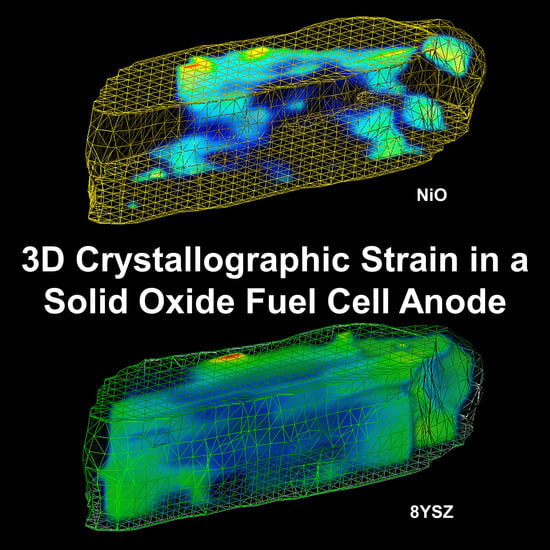
The Detection of Monoclinic Zirconia and Non-Uniform 3D Crystallographic Strain in a Re-Oxidized Ni-YSZ Solid Oxide Fuel Cell Anode
Abstract
:1. Introduction
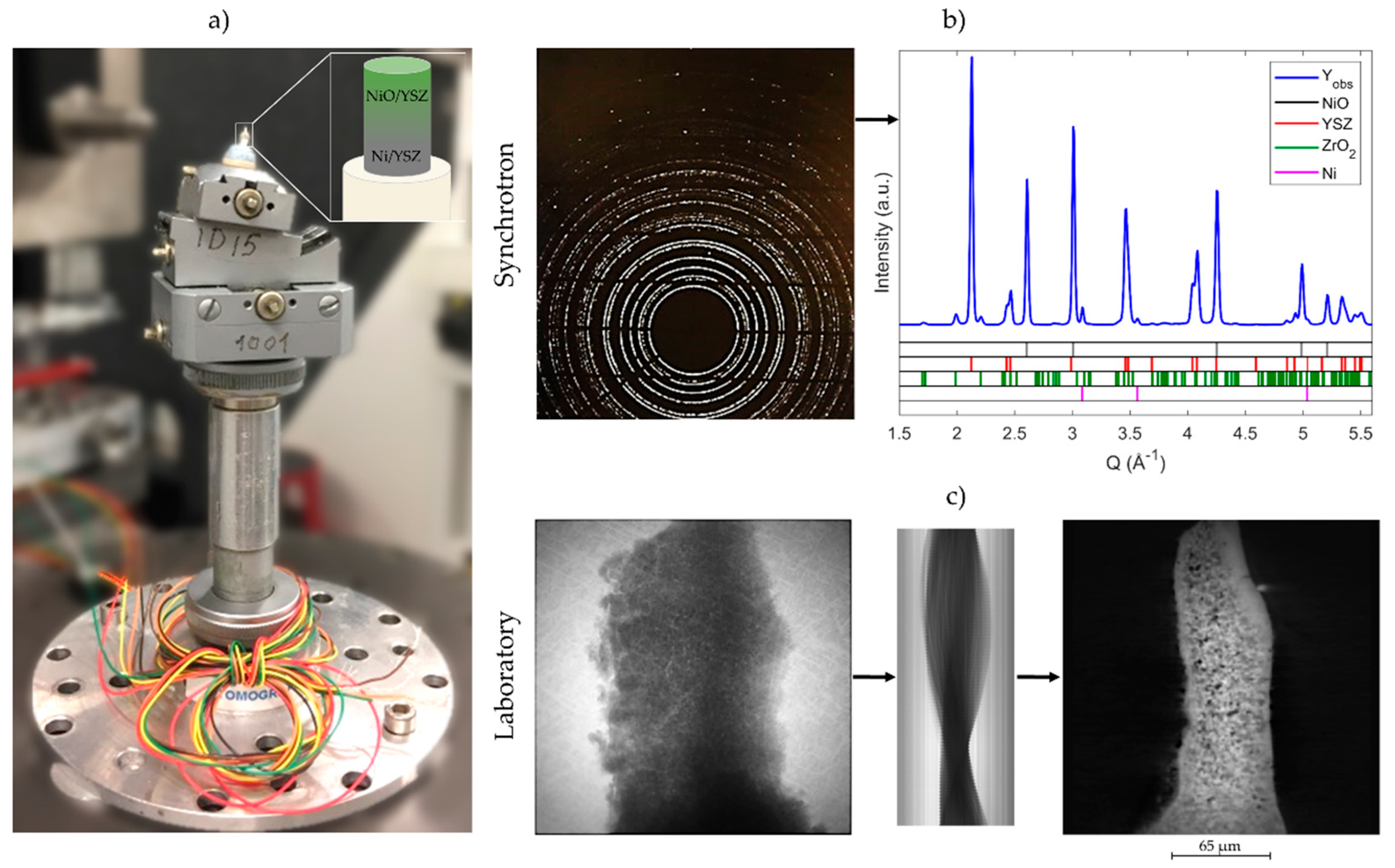
2. Materials and Methods
3. Results
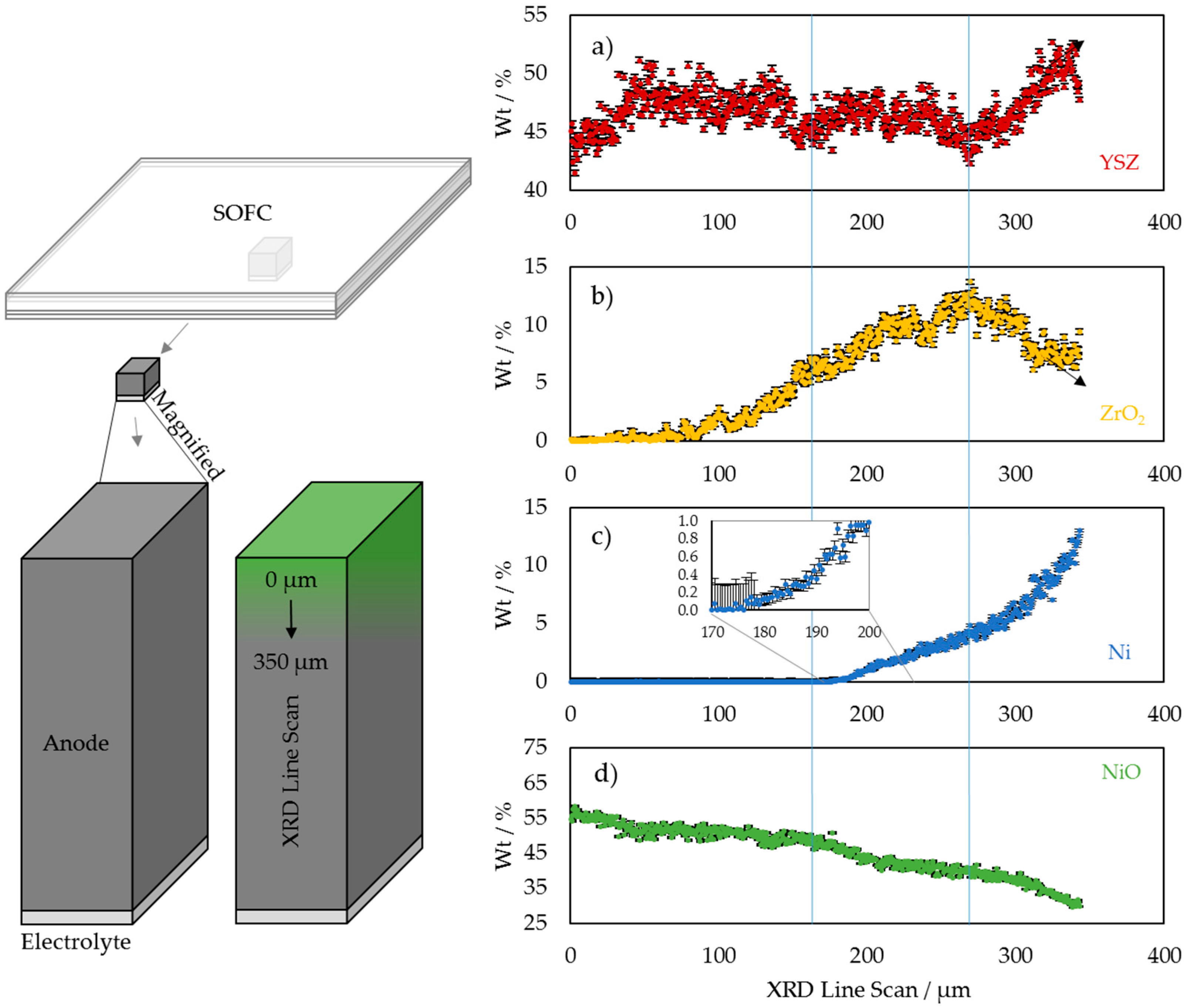
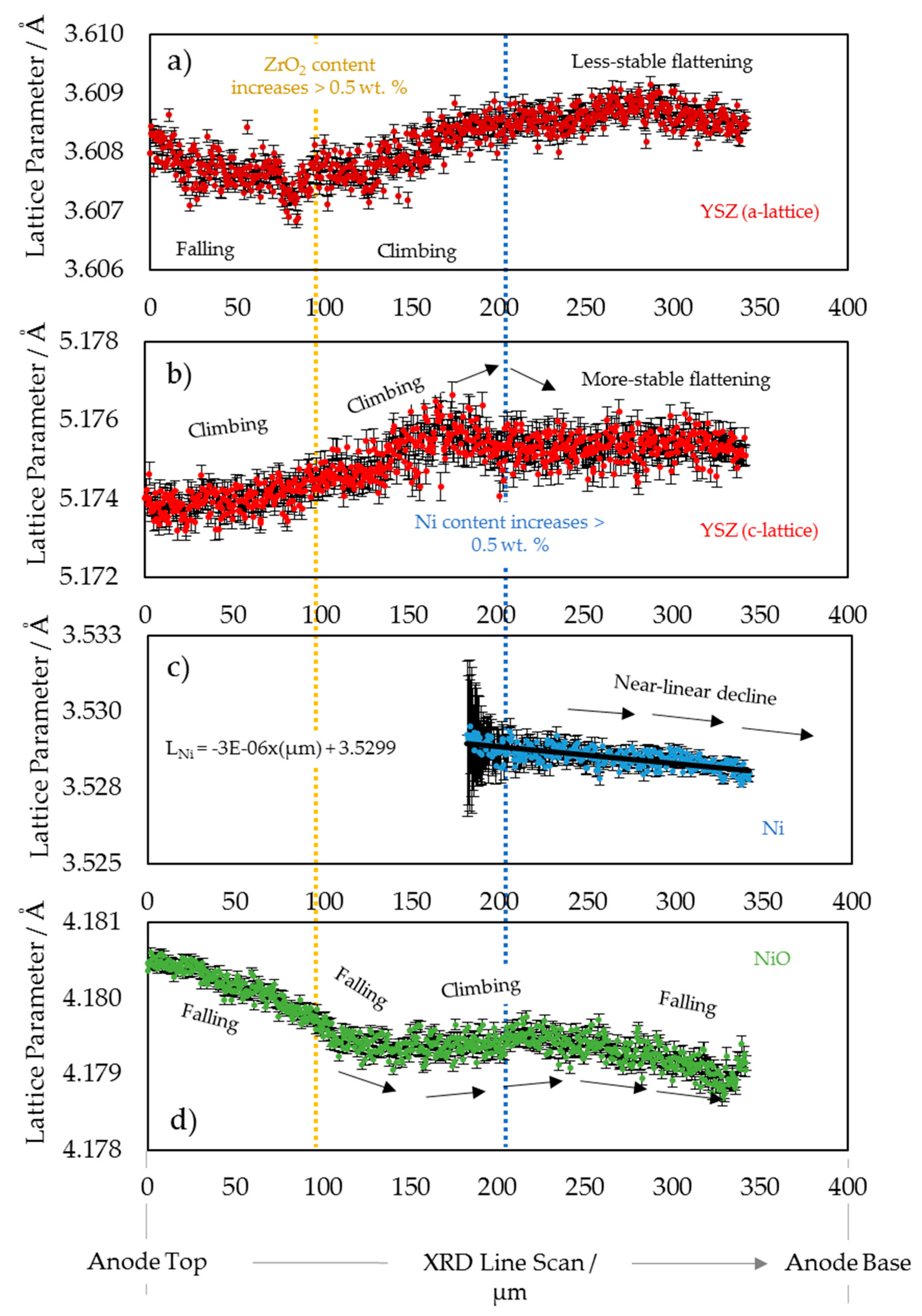
3.1. 2D Crystallography
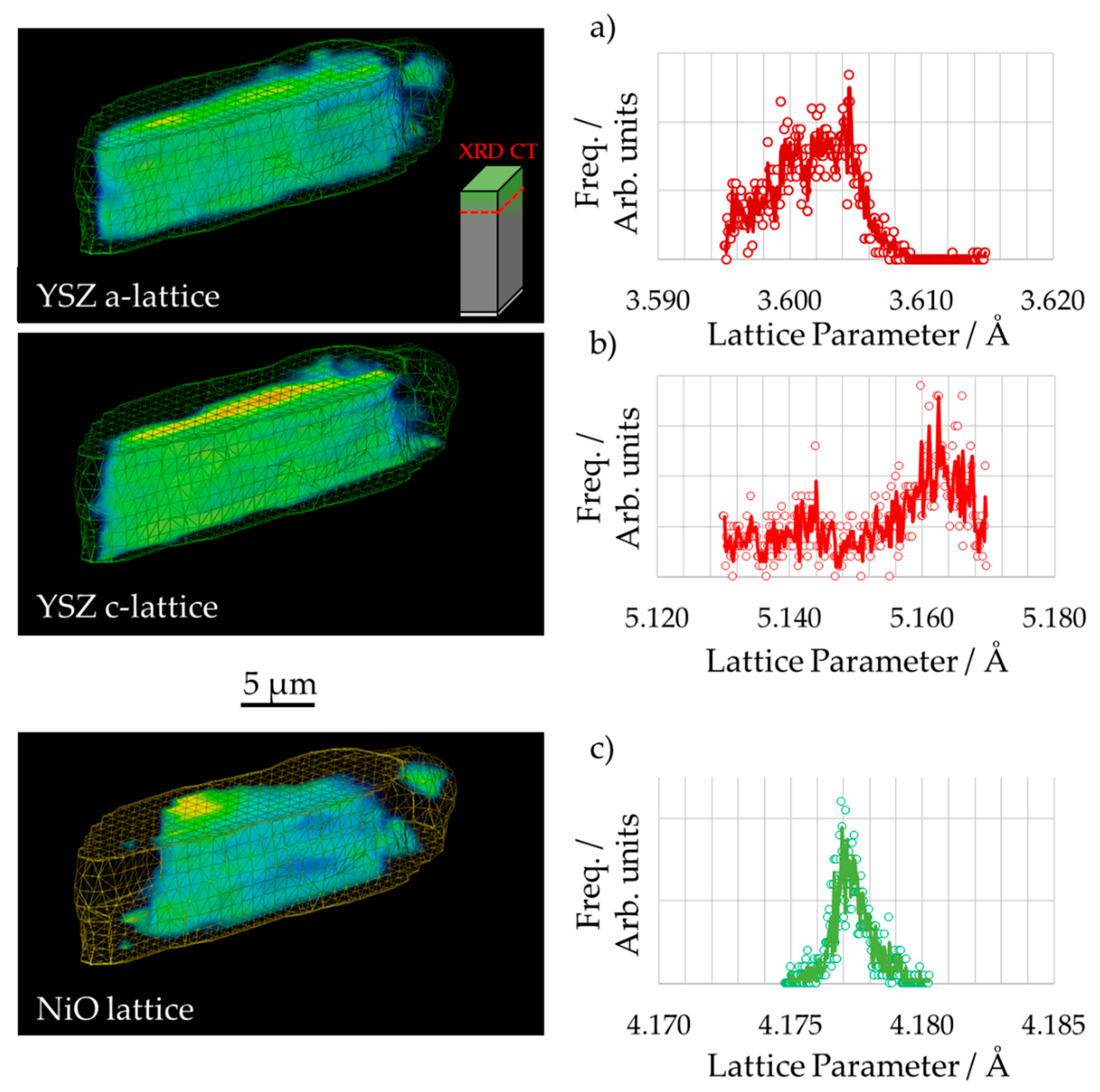
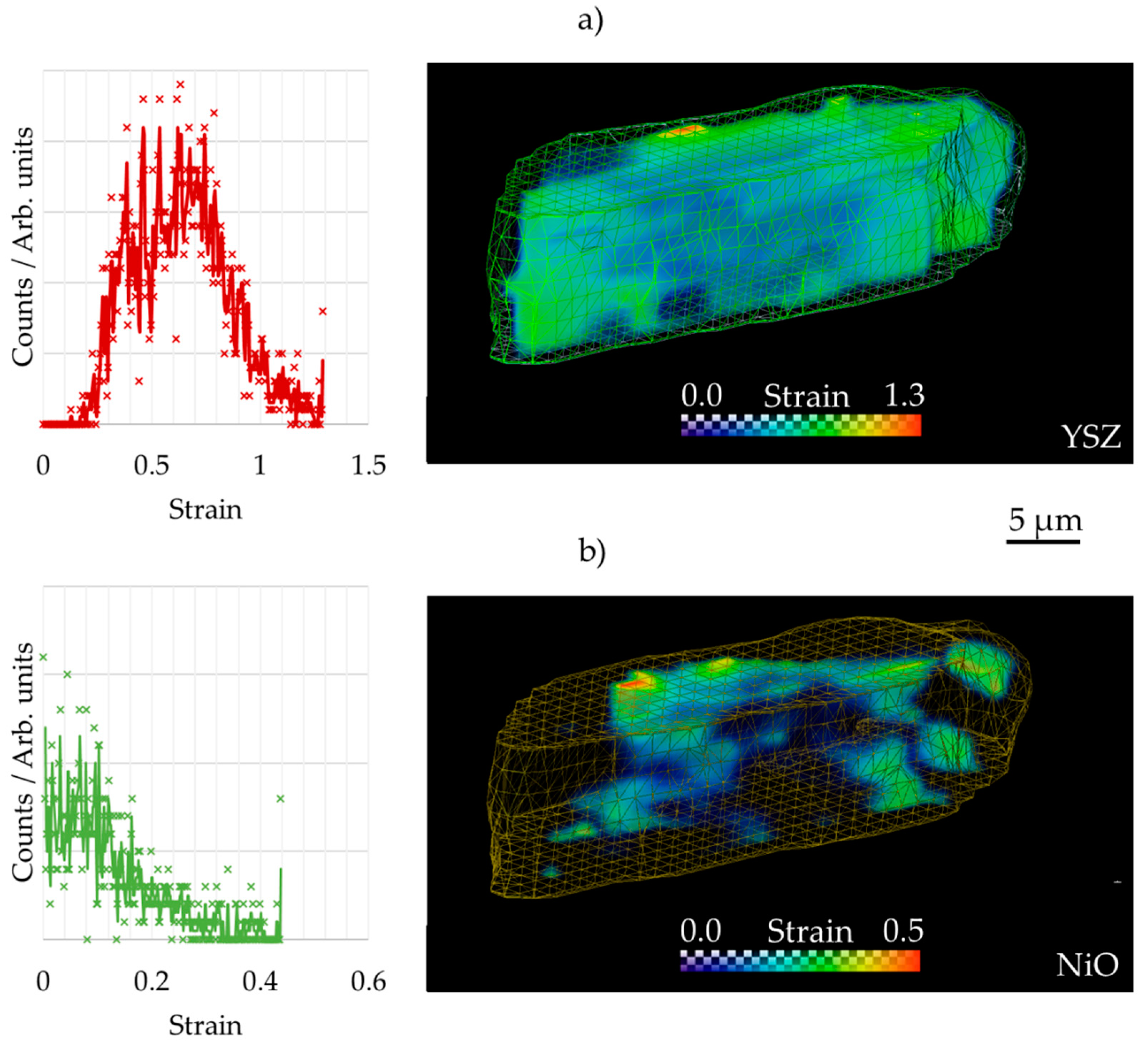
3.2. 3D Crystallography
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Singhal, S.C.; Kendall, K. (Eds.) High-Temperature Solid Oxide Fuel Cells: Fundamentals, Design and Applications; Elsevier: Oxford, UK, 2003. [Google Scholar]
- Khan, M.S.; Lee, S.-B.; Song, R.-H.; Lee, J.-W.; Lim, T.-H.; Park, S.-J. Fundamental mechanisms involved in the degradation of nickel–yttria stabilized zirconia (Ni–YSZ) anode during solid oxide fuel cells operation: A review. Ceram. Int. 2016, 42, 35–48. [Google Scholar] [CrossRef]
- Witz, G.; Shklover, V.; Steurer, W.; Bachegowda, S.; Bossmann, H.-P. Phase Evolution in Yttria-Stabilized Zirconia Thermal Barrier Coatings Studied by Rietveld Refinement of X-ray Powder Diffraction Patterns. J. Am. Ceram. Soc. 2007, 90, 2935–2940. [Google Scholar] [CrossRef]
- Asadikiya, M.; Sabarou, H.; Chen, M.; Zhong, Y. Phase diagram for a nano-yttria-stabilized zirconia system. RSC Adv. 2016, 6, 17438–17445. [Google Scholar] [CrossRef] [Green Version]
- Kondo, H.; Sekino, T.; Kusunose, T.; Nakayama, T.; Yamamoto, Y.; Niihara, K. Phase stability and electrical property of NiO-doped yttria-stabilized zirconia. Mater. Lett. 2003, 57, 1624–1628. [Google Scholar] [CrossRef]
- Kishimoto, H.; Suzuki, A.; Shimonosono, T.; Brito, M.E.; Yamaji, K.; Horita, T.; Munakata, F.; Yokokawa, H. Agglomeration behavior of nickel particles on YSZ electrolyte. Solid State Ion. 2012, 225, 65–68. [Google Scholar] [CrossRef]
- Heenan, T.M.M.; Lu, X.; Robinson, J.B.; Iacoviello, F.; Brett, D.J.L.; Shearing, P.R. Thermally Driven SOFC Degradation in 4D: Part II. Macroscale. J. Electrochem. Soc. 2018, 165, F932–F941. [Google Scholar] [CrossRef] [Green Version]
- Sarantaridis, D.; Atkinson, A. Redox Cycling of Ni-Based Solid Oxide Fuel Cell Anodes: A Review. Fuel Cells 2007, 7, 246–258. [Google Scholar] [CrossRef]
- Heenan, T.M.M.; Nabavi, S.A.; Erans, M.; Robinson, J.B.; Kok, M.D.R.; Maier, M.; Brett, D.J.L.; Shearing, P.R.; Manovic, V. The Role of Bi-Polar Plate Design and the Start-up Protocol in the Spatiotemporal Dynamics during Solid Oxide Fuel Cell Anode Reduction. Energies 2020, 13, 3552. [Google Scholar] [CrossRef]
- Shearing, P.R.; Bradley, R.; Gelb, J.; Tariq, F.; Withers, P.J.; Brandon, N.J. Exploring microstructural changes associated with oxidation in Ni–YSZ SOFC electrodes using high resolution X-ray computed tomography. Solid State Ion. 2012, 216, 69–72. [Google Scholar] [CrossRef]
- Clague, R.; Shearing, P.R.; Lee, P.; Zhang, Z.; Brett, D.J.L.; Marquis, A.; Brandon, N. Stress analysis of solid oxide fuel cell anode microstructure reconstructed from focused ion beam tomography. J. Power Sources 2011, 196, 9018–9021. [Google Scholar] [CrossRef]
- Heenan, T.M.M.; Lu, X.; Iacoviello, F.; Robinson, J.B.; Brett, D.J.L.; Shearing, P.R. Thermally Driven SOFC Degradation in 4D: Part I. Microscale. J. Electrochem. Soc. 2018, 165, F921–F931. [Google Scholar] [CrossRef] [Green Version]
- Stock, S. X-ray microtomography of materials. Int. Mater. Rev. 1999, 44, 141–164. [Google Scholar] [CrossRef]
- Maire, É.; Withers, P.J. Quantitative X-ray tomography. Int. Mater. Rev. 2013, 59, 1–43. [Google Scholar] [CrossRef] [Green Version]
- Villanova, J.; Sicardy, O.; Fortunier, R.; Micha, J.S.; Bleuet, P. X-ray Diffraction Determination of Macro and Micro Stresses in SOFC Electrolyte and Evolution with Redox Cycling of the Anode. Mater. Sci. Forum 2011, 681, 25–30. [Google Scholar] [CrossRef]
- Sanchez, D.F.; Villanova, J.; Laurencin, J.; Micha, J.-S.; Montani, A.; Gergaud, P.; Bleuet, P. X-ray micro Laue diffraction tomography analysis of a solid oxide fuel cell. J. Appl. Crystallogr. 2015, 48, 357–364. [Google Scholar] [CrossRef] [Green Version]
- Li, T.; Heenan, T.M.M.; Rabuni, M.F.; Wang, B.; Farandos, N.M.; Kelsall, G.H.; Matras, D.; Tan, C.; Lu, X.; Jacques, S.D.M.; et al. Design of next-generation ceramic fuel cells and real-time characterization with synchrotron X-ray diffraction computed tomography. Nat. Commun. 2019, 10, 1497. [Google Scholar] [CrossRef]
- Vamvakeros, A.; Jacques, S.D.M.; Di Michiel, M.; Matras, D.; Middelkoop, V.; Ismagilov, I.Z.; Matus, E.V.; Kuznetsov, V.V.; Drnec, J.; Senecal, P.; et al. 5D operando tomographic diffraction imaging of a catalyst bed. Nat. Commun. 2018, 9, 4751. [Google Scholar] [CrossRef] [Green Version]
- Heenan, T.M.M.; Finegan, D.P.; Tjaden, B.; Lu, X.; Iacoviello, F.; Millichamp, J.; Brett, D.; Shearing, P.R. 4D nano-tomography of electrochemical energy devices using lab-based X-ray imaging. Nano Energy 2018, 47, 556–565. [Google Scholar] [CrossRef]
- Vaughan, G.; Baker, R.; Barret, R.; Bonnefoy, J.; Buslaps, T.; Checchia, S.; Duran, D.; Fihman, F.; Got, P.; Kieffer, J.; et al. ID15A at the ESRF—A beamline for high speed operando X-ray diffraction, diffraction tomography and total scattering. J. Synchrotron Radiat. 2020, 27, 515–528. [Google Scholar] [CrossRef]
- Ashiotis, G.; Deschildre, A.; Nawaz, Z.; Wright, J.P.; Karkoulis, D.; Picca, F.E.; Kieffer, J. The fast azimuthal integration Python library:pyFAI. J. Appl. Crystallogr. 2015, 48, 510–519. [Google Scholar] [CrossRef]
- Vamvakeros, A.; Jacques, S.D.; Di Michiel, M.; Middelkoop, V.; Egan, C.K.; Cernik, R.; Beale, A.M. Removing multiple outliers and single-crystal artefacts from X-ray diffraction computed tomography data. J. Appl. Crystallogr. 2015, 48, 1943–1955. [Google Scholar] [CrossRef]
- Kieffer, J.; Petitdemange, S.; Vincent, T. Real-time diffraction computed tomography data reduction. J. Synchrotron Radiat. 2018, 25, 612–617. [Google Scholar] [CrossRef] [PubMed]
- Vamvakeros, A. nDTomo Software Suite; Github: San Francisco, CA, USA, 2018. [Google Scholar]
- Coelho, A.A. TOPASandTOPAS-Academic: An optimization program integrating computer algebra and crystallographic objects written in C++. J. Appl. Crystallogr. 2018, 51, 210–218. [Google Scholar] [CrossRef] [Green Version]
- Heenan, T.M.M.; Robinson, J.B.; Lu, X.; Tjaden, B.; Cervellino, A.; Bailey, J.J.; Brett, D.J.L.; Shearing, P.R. Understanding the thermo-mechanical behaviour of solid oxide fuel cell anodes using synchrotron X-ray diffraction. Solid State Ion. 2018, 314, 156–164. [Google Scholar] [CrossRef] [Green Version]
- Malzbender, J.; Steinbrech, R. Advanced measurement techniques to characterize thermo-mechanical aspects of solid oxide fuel cells. J. Power Sources 2007, 173, 60–67. [Google Scholar] [CrossRef]
- Sarantaridis, D.; Rudkin, R.A.; Atkinson, A. On the Redox Cycling of Anode-Supported SOFCs: Mechanical Properties and Damage Mechanisms. ECS Trans. 2019, 7, 1491–1499. [Google Scholar] [CrossRef]
- Hatae, T.; Matsuzaki, Y.; Yamashita, S.; Yamazaki, Y. Destruction Modes of Anode-Supported SOFC Caused by Degrees of Electrochemical Oxidation in Redox Cycle. J. Electrochem. Soc. 2010, 157, B650–B654. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Heenan, T.M.M.; Vamvakeros, A.; Tan, C.; Finegan, D.P.; Daemi, S.R.; Jacques, S.D.M.; Beale, A.M.; Di Michiel, M.; Brett, D.J.L.; Shearing, P.R. The Detection of Monoclinic Zirconia and Non-Uniform 3D Crystallographic Strain in a Re-Oxidized Ni-YSZ Solid Oxide Fuel Cell Anode. Crystals 2020, 10, 941. https://doi.org/10.3390/cryst10100941
Heenan TMM, Vamvakeros A, Tan C, Finegan DP, Daemi SR, Jacques SDM, Beale AM, Di Michiel M, Brett DJL, Shearing PR. The Detection of Monoclinic Zirconia and Non-Uniform 3D Crystallographic Strain in a Re-Oxidized Ni-YSZ Solid Oxide Fuel Cell Anode. Crystals. 2020; 10(10):941. https://doi.org/10.3390/cryst10100941
Chicago/Turabian StyleHeenan, Thomas M. M., Antonis Vamvakeros, Chun Tan, Donal P. Finegan, Sohrab R. Daemi, Simon D. M. Jacques, Andrew M. Beale, Marco Di Michiel, Dan J. L. Brett, and Paul R. Shearing. 2020. "The Detection of Monoclinic Zirconia and Non-Uniform 3D Crystallographic Strain in a Re-Oxidized Ni-YSZ Solid Oxide Fuel Cell Anode" Crystals 10, no. 10: 941. https://doi.org/10.3390/cryst10100941