Abstract
The catalyst activities of various heterogeneous palladium catalysts supported by anatase-, rutile- and brookite-type titanium oxide for ligand-free Suzuki–Miyaura cross-coupling reactions of aryl chlorides were evaluated. Palladium acetate [Pd(OAc)2], supported on anatase-type titanium oxide (TiO2) via acetonitrile solution impregnation process without reduction [Pd/TiO2 (anatase-type)], demonstrated the highest catalyst activity in comparison to those of other titanium oxide (rutile- or brookite-type) supported Pd(OAc)2 without reduction and reduced Pd/TiO2 (anatase-type) [Pd(red)/TiO2 (anatase-type)]. Various aryl chloride and bromide derivatives were smoothly coupled with arylboronic acids including heteroarylboronic acids in the presence of 5–10 mol% Pd/TiO2 (anatase-type) without the addition of any ligands. Although the fresh Pd/TiO2 (anatase-type) catalyst was surprisingly comprised of ca. 1:2 mixture of palladium(II) and palladium(0) species according to X-ray photoelectron spectroscopy (XPS), in spite of no reduction process, significant further increment of palladium(0) species was observed during the Suzuki–Miyaura coupling reaction, and Pd/TiO2 (anatase-type) was converted into a catalyst, which contained palladium(0) species as the main component [ca. 1:5 mixture of palladium(II) and palladium(0) species]. Therefore, the reduction via the electron donation process to the palladium(II) species may have occurred during the reaction on anatase-type titanium oxide.
1. Introduction
Palladium (Pd)-catalyzed Suzuki–Miyaura coupling reaction [1,2,3,4,5,6,7,8,9,10,11,12] of aryl halides and arylbolonic acid derivatives is one of the most reliable and useful synthetic methods for constructing biaryl derivatives as fundamental skeletons of various biologically active compounds [2,4,6], pharmaceuticals [2,4] and functional materials [5,6]. Various heterogeneous catalysts, including heterogeneous Pd catalysts, have been developed from the perspective of sustainability and process chemistry based on their advantages, such as recoverability, reusability and low residual metal property [7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60]. Heterogeneous Pd-catalyzed ligand-free Suzuki–Miyaura coupling reactions have attracted attention as environmentally friendly, metal-contamination-free (or low) and cost-effective methods over the past couple of decades [7,8,9,10,11,12,17,18,19]. Particularly, aryl chlorides, which are easily available and less expensive organic compounds in comparison with aryl iodides and bromides, are preferred substrates for the Suzuki–Miyaura coupling reaction, although special ingenuity is necessary for the effective activation of the low-reactive carbon-chloride bond. Therefore, various heterogeneous catalysts have been developed to achieve the heterogeneously catalyzed Suzuki–Miyaura coupling reaction using aryl chlorides [21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40].
Incidentally, the supports for the heterogeneous Pd catalysts are roughly categorized into two. One is ligand-like nitrogen- [21,22,23,24,39,40] or phosphine- [25,26,27,28,29,30] substituted polystyrene-polymers for the purpose of stabilizing the Pd-arene complexes, and/or the enhancement of the catalyst activity based on the coordination effect of the substituent. Furthermore, we developed heterogeneous Pd catalysts immobilized on a ligand-like tertiary amine polystyrene-divinylbenzene-based polymer for the ligand-free Suzuki–Miyaura coupling reaction of aryl chlorides [39,40]. The other category is polymers [34] or inorganics [31,32,33,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50] without additional and coordinable functionalities, such as activated carbon [31,32,33], polystyrene [34], metal-organic frameworks [35], polyoxometalate [36] and titanium dioxide (TiO2) [42,43,44,45,46,47,48,49,50].
Some TiO2-supported Pd catalysts have been applied to various organic and inorganic reactions such as the Suzuki–Miyaura reaction [42,43,44,45,46,47,48,49,50], hydrogenation [51,52,53,54,55,56,57,58] and Heck reaction [59]. However, the effect on the catalyst activity, depending on the difference of the TiO2 crystal forms of TiO2-supported Pd catalysts toward cross-coupling reactions, has never been systematically evaluated, except for a report related to the oxidative homo coupling reaction of 4-methylpyridines in the presence of Pd oxide-loaded TiO2 (PdO/TiO2) as a catalyst [60].
In this study, we prepared and investigated the physical properties of the anatase-, rutile- and brookite-type TiO2-supported Pd catalysts by using X-ray photoelectron spectroscopy (XPS) and transmission electron microscopy (TEM), and comprehensively investigated the effect on the catalyst activities depending on the crystal form of TiO2 to the TiO2-supported Pd-catalyzed ligand-free Suzuki–Miyaura coupling reaction. Consequently, the anatase-type TiO2-supported Pd catalyst was found to have quite an efficient catalyst activity toward the Suzuki–Miyaura coupling reaction, including the coupling of aromatic chlorides due to the quite highly distributed small Pd nanoparticles (1–2 nm) on the anatase-type TiO2.
2. Results and Discussion
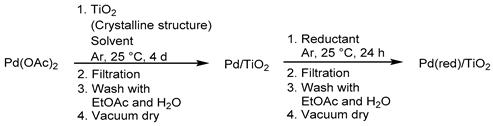
Initially, nine kinds of heterogeneous TiO2-supported Pd catalysts (Table 1, Categories Cat. A–I) were prepared in a manner analogous to the previously established preparation method in our laboratory for a chelate resin-supported heterogeneous Pd catalyst (7% Pd/WA30) [39,40,41]. Pd(OAc)2, which was 5 wt% of Pd metal equivalent of the usage of TiO2, was embedded on the colorless anatase-, rutile- or brookite-type TiO2 powder by gentle stirring in acetonitrile (MeCN) or ethyl acetate (EtOAc) at 25 °C for 4 days under argon atmosphere. Colorless TiO2 powders gradually turned to light yellow due to the interaction between Pd(OAc)2 and TiO2; subsequently, the resulting insoluble powder was filtered, washed with EtOAc and H2O and dried in vacuo to afford a light yellowish powder (Entries 4–9, Pd/TiO2, Cat. D–I). The Pd content in Pd/TiO2 (Cat. A–D and F–I) and Pd/TiO2 (Cat. E) were determined to be approximately 5 wt% and 4 wt% by the atomic absorption analysis of the residual Pd species in the collected organic and aqueous filtrates, respectively.

Table 1.
Preparation of Pd/TiO2 and Pd(red)/TiO2.
The filtered Pd/TiO2 prepared in MeCN suspension (Entry 4, Cat. D, anatase-type) was stirred at 25 °C for 24 h in H2O accompanied by hydrazine monohydrate or sodium borohydride, or in EtOAc under hydrogen atmosphere. The filtered resulting powder was sequentially washed with EtOAc and H2O, and dried under reduced pressure to afford grayish white Pd(red)/TiO2 catalysts (Entries 1–3, Cat. A–C, anatase-type).
The XPS analysis of the Pd/TiO2 catalysts (Cat. E–I) prepared without reduction processes surprisingly indicated an approximately 2:1 mixture of Pd(0) species and Pd(II) ions (Figure 1). The characteristic peaks of Pd3d3/2 and Pd3d5/2 and the area % of Pd(0) species and Pd(II) ions are shown in the Supporting Information (Figure S1).
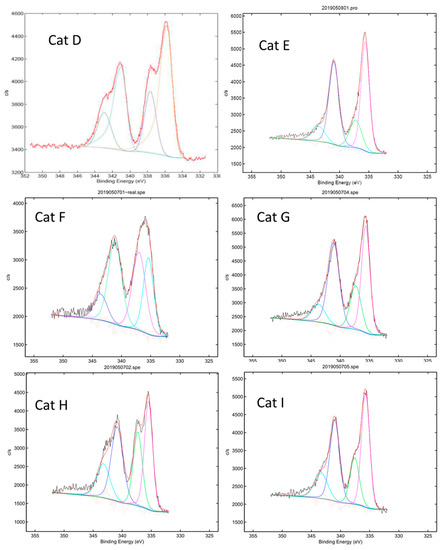
Figure 1.
X-ray photoelectron spectroscopy (XPS) spectra of 5% Pd/TiO2 (Cat. D and F–I) and 4% Pd/TiO2 (Cat. E).
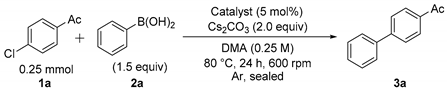
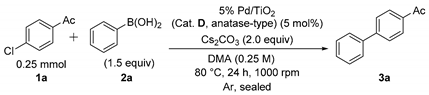
The catalyst activities of various TiO2-supported Pd catalysts (Cat. A–I) for the Suzuki–Miyaura coupling reaction using 4’-chloroacetophenone (1a) and phenylboronic acid (2a) as substrates were evaluated. A portion of each of the catalysts (5 mol%) was added to the mixture of 1a (0.25 mmol), 2a (1.5 equiv) and cesium carbonate (Cs2CO3; 2.0 equiv) in dimethylacetamide (DMA; 1 mL) and stirred at 600 rpm for 24 h at 80 °C under argon atmosphere (Table 2, Entries 1–10). While the reduced TiO2-supported Pd catalysts [Pd(red)/TiO2, (Cat. A–C, anatase-type)] using hydrazine monohydrate, sodium borohydride or hydrogen gas indicated less catalyst efficiencies for the Suzuki–Miyaura coupling reaction (Entries 1–3), the use of Pd/TiO2 (Cat. D and E, anatase-type), without the reduction process, afforded biaryls (3a) in relatively good conversion ratios (Entries 4 and 5). The catalyst activities of other Pd/TiO2 catalysts without reduction process (Cat. F and G, rutile-type, and Cat. I, brookite-type) indicated low to moderate conversion ratios (Entries 6–8), except for Cat. H (brookite-type), which had a good conversion ratio (65%, Entry 9). 1a was completely converted to the corresponding 4-acetylbiphenyl (3a) in the presence of 5% Pd/TiO2 (Cat. D, anatase-type, 5 mol%) by enhancing the stirring speed to 1000 rpm from 600 rpm (Entry 10), while the coupling reaction was never completed using 5% Pd/TiO2 (Cat. I, brookite-type, 5 mol%) at 1000 rpm (Entry 11). Based on these results, the catalytic activity depends in part on the difference of TiO2 crystal forms based on the different interaction properties between Pd species and TiO2 crystal forms, and the catalytic activities are approximately catalysts D and E (anatase type) > catalysts F and G (rutile type) > catalysts H and I (brookite type) in this order [61,62,63]. Therefore, 5% Pd/TiO2 (Cat. D, anatase-type) indicated quite a high catalyst activity toward the ligand-free Suzuki–Miyaura coupling reaction of aryl chlorides.

Table 2.
Catalyst activity of TiO2-supported Pd catalysts for the Suzuki–Miyaura coupling reaction of 4′-chloroacetophenone (1a) with phenylboronic acid (2a). a
The catalyst activity of the 5% Pd/TiO2 (Cat. D, anatase-type) was compared with other heterogeneous and homogeneous Pd catalysts (Table 3). The quantitative conversion of 1a could be achieved by using 5 mol% of 7% Pd/WA30 developed in our research group [39,40,41], although high speed and vigorous stirring (1600 rpm) conditions were required (Entry 2). On the other hand, 10% Pd on carbon (Pd/C) (5 mol%) indicated ineffective catalyst activity (Entry 3). Furthermore, some homogeneous Pd catalysts are known to have high catalyst activity in Suzuki–Miyaura coupling reaction [64,65], however, the use of 5 mol% of Pd(OAc)2 as a homogeneous catalyst gave only moderate conversion (Entry 4). Therefore, 5% Pd/TiO2 (Cat. D) is suitable as an active catalyst for the Suzuki–Miyaura coupling reaction for aryl chlorides.

Table 3.
Comparison of the catalyst activity. a
Next, we investigated the effects of the reaction temperature and atmospheric conditions in a test tube as a reaction vessel on 5% Pd/TiO2 (Cat. D, anatase-type)-catalyzed Suzuki–Miyaura coupling reaction (Table 4). The reaction did not proceed at 25 °C (Entry 2). While the reaction quantitatively proceeded in a test tube sealed with a septum under argon atmosphere at 80 °C for 24 h in Entry 1, the reaction efficiency was drastically decreased under atmospheric exposure (Entry 3). Therefore, the elimination of oxygen from the reaction vessel is strongly required for the Pd/TiO2 (Cat. D, anatase-type)-catalyzed ligand-free Suzuki–Miyaura coupling reaction of aryl chloride. Further detailed optimization of the base, solvent and reaction time are indicated in the Supporting Information (Tables S1–S3).

Table 4.
Effect of various reaction conditions.
The substrate applicability was investigated using 5 mol% of 5% Pd/TiO2 (Cat. D, anatase-type) and Cs2CO3 (2 equiv) in DMA at 80 °C for 24 h at 1000 rpm (Scheme 1). Aryl chlorides bearing an electron withdrawing group such as Ac and EtO2C on the aromatic ring (1a–1d) efficiently reacted with 2a or 4-acetylphenylboronic acid (2b) regardless of their substitution pattern (3b–3e). Although the coupling of chlorobenzene (1e) with 4-methoxyphenylboronic acid (2c) was inefficient and only a 17% yield of 3f was obtained, the reaction efficiency was significantly improved using 2.0 equiv of KOtBu instead of Cs2CO3. Furthermore, 1a was reacted with 4’-, 3’- or 2’-methoxyphenylboronic acid (2c, 2d, or 2e) in moderate to good yields (3g, 3h, or 3i). The direct formation of heterobiaryl skeletons is important for the construction of partial scaffolds of biologically active compounds [66,67,68,69,70,71,72] and functional materials [73,74]. The coupling of 4’-chloroacetophenone (1a) with heteroarylboronic acid derivatives afforded the corresponding heterobiaryl derivatives (3j and 3k) in moderate to excellent yields. Although 3f was obtained in a relatively low yield (24%) using 4-chloroanisole (1f) possessing an electron donating methoxy group on the aromatic ring even by the increased usage of 5% Pd/TiO2 (10 mol%, Cat. D, anatase-type), phenylboronic acid (2a, 2.0 equiv) and Cs2CO3 (3.0 equiv), the use of 4’-methoxyphenylbromide (4a) as an aryl bromide afforded a high yield (85%) of 3e under the original reaction conditions [5% Pd/TiO2 (Cat. D, anatase-type, 5 mol%), 2a (1.5 equiv) and Cs2CO3 (2.0 equiv), Equation (1)].
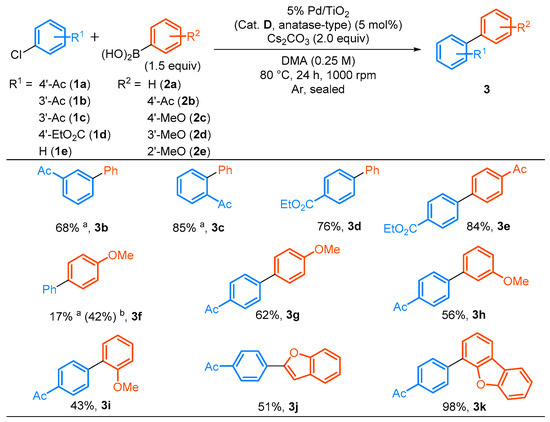
Scheme 1.
Scope and limitations of the Suzuki–Miyaura coupling reactions. a 2.0 equiv of an arylboronic acid, 10 mol% of 5% Pd/TiO2 (Cat. D anatase-type) and 3.0 equiv of Cs2CO3 were used. b 2.0 equiv of KOtBu was used instead of Cs2CO3.
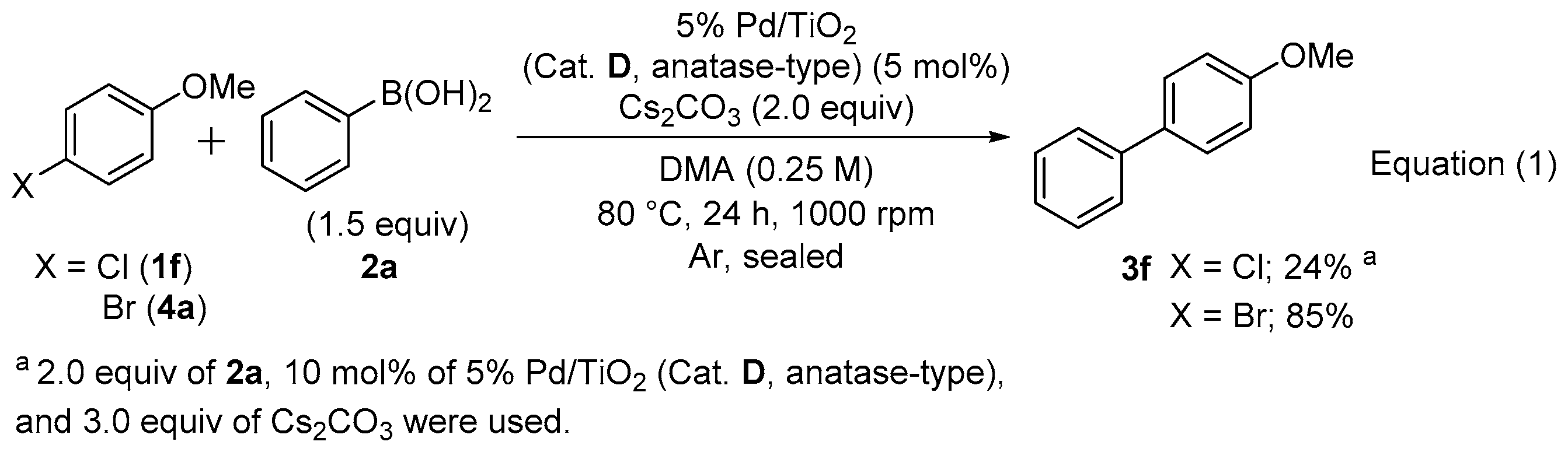
Although one of the advantages of the heterogeneous catalyst is reusability, the catalyst efficiency of 5% Pd/TiO2 (Cat. D, anatase-type) recovered after the first Suzuki–Miyaura coupling reaction using 1a and 2a significantly decreased, and the coupling reaction was never completed at 80 °C even after 24 h [Equation (2)].
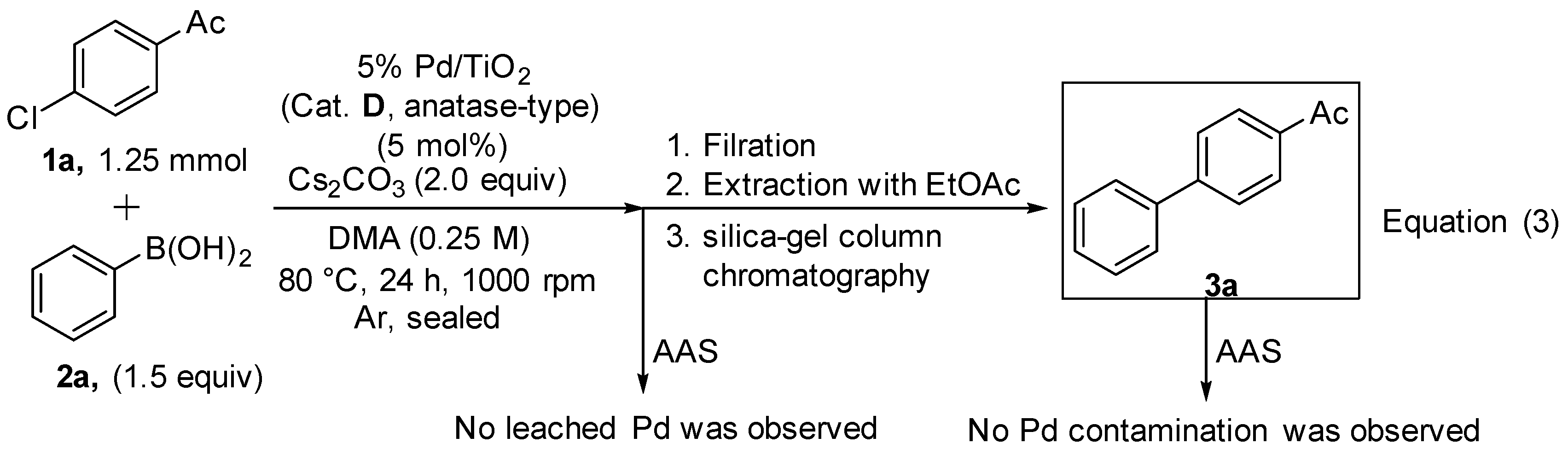
The Pd leaching behavior into the Suzuki–Miyaura coupling reaction medium and the trace Pd contamination in the coupling product after the purification process were examined using atomic absorption spectrometry [AAS, Equation (3)]. As a result, Pd species were never observed in both the reaction media after reaction and the coupling product 3a after purification including filtration, extraction with EtOAc and silica-gel column chromatography.
Although the fresh 5% Pd/TiO2 (Cat. D, anatase-type) without any reduction processes during the preparation amazingly consisted of ca. 1:2 mixture of Pd(II) ion (characteristic peaks at ca. 342.9 and 337.7 eV for Pd3d3/2 and Pd3d5/2) and Pd(0) species (characteristic peaks at ca. 341.0 and 335.7 eV for Pd3d3/2 and Pd3d5/2) according to XPS (Figure 2a), a significant increment in Pd(0) species (characteristic peaks at ca. 340.1 and 334.9 eV for Pd3d3/2 and Pd3d5/2) was observed and the recovered 5% Pd/TiO2 (Cat. D, anatase-type) was composed of ca. 1:5 mixture of Pd(II) ion (characteristic peaks at ca. 341.6 and 336.4 eV for Pd3d3/2 and Pd3d5/2) and Pd(0) species after the coupling reaction [Figure 2b]. From these results, Pd(II) species might be reduced by the significant electron donation during the Suzuki–Miyaura coupling reaction, probably due to the interaction between Pd species and a mass of TiO2 via metal-support interaction [strong metal support interaction (SMSI) or weak metal support interaction (WMSI) reported in the literature] [61,62,63]. Therefore, 5% Pd/TiO2 (Cat. D, anatase-type) indicated efficient catalyst activity toward the Suzuki–Miyaura coupling reaction, while TiO2 possessed only a small specific surface area (from 110 m2g−1 of the fresh catalyst to 22 m2g−1).
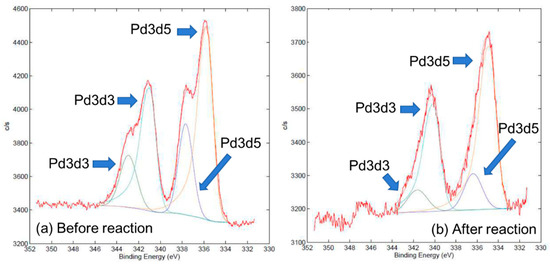
Figure 2.
XPS spectra of 5% Pd/TiO2 (Cat. D, anatase-type) before (a) and after (b) the Suzuki–Miyaura coupling reaction.
The average palladium particle sizes on 5% Pd/TiO2 (Cat. D, anatase-type) before and after the Suzuki-Miyaura coupling reaction (Figure 3a and 3b) were each roughly estimated to be 1 to 2 and 3 to 4 nm based on TEM images. These results strongly suggested that the high catalyst activity of fresh 5% Pd/TiO2 (Cat. D, anatase-type) might be induced by the extensively distributed small Pd nanoparticles (Figure 3a) while the gradual increase of the Pd particle size during the Suzuki–Miyaura coupling reaction probably caused the decreasing of catalytic activity [Figure 3b and Equation (2)].
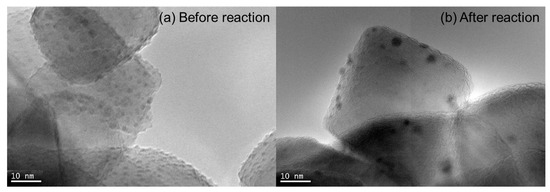
Figure 3.
Transmission electron microscopy (TEM) images of 5% Pd/TiO2 (Cat. D, anatase-type) before (a) and after (b) the Suzuki–Miyaura coupling reaction.
Furthermore, the chemisorption behavior of carbon monoxide on 5% Pd/TiO2 (Cat. D, anatase-type) indicated the significant degradation of the specific surface area from 110 m2g−1 of the fresh catalyst to 22 m2g−1 after the Suzuki–Miyaura coupling reaction due to the remarkable Pd sintering. Based on these experimental and analytical investigations, the decreasing of catalytic activity of the recovered 5% Pd/TiO2 (Cat. D, anatase-type) should be caused by the deterioration of the specific surface area due to the Pd sintering. Therefore, 5% Pd/TiO2 (Cat. D, anatase-type) was unfortunately hard to reuse, while the fresh catalyst was suitable for the ligand-free Suzuki–Miyaura coupling reaction of aryl chlorides.
3. Materials and Methods
3.1. General
All reagents and solvents were obtained from commercial sources and used without further purification. Pd(OAc)2 was obtained from N.E. Chemcat Co. (Tokyo, Japan). The 1H NMR and 13C NMR spectra were recorded on a JEOL ECZ-400 (400 MHz for 1H NMR and 100 MHz for 13C NMR) spectrometer. CDCl3 was used as the solvent for NMR measurement. Chemical shifts (d) are expressed in part per million and internally referenced (0.00 ppm for tetramethylsilane or 7.26 ppm for CHCl3 for 1H NMR for CDCl3 and 77.0 ppm for CDCl3 for 13C NMR). SHIMADZU AA-7000 was used for the atomic absorption spectrometry (AAS). All of 1H and 13C NMR spectra of known products were identical with those in the literature.
3.2. Experimentals
3.2.1. Preparation of 5% Pd/TiO2 (Cat. D, anatase-type)
A suspension of anatase-type TiO2 (3.00 g, colorless powder) in MeCN solution (30 mL) of Pd(OAc)2 [316 mg, 1.41 mmol (150 mg, palladium quantity)] was stirred under argon atmosphere at 25 °C for 4 days. The resulting light yellow solid was collected by filtration (1 µm filter paper), washed with H2O (5 mL × 5) and EtOAc (5 mL × 5) and dried in vacuo for 24 h to afford the Pd/TiO2 (3.15 g, Cat. D, anatase-type). The filtrate was concentrated in vacuo, and then transferred to a 100 mL volumetric flask with H2O and 15.58 ppm (1.56 mg) of palladium species were observed in the diluted filtrate by atomic absorption spectrometry (SHIMADZU AA-7000, Kyoto, Japan). Since the total palladium species which was not absorbed on anatase-type TiO2 was 1.56 mg, the palladium content of the anatase-type Pd/TiO2 (Cat. D) was approximately 5% (4.7%) [(150 − 1.56)/3150 × 100].
3.2.2. Preparation of 5% Pd(red)/TiO2 (Cat. A, Anatase-Type)
The collected anatase-type 5% Pd/TiO2 (Cat. D, 500.0 mg) was stirred with NH2NH2·H2O (29.2 µL, 0.6 mmol) in H2O (40 mL) under argon atmosphere at 25 °C for 24 h. The grayish white solid was collected by filtration (1 µm filter paper), washed with H2O (10 mL × 5) and EtOAc (10 mL × 5) and dried in vacuo for 12 h to give 5% Pd(red)/TiO2 (343 mg, Cat. A, anatase-type).
3.2.3. Typical Procedure for the Coupling Reaction between Aryl Chlorides and Arylboronic Acids
A mixture of 5% Pd/TiO2 (30.0 mg, 12.5 μmol), an aryl chloride (250 μmol), an arylboronic acid (375 μmol) and Cs2CO3 (163 mg, 500 μmol) in DMA (1 mL) was stirred at 80 °C in a test tube tightly sealed under argon atmosphere. After 24 h, the mixture was cooled to 25 °C, diluted with EtOAc (10 mL) and H2O (10 mL), and filtered through a membrane filter (pore size: 0.2 µm). The catalyst on the filter was washed with EtOAc (10 mL) and H2O (10 mL), and combined filtrates were separated to two layers. The aqueous layer was extracted with EtOAc (15 mL × 3), and combined EtOAc layers were washed with H2O (20 mL × 3) and brine (20 mL), dried over Na2SO4, filtered, and concentrated in vacuo. The residue was purified by silica-gel column chromatography using hexane/EtOAc (10:1) as eluents to afford the corresponding biaryl derivative.
3.2.4. Procedure for Reuse Test of Recovered 5% Pd/TiO2 (Cat. D, anatase-type) (Equation (2))
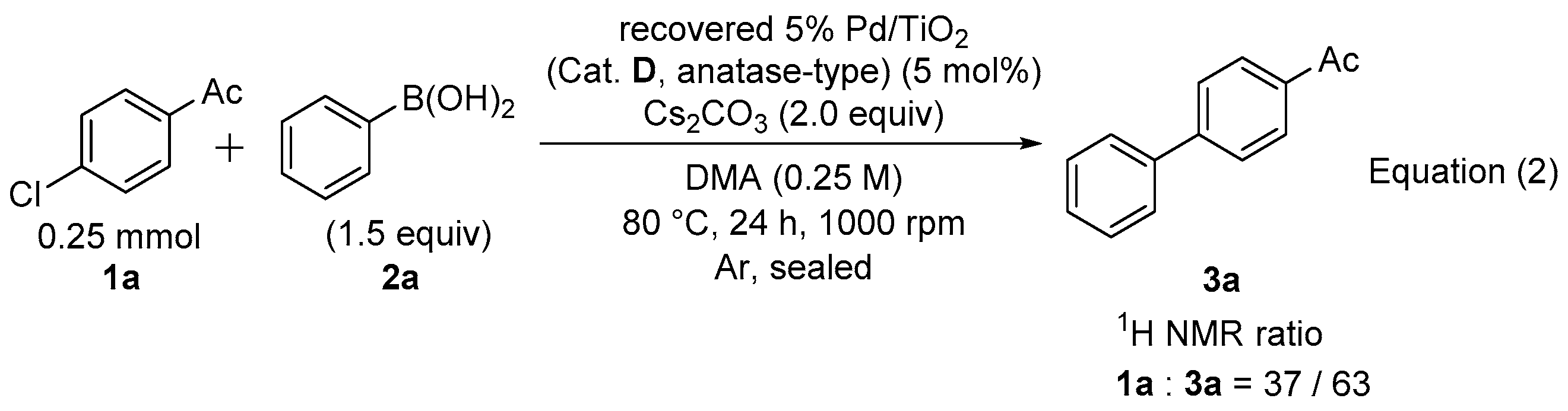
Three exactly the same reaction mixtures containing 5% Pd/TiO2 (Cat. D, anatase-type) (30.0 mg, 12.5 μmol), 4’-chloroacetophenone (1a, 38.6 mg, 250 μmol), a phenylboronic acid (2a, 45.7 mg, 375 μmol), Cs2CO3 (163 mg, 500 μmol) in DMA (1 mL) were stirred at 80 °C in each separate sealed test tube under argon atmosphere. After 24 h, the combined reaction mixture was filtered using a funnel (1 μm filter paper). The catalyst on the filter was washed with EtOAc (3 mL × 5), and the recovered catalyst was dried at room temperature under reduced pressure overnight, then weighed (90.0 mg, 100 %). The reaction of the second run was carried out in the same manner as the first run except for using the recovered 5% Pd/TiO2 (30.0 mg, 12.5 μmol) and only one reaction mixture in a test tube. The 1H NMR ratio of 1a and 3a indicated 37:63.
3.2.5. Procedure for the Confirmation Experiment of Pd Leaching in the Reaction Medium and Pd Contamination of the Coupling Product after Purification Processes (Equatiopn (3))
A mixture of 5% Pd/TiO2 (Cat. D, anatase-type) (150.0 mg, 62.5 μmol), 4’-Chloroacetophenone (1a, 193.0 mg, 1.25 mmol), a phenylboronic acid (2a, 229.1 mg, 1.88 mmol) and Cs2CO3 (815 mg, 2.5 mmol) in DMA (5 mL) was stirred at 80 °C in a test tube sealed under argon. After 24 h, the mixture was filtered using a funnel (1 μm filter paper). The catalyst on the filter paper was washed with EtOAc (15 mL × 5). The filtrate was concentrated in vacuo and transferred to a 100 mL volumetric flask with EtOAc. No palladium species were observed [0.0 ppm (0.0 mg)] in the diluted filtrate by measurement of atomic absorption spectrometry (SHIMADZU AA-7000). The organic layer was washed with H2O (20 mL × 3) and brine (20 mL), dried over Na2SO4, filtered and concentrated in vacuo. The residue was purified by silica-gel column chromatography using hexane/EtOAc (10:1) as eluents to afford the corresponding analytically pure 4-acetylbiphenyl (3a). The obtained 3a was transferred to a 100 mL volumetric flask with EtOAc, and no palladium species were observed [0.0 ppm (0.0 mg)] in the diluted filtrate by measurement of atomic absorption spectrometry.
4. Conclusions
We have prepared TiO2-supported catalysts possessing different crystalline structure of TiO2-and evaluated the significant effect based on the crystalline types on the ligand-free Suzuki–Miyaura cross coupling reaction mainly of aryl chlorides for the first time. The anatase-type TiO2 supported Pd catalyst [Pd/TiO2 (Cat. D, anatase-type)] demonstrated efficient catalyst activity toward the ligand-free Suzuki–Miyaura cross coupling reaction of aryl chlorides. The quite high catalyst activity would be due to the extensively distributed small Pd nanoparticles on the anatase-type TiO2 and the appropriate interaction between the Pd species and anatase-type TiO2 based on the reduction of the Pd(II) to Pd(0) species, although details of the actual electron donating process have not been clarified. Various aryl chlorides possessing electron withdrawing substituents, such as acetyl and ester functionalities, on the aromatic ring were smoothly coupled with aryl and heteroarylboronic acids in moderate to excellent yields. For aryl halides possessing an electron donating substrate on the aromatic ring, the coupling reaction could be performed using the corresponding aryl bromide.
Supplementary Materials
The following are available online at https://www.mdpi.com/2073-4344/9/5/461/s1, Figure S1. XPS image and curve-fitting of Pd peaks (Cat. D–I). Figure S2. XPS image and curve-fitting of the Pd peaks of 5% Pd/TiO2 (Cat. D, anatase-type) before and after use. Table S1. Base efficiency. Table S2. Solvent efficiency. Table S3. Time course study.
Author Contributions
Writing—original draft preparation T.Y.; designing the research, T.Y., T.I., K.P., Y.S. and H.S.; investigation and analysis, H.M., T.T., M.Y., N.I., Y.O. and Y.T.; project administration, supervision and polishing up the manuscript, H.S.
Funding
This research received no external funding.
Acknowledgments
We sincerely appreciate the N.E. Chemcat Co. for providing the Pd(OAc)2.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Miyaura, N.; Suzuki, A. Palladium-Catalyzed Cross-Coupling Reactions of Organoboron Compounds. Chem. Rev. 1995, 95, 2457–2483. [Google Scholar] [CrossRef]
- Hassan, J.; Sèvignon, M.; Gozzi, C.; Schulz, E.; Lemaire, M. Aryl-Aryl Bond Formation One Century after the Discovery of the Ullmann Reaction. Chem. Rev. 2002, 102, 1359–1470. [Google Scholar] [PubMed]
- Ackermann, L. Modern Arylation Methods; Wiley: Weinheim, Germany, 2009. [Google Scholar]
- Bringmann, G.; Gulder, T.; Gulder, T.A.M.; Breuning, M. Atroposelective Total Synthesis of Axially Chiral Biaryl Natural Products. Chem. Rev. 2011, 111, 563–639. [Google Scholar] [CrossRef]
- Wu, J.-S.; Cheng, S.-W.; Cheng, Y.-J.; Hsu, C.-S. Donor–acceptor conjugated polymers based on multifused ladder-type arenes for organic solar cells. Chem. Soc. Rev. 2015, 44, 1113–1154. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Lan, J.; You, J. Oxidative C−H/C−H Coupling Reactions between Two (Hetero)arenes. Chem. Rev. 2017, 117, 8787–8863. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.; Liebscher, J. Carbon-Carbon Coupling Reactions Catalyzed by Heterogeneous Palladium Catalysts. Chem. Rev. 2007, 107, 133–173. [Google Scholar] [CrossRef] [PubMed]
- Fihri, A.; Bouhrara, M.; Nekoueishahraki, B.; Basset, J.-M.; Polshettiwar, V. Nanocatalysts for Suzuki cross-coupling reactions. Chem. Soc. Rev. 2011, 40, 5181–5203. [Google Scholar] [CrossRef]
- Monguchi, Y.; Fujita, Y.; Hashimoto, S.; Ina, M.; Takahashi, T.; Ito, R.; Nozaki, K.; Maegawa, T.; Sajiki, H. Palladium on carbon-catalyzed solvent-free and solid-phase hydrogenation and Suzuki–Miyaura reaction. Tetrahedron 2011, 67, 8628–8634. [Google Scholar]
- Kitamura, Y.; Sako, S.; Udzu, T.; Tsutsui, A.; Maegawa, T.; Monguchi, Y.; Sajiki, H. Ligand-free Pd/C-catalyzed Suzuki–Miyaura coupling reaction for the synthesis of heterobiaryl derivatives. Chem. Commun. 2007, 5069–5071. [Google Scholar] [CrossRef] [PubMed]
- Maegawa, T.; Kitamura, Y.; Sako, S.; Udzu, T.; Sakurai, A.; Tanaka, A.; Kobayashi, Y.; Endo, K.; Bora, U.; Kurita, T.; et al. Heterogeneous Pd/C-Catalyzed Ligand-Free, Room-Temperature Suzuki–Miyaura Coupling Reactions in Aqueous Media. Chem. Eur. J. 2007, 13, 5937–5943. [Google Scholar] [CrossRef] [PubMed]
- Kitamura, Y.; Sakurai, A.; Udzu, T.; Maegawa, T.; Monguchi, Y.; Sajiki, H. Heterogeneous Pd/C-catalyzed ligand-free Suzuki–Miyaura coupling reaction using aryl boronic esters. Tetrahedron 2007, 6, 10596–10602. [Google Scholar] [CrossRef]
- Shokouhimehr, M.; Asl, M.S.; Mazinani, B. Modulated large-pore mesoporous silica as an efficient base catalyst for the Henry reaction. Res. Chem. Intermed. 2018, 44, 1617–1626. [Google Scholar] [CrossRef]
- Shokouhimehr, M. Magnetically Separable and Sustainable Nanostructured Catalysts for Heterogeneous Reduction of Nitroaromatics. Catalysts 2015, 5, 534–560. [Google Scholar] [CrossRef]
- Zhang, K.; Hong, K.; Suh, J.M.; Lee, T.H.; Kwon, O.; Shokouhimehr, M.; Jang, H.W. Facile synthesis of monodispersed Pd nanocatalysts decorated on graphene oxide for reduction of nitroaromatics in aqueous solution. Res. Chem. Intermediat. 2019, 45, 599–611. [Google Scholar] [CrossRef]
- Shokouhimehr, M.; Hong, K.; Lee, T.H.; Moon, C.W.; Hong, S.P.; Zhang, K.; Suh, J.M.; Choi, K.S.; Varma, R.S.; Jang, H.W. Magnetically retrievable nanocomposite adorned with Pd nanocatalysts: Efficient reduction of nitroaromatics in aqueous media. Green Chem. 2018, 20, 3809–3817. [Google Scholar] [CrossRef]
- Choi, K.-H.; Shokouhimehr, M.; Sung, Y.-E. Heterogeneous Suzuki Cross-Coupling Reaction Catalyzed by Magnetically Recyclable Nanocatalyst. Bull. Korean Chem. Soc. 2013, 34, 1477–1480. [Google Scholar] [CrossRef]
- Kim, A.; Rafiaei, S.M.; Abolhosseini, S.; Shokouhimehr, M. Palladium Nanocatalysts Confined in Mesoporous Silica for Heterogeneous Reduction of Nitroaromatics. Energy Environ. Focus 2015, 4, 18–23. [Google Scholar] [CrossRef]
- Shokouhimehr, M.; Lee, J.E.; Han, S.I.; Hyeon, T. Magnetically recyclable hollow nanocomposite catalysts for heterogeneous reduction of nitroarenes and Suzuki reactions. Chem. Commun. 2013, 49, 4779–4781. [Google Scholar] [CrossRef]
- Shokouhimehr, M.; Kim, T.; Jun, S.W.; Shin, K.; Jang, Y.; Kim, B.H.; Kim, J.; Hyeon, T. Magnetically separable carbon nanocomposite catalysts for efficient nitroarene reduction and Suzuki reactions. Appl. Catal. A General 2014, 476, 133–139. [Google Scholar] [CrossRef]
- Li, G.; Yang, H.; Li, W.; Zhang, G. Rationally designed palladium complexes on a bulky N-heterocyclic carbene-functionalized organosilica: An efficient solid catalyst for the Suzuki–Miyaura coupling of challenging aryl chlorides. Green Chem. 2011, 13, 2939–2947. [Google Scholar] [CrossRef]
- Karimi, B.; Akhavan, P.F. A Study on Applications of N-Substituted Main-Chain NHC-Palladium Polymers as Recyclable Self-Supported Catalysts for the Suzuki–Miyaura Coupling of Aryl Chlorides in Water. Inorg. Chem. 2011, 50, 6063–6072. [Google Scholar] [CrossRef]
- Das, P.; Sharma, D.; Shil, A.K.; Kumari, A. Solid-supported palladium nano and microparticles: An efficient heterogeneous catalyst for ligand-free Suzuki–Miyaura cross coupling reaction. Tetrahedron Lett. 2011, 52, 1176–1178. [Google Scholar] [CrossRef]
- Yamada, Y.M.A.; Sarkar, S.M.; Uozumi, Y. Self-Assembled Poly(imidazole-palladium): Highly Active, Reusable Catalyst at Parts per Million to Parts per Billion Levels. J. Am. Chem. Soc. 2012, 134, 3190–3198. [Google Scholar] [CrossRef]
- Wu, L.; Li, B.-O.; Huang, Y.-Y.; Zhou, H.-F.; He, Y.-M.; Fan, Q.-H. Phosphine Dendrimer-Stabilized Palladium Nanoparticles, a Highly Active and Recyclable Catalyst for the Suzuki−Miyaura Reaction and Hydrogenation. Org. Lett. 2006, 8, 3605–3608. [Google Scholar] [CrossRef] [PubMed]
- Schweizer, S.; Becht, J.-M.; Drian, C.L. Highly Efficient and Reusable Supported Pd Catalysts for Suzuki−Miyaura Reactions of Aryl Chlorides. Org. Lett. 2007, 9, 3777–3780. [Google Scholar] [CrossRef] [PubMed]
- Sayah, R.; Glegoła, K.; Framery, E.; Dufaud, V. Suzuki–Miyaura Reactions of Aryl Chloride Derivatives with Arylboronic Acids using Mesoporous Silica-Supported Aryldicyclohexylphosphine. Adv. Synth. Catal. 2007, 349, 373–381. [Google Scholar] [CrossRef]
- Pandarus, V.; Desplantier-Giscard, D.; Gingras, G.; Béland, F.; Ciriminna, R.; Pagliaro, M. Greening the Valsartan Synthesis: Scale-up of Key Suzuki–Miyaura Coupling over SiliaCat DPP-Pd. Org. Process Res. Dev. 2013, 17, 1492–1497. [Google Scholar] [CrossRef]
- Lee, D.-H.; Choi, M.; Yu, B.-W.; Ryoo, R.; Taher, A.; Hossain, S.; Jin, M.-J. Expanded Heterogeneous Suzuki–Miyaura Coupling Reactions of Aryl and Heteroaryl Chlorides under Mild Conditions. Adv. Synth. Catal. 2009, 351, 2912–2920. [Google Scholar] [CrossRef]
- Jin, M.-J.; Lee, D.-H. A Practical Heterogeneous Catalyst for the Suzuki, Sonogashira, and Stille Coupling Reactions of Unreactive Aryl Chlorides. Angew. Chem. Int. Ed. 2010, 49, 1119–1122. [Google Scholar] [CrossRef]
- LeBlond, C.R.; Andrews, A.T.; Sun, Y.; Sowa, J.R., Jr. Activation of Aryl Chlorides for Suzuki Cross-Coupling by Ligandless, Heterogeneous Palladium. Org. Lett. 2001, 3, 1555–1557. [Google Scholar] [CrossRef]
- Tagata, T.; Nishida, M. Palladium Charcoal-Catalyzed Suzuki–Miyaura Coupling to Obtain Arylpyridines and Arylquinolines. J. Org. Chem. 2003, 68, 9412–9415. [Google Scholar] [CrossRef] [PubMed]
- Arvela, R.K.; Leadbeater, N.E. Suzuki Coupling of Aryl Chlorides with Phenylboronic Acid in Water, Using Microwave Heating with Simultaneous Cooling. Org. Lett. 2005, 7, 2101–2104. [Google Scholar] [CrossRef] [PubMed]
- Ohtaka, A.; Sakaguchi, E.; Yamaguchi, T.; Hanasaka, G.; Uozumi, Y.; Shimomura, O.; Nomura, R. A Recyclable “Boomerang” Linear Polystyrene-Stabilized Pd Nanoparticles for the Suzuki Coupling Reaction of Aryl Chlorides in Water. ChemCatChem 2013, 5, 2167–2169. [Google Scholar] [CrossRef]
- Yuan, B.; Pan, Y.; Li, Y.; Yin, B.; Jiang, H.A. Highly Active Heterogeneous Palladium Catalyst for the Suzuki–Miyaura and Ullmann Coupling Reactions of Aryl Chlorides in Aqueous Media. Angew. Chem. Int. Ed. 2010, 49, 4054–4058. [Google Scholar] [CrossRef] [PubMed]
- Kogan, V.; Aizenshtat, Z.; Popovitz-Biro, R.; Newmann, R. Carbon-Carbon and Carbon-Nitrogen Coupling Reactions Catalyzed by Palladium Nanoparticles Derived from a Palladium Substituted Keggin-Type Polyoxometalate. Org. Lett. 2002, 4, 3529–3532. [Google Scholar] [CrossRef] [PubMed]
- Choudary, B.M.; Madhi, S.; Chowdari, N.S.; Kantam, M.L.; Sreedhar, B. Layered Double Hydroxide Supported Nanopalladium Catalyst for Heck-, Suzuki-, Sonogashira-, and Stille-Type Coupling Reactions of Chloroarenes. J. Am. Chem. Soc. 2002, 124, 14127–14136. [Google Scholar] [CrossRef] [PubMed]
- Ciriminna, R.; Pandarus, V.; Gingras, G.; Béland, F.; Demma, P.; Pagliaro, M. Heterogeneously catalyzed Suzuki–Miyaura conversion of broad scope. RSC Adv. 2012, 2, 10798–10804. [Google Scholar] [CrossRef]
- Monguchi, Y.; Ichikawa, T.; Netsu, M.; Hattori, T.; Mizusaki, T.; Sawama, Y.; Sajiki, H. Tertiary-Amino-Functionalized Resin-Supported Palladium Catalyst for the Heterogeneous Suzuki–Miyaura Reaction of Aryl Chlorides. Synlett 2015, 26, 2014–2018. [Google Scholar] [CrossRef]
- Ichikawa, T.; Netsu, M.; Mizuno, M.; Mizusaki, T.; Takagi, Y.; Sawama, Y.; Monguchi, Y.; Sajiki, H. Development of a Unique Heterogeneous Palladium Catalyst for the Suzuki–Miyaura Reaction using (Hetero)aryl Chlorides and Chemoselective Hydrogenation. Adv. Synth. Catal. 2017, 359, 2269–2279. [Google Scholar] [CrossRef]
- Ichikawa, T.; Mizuno, M.; Ueda, S.; Ohneda, N.; Odajima, H.; Sawama, Y.; Monguchi, Y.; Sajiki, H. A practical method for heterogeneously-catalyzed Mizoroki–Heck reaction: Flow system with adjustment of microwave resonance as an energy source. Tetrahedron 2018, 74, 1810–1816. [Google Scholar] [CrossRef]
- Sreedhar, B.; Yada, D.; Reddy, P.S. Nanocrystalline Titania-Supported Palladium(0) Nanoparticles for Suzuki–Miyaura Cross-Coupling of Aryl and Heteroaryl Halides. Adv. Synth. Catal. 2011, 353, 2823–2836. [Google Scholar] [CrossRef]
- Nasrollahzadeh, M.; Sajadi, S.M. Green synthesis, characterization and catalytic activity of the Pd/TiO2 nanoparticles for the ligand-free Suzuki–Miyaura coupling reaction. J. Colloid Interf. Sci. 2016, 465, 121–127. [Google Scholar] [CrossRef]
- Mondal, P.; Khatun, R.; Bhanja, P.; Bhaumik, A.; Das, D.; Islam, S.M. Palladium nanoparticles embedded on mesoporous TiO2 material (Pd@MTiO2) as an efficient heterogeneous catalyst for Suzuki-Coupling reactions in water medium. J. Colloid Interf. Sci. 2017, 508, 378–386. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Niu, Y.; Li, Y.; Yang, F.; Guo, J.; Wang, Q.; Jing, P.; Zhang, J.; Yun, G. A mesoporous “shell-in-shell” structured nanocatalyst with large surface area, enhanced synergy, and improved catalytic performance for Suzuki–Miyaura coupling reaction. Chem. Commun. 2014, 50, 12356–12359. [Google Scholar] [CrossRef]
- Kamari, Y.; Ghiaci, M. Incorporation of TiO2 coating on a palladium heterogeneous nanocatalyst. A new method to improve reusability of a catalyst. Catal. Commun. 2016, 84, 16–20. [Google Scholar] [CrossRef]
- Koohgard, M.; Hosseini-Sarvari, M. Enhancement of Suzuki–Miyaura coupling reaction by photocatalytic palladium nanoparticles anchored to TiO2 under visible light irradiation. Catal. Commun. 2018, 111, 10–15. [Google Scholar] [CrossRef]
- Shiraishi, Y.; Takeda, Y.; Sugano, Y.; Ichikawa, S.; Tanaka, S.; Hirai, T. Highly efficient photocatalytic dehalogenation of organic halides on TiO2 loaded with bimetallic Pd–Pt alloy nanoparticles. Chem. Commun. 2011, 47, 7863–7865. [Google Scholar] [CrossRef]
- Yoshida, H.; Fujimura, Y.; Yuzawa, H.; Kumagai, J.; Yoshida, M. A heterogeneous palladium catalyst hybridised with a titanium dioxide photocatalyst for direct C–C bond formation between an aromatic ring and acetonitrile. Chem. Commun. 2013, 49, 3793–3795. [Google Scholar] [CrossRef] [PubMed]
- Sikhwivhilu, L.M.; Coville, N.J.; Naresh, D.; Chary, K.V.R.; Vishwanathan, V. Nanotubular titanate supported palladium catalysts: The influence of structure and morphology on phenol hydrogenation activity. Appl. Catal. A General 2007, 324, 52–61. [Google Scholar] [CrossRef]
- Yang, J.; Cao, L.-X.; Wang, G.-C. Acetylene hydrogenation on anatase TiO2(101) supported Pd4 cluster: Oxygen deficiency effect. J. Mol. Model. 2012, 18, 3329–3339. [Google Scholar] [CrossRef] [PubMed]
- Ortel, E.; Sokolov, S.; Zielke, C.; Lauermann, I.; Selve, S.; Weh, K.; Paul, B.; Polte, J.; Kraehnert, R. Supported Mesoporous and Hierarchical Porous Pd/TiO2 Catalytic Coatings with Controlled Particle Size and Pore Structure. Chem. Mater. 2012, 24, 3828–3838. [Google Scholar] [CrossRef]
- Khatun, R.; Bhanja, P.; Mondal, P.; Bhaumik, A.; Das, D.; Islam, S.M. Palladium nanoparticles embedded over mesoporous TiO2 for chemical fixation of CO2 under atmospheric pressure and solvent-free conditions. New J. Chem. 2017, 41, 12937–12946. [Google Scholar] [CrossRef]
- Melchionna, M.; Beltram, A.; Montini, T.; Monai, M.; Nasi, L.; Fornasiero, P.; Prato, M. Highly efficient hydrogen production through ethanol photoreforming by a carbon nanocone/ Pd@TiO2 hybrid catalyst. Chem. Commun. 2016, 52, 764–767. [Google Scholar] [CrossRef] [PubMed]
- Sayed, F.N.; Jayakumar, O.D.; Sasikala, R.; Kadam, R.M.; Bharadwaj, S.R.; Kienle, L.; Schürmann, U.; Kaps, S.; Adelung, R.; Mittal, J.P.; Tyagi, A.K. Photochemical Hydrogen Generation Using Nitrogen-Doped TiO2−Pd Nanoparticles: Facile Synthesis and Effect of Ti3+ Incorporation. J. Phys. Chem. C 2012, 116, 12462–12467. [Google Scholar] [CrossRef]
- Bahruji, H.; Bowkera, M.; Brookes, C.; Davies, P.R.; Wawata, I. The adsorption and reaction of alcohols on TiO2 and Pd/TiO2 catalysts. Appl. Catal. A General 2013, 454, 66–73. [Google Scholar] [CrossRef]
- Cao, C.; Yan, Y.; Yu, Y.; Yang, X.; Liu, W.; Cao, Y. Modification of Pd and Mn on the Surface of TiO2 with Enhanced Photocatalytic Activity for Photoreduction of CO2 into CH4. J. Phys. Chem. C 2017, 121, 270–277. [Google Scholar] [CrossRef]
- Sayed, F.N.; Sasikala, R.; Jayakumar, O.D.; Rao, R.; Betty, C.A.; Chokkalingam, A.; Kadam, R.M.; Jagannath; Bharadwaj, S.R.; Vinuc, A.; Tyagi, A.K. Photocatalytic hydrogen generation from water using a hybrid of graphene nanoplatelets and self doped TiO2–Pd. RSC Adv. 2014, 4, 13469–13476. [Google Scholar] [CrossRef]
- Nasrollahzadeh, M.; Azarian, A.; Ehsani, A.; Khalaj, M. Preparation, optical properties and catalytic activity of TiO2@Pd nanoparticles as heterogeneous and reusable catalysts for ligand-free Heck coupling reaction. J. Mol. Catal. A Chem. 2014, 394, 205–210. [Google Scholar] [CrossRef]
- Dodson, J.J.; Hagelin-Weaver, H.E. Effect of titania structure on palladium oxide catalysts in the oxidativecoupling of 4-methylpyridine. J. Mol. Catal. A Chem. 2015, 410, 271–279. [Google Scholar] [CrossRef]
- Sá, J.; Bernardi, J.; Anderson, J.A. Imaging of low temperature induced SMSI on Pd/TiO2 catalysts. Catal. Lett. 2007, 114, 91–95. [Google Scholar] [CrossRef]
- Colmenares, J.C.; Magdziarz, A.; Aramendia, M.A.; Marinas, A.; Marinas, J.M.; Urbano, F.J.; Navio, J.A. Influence of the strong metal support interaction effect (SMSI) of Pt/TiO2 and Pd/TiO2 systems in the photocatalytic biohydrogen production from glucose solution. Catal. Commun. 2011, 16, 1–6. [Google Scholar] [CrossRef]
- Pan, C.-J.; Tsai, M.-C.; Su, W.-N.; Rick, J.; Akalework, N.G.; Agegnehu, A.K.; Cheng, S.-Y.; Hwang, B.-J. Tuning/exploiting Strong Metal-Support Interaction (SMSI) in Heterogeneous Catalysis. J. Taiwan Inst. Chem. E 2017, 74, 154–186. [Google Scholar] [CrossRef]
- O’Brien, C.J.; Kantchev, E.A.B.; Valente, C.; Hadei, N.; Chass, G.A.; Lough, A.; Hopkinson, A.C.; Organ, M.G. Easily Prepared Air-and Moisture-Stable Pd–NHC (NHC=N-Heterocyclic Carbene) Complexes: A Reliable, User-Friendly, Highly Active Palladium Precatalyst for the Suzuki–Miyaura Reaction. Chem. Eur. J. 2006, 12, 4743–4748. [Google Scholar] [CrossRef]
- Valente, C.; Çalimsiz, S.; Hoi, K.H.; Mallik, D.; Sayah, M.; Organ, M.G. The Development of Bulky Palladium NHC Complexes for the Most-Challenging Cross-Coupling Reactions. Angew. Chem. Int. Ed. 2012, 51, 3314–3332. [Google Scholar] [CrossRef] [PubMed]
- Petrassi, H.M.; Johnson, S.M.; Purkey, H.E.; Chiang, K.P.; Walkup, T.; Jiang, X.; Powers, E.T.; Kelly, J.W. Potent and Selective Structure-Based Dibenzofuran Inhibitors of Transthyretin Amyloidogenesis: Kinetic Stabilization of the Native State. J. Am. Chem. Soc. 2005, 127, 6662–6671. [Google Scholar] [CrossRef]
- Oliveira, A.M.A.G.; Raposo, M.M.M.; Oliveira-Campos, A.M.F.; Machado, A.E.H.; Puapairoj, P.; Pedro, M.; Nascimento, M.S.J.; Portela, C.; Afonso, C.; Pinto, M. Psoralen analogues: Synthesis, inhibitory activity of growth of human tumor cell lines and computational studies. Eur. J. Med. Chem. 2006, 41, 367–372. [Google Scholar] [CrossRef] [PubMed]
- Lusic, H.; Uprety, R.; Deiters, A. Improved Synthesis of the Two-Photon Caging Group 3-Nitro-2-Ethyldibenzofuran and Its Application to a Caged Thymidine Phosphoramidite. Org. Lett. 2010, 12, 916–919. [Google Scholar] [CrossRef] [PubMed]
- Love, B.E. Isolation and synthesis of polyoxygenated dibenzofurans possessing biological activity. Eur. J. Med. Chem. 2015, 97, 377–387. [Google Scholar] [CrossRef]
- Huang, W.; Xu, J.; Liu, C.; Chen, Z.; Gu, Y. Lewis Acid-Catalyzed Synthesis of Benzofurans and 4,5,6,7-Tetrahydrobenzofurans from Acrolein Dimer and 1,3-Dicarbonyl Compounds. J. Org. Chem. 2019, 84, 2941–2950. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Jain, P.; Lin, B.; Ferrer, M.; Hu, Z.; Southall, N.; Hu, X.; Zheng, W.; Neuenswander, B.; Cho, C.-H.; et al. High-Throughput Screening, Discovery, and Optimization To Develop a Benzofuran Class of Hepatitis C Virus Inhibitors. ACS Comb. Sci. 2015, 17, 641–652. [Google Scholar] [CrossRef]
- Chong, P.Y.; Shotwell, J.B.; Miller, J.; Price, D.J.; Maynard, A.; Voitenleitner, C.; Mathis, A.; Williams, S.; Pouliot, J.; Creech, K.; et al. Design of N-Benzoxaborole Benzofuran GSK8175–Optimization of Human PK Inspired by Metabolites of a Failed Clinical HCV Inhibitor. J. Med. Chem. 2019, 62, 3254–3267. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhang, Y.-X.; Hu, Y.; Shi, X.-B.; Jiang, Z.-Q.; Wang, Z.-K.; Liao, L.-S. Highly Efficient Blue Phosphorescent Organic Light-Emitting Diodes Employing a Host Material with Small Bandgap. ACS Appl. Mater. Interfaces 2016, 8, 16186–16191. [Google Scholar] [CrossRef] [PubMed]
- Solórzano, P.C.; Brigante, F.; Pierini, A.B.; Jimenez, L.B. Photoinduced Synthesis of Dibenzofurans: Intramolecular and Intermolecular Comparative Methodologies. J. Org. Chem. 2018, 83, 7867–7877. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).