detected analyzing XRD patterns (see Figure 3) may also contain aluminum, although no especial studies have been carried out in this work. Although the refined composition of the grown crystals is very rich in aluminum (VAl1.534(3)Ge0.466(3)), this compound crystallizes in the CrSi2 structure type, whereas isostructural VAl0.66Si1.34, on the contrary, has a reduced content of a group 13 metal [41]. Thus, the transition from the TiSi2 structure type to the CrSi2-type strongly depends on both the nominal composition and synthetic conditions. At 750 °C, VAl0.72(2)Ge1.28(2) that crystallizes in the TiSi2 structure type is synthesized, while the increasing of the germanium content leads to “VGe2” with the CrSi2 structure, which probably contains Al. Crystal growth from VAl0.72(2)Ge1.72(2) powder with iodine as a transport agent at 400 °C leads to the formation of VAl1.534(3)Ge0.466(3) that crystallizes in the CrSi2 structure type. Note that the temperature of 400 °C is too low to achieve interaction in the V-Al-Ge system.
The atomic parameters for the VAl1.534(3)Ge0.466(3) crystal structure are presented in Table 4.

Table 4.
Atomic coordinates and displacement parameters for VAl1.534(3)Ge0.466(3).
As a matter of fact, both structure types are very close to each other. A comparison of the two structures is shown in Figure 4a–c. Clearly, the CrSi2 structure also contains a “chimney” formed by vanadium atoms, inside which the ladder of silicon atoms is located (Figure 4b). The difference lies in the shift of these “chimneys” relative to each other along the a axis by half of the a parameter. Other structural differences are minimal. In both VAl2−xGex modifications—VAl0.72(2)Ge1.28(2) (TiSi2-type) and VAl1.534(3)Ge0.466(3) (CrSi2-type)—the environment of the vanadium atoms is equivalent and is shown in Figure 4c. Analysis of the crystal structures indicates the presence of four V–V interactions per vanadium atom in both structures, while the systems of p-element bonds orthogonal to the transition metal are absent. According to the 18–n rule, both structure types should have a valence electron count of 14 to be stable and demonstrate properties of a semiconductor [26]. New compounds possess VEC values smaller than 14: 12.28 for VAl0.72(2)Ge1.28(2) (TiSi2-type) and 11.47 for VAl1.534(3)Ge0.466(3) (CrSi2-type). Nevertheless, for both structure types, a reduced VEC value is not uncommon, and the compounds exhibit a metallic character of conductivity. Moreover, to the best of our knowledge, VAl1.534(3)Ge0.466(3) is the first representative of the CrSi2 structure type that has a VEC lower than 12.
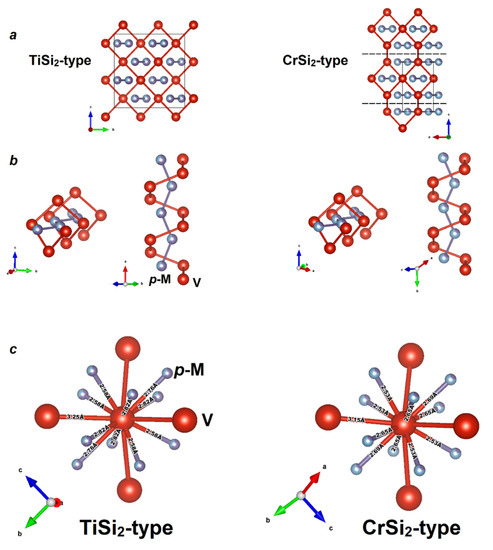
Figure 4.
Comparison of the crystal structures of the VAl2−xGex—TiSi2-type for x = 1.28(2) and CrSi2-type for x = 0.466(3): (a) a general view of the crystal structures; (b) equivalence of the d- and p-metal helices in both structures; (c) local environment of vanadium atoms.
According to the values of VEC, the new compounds VAl0.72(2)Ge1.28(2) and VAl1.534(3)Ge0.466(3) should be metallic conductors. To confirm this assumption, we performed electronic structure calculations. It should be noted that the ideal composition VAlGe was used for the calculations because it was not possible to apply the virtual crystal approximation (VCA) for accounting for the non-integer occupancy factors of two p-elements from different periods.
The calculated electronic structure near the Fermi level for VAlGe (TiSi2-type) is presented in Figure 5 for all ordered models. All the selected ordered models show qualitatively identical results. Clearly, VAlGe should have a metallic type of electrical conductivity, since a nonzero density of states (DOS) is found at the Fermi level. As expected, the band gap is located above the Fermi level, the value of which varies from 0.37 to 0.43 eV, depending on the ordering model. The main contribution to the DOS around the gap is made by the states of vanadium and, to a lesser extent, the states of aluminum and germanium. The same can be said about the states near the Fermi level: vanadium makes the main contribution to the DOS, and approximately equal contributions are observed from aluminum and germanium; the latter are approximately 2–3 times smaller than those from vanadium.
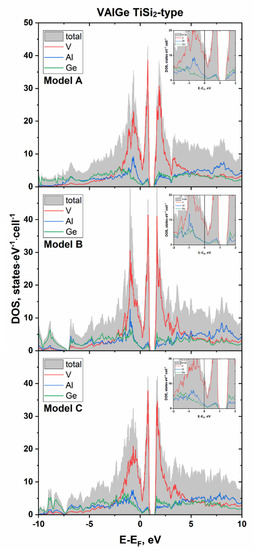
Figure 5.
Calculated total and projected density of states (DOS) near Fermi level for ordered models of VAlGe with the TiSi2 structure type.
The integration of the DOS region from the Fermi level to the band gap gives exactly two electrons per formula unit, yielding an expected 14-ē phase, which is consistent with the 18–n rule. Since the VAlGe ideal composition has a VEC value of 12, according to the 18–n rule, the VEC should be equal to 14 in order to realize a semiconducting state.
An interesting feature is the presence of a pronounced deep minimum located just above the Fermi level and well below the gap (see the insets in Figure 5). The integration of the DOS region between the dip and the Fermi level yields 0.24–0.26 electrons per formula for different models, which nicely matches the actual composition VAl0.72(2)Ge1.28(2) (12 + 0.28 electrons). We can assume that the deviation of the VAl2−xGex actual composition from the ideal 12-ē configuration and the very narrow homogeneity range stem from the tendency to reach the local energy minimum that corresponds to the dip in the DOS.
Apparently, the presence of such a minimum (pseudogap) below the band gap ensures the existence of many electron-poor phases that demonstrate a VEC lower than that predicted by the 18–n rule [26]. The states slightly below the energy gap are usually non-bonding orbitals of the transition metal in such compounds; therefore, a large number of electron-poor phases becomes unsurprising. We especially observe this fact for related structure types of disilicides: TiSi2-type (TiSi2, TiGe2, and ZrSn2 VEC = 12; CrAlGe VEC = 13), CrSi2-type (VSi2, VGe2, NbSi2, NbGe2, TaSi2, TaGe2, CrAlSi, MoAlGe, and MoAlSi VEC = 13), and ZrSi2-type (ScGe2 and YGe2 VEC = 11; TiSi2, ZrSi2, ZrGe2, and HfSi2 VEC = 12) [14,17,20,21,41,42,43,44,45,46,47,48,49,50,51].
Similar results were obtained from the electronic structure calculations for VAlGe with the CrSi2-type (see Figure 6). The Fermi level is located in the valence band, where the main contribution comes from the states of vanadium. Exactly two electrons per formula fit between the Fermi level and the energy gap of the 12-ē VAlGe, which also agrees with the 18–n rule applied to this structure type [26]. Thus, the 14-ē compounds CrSi2, MoSi2, and WSi2 with the CrSi2-type exhibit semiconducting behavior according to theoretical calculations as well as an experimental investigation in the case of CrSi2 [45,52].
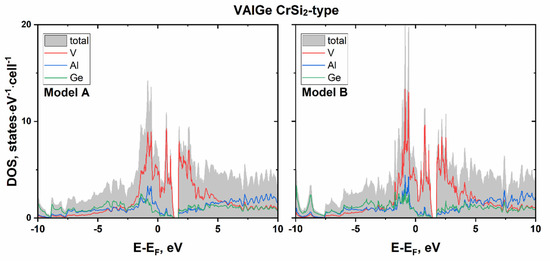
Figure 6.
Calculated total and projected DOS near Fermi level for VAlGe ordered models in CrSi2-type.
We also examined the VGa2−xGex cut in detail and did not detect the existence of an expected phase with the NCL phase crystal structure (the investigated range of x is given in Section 2.1). Since the aim of this study was precisely the target synthesis of the NCL phase, we do not give a detailed description of the phase relations in this system. In all cases, mixtures of known vanadium germanides and vanadium aluminides were obtained according to PXRD data.
3.2. NbE2−xGex (E = Al, Ga): Phase Equilibria, Crystal and Electronic Structure
The same approach was applied to the search for new compounds with the NCL phase structure (TiSi2-type) in the systems where niobium was used as a transition metal instead of vanadium, and the combinations of Al/Ge and Ga/Ge were used as a p-metal/semimetal.
The formation of a new compound, reflections of which are indexed in an orthorhombic unit cell (Sp. Gr. Fddd), was detected in the Nb-Al-Ge system for a wide range of x. Although niobium digermanide NbGe2 [43] is a main phase at x ≥ 1.1, there are also some amounts of a target phase along with a second side-phase NbAl3 [26,53]. The new compound and NbAl3 become the main phases at x ≤ 1, while the maximum intensity of reflections of the target phase is observed at x = 1. The phase composition change across the NbAl2−xGex series is shown in Figure 7. As can be seen, the NbAl2−xGex cut does not show a region of single-phase NCL phase existence, which might indicate a deficiency of the p-elements in the desired compound. According to the EDX data, the measured composition of the NCL phase was Nb1.11(3)Al0.57(6)Ge1.32(3) for the sample with the NbAlGe nominal composition (for details, see Table S1 and Figure S1), which might indicate an exit from the NbAl2−xGex cut. We note that our attempts to optimize the synthetic conditions by changing the initial composition and varying the temperature did not lead to a better yield of the target phase, and the best yield was obtained for the nominal composition NbAlGe. The inset in Figure 7 shows the dependence of the unit cell volume obtained by indexing peaks attributed to the NCL phase. It should be borne in mind that the given composition “NbAl2−xGex” differs from the real one. Nevertheless, the fundamental possibility of obtaining a compound with a crystal structure of the NCL phase (TiSi2-type) in the Nb-Al-Ge system was shown.
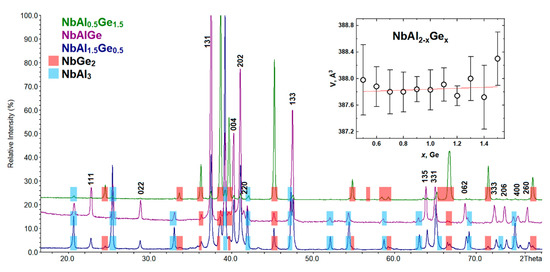
Figure 7.
PXRD patterns of NbAl2−xGex (x = 0.5, 1, and 1.5). Inset: the unit cell volume of the “NbAl2−xGex” (Fddd) vs. nominal Ge content.
For the NbGa2−xGex cut, a single-phase sample was synthesized with a NbGa0.8Ge1.2 composition and an orthorhombic unit cell (Sp. Gr. Fddd). The measured composition according to the EDX analysis (Nb0.99(3)Ga0.81(3)Ge1.20(3)) perfectly matches the nominal one. NbGe2 [43] is formed as a main phase at x ≥ 1.3, where the content of a new compound decreases. An increase in the nominal gallium content in the sample leads to the appearance of NbGa3 [53,54] as an admixture along with an unidentified compound, the intensity of the peaks of which is quite high. The PXRD patterns of the NbGa2−xGex samples are presented in Figure 8. The unit cell parameters of the new compound slightly decrease with increasing nominal germanium content. We can conclude that the NbGa2−xGex (Fddd) homogeneity range is very narrow.
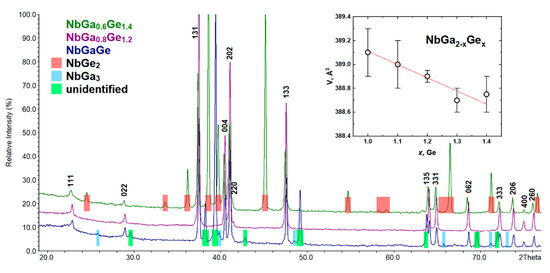
Figure 8.
PXRD patterns of NbGa2−xGex (x = 1, 1.2, and 1.4). Inset: the unit cell volume of the NbGa2−xGex (Fddd) vs. nominal Ge content.
For the phase-pure sample of NbGa0.8Ge1.2, the crystal structure was refined using synchrotron PXRD data (for details, see Table 1). Crystal structure refinement confirmed the TiSi2 structure type. The occupancies of the unique p-element site containing gallium and germanium atoms were fixed according to the nominal composition NbGa0.8Ge1.2. The atomic parameters for the crystal structure of NbGa0.8Ge1.2 are presented in Table 5.

Table 5.
Atomic coordinates and thermal displacement parameters for NbGa0.8Ge1.2.
The crystal structure parameters of NbGa0.8Ge1.2 were used for electronic structure calculations. The calculations were performed for the same models of p-elements’ ordering as for VAlGe (see Figure 2); in addition, the proximity of gallium and germanium allowed us to carry out the band structure calculation in the VCA approximation (both for the ideal composition NbGaGe and for the actual composition NbGa0.8Ge1.2). The calculated DOS curves are shown in Figure 9.
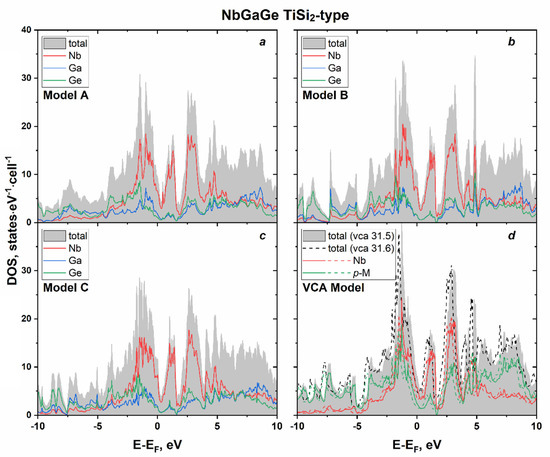
Figure 9.
Calculated total and projected DOS near Fermi level of NbGaGe (TiSi2-type) for ordered models (a–c) and within the virtual crystal approximation (VCA) (d) with p-M charge 31.5 (NbGaGe) and 31.6 (NbGa0.8Ge1.2).
According to the calculation results, NbGaGe is the expected metallic conductor. However, the main feature of the NbGaGe band structure for all the models is the absence of an open energy gap. Unlike in the isostructural VAlGe (see above), the opened gap collapses and turns into a pseudogap in NbGaGe. Similar changes in the band pattern are observed with the increasing atomic mass (replacement of vanadium by niobium and aluminum by gallium in our case). For instance, when passing from Ru2Ge3 to Ru2Sn3 (isostructural NCL phases), the gap also closes with the formation of a pseudogap at the Fermi level [9].
The number of electrons found by integrating the DOS region from the Fermi level to the pseudogap is still two (for ordered models and the VCA model with p-M charge 31.5), which is predicted by the 18–n rule.
4. Conclusions
Three new ternary compounds in the V-Al-Ge, Nb-Al-Ge, and Nb-Ga-Ge systems were synthesized by the standard ampoule technique. All phases belong to the family of Nowotny chimney ladder phases and crystallize in the TiSi2 structure type. Two compounds, VAl0.7Ge1.3 and NbGa0.8Ge1.2, were obtained as phase-pure powders, and their crystal structures were refined using synchrotron PXRD data. No superstructure was detected, which demonstrates the statistical occupation of a single p-element site by atoms of groups 13 and 14 without any tendency for ordering. As the respective binary NCL phases are absent, it is plausible to conclude that the formation of new phases is ensured primarily by combining elements of different groups with vanadium or niobium so that the correct VEC is achieved. Crystal growth from the VAl0.7Ge1.3 single-phase powder by means of a chemical transport reaction reveals a change in the compound composition (VAl1.5Ge0.5) with a concomitant transition to the CrSi2 structure type that is genetically very close to the NCL TiSi2-type. The calculations of the electronic structures demonstrate metallic properties for all the new compounds, which is in line with the 18–n rule and the corresponding VEC of the new compounds, which is close to 12. In addition, the energy gap in VAlGe, which is also predicted by the 18–n rule, degenerates into a pseudogap upon transition to the compound with heavier elements in the case of NbGaGe.
Supplementary Materials
The following data are available online at https://www.mdpi.com/2073-4352/10/8/670/s1. Table S1: EDX analysis results for VAl2−xGex, NbGa2−xGex, and NbAl2−xGex samples. Figure S1: EDX mapping of the samples with the nominal compositions VAl0.7Ge1.3 (a), NbGa0.8Ge1.2 (b), and NbAlGe (c). (d) comparison of the nominal composition with measured one. Figure S2: Powder X-ray diffraction patterns of VAl0.72(2)Ge1.28(2) (a) and NbGa0.8Ge1.2 (b).
Author Contributions
Conceptualization, M.S.L. and A.V.S.; formal analysis, M.S.L., N.V.S. and A.V.S.; investigation, M.S.L., N.V.S. and Z.W.; writing—original draft preparation, M.S.L.; writing—review and editing, E.V.D. and A.V.S.; visualization, M.S.L.; supervision, A.V.S.; project administration, A.V.S.; funding acquisition, E.V.D. and A.V.S. All authors have read and agreed to the published version of the manuscript.
Funding
The work was supported by the Russian Science Foundation, grant No. 17-13-01033. Z.W. and E.D. thank the National Science Foundation for supporting the structural studies under grant no. CHE-1955585.
Acknowledgments
We thank V. Yu. Verchenko for carrying out EDX analysis. We acknowledge ESRF for granting the beam time at ID22 (HC-3841) and thank W. Mogodi for his kind support during the high-resolution XRD measurements.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Steurer, W.; Dshemuchadse, J. Intermetallics: Structures, Properties, and Statistics; Oxford University Press: Oxford, UK, 2016. [Google Scholar]
- Akhmetshina, T.G.; Blatov, V.A.; Proserpio, D.M.; Shevchenko, A.P. Topology of intermetallic structures: From statistics to rational design. Acc. Chem. Res. 2018, 51, 21–30. [Google Scholar] [CrossRef]
- Oliynyk, A.O.; Mar, A. Discovery of intermetallic compounds from traditional to machine-learning approaches. Acc. Chem. Res. 2018, 51, 59–68. [Google Scholar] [CrossRef]
- Buschow, K.H.J. The importance of ternary intermetallic compounds in science and technology. J. Alloys Compd. 1993, 193, 223–230. [Google Scholar] [CrossRef]
- Fredrickson, D.C.; Miller, G.J. Intermetallic Chemistry: New Advances in Humanity’s Age-Old Exploration of Metals and Alloys. Acc. Chem. Res. 2018, 51, 213. [Google Scholar] [CrossRef]
- Fredrickson, D.C. Parallels in Structural Chemistry between the Molecular and Metallic Realms Revealed by Complex Intermetallic Phases. Acc. Chem. Res. 2018, 51, 248–257. [Google Scholar] [CrossRef] [PubMed]
- Mizutani, U. Hume-Rothery Rules for Structurally Complex Alloy Phases; CRC Press: New York, NY, USA, 2010. [Google Scholar]
- Shevelkov, A.V.; Kovnir, K. Zintl Clathrates: Structure and Bonding; Springer: Berlin/Heidelberg, Germany, 2011; Volume 139, pp. 97–142. [Google Scholar]
- Imai, Y.; Watanabe, A. Consideration of the validity of the 14 valence electron rule for semiconducting chimney-ladder phase compounds. Intermetallics 2005, 13, 233–241. [Google Scholar] [CrossRef]
- Verchenko, V.Y.; Wei, Z.; Tsirlin, A.A.; Callaert, C.; Jesche, A.; Hadermann, J.; Dikarev, E.V.; Shevelkov, A.V. Crystal growth of the Nowotny chimney ladder phase Fe2Ge3: Exploring new Fe-based narrow-gap semiconductor with promising thermoelectric performance. Chem. Mater. 2017, 29, 9954–9963. [Google Scholar] [CrossRef]
- Sato, N.; Ouchi, H.; Takagiwa, Y.; Kimura, K. Glass-like Lattice Thermal Conductivity and Thermoelectric Properties of Incommensurate Chimney-Ladder Compound FeGeγ. Chem. Mater. 2016, 28, 529–533. [Google Scholar] [CrossRef]
- Terada, T.; Ishibe, T.; Watanabe, K.; Nakamura, Y. Growth of epitaxial FeGeγ nanocrystals with incommensurate Nowotny chimney-ladder phase on Si substrate. Jpn. J. Appl. Phys. 2018, 57, 08NB01. [Google Scholar] [CrossRef]
- Boström, M.; Lind, H.; Lidin, S.; Niewa, R.; Grin, Y. Synthesis, crystal structure, phase relations and chemical bonding analysis of the new Nowotny chimney-ladder compound ZrBi1.62. Solid State Sci. 2006, 8, 1173–1180. [Google Scholar] [CrossRef]
- Jeitschko, W. Refinement of the crystal structure of TiSi2 and some comments on bonding in TiSi2 and related compounds. Acta Crystallogr. B 1977, 33, 2347–2348. [Google Scholar] [CrossRef]
- Fredrickson, D.C.; Lee, S.; Hoffmann, R. The Nowotny chimney ladder phases: Whence the 14 electron rule? Inorg. Chem. 2004, 43, 6159–6167. [Google Scholar] [CrossRef] [PubMed]
- Fredrickson, D.C.; Lee, S.; Hoffmann, R.; Lin, J. The Nowotny chimney ladder phases: Following the cpseudo clue toward an explanation of the 14 electron rule. Inorg. Chem. 2004, 43, 6151–6158. [Google Scholar] [CrossRef] [PubMed]
- Milyan, V.V.; Kuzma, Y.B. Phase equilibria in the Cr-Al-Ge system. Russ. Metall. 1987, 4, 191–193. [Google Scholar]
- Kusma, J.B.; Nowotny, H. Untersuchungen im Dreistoff: Mn-Al-Si. Monatsh. Chem. 1964, 95, 1266–1271. [Google Scholar] [CrossRef]
- Nowotny, H.; Benesovsky, F.; Brukl, C.E. Der Dreistoff: Niob-Aluminium-Silicium. Monatsh. Chem. 1961, 92, 193–196. [Google Scholar] [CrossRef]
- Kuzma, Y.B.; Milyan, V.V. New compounds in the system Mo-Al-Ge. Inorg. Mater. 1979, 15, 11–13. [Google Scholar]
- Brukl, C.E.; Nowotny, H.; Schob, O.; Benesovsky, F. Die Kristallstrukturen von TiSi, Ti(Al,Si)2 und Mo(Al,Si)2. Monatsh. Chem. 1961, 92, 781–788. [Google Scholar] [CrossRef]
- Völlenkle, H.; Wittmann, A.; Nowotny, H. Abkömmlinge der TiSi2-Struktur—Ein neues Bauprinzip. Monatsh. Chem. 1966, 97, 506–516. [Google Scholar] [CrossRef]
- Miyazaki, Y.; Nakajo, T.; Kikuchi, Y.; Hayashi, K. Crystal structure and thermoelectric properties of the incommensurate chimney–ladder compound RhGeγ (γ ~ 1.293). J. Mater. Res. 2015, 30, 2611–2617. [Google Scholar] [CrossRef]
- Larchev, V.I.; Popova, S.V. The new chimney ladder phases Co2Si3 and Re4Ge7 formed by treatment at high temperatures and pressures. J. Less-Common Met. 1982, 84, 87–91. [Google Scholar] [CrossRef]
- Yannello, V.J.; Fredrickson, D.C. Orbital origins of helices and magic electron counts in the Nowotny chimney ladders: The 18-n rule and a path to incommensurability. Inorg. Chem. 2014, 53, 10627–10631. [Google Scholar] [CrossRef] [PubMed]
- Yannello, V.J.; Fredrickson, D.C. Generality of the 18-n rule: Intermetallic structural chemistry explained through isolobal analogies to transition metal complexes. Inorg. Chem. 2015, 54, 11385–11398. [Google Scholar] [CrossRef] [PubMed]
- Hamada, H.; Kikuchi, Y.; Hayashi, K.; Miyazaki, Y. Crystal structure and thermoelectric properties of the incommensurate chimney–ladder compound VGeγ (γ ~ 1.82). J. Electron. Mater. 2016, 45, 1365–1368. [Google Scholar] [CrossRef]
- Petricek, V.; Dusek, M.; Palatinus, L. Crystallographic computing system JANA2006: General features. Z. Kristallogr. Cryst. Mater. 2014, 229, 345–352. [Google Scholar] [CrossRef]
- Bruker. SAINT (Version 8.38A); Bruker AXS Inc.: Madison, WI, USA, 2017. [Google Scholar]
- Krause, L.; Herbst-Irmer, R.; Sheldrick, G.M.; Stalke, D. Comparison of Silver and Molybdenum Microfocus X-ray Sources for Single-crystal Structure Determination. J. Appl. Cryst. 2015, 48, 3–10. [Google Scholar] [CrossRef]
- Bruker. SADABS v. 2014/5; Bruker AXS Inc.: Madison, WI, USA, 2015. [Google Scholar]
- Palatinus, L.; Chapuis, G. Superflip—A computer program for the solution of crystal structures by charge flipping in arbitrary dimensions. J. Appl. Crystallogr. 2007, 40, 786–790. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal Structure Refinement with SHELXL. Acta Crystallogr. C 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Momma, K.; Izumi, F. VESTA|3 for three-dimensional visualization of crystal, volumetric and morphology data. J. Appl. Crystallogr. 2011, 44, 1272–1276. [Google Scholar] [CrossRef]
- Koepernik, K.; Eschrig, H. Full-Potential Nonorthogonal Local-Orbital Minimum-Basis Band-Structure Scheme. Phys. Rev. B 1999, 59, 1743. [Google Scholar] [CrossRef]
- Perdew, J.P.; Wang, Y. Accurate and Simple Analytic Representation of the Electron-Gas Correlation Energy. Phys. Rev. B 1992, 45, 13244. [Google Scholar] [CrossRef] [PubMed]
- Blöchl, P.E.; Jepsen, O.; Andersen, O.K. Improved Tetrahedron Method for Brillouin-Zone Integrations. Phys. Rev. B 1994, 49, 16223. [Google Scholar] [CrossRef] [PubMed]
- Takizawa, H.; Sato, T.; Endo, T.; Shimada, M. High-Pressure Synthesis and Crystal Structure of VGe2 and Cr4Ge7. J. Solid State Chem. 1988, 73, 427–432. [Google Scholar] [CrossRef]
- Rudometkina, M.V.; Seropegin, Y.D.; Schvyryaeva, E.E. Investigation of the Zr-V-Ge system alloys. J. Less-Common Met. 1988, 138, 263–269. [Google Scholar] [CrossRef]
- Maas, J.; Bastin, G.F.; Van Loo, F.J.J.; Metselaar, R. The Texture in Diffusion-Grown Layers of Trialuminides MeAl3 (Me = Ti, V, Ta, Nb, Zr, Hf) and VNi3. Z. Metallkd. 1983, 74, 294–299. [Google Scholar]
- Brukl, C.E.; Nowotny, H.; Benesovsky, F. Untersuchungen in den Dreistoffsystemen: V-Al-Si, Nb-Al-Si, Cr-Al-Si, Mo-Al-Si bzw. Cr(Mo)-Al-Si. Monatsh. Chem. 1961, 92, 967–980. [Google Scholar] [CrossRef]
- Tanaka, K.; Nawata, K.; Inui, H.; Yamaguchi, M.; Koiwa, M. Refinement of Crystallographic Parameters in Refractory Metal Disilicides. Mater. Res. Soc. Symp. Proc. 2000, 646, N4.3.1. [Google Scholar] [CrossRef]
- Rudometkina, M.V.; Seropegin, Y.D.; Gribanov, A.V.; Gusei, L.S. Phase equilibria in the Ti-Nb-Ge system at 1170K. J. Less-Common Met. 1989, 147, 239–247. [Google Scholar] [CrossRef]
- Kubiak, R.; Horyn, R.; Broda, H.; Lukaszewicz, K. Refinement of the Crystal Structure of NbSi2, NbGe2 and TaGe2. Bull. Acad. Pol. Sci. (Ser. Sci. Chim.) 1972, 20, 429–436. [Google Scholar]
- Mattheiss, L.F. Calculated structural properties of CrSi2, MoSi2, and WSi2. Phys. Rev. B Condens. Matter Mater. Phys. 1992, 45, 3252–3259. [Google Scholar] [CrossRef]
- Baykov, V.I.; Jerlerud Perez, R.; Korzhavyi, P.A.; Sundman, B.; Johansson, B. Structural stability of intermetallic phases in the Zr-Sn system. Scr. Mater. 2006, 55, 485–488. [Google Scholar] [CrossRef]
- Kotur, B.Y. The Sc-Hf-Ge phase diagram for 1070K. Russ. Metall. (Engl. Transl.) 1991, 3, 211–214. [Google Scholar]
- Jongste, J.F.; Loopstra, O.B.; Janssen, G.C.A.M.; Radelaar, S. Elastic constants and thermal expansion coefficient of metastable C49 TiSi2. J. Appl. Phys. 1993, 73, 2816–2820. [Google Scholar] [CrossRef]
- Setton, M.; Van Der Spiegel, J. Structural and electrical properties of ZrSi2 and Zr2CuSi4 formed by rapid thermal processing. J. Appl. Phys. 1991, 70, 193–197. [Google Scholar] [CrossRef]
- Weitzer, F.; Rogl, P.; Noel, H. The ternary system: Hafnium-silicon-uranium. J. Alloys Compd. 2005, 387, 246–250. [Google Scholar] [CrossRef]
- Mudryk, Y.S.; Romaka, L.P.; Stadnyk, Y.V.; Bodak, O.I.; Fruchart, D. X-ray investigation of the R-Fe-Sn ternary systems (R = Y, Gd). J. Alloys Compd. 2004, 383, 162–165. [Google Scholar] [CrossRef]
- Dasgupta, T.; Etourneau, J.; Chevalier, B.; Matar, S.F.; Umarji, A.M. Structural, thermal, and electrical properties of CrSi2. J. Appl. Phys. 2008, 103, 113516. [Google Scholar] [CrossRef]
- Lue, C.S.; Su, T.H.; Xie, B.X.; Cheng, C. Comparative NMR study of hybridization effect and structural stability in D022-type NbAl3 and NbGa3. Phys. Rev. B 2006, 74, 094101. [Google Scholar] [CrossRef]
- Kilduff, B.J.; Yanello, V.J.; Fredrickson, D.C. Defusing Complexity in Intermetallics: How Covalently Shared Electron Pairs Stabilize the FCC Variant Mo2CuxGa6–x (x ≈ 0.9). Inorg. Chem. 2015, 54, 8103–8110. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).