Abstract
A highly efficient and enantioselective approach to the synthesis of functionalized benzofuran-3(2H)-ones is presented. It proceeds via an intramolecular Stetter reaction using β,β-disubstituted Michael acceptors in the construction of five-membered rings with fully-substituted quaternary stereogenic centers and is promoted by terpene-derived triazolium salts. As a result, a series of chiral 2,2-disubstituted benzofuran-3(2H)-one derivatives with linear, branched, and cyclic aliphatic substitutions on the quaternary stereogenic center were obtained in high yields and with excellent enantioselectivities of up to 99% ee.
1. Introduction
The development of stereocontrolled strategies leading to molecules of biological interest is of vital importance in contemporary organic chemistry [1,2]. 3-Coumaranones (benzofuran-3(2H)-one) and naphthofuranone derivatives constitute an interesting class of heterocycles because of their presence in many naturally occurring and biologically interesting compounds and they are regarded as having a “privileged” structure in medicinal chemistry [3,4,5,6,7,8,9]. They have also been found to be important building blocks in the synthesis of valuable biologically active heterocycles and possess interesting cytotoxic and pharmacological properties such as antifungal, anticancer, and antipsychotic [10,11,12,13,14,15]. In one particularly valuable context, 2,2-disubstituted coumaranones bearing a fully substituted quaternary stereogenic center act as synthetic intermediates, since the core skeleton is present in several natural products, including pterocarpans, lignans, and other biologically active agents such as Geodin [16], griseofulvin (an antifungal agent) [17], linobiflavonoid (an anticancer agent) [18], and Sch 202,596 (which combats Alzheimer’s disease) [19] (Figure 1). The quaternary stereogenic centers are present in many natural products, but their construction represents a synthetic challenge, especially with the need for stereoselective synthesis [20]. Therefore, efficient and enantioselective methods to construct such scaffolds are desirable. Progress in this area has mainly come from transition-metal-catalyzed C–H bond activations [21,22,23]. Metal-free catalytic approaches have been much less explored.
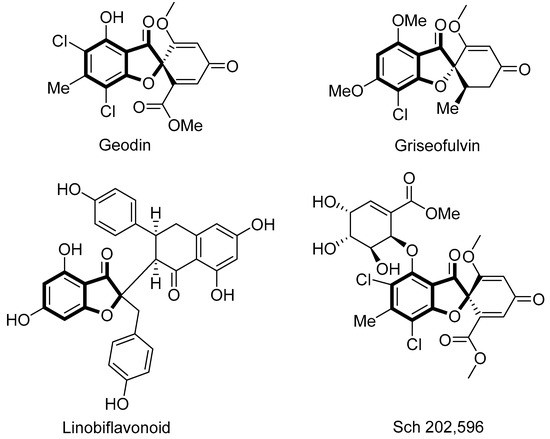
Figure 1.
Selected benzofuran-3(2H)-one-derived natural products.
Over the past decade, N-heterocyclic carbene (NHC) organocatalysts have received considerable attention due to their unique ability to catalyze a wide range of synthetic transformations [24,25,26,27,28,29]. The ability of NHCs to reverse natural reactivity of a functional group has led to intensive research on them, leading to unprecedented access to designed target molecules and a set of umpolung reactions [30,31,32]. As a powerful carbon–carbon bond-forming reaction, the Stetter reaction is undoubtedly one of the most attractive and important umpolung processes and it enables access to many 1,4-dicarbonyl compounds through 1,4-addition to electron-deficient olefins [33,34,35,36,37,38,39,40]. N-Heterocyclic carbenes (NHCs) have been demonstrated to be useful catalysts for the synthesis of 2,2-disubstituted benzofuranone compounds [34,35,41,42]. The pioneering work by Rovis showcased how the Stetter reaction involves the addition of an aldehyde to a β,β-disubstituted Michael acceptor and is an excellent way to access five-membered rings with fully-substituted quaternary stereogenic centers. A series of enantioenriched thiobenzofuranones and aliphatic heterocycles have been obtained in this way. However, in the case of benzofuran-3-one analogues, this approach is so far limited to two examples [34,35].
Recently, Rovis and coworkers described an elegant procedure for the enantioselective preparation of benzofuran-3-one products, utilizing a one-pot Michael/Stetter protocol. However, their methodology only gave adequate enantioselectivity for dimethyl acetylenedicarboxylate [43]. Application of unsymmetrical alkynes significantly decreased yield and selectivity of the products. More recently, Glorius uncovered a non-enantioselective approach to the synthesis of 2,2-disubstituted benzofuran-3-ones using a multicatalytic process that involves intramolecular hydroacylation of unactivated alkynes, followed by an intermolecular Stetter reaction and a subsequent base-catalyzed rearrangement (Figure 2) [42]. Despite these elegant contributions, the asymmetric synthesis of chiral 2,2-disubstituted benzofuran-3-one derivatives is still in its infancy and novel catalytic processes are highly desirable.
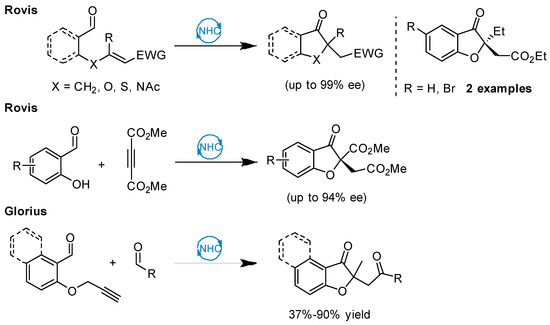
Figure 2.
N-heterocyclic carbene (NHC)-catalyzed approach to the 2,2-disubstituted benzofuran-3-ones.
Due to the ongoing interest in the development of chiral terpene-based N-heterocyclic carbene catalysts and their applications to organocatalytic reactions [44,45,46,47,48], we report the NHC-catalyzed stereoselective synthesis of functionalized 3-coumaranones and naphthofuranones via an intramolecular Stetter reaction.
2. Results and Discussion
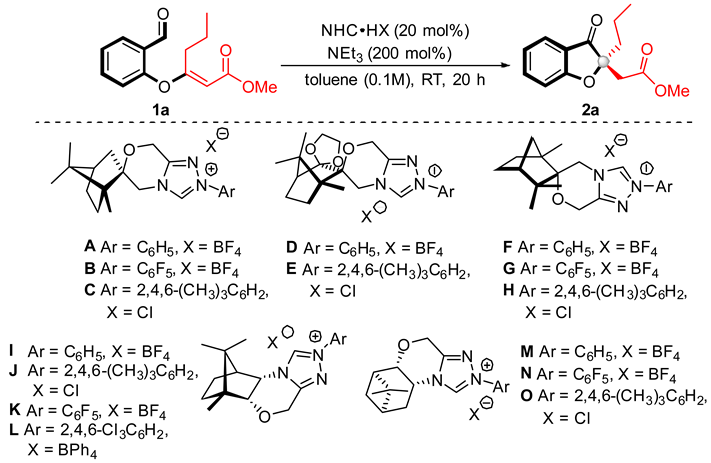
To examine the feasibility of the envisaged intramolecular Stetter reaction, we started by surveying a variety of chiral terpene-derived triazolium salts, A–O, as N-heterocyclic carbene precursors, with the use of salicylaldehyde-derived 1a as a model substrate. It was found that the devised strategy is possible under umpolung activation by using different terpene-derived carbene precursors. To our delight, in most cases, the reaction product 2a could be obtained. Catalysts with an N-phenyl substituent were ineffective (Table 1, entries 1, 4, 6, 9, and 13), likely due to the orthosubstitution effects [49]. Similar observations with NHCs lacking orthosubstituted aromatics were reported in several studies [50,51]. We were encouraged to find that, in the presence of spirocyclic camphor-derived pre-NHCs (C, D), the desired coumaranone with fully substituted quaternary stereogenic centers 2a could be obtained with moderate yield and selectivity (Table 1, entries 3 and 5). Replacing the N-mesityl substituent of the NHC catalyst C with a pentafluorophenyl unit led to the formation of 2a with excellent yield, though slight erosion of enantiomeric excess was observed (Table 1, entry 2). Similar results were obtained using fenchone-derived NHCs (F, G, H) (Table 1, entries 6–8). We next evaluated triazolium NHC catalysts derived from camphor J–L, which displayed significantly better outcomes (Table 1, entries 10–12). Gratifyingly, the desired product 2a was obtained in 98% yield with 84% ee when the precatalyst L, bearing a 2,4,6-trichlorophenyl N-substituent, was employed (entry 12). Pinene-derived triazolium salt N with a pentafluorophenyl moiety promoted the intramolecular Stetter reaction to give 2,2-disubstituted coumaranone 2a with excellent control of the enantioselectivity of the process (Table 1, entry 14).

Table 1.
NHC catalyst screening a.
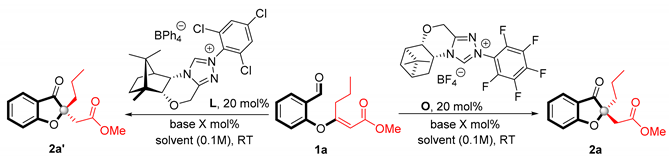
Taking into account the very promising results in terms of stereoselectivity and the fact that precatalysts L and O gave products with opposite stereochemical configurations, further work was carried out using camphor-derived NHC L and O pinene-derived NHC precursors.
With the catalyst screening accomplished, further optimization studies were undertaken (Table 2). Various reaction parameters, including base (Table 2, entries 1–23), solvent (entries 24–37), and reaction time, were evaluated.

Table 2.
Optimization of the reaction conditions a.
As can be seen from Table 2, all bases used in this model reaction were well tolerated, giving the benzofuran-3(2H)-one derivative 2a and 2a’ in high yields with excellent enantioselectivities. Initially, the reaction was conducted with 200 mol% of triethylamine. The reaction product 2a was formed in full conversion after 6 h for the precatalyst L and after 20 h for the precatalyst O (Table 1, entry 1, 2). Fortunately, for both triazolium salts, L and O, the base loading could be reduced to 20 mol% and so undesired reactions with bases stronger than triethylamine were avoided. The corresponding benzofuranones, 2a and 2a’, were obtained with the same level of yield and enantioselectivity (Table 2, entries 3 and 4). It is worth noting that for both triazolium salts, the use of organic bases (such as P2-Et, KHMDS, BEMP, t-BuOK) had a significant effect on reaction time, affording the desired Stetter products, 2a and 2a’, within 30 min, without erosion of the ee value (Table 2, entries 14–21). Among the various solvents screened, the reactions in nonpolar solvents, such as TAME, MTBE, and CMPE, resulted in comparable results (entries 28–31, 34, 35), whereas after reaction in polar ethanol, the desired coumaranone-type products were not observed. Gratifyingly, the enantioselectivity slightly increased to 97% ee for both NHC precatalysts with cyclohexane as a solvent. These observations enabled us to identify the final reaction parameters (Table 2, entries 32, 33).
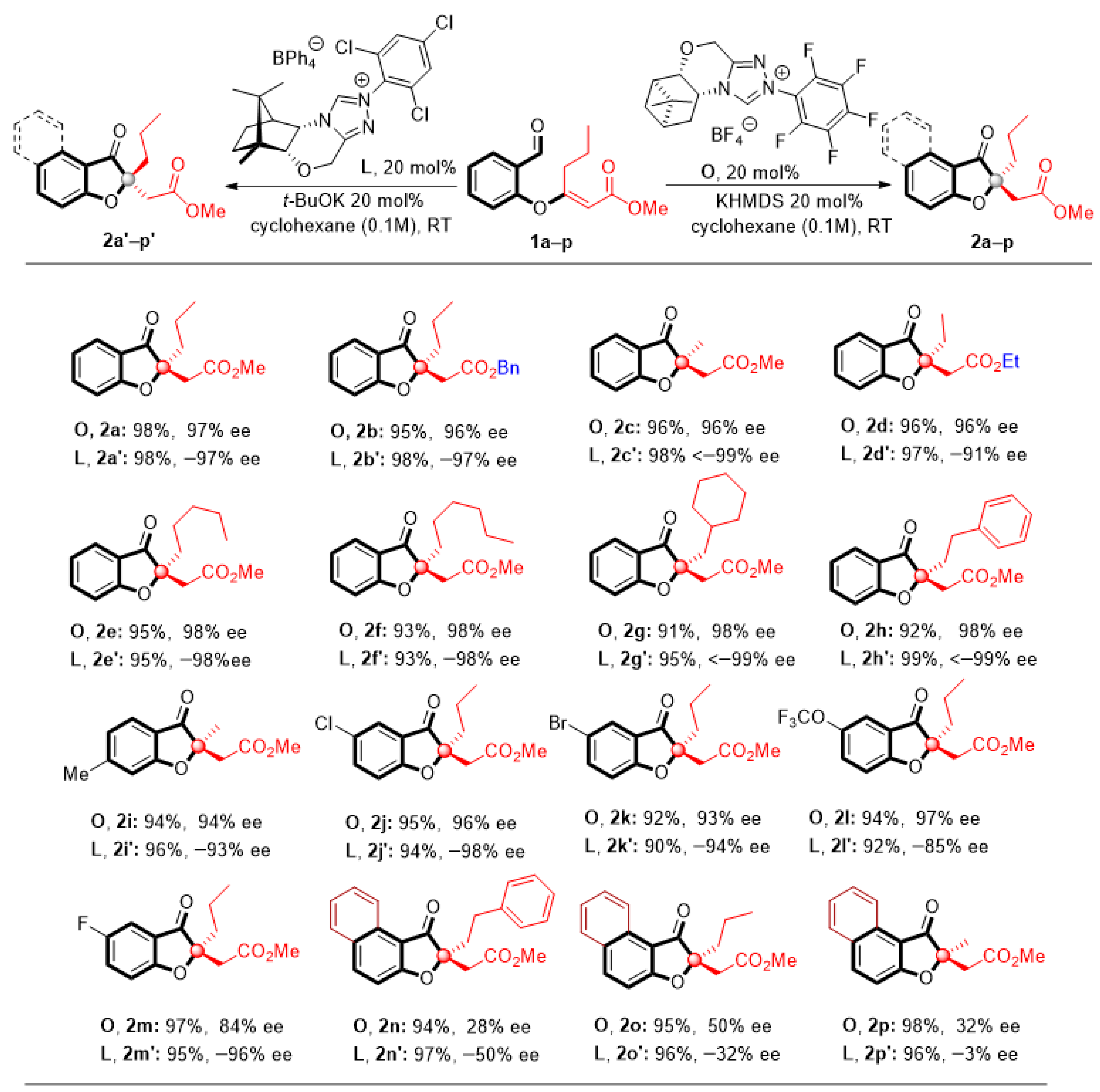
Next, the generalizability of the reaction was verified by engaging a variety of coumarone-type products. Generally, the reaction reached completion within 3 h and gave the products in high yields, with good to excellent enantioselectivity at room temperature. As shown in Table 3 (2a–2h, 2a’–2h’), a wide range of aliphatic substituents located in the double bonds of the substrates could be converted into products of uniformly high efficiency and selectivity. In addition, different ester groups, such as benzyl and ethyl, were also accommodated (Table 3, 2b, 2b’, 2d, 2d’). Furthermore, various substituted salicylaldehyde-derived substrates 1i–1m, including those bearing electron-withdrawing and electron-donating substituents at different positions on the aromatic ring, could be tolerated and gave the corresponding compounds 2i–2m, 2i’–2m’ in high yields and with excellent enantioselectivities. The electronic effects of the substituents did not have much influence on the outcome of the reaction. Of particular interest is the fact that simply changing the carbenes generated in situ from triazolium salts L or O, it is possible to affect chirality switching and select the product with the desired stereochemical configuration. We were surprised to find that, despite the structural differences between the camphor-derived L and pinene-derived O triazolium salts, both catalyzed the Stetter reaction and promoted opposite but high enantiofacial selectivity. The catalytic system also proved to be efficient when 2n–p, 2n’–p’ contained a naphthyl substituent, producing distinct naphthofuranones with high yields (94–98%), albeit with reduced enantioselectivity. Interestingly, for the NHC precatalyst L, reduction of the enantiomeric excess occurred along with a decrease in the substituent at the double bond. In particular, 3% ee was observed where the product 2p’ had a methyl substituent. This method represents a unique NHC-catalyzed asymmetric intramolecular Stetter reaction with the use of β,β-disubstituted Michael acceptors to access enantioenriched all-carbon quaternary 2,2-disubstituted coumaranones and naphthofuranones.

Table 3.
Scope of coumaranones and naphthofuranones a.
3. Materials and Methods
Reactions involving moisture-sensitive reagents were carried out under an argon atmosphere using standard vacuum line techniques. All glassware used was flame-dried and cooled under a vacuum. All solvents were dried using an Innovative Technologies PureSolv Solvent Purification System (INERT) and degassed via three freeze–pump–thaw cycles. All other commercial reagents were used as supplied without further purification, unless stated otherwise. The crude compounds were purified by a Combiflash Rf chromatography system (Teledyne Technologies, Inc., Thousand Oaks, CA, USA) unless specified otherwise. Analytical thin-layer chromatography was performed on pre-coated aluminum plates (Kieselgel 60 F254 silica). TLC visualization was carried out with ultraviolet light (254 nm), followed by staining with a 1% aqueous KMnO4 solution. NMR spectra were recorded on Bruker AMX 400 and 700 (Bruker, Karlruhe, Germany) spectrometers and referenced to the solvent residual peak. Elemental analyses were performed on a Vario MACRO CHN analyzer (Elementar Analysensysteme GmbH, Langenselbold, Germany). Optical rotations ([α]D) were measured on a PolAAr 3000 (Optical Activity Ltd., Cambridgeshire, UK) polarimeter. IR spectra were recorded on a Bruker Alfa spectrometer and are reported in terms of frequency of absorption cm−1. Mass spectra were collected on a Shimadzu HPLC Chromatograph/Mass Spectrometer LCMS-8030 (Shimadzu, Kyoto, Japan), (ESI, operating in positive mode). Enantiomeric excesses were determined by HPLC analysis on chiral stationary phase using 4.6 mm × 250 mm Phenomenex Lux Cellulose-1 and Luz Amylose-1 with n-hexane, 2-propanol as eluent.
All other reagents were purchased from commercial suppliers. Catalysts A–O were prepared according to the known method [44,45,46,47]. Salicylaldehyde-derived substrates 1a–1p were prepared by using two-step procedure (Scheme 1). The spectra of NMR and HPLC are in Supplementary Materials.
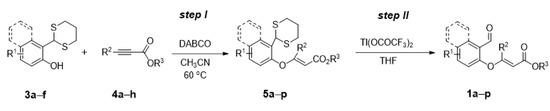
Scheme 1.
Synthesis of salicylaldehyde-derived substrates 1a–p.
3.1. General Procedure for the Preparation of Compounds 1a–p
Step I: To a solution of dithiane [52] (5.2 mmol) in acetonitrile (52 mL), DABCO (0.58 g, 5.2 mmol) was added. After stirring for 10 min, alkyne derivative (10.4 mmol, 2 equiv.) was added and the mixture was stirred at 60 °C overnight. After completion (monitored by TLC), most of the acetonitrile was evaporated, then water was added to the solution and the mixture was extracted with ethyl acetate. The combined ethyl acetate extract was washed with brine, dried over anhydrous MgSO4 and then concentrated under reduced pressure. The crude product was purified by flash chromatography.
Step II: To a solution of dithane (3.0 mmol) in tetrahydrofuran (25 mL), thallium trifluoroacetate (3.3 g, 6.0 mmol, 2 equiv.) was added and the resulting cloudy solution was stirred until reaction was judged to be complete by TLC. The reaction mixture was then filtered through a Cellite pad and the resulting solution was concentrated in vacuo. The desired product was purified by decanting from a solution of petroleum ether, or a mixture of pentane and diethyl ether.
Methyl (E)-3-(2-(1,3-dithian-2-yl)phenoxy)hex-2-enoate (5a). The title compound was prepared according to the general procedure (Step I) using 2-(1,3-dithian-2-yl)phenol (3a) (5.0 g, 21.2 mmol) and methyl hex-2-ynoate (4a). The product was purified by flash chromatography (petroleum ether/EtOAc 8:2) to give 5a (4.60 g, 64%) as a white solid. M.p. = 63–65 °C. 1H NMR (400 MHz, CDCl3) δ: 1.13 (t, J = 8.0 Hz, 3H), 1.81–1.90 (m, 2H), 1.92–1.99 (m, 1H), 2.15–2.20 (m, 1H), 2.88–2.90 (m, 1H), 2.92–2.94 (m, 1H), 3.00–3.07 (m, 4H), 3.63 (s, 3H), 4.85 (s, 1H), 5.30 (s, 1H), 6.97 (dd, J = 8.0, 4.0 Hz, 1H), 7.20 (dt, J = 7.2, 1.6 Hz, 1H), 7.33 (dt, J = 8.0, 2.0 Hz, 1H), 7.71 (dd, J = 7.6, 1.6 Hz, 1H). 13C NMR (100 MHz, CDCl3) δ: 13.9, 21.0, 25.1, 32.3 (2C), 33.0, 44.2, 50.8, 96.1, 122.1, 126.4, 129.7, 129.9, 131.6, 149.6, 167.6, 175.7. IR (ATR) ν (cm−1): 2958, 2933, 2896, 1710, 1632, 1484, 1432, 1373, 1276, 1246, 1129, 1088, 1041, 938, 830, 822, 750, 670. LRMS (ESI): Mass calcd. for [M + Na]+ C17H22O3S2Na: 361.1; found 361.2. Anal. Calcd. for C17H22O3S2: C, 60.32; H, 6.55; found: C, 60.37; H, 6.63.
Benzyl (E)-3-(2-(1,3-dithian-2-yl)-5-phenoxy)but-2-enoate (5b). The title compound was prepared according to the general procedure (Step I) using 2-(1,3-dithian-2-yl)phenol (3a) (5.0 g, 21.2 mmol) and benzyl hex-2-ynoate (4b). The product was purified by flash chromatography (petroleum ether/EtOAc 8:2) to give 5b (4.67 g, 53%) as a yellow oil. 1H NMR (400 MHz, CDCl3) δ: 1.16 (t, J = 8.0 Hz, 3H), 1.86–1.95 (m, 3H), 2.12–2.18 (m, 1H), 2.90 (dt, J = 13.6, 4.0 Hz, 2H), 2.99–3.09 (m, 4H), 4. 97 (s, 1H), 5.12 (s, 2H), 5.34 (s, 1H), 7.00 (dd, J = 8.0, 1.2 Hz, 1H), 7.26 (dt, J = 6.4, 1.2 Hz, 1H), 7.29–7.36 (m, 6H), 7.74 (dd, J = 7.6, 1.6 Hz, 1H). 13C NMR (100 MHz, CDCl3) δ: 14.0, 21.0, 25.2, 32.4 (2C), 33.2, 44.3, 65.5, 96.3, 122.1, 126.5, 128.0, 128.2 (2C), 128.5 (2C), 129.8, 130.0, 131.6, 136.4, 149.6, 167.1, 175.9. IR (ATR) ν (cm−1): 2959, 2932, 1709, 1629, 1484, 1451, 1383, 1258, 1223, 1121, 1090, 1023, 833, 732, 696. LRMS (ESI): Mass calcd. for [M + Na]+ C23H26O3S2Na: 437.1; found 437.2. Anal. Calcd. for C23H26O3S2: C, 66.63; H, 6.32; found: C, 66.71; H, 6.42.
Ethyl (E)-3-(2-(1,3-dithian-2-yl)phenoxy)pent-2-enoate (5d). The title compound was prepared according to the general procedure (Step I) using 2-(1,3-dithian-2-yl)phenol (3a) (5.0 g, 21.2 mmol) and ethyl pent-2-ynoate (4d). The product was purified by flash chromatography (petroleum ether/EtOAc 8:2) to give 5d (5.53 g, 77%) as a yellow oil. 1H NMR (400 MHz, CDCl3) δ: 1.22 (t, J = 7.2 Hz, 3H), 1.38 (t, J = 7.6 Hz, 3H), 1.88–1.98 (m, 1H), 2.14–2.19 (m, 1H), 2.88–2.93 (m, 2H), 3.00–3.07 (m, 4H), 4.10 (q, J = 7.2 Hz, 2H), 4.84 (s, 1H), 5.31 (s, 1H), 6.99 (dd, J = 8.4, 1.6 Hz, 1H), 7.25 (dt, J = 7.6, 1.2 Hz, 1H), 7.32 (dt, J = 7.6, 1.6 Hz, 1H), 7.71 (dd, J = 8.0, 1.6 Hz, 1H). 13C NMR (100 MHz, CDCl3) δ: 12.1, 14.3, 24.8, 25.1, 32.3 (2C), 44.3, 59.6, 95.8, 122.1, 126.4, 129.7, 129.9, 131.6, 149.7, 167.2, 176.7. IR (ATR) ν (cm−1): 2975, 2936, 1706, 1629, 1448, 1378, 1275, 1227, 1211, 1127, 1086, 1041, 1003, 832. LRMS (ESI): Mass calcd. for [M + Na]+ C17H22O3S2Na: 361.1; found 361.3. Anal. Calcd. for C17H22O3S2: C, 60.32; H, 6.55; found: C, 60.41; H, 6.50.
Methyl (E)-3-(2-(1,3-dithian-2-yl)phenoxy)oct-2-enoate (5e). The title compound was prepared according to the general procedure (Step I) using 2-(1,3-dithian-2-yl)phenol (3a) (5.0 g, 21.2 mmol) and methyl oct-2-ynoate (4e). The product was purified by flash chromatography (petroleum ether/EtOAc 8:2) to give 5e (3.96 g, 51%) as a yellow oil. 1H NMR (400 MHz, CDCl3) δ: 0.97 (t, J = 8.0 Hz, 3H), 1.41–1.52 (m, 4H), 1.78–1.86 (m, 2H), 1.88–1.98 (m, 1H), 2.14–2.19 (m, 1H), 2.87–2.93 (m, 2H), 2.99–3.06 (m, 4H), 3.62 (s, 3H), 4.84 (s, 1H), 5.30 (s, 1H), 6.97 (dd, J = 8.0, 4.0 Hz, 1H), 7.25 (dt, J = 8.0, 1.6 Hz, 1H), 7.31 (dt, J = 7.6, 1.6 Hz, 1H), 7.70 (dd, J = 7.6, 1.6 Hz, 1H). 13C NMR (100 MHz, CDCl3) δ: 14.0, 22.6, 25.1, 27.4, 31.2, 31.6, 32.3 (2C), 44.2, 50.9, 95.6, 122.1, 126.4, 129.7, 129.9, 131.6, 149.7, 167.7, 176.0. IR (ATR) ν (cm−1): 2953, 2928, 2854, 1711, 1634, 1428, 1367, 1244, 1213, 1134, 1093, 825. LRMS (ESI): Mass calcd. for [M + Na]+ C19H26O3S2Na: 389.1; found 389.3. Anal. Calcd. for C19H26O3S2: C, 58.59; H, 6.73; found: C, 58.66; H, 6.82.
Methyl (E)-3-(2-(1,3-dithian-2-yl)phenoxy)non-2-enoate (5f). The title compound was prepared according to the general procedure (Step I) using 2-(1,3-dithian-2-yl)phenol (3a) (5.0 g, 21.2 mmol) and methyl non-2-ynoate (4f). The product was purified by flash chromatography (petroleum ether/EtOAc 8:2) to give 5f (3.53 g, 41%) as a yellow oil. 1H NMR (400 MHz, CDCl3) δ: 0.93 (t, J = 8.0 Hz, 3H), 1.37–1.41 (m, 4H), 1.48–1.55 (m, 2H), 1.77–1.85 (m, 2H), 1.92–1.98 (m, 1H), 2.14–2.20 (m, 1H), 2.88–2.93 (m, 2H), 2.99–3.06 (m, 4H), 3.62 (s, 3H), 4.83 (s, 1H), 5.29 (s, 1H), 6.96 (dd, J = 8.0, 4.0 Hz, 1H), 7.25 (dt, J = 8.0, 1.6 Hz, 1H), 7.31 (dt, J = 7.6, 1.6 Hz, 1H), 7.70 (dd, J = 7.6, 1.6 Hz, 1H). 13C NMR (100 MHz, CDCl3) δ: 14.0, 22.6, 25.1, 27.4, 31.2, 31.6, 32.3 (2C), 44.2, 50.9, 95.6, 122.1, 126.4, 129.7, 129.9, 131.6, 149.7, 167.7, 176.0. IR (ATR) ν (cm−1): 2950, 2924, 2850, 1709, 1636, 1427, 1366, 1240, 1212, 1134, 1093, 825. LRMS (ESI): Mass calcd. for [M + Na]+ C20H28O3S2Na: 403.1; found 403.2. Anal. Calcd. for C20H28O3S2: C, 63.12; H, 7.42; found: C, 63.27; H, 7.58.
Methyl (E)-3-(2-(1,3-dithian-2-yl)phenoxy)-4-cyclohexylbut-2-enoate (5g). The title compound was prepared according to the general procedure (Step I) using 2-(1,3-dithian-2-yl)phenol (3a) (5.0 g, 21.2 mmol) and methyl 4-cyclohexylbut-2-ynoate (4g). The product was purified by flash chromatography (petroleum ether/EtOAc 8:2) to give 5g (3.53 g, 41%) as a yellow oil. 1H NMR (400 MHz, CDCl3) δ: 1.19–1.36 (m, 5H), 1.68–1.74 (m, 2H), 1.78–1.84 (m, 2H), 1.89–1.99 (m, 3H), 2.15–2.21 (m, 1H), 2.88–3.06 (m, 6H), 3.62 (s, 3H), 4.89 (s, 1H), 5.31 (s, 1H), 6.97 (dd, J = 8.0, 1.6 Hz, 1H), 7.26 (dt, J = 7.6, 1.6 Hz, 1H), 7.30 (dt, J = 7.6, 1.6 Hz, 1H), 7.72 (dd, J = 7.6, 1.6 Hz, 1H). 13C NMR (100 MHz, CDCl3) δ: 25.1, 26.4, 26.4 (2C), 32.3 (2C), 33.0 (2C), 36.7, 38.4, 44.1, 50.8, 96.4, 122.1, 126.4, 129.8, 130.0, 131.6, 149.6, 167.7, 174.7. IR (ATR) ν (cm−1): 2920, 2850, 1713, 1630, 1447, 1430, 1373, 1274, 1258, 1194, 1170, 1134, 1104, 1040, 830. LRMS (ESI): Mass calcd. for [M + Na]+ C21H28O3S2Na: 415.1; found 415.3. Anal. Calcd. for C21H28O3S2: C, 64.25; H, 7.19; found: C, 64.31; H, 7.27.
Methyl (E)-3-(2-(1,3-dithian-2-yl)phenoxy)-5-phenylpent-2-enoate (5h). The title compound was prepared according to the general procedure (Step I) using 2-(1,3-dithian-2-yl)phenol (3a) (5.0 g, 21.2 mmol) and methyl 5-phenylpent-2-ynoate (4h). The product was purified by flash chromatography (petroleum ether/EtOAc 8:2) to give 5h (3.65 g, 43%) as a yellow solid. M.p. = 97–99 °C. 1H NMR (700 MHz, CDCl3) δ: 1.90 (m, 1H), 2.13 (dspt, J = 14.2, 2.4 Hz, 1H), 2.87 (ddd, J = 14.6, 3.2, 0.9 Hz, 2H), 2.97 (ddd, J = 14.7, 12.5, 2.5 Hz, 2H), 3.10–3.14 (m, 2H), 3.28–3.40 (m, 2H), 3.63 (s, 3H), 4.86 (s, 1H), 5.24 (s, 1H), 6.87 (dd, J = 8.0, 1.3 Hz, 1H), 7.22–7.26 (m, 2H), 7.28–7.31 (m, 1 H), 7.32–7.35 (m, 2H), 7.36–7.39 (m, 2H), 7.70 (dd, J = 7.7, 1.7 Hz, 1H). 13C NMR (176 MHz, CDCl3) δ: 24.68, 31.86, 32.59, 33.18, 43.75, 50.60, 96.11, 121.64, 125.82, 126.14, 128.09, 128.19, 129.40, 129.60, 131.21, 140.49, 149.22, 167.16, 174.33. IR (ATR) ν (cm−1): 2920, 2850, 1713, 1630, 1447, 1430, 1373, 1274, 1258, 1194, 1170, 1134, 1104, 1040, 830. LRMS (ESI): Mass calcd. for [M + Na]+ C22H24O3S2Na: 423.1; found 423.0. Anal. Calcd. for C22H24O3S2: C, 65.97; H, 6.04; found: C, 66.01 H, 6.11.
Methyl (E)-3-(2-(1,3-dithian-2-yl)-5-methylphenoxy)but-2-enoate (5i). The title compound was prepared according to the general procedure (Step I) using 2-(1,3-dithian-2-yl)-5-methylphenol (3b) (5.0 g, 21.2 mmol) and methyl but-2-ynoate (4c). The product was purified by flash chromatography (petroleum ether/EtOAc 8:2) to give 5i (6.17 g, 85%) as a yellow solid. M.p. = 108–109 °C. 1H NMR (400 MHz, CDCl3) δ: 1.88–1.99 (m, 1H), 2.15–2.22 (m, 1H), 2.36 (s, 3H), 2.55 (s, 3H), 2.88–2.93 (m, 2H), 3.02–3.10 (m, 2H), 3.64 (s, 3H), 4.96 (s, 1H), 5.24 (s, 1H), 6.86 (d, J = 8.4 Hz, 1H), 7.10–7.12 (m, 1H), 7.50 (d, J = 2.0 Hz, 1H). 13C NMR (100 MHz, CDCl3) δ: 18.2, 20.8, 25.2, 32.4 (2C), 44.3, 50.9, 96.6, 121.8, 130.3, 130.4, 131.0, 136.3, 147.4, 168.0, 172.4. IR (ATR) ν (cm−1): 2949, 2927, 2822, 1706, 1629, 1493, 1434, 1383, 1340, 1257, 1122, 1094, 1041, 1004, 933, 876, 822. LRMS (ESI): Mass calcd. for [M + Na]+ C16H20O3S2Na: 347.1; found 347.2. Anal. calcd. for C16H20O3S2: C, 59.23; H, 6.21; found: C, 59.31; H, 6.34.
Methyl (E)-3-(4-chloro-2-(1,3-dithian-2-yl)phenoxy)hex-2-enoate (5j). The title compound was prepared according to the general procedure (Step I) using 4-chloro-2-(1,3-dithian-2-yl)phenol (3c) (5.0 g, 21.2 mmol) and methyl hex-2-ynoate (4a). The product was purified by flash chromatography (petroleum ether/EtOAc 8:2) to give 5j (6.17 g, 85%) as a white solid. M.p. 103–104 °C. 1H NMR (400 MHz, CDCl3) δ: 1.11 (t, J = 7.2 Hz, 3H), 1.78–1.87 (m, 2H), 1.91–1.99 (m, 1H), 2.14–2.20 (m, 1H), 2.86–2.94 (m, 2H), 2.97–3.05 (m, 4H), 3.65 (s, 3H), 4.84 (s, 1H), 5.23 (s, 1H), 6.92 (d, J = 8.8 Hz, 1H), 7.28 (dd, J = 8.4, 2.4 Hz, 1H), 7.69 (d, J = 2.4 Hz, 1H). 13C NMR (100 MHz, CDCl3) δ: 13.8, 21.0, 25.0, 32.2 (2C), 32.9, 43.7, 51.0, 96.5, 123.4, 129.9, 130.0, 131.8, 133.4, 148.2, 167.4, 175.4. IR (ATR) ν (cm−1): 2954, 2888, 1711, 1625, 1479, 1436, 1370, 1226, 1210, 1126, 1102, 1080, 1037, 936, 828. LRMS (ESI): Mass calcd. for [M + Na]+ C17H21ClO3S2Na: 395.0; found 395.1. Anal. Calcd. for C17H21ClO3S2: C, 54.75; H, 5.68; found: C, 54.88; H, 5.72.
Methyl (E)-3-(4-bromo-2-(1,3-dithian-2-yl)phenoxy)hex-2-enoate (5k). The title compound was prepared according to the general procedure (Step I) using 4-bromo-2-(1,3-dithian-2-yl)phenol (3d) (5.0 g, 21.2 mmol) and methyl hex-2-ynoate (4a). The product was purified by flash chromatography (petroleum ether/EtOAc 8:2) to give 5k (6.19 g, 70%) as a white solid. M.p. 87–89 °C. 1H NMR (700 MHz, CDCl3) δ: 1.12 (t, J = 7.7 Hz, 3H), 1.82–1.97 (m, 1H), 2.18–2.20 (m, 1H), 2.92–2.95 (m, 2H), 3.00–3.05 (m, 4H), 3.65 (s, 3H), 4.85 (s, 1H), 5.24 (s, 1H), 6.87 (d, J = 8.4 Hz, 1H), 7.44–7.46 (m, 1H), 7.85 (d, J = 2.1 Hz, 1H). 13C NMR (176 MHz, CDCl3) δ: 14.9, 22.0, 26.0, 33.2 (2C), 33.9, 44.6, 52.0, 97.6, 120.4, 124.8, 133.8, 134.0, 134.8, 149.8, 168.4, 176.4. IR (ATR) ν (cm−1): 2957, 2933, 1710, 1626, 1477, 1436, 1369, 1226, 1209, 1125, 1102, 1079, 1035, 934, 829. LRMS (ESI): Mass calcd. for [M + Na]+ C17H21BrO3S2Na: 439.0; found 439.1. Anal. Calcd. for C17H21BrO3S2: C, 49.92; H, 5.07; found: C, 50.02; H, 5.17.
Methyl (E)-3-(2-(1,3-dithian-2-yl)-4-(trifluoromethoxy)phenoxy)hex-2-enoate (5l). The title compound was prepared according to the general procedure (Step I) using 4-trifluoromethoxy-2-(1,3-dithian-2-yl)phenol (3e) (5.0 g, 21.2 mmol) and methyl hex-2-ynoate (4a). The product was purified by flash chromatography (petroleum ether/EtOAc 8:2) to give 5l (3.77 g, 42%) as a white solid. M.p. 57–60 °C. 1H NMR (700 MHz, CDCl3) δ: 1.13 (t, J = 7.7 Hz, 3H), 1.82–1.88 (m, 2H), 1.92–1.96 (m, 1H), 2.17–2.20 (m, 1H), 2.91–2.94 (m, 2H), 3.00–3.06 (m, 4H), 3.65 (s, 3H), 4.86 (s, 1H), 5.27 (s, 1H), 7.01 (d, J = 8.4 Hz, 1H), 7.18–7.20 (m, 1H), 7.59 (d, J = 2.1 Hz, 1H). 13C NMR (176 MHz, CDCl3) δ: 14.8, 22.0, 25.9, 33.1 (2C), 33.9, 44.6, 52.0, 97.6, 121.0 (q, J = 258 Hz), 123.2, 123.7, 124.3, 134.6, 147.8, 148.9, 168.4, 176.4. IR (ATR) ν (cm−1): 2959, 2934, 2901, 1714, 1634, 1490, 1434, 1372, 1255, 1208, 1164, 1128, 1093, 1038, 937, 883, 834. LRMS (ESI): Mass calcd. for [M + Na]+ C18H21F3O4S2Na: 445.1; found 445.1. Anal. Calcd. for C18H21F3O4S2: C, 51.17; H, 5.01; found: C, 51.27; H, 5.10.
Methyl (E)-3-(4-fluoro-2-(1,3-dithian-2-yl)phenoxy)hex-2-enoate (5m). The title compound was prepared according to the general procedure (Step I) using 4-fluoro-2-(1,3-dithian-2-yl)phenol (3g) (5.0 g, 21.1 mmol) and methyl hex-2-ynoate (4a). The product was purified by flash chromatography (petroleum ether/EtOAc 8:2) to give 5m (3.56 g, 47%) as a white solid. M.p. 75–78 °C. 1H NMR (700 MHz, CDCl3) δ: 1.11 (t, J = 7.2 Hz, 3H), 1.78–1.87 (m, 2H), 1.91–1.99 (m, 1H), 2.14–2.20 (m, 1H), 2.86–2.94 (m, 2H), 2.97–3.05 (m, 4H), 3.65 (s, 3H), 4.84 (s, 1H), 5.23 (s, 1H), 6.92 (d, J = 8.8 Hz, 1H), 7.28 (dd, J = 8.4, 2.4 Hz, 1H), 7.69 (d, J = 2.4 Hz, 1H). 13C NMR (100 MHz, CDCl3) δ: 13.8, 21.0, 25.0, 32.2 (2C), 32.9, 43.7, 51.0, 96.5, 123.4, 129.9, 130.0, 131.8, 133.4, 148.2, 167.4, 175.4. IR (ATR) ν (cm−1): 2956, 2915, 1714, 1622, 1490, 1432, 1221, 1206, 1163, 1125, 1085, 1071, 1036, 1002, 965, 933, 874, 831. LRMS (ESI): Mass calcd. for [M + Na]+ C17H21FO3S2Na: 379.1; found 379.2. Anal. Calcd. for C17H21FO3S2: C, 57.28; H, 5.94; found: C, 57.40; H, 5.99.
Methyl (E)-3-((1-(1,3-dithian-2-yl)naphthalen-2-yl)oxy)-5-phenylpent-2-enoate (5n). The title compound was prepared according to the general procedure (Step I) using 1-(1,3-dithian-2-yl)naphthalen-2-ol (3f) (5.0 g, 19.1 mmol) and methyl 5-phenylpent-2-ynoate (4h). The product was purified by flash chromatography (petroleum ether/EtOAc 8:2) to give 5n (3.93 g, 53%) as a white solid. M.p. 140–144 °C. 1H NMR (400 MHz, CDCl3) δ: 2.05–2.10 (m, 1H), 2.21–2.25 (m, 1H), 2.97 (t, J = 2.0 Hz, 1H), 2.99 (t, J = 2.0 Hz, 1H), 3.05–3.08 (m, 2H), 3.23–3.25 (m, 2H), 3.44–3.46 (m, 2H), 3.65 (s, 3H), 4.90 (s, 1H), 5.96 (s, 1H), 7.03 (d, J = 5.3 Hz, 1H), 7.30 (t, J = 4.4 Hz, 1H), 7.39–7.41 (m, 2H), 7.46 (m, 2H), 7.53–7.55 (m, 1H), 7.63–7.65 (m, 1H), 7.82 (d, J = 5.2 Hz, 1H), 7.85 (d, J = 4.4 Hz, 1H), 9.24 (d, J = 4.8 Hz, 1H). 13C NMR (100 MHz, CDCl3) δ: 25.7, 33.0, 33.2 (2C), 33.8, 45.4, 51.0, 97.4, 120.7, 125.7, 126.0, 126.1, 126.3, 127.4, 128.4, 128.5 (2C), 128.6 (2C), 130.9, 132.4, 132.5, 140.8, 147.0, 167.5, 174.2. IR (ATR) ν (cm−1): 3026, 2980, 2899, 1710, 1632, 1432, 1365, 1232, 1215, 1185, 1161, 1150, 1113, 1046, 1008, 931, 838, 828. LRMS (ESI): Mass calcd. for [M + Na]+ C26H26O3S2Na: 473.1; found 473.3. Anal. Calcd. for C26H26O3S2: C, 69.30; H, 5.82; found: C, 69.41; H, 5.87.
Methyl (E)-3-((1-(1,3-dithian-2-yl)naphthalen-2-yl)oxy)hex-2-enoate (5o). The title compound was prepared according to the general procedure (Step I) using 1-(1,3-dithian-2-yl)naphthalen-2-ol (3f) (5.0 g, 19.1 mmol) and methyl hex-2-ynoate (4a). The product was purified by flash chromatography (petroleum ether/EtOAc 8:2) to give 5o (2.37 g, 32%) as a yellow solid. M.p. 108–109 °C. 1H NMR (700 MHz, CDCl3) δ: 1.19 (t, J = 7.7 Hz, 3H), 1.95–1.97 (m, 2H), 2.08–2.12 (m, 1H), 2.26–2.28 (m, 1H), 2.98–3.02 (m, 2H) 3.08–3.13 (m, 4H), 3.62 (s, 3H), 4.86 (s, 1H), 5.97 (s, 1H), 7.11 (d, J = 9.1 Hz, 1H), 7.51–7.54 (m, 1H), 7.61–7.63 (m, 1H), 7.82 (d, J = 8.4 Hz, 1H), 7.84 (d, J = 8.4 Hz, 1H), 9.20 (d, J = 8.4 Hz, 1H). 13C NMR (100 MHz, CDCl3) δ: 15.0, 22.2, 26.7, 34.0, 34.2 (2C), 46.4, 51.9, 98.0, 121.8, 126.7, 127.0, 127.1, 128.4, 129.4, 131.9, 133.4, 133.5, 148.0, 168.6, 176.2. IR (ATR) ν (cm−1): 2967, 2939, 1710, 1625, 1509, 1431, 1368, 1273, 1257, 1129, 1080, 830. LRMS (ESI): Mass calcd. for [M + Na]+ C21H24O3S2Na: 411.1; found 411.2. Anal. Calcd. for C21H24O3S2: C, 64.92; H, 6.23; found: C, 65.01; H, 6.37.
Methyl (E)-3-((3-(1,3-dithian-2-yl)naphthalen-2-yl)oxy)but-2-enoate (5p). The title compound was prepared according to the general procedure (Step I) using 1-(1,3-dithian-2-yl)naphthalen-2-ol (3f) (5.0 g, 19.1 mmol) and methyl but-2-ynoate (4c). The product was purified by flash chromatography (petroleum ether/EtOAc 8:2) to give 5p (3.02 g, 44%) as a yellow oil. 1H NMR (400 MHz, CDCl3) δ: 2.03–2.13 (m, 1H), 2.21–2.29 (m, 1H), 2.62 (s, 3H), 2.96–3.02 (m, 2H), 3.10–3.16 (m, 2H), 3.63 (s, 3H), 4.96 (s, 1H), 5.94 (s, 1H), 7.12 (d, J = 8.8 Hz, 1H), 7.49–7.53 (m, 1H), 7.59–7.63 (m, 1H), 7.80–7.84 (m, 2H), 9.21 (d, J = 8.4 Hz, 1H). 13C NMR (100 MHz, CDCl3) δ: 18.2, 25.7, 33.3 (2C), 45.5, 50.9, 97.7, 120.8, 125.6, 125.9, 126.0, 127.4, 128.4, 130.8, 132.3, 132.4, 147.2, 167.9, 171.6. IR (ATR) ν (cm−1): 2951, 2908, 1706, 1638, 1620, 1508, 1345, 1248, 1213, 1144, 1128, 1016, 930 856, 831. LRMS (ESI): Mass calcd. for [M + Na]+ C19H20O3S2Na: 383.1; found 383.2. Anal. Calcd. for C19H20O3S2: C, 63.31; H, 5.59; found: C, 63.40; H, 5.70.
Methyl (E)-3-(2-formylphenoxy)hex-2-enoate (1a). The title compound was prepared according to the general procedure (Step II) using methyl (E)-3-(2-(1,3-dithian-2-yl)phenoxy)hex-2-enoate (5a) (1.0 g, 3.0 mmol) and thallium (III) trifluoroacetate. The product was obtained as a yellow oil in 94% yield (0.70 g). 1H NMR (400 MHz, CDCl3) δ: 1.09 (t, J = 8.0 Hz, 3H), 1.77–1.86 (m, 2H), 2.99–3.03 (m, 2H), 3.62 (s, 3H), 4.81 (s, 1H), 7.10 (dd, J = 8.0, 0.8 Hz, 1H), 7.35–7.39 (m, 1H), 7.65 (dddd, J = 15.6, 9.2, 7.6, 2.0 Hz, 1H), 7.95 (dd, J = 8.0, 2.0 Hz, 1H), 10.16 (d, J = 0.8 Hz, 1H). 13C NMR (100 MHz, CDCl3) δ: 13.9, 20.8, 33.0, 51.0, 97.3, 122.8, 126.2, 128.4, 129.0, 135.9, 155.7, 167.0, 176.4, 188.1. IR (ATR) ν (cm−1): 2951, 2929, 1715, 1696, 1629, 1600, 1455, 1434, 1371, 1236, 1187, 1097, 1042, 833. LRMS (ESI): Mass calcd. for [M + Na]+ C14H16O4Na: 271.1; found 271.2. Anal. Calcd. for C14H16O4: C, 67.73; H, 6.50; found: C, 67.82; H, 6.66.
Benzyl (E)-3-(2-formylphenoxy)hex-2-enoate (1b). The title compound was prepared according to the general procedure (Step II) using benzyl (E)-3-(2-(1,3-dithian-2-yl)-5-phenoxy)but-2-enoate (5b) (1.2 g, 3.0 mmol) and thallium (III) trifluoroacetate. The product was obtained as a yellow oil in 90% yield (0.70 g). 1H NMR (700 MHz, CDCl3) δ: 1.08 (t, J = 7.4 Hz, 4H), 1.81 (sxtt, J = 7.6, 1.7 Hz, 2H), 2.99–3.03 (m, 2H), 4.84 (s, 1H), 5.07 (s, 2H), 7.08 (dd, J = 8.1, 1.0 Hz, 1H), 7.29–7.34 (m, 6H), 7.63 (ddd, J = 8.2, 7.3, 1.7 Hz, 1H), 7.94 (ddd, J = 7.7, 1.8, 0.3 Hz, 1H), 10.15 (d, J = 0.8 Hz, 1H). 13C NMR (176 MHz, CDCl3) δ: 13.6, 20.5, 32.8, 65.4, 96.9, 122.5, 125.9, 127.8, 128.0, 128.2, 128.7, 135.6, 135.7, 155.2, 166.1, 176.4, 187.6, 187.7. IR (ATR) ν (cm−1): 2946, 2932, 1711, 1692, 1633, 1598, 1455, 1430, 1373, 1235, 1189, 1100, 1042, 833. LRMS (ESI): Mass calcd. for [M + Na]+ C20H20O4Na: 347.1; found 347.2. Anal. Calcd. for C20H20O4: C, 74.06; H, 6.22; found: C, 74.15; H, 6.34.
Methyl (E)-3-(2-formylphenoxy)but-2-enoate (1c). The title compound was prepared according to the general procedure (Step II) using methyl (E)-3-(2-(1,3-dithian-2-yl)phenoxy)but-2-enoate (5c) (0.93 g, 3.0 mmol) and thallium (III) trifluoroacetate. The product was obtained as a yellow oil in 95% yield (0.63 g). 1H NMR (700 MHz, CDCl3) δ: 2.57 (d, J = 0.6 Hz, 3H), 3.63 (s, 3H), 4.84 (d, J = 0.6 Hz, 1H), 7.11 (dd, J = 8.2, 1.0 Hz, 1H), 7.36–7.39 (m, 1H), 7.65 (ddd, J = 8.1, 7.4, 1.7 Hz, 1H), 7.94 (dd, J = 7.7, 1.8 Hz, 1H), 10.12–10.14 (m, 1H). 13C NMR (176 MHz, CDCl3) δ: 17.7, 50.7, 97.2, 122.4, 126.0, 127.8, 128.9, 135.5, 155.0, 166.9, 172.5, 187.7, 187.8. IR (ATR) ν (cm−1): 2946, 1713, 1694, 1636, 1602, 1457, 1428, 1375, 1232, 1189, 1100, 1045, 834. LRMS (ESI): Mass calcd. for [M + Na]+ C12H12O4Na: 243.1; found 243.2. Anal. Calcd. for C12H12O4: C, 65.45; H, 6.49; found: C, 65.54; H, 6.48.
Ethyl (E)-3-(2-formylphenoxy)pent-2-enoate (1d). The title compound was prepared according to the general procedure (Step II) using ethyl (E)-3-(2-(1,3-dithian-2-yl)phenoxy)pent-2-enoate (5d) (1.0 g, 3.0 mmol) and thallium (III) trifluoroacetate. The product was obtained as a yellow oil in 92% yield (0.68 g). 1H NMR (700 MHz, CDCl3) δ: 1.19–1.22 (m, 3H), 1.31–1.35 (m, 3H), 3.03 (q, J = 7.5 Hz, 2H), 4.08 (q, J = 7.1 Hz, 2H), 4.74 (s, 1H), 7.10 (dd, J = 8.2, 0.9 Hz, 1H), 7.35–7.39 (m, 1H), 7.65 (ddd, J = 8.2, 7.3, 1.7 Hz, 1H), 7.95 (dd, J = 7.7, 1.7 Hz, 1H), 10.16 (d, J = 0.9 Hz, 1H). 13C NMR (176 MHz, CDCl3) δ: 11.4, 13.8, 24.4, 59.4, 96.6, 122.4, 125.9, 128.0, 128.5, 135.6, 155.4, 166.2, 177.1, 187.8. IR (ATR) ν (cm−1): 2940, 1710, 1691, 1629, 1600, 1453, 1428, 1373, 1232, 1189, 1110, 1045, 834. LRMS (ESI): Mass calcd. for [M + Na]+ C14H16O4Na: 271.1; found 271.1. Anal. Calcd. for C14H16O4: C, 67.73; H, 6.50; found: C, 67.70; H, 6.59.
Methyl (E)-3-(2-formylphenoxy)oct-2-enoate (1e). The title compound was prepared according to the general procedure (Step II) using methyl (E)-3-(2-(1,3-dithian-2-yl)phenoxy)oct-2-enoate (5e) (1.0 g, 2.72 mmol) and thallium (III) trifluoroacetate. The product was obtained as a yellow oil in 85% yield (0.64 g). 1H NMR (700 MHz, CDCl3) δ: 0.93 (t, J = 7.2 Hz, 3H), 1.37–1.47 (m, 4H), 1.74–1.79 (m, 2H), 2.98–3.02 (m, 2H), 3.61 (s, 3H), 4.78 (s, 1H), 7.08 (dd, J = 8.2, 1.0 Hz, 1H), 7.35–7.38 (m, 1H), 7.64 (ddd, J = 8.2, 7.4, 1.8 Hz, 1H), 7.95 (dd, J = 7.8, 1.8 Hz, 1H), 10.15 (d, J = 0.9 Hz, 1H). 13C NMR (101 MHz, CDCl3) δ: 14.0, 22.4, 27.1, 31.2, 31.6, 51.0, 97.1, 122.8, 126.3, 128.4, 128.9, 135.9, 155.8, 167.0, 176.8, 188.1. IR (ATR) ν (cm−1): 2951, 2929, 2857, 1715, 1696, 1629, 1600, 1455, 1434, 1371, 1272, 1236, 1187, 1110, 1045, 833. LRMS (ESI): Mass calcd. for [M + Na]+ C16H20O4Na: 299.1; found 299.1. Anal. Calcd. for C14H16O4: C, 69.55; H, 7.30; found: C, 69.60; H, 7.42.
Methyl (E)-3-(2-formylphenoxy)non-2-enoate (1f). The title compound was prepared according to the general procedure (Step II) using methyl (E)-3-(2-(1,3-dithian-2-yl)phenoxy)non-2-enoate (5f) (1.0 g, 2.63 mmol) and thallium (III) trifluoroacetate. The product was obtained as a yellow oil in 82% yield (0.63 g). 1H NMR (400 MHz, CDCl3) δ: 0.93 (t, J = 7.1 Hz, 3H), 1.35–1.40 (m, 4H), 1.43–1.53 (m, 2H), 1.73–1.82 (m, 2H), 2.99–3.05 (m, 2H), 3.63 (s, 3H), 4.80 (s, 1H), 7.10 (dd, J = 8.1, 1.0 Hz, 1H), 7.35–7.40 (m, 1H), 7.66 (ddd, J = 8.2, 7.3, 1.8 Hz, 1H), 7.96 (dd, J = 7.8, 1.7 Hz, 1H), 10.17 (d, J = 0.7 Hz, 1H). 13C NMR (101 MHz, CDCl3) δ: 14.0, 22.6, 27.4, 29.1, 31.2, 31.6, 51.0, 97.2, 122.8, 126.2, 128.4, 129.0, 135.9, 155.8, 167.0, 176.8, 188.1. IR (ATR) ν (cm−1): 2950, 2929, 2855, 1717, 1694, 1632, 1602, 1455, 1434, 1374, 1272, 1236, 1187, 1113, 1045, 833. LRMS (ESI): Mass calcd. for [M + Na]+ C17H22O4Na: 313.1; found 313.2. Anal. Calcd. for C17H22O4: C, 70.32; H, 7.64; found: C, 70.28; H, 7.74.
Methyl (E)-4-cyclohexyl-3-(2-formylphenoxy)but-2-enoate (1g). The title compound was prepared according to the general procedure (Step II) methyl (E)-3-(2-(1,3-dithian-2-yl)phenoxy)-4-cyclohexylbut-2-enoate (5g) (1.0 g, 2.55 mmol) and thallium (III) trifluoroacetate. The product was obtained as a yellow oil in 87% yield (0.67 g). 1H NMR (700 MHz, CDCl3) δ: 0.90 (t, J = 7.0 Hz, 3H), 1.13–1.30 (m, 5H), 1.65–1.89 (m, 5H), 2.93 (d, J = 7.0 Hz, 2H), 3.60 (s, 3H), 4.85 (s, 1H), 7.07 (d, J = 7.0, 1H), 7.34 (m, 1H), 7.63 (m, 1H), 7.93 (dd, J = 14.0, 7.0 Hz, 1H), 10.01 (s, 1H). 13C NMR (176 MHz, CDCl3) δ: 25.8 (2C), 25.9, 32.7 (2C), 36.2, 37.8, 50.6, 97.8, 122.3, 125.8, 127.9, 128.5, 135.5, 155.4, 166.7, 175.0, 187.8. IR (ATR) ν (cm−1): 2950, 2929, 1710, 1691, 1632, 1602, 1457, 1435, 1372, 1274, 1236, 1187, 1113, 1047, 830. LRMS (ESI): Mass calcd. for [M + Na]+ C18H22O4Na: 325.1; found 325.3. Anal. Calcd. for C18H22O4: C, 71.50; H, 7.33; found: C, 71.56; H, 7.45.
Methyl (E)-3-(2-formylphenoxy)-5-phenylpent-2-enoate (1h). The title compound was prepared according to the general procedure (Step II) methyl (E)-3-(2-(1,3-dithian-2-yl)phenoxy)-5-phenylpent-2-enoate (5h) (1.0 g, 2.50 mmol) and thallium (III) trifluoroacetate. The product was obtained as a yellow oil in 93% yield (0.72 g). 1H NMR (400 MHz, CDCl3) δ: 3.11 (dd, J = 9.0, 6.6 Hz, 8H), 3.36 (dd, J = 9.8, 6.6 Hz, 8H), 3.65 (s, 3H), 4.81 (s, 1H), 6.97 (dd, J = 8.1, 1.0 Hz, 1H), 7.23–7.28 (m, 1H), 7.33–7.40 (m, 5H), 7.64 (ddd, J = 8.2, 7.3, 1.8 Hz, 1H), 7.95 (dd, J = 7.8, 1.7 Hz, 1H), 10.00 (d, J = 0.7 Hz, 1H). 13C NMR (101 MHz, CDCl3) δ: 33.0, 33.4, 51.1, 97.6, 122.8, 126.3, 126.4, 128.5, 128.6, 129.1, 136.0, 140.5, 155.5, 166.9, 175.5, 188.1. IR (ATR) ν (cm−1): 2954, 2932, 1714, 1689, 1634, 1600, 1455, 1433, 1373, 1272, 1236, 1187, 1111, 1044, 833. LRMS (ESI): Mass calcd. for [M + Na]+ C19H18O4Na: 333.1; found 333.1. Anal. Calcd. for C19H18O4: C, 73.53; H, 5.85; found: C, 73.61; H, 5.89.
Methyl (E)-3-(2-formyl-5-methoxyphenoxy)but-2-enoate (1i). The title compound was prepared according to the general procedure (Step II) methyl (E)-3-(2-(1,3-dithian-2-yl)-5-methoxyphenoxy)but-2-enoate (5i) (1.0 g, 2.94 mmol) and thallium (III) trifluoroacetate. The product was obtained as a yellow oil in 95% yield (0.70 g). 1H NMR (400 MHz, CDCl3) δ: 2.42 (s, 3H), 2.57 (d, J = 0.5 Hz, 3H), 3.64 (s, 3H), 4.84 (d, J = 0.5 Hz, 1H), 7.01 (d, J = 8.3 Hz, 1H), 7.41–7.50 (m, 1H), 7.74 (d, J = 2.2 Hz, 1H), 10.10 (s, 1H). 13C NMR (101 MHz, CDCl3) δ: 18.1, 20.7, 51.0, 97.3, 122.7, 127.8, 129.3, 136.4, 136.7, 153.3, 167.4, 173.2, 188.4. IR (ATR) ν (cm−1): 2955, 2931, 1711, 1691, 1632, 1601, 1456, 1433, 1373, 1269, 1234, 1187, 1113, 1045, 834. LRMS (ESI): Mass calcd. for [M + Na]+ C13H14O5Na: 273.1; found 273.1. Anal. Calcd. for C13H14O5: C, 62.39; H, 5.64; found: C, 62.47; H, 5.72.
Methyl (E)-3-(4-chloro-2-formylphenoxy)hex-2-enoate (1j). The title compound was prepared according to the general procedure (Step II) methyl (E)-3-(4-chloro-2-(1,3-dithian-2-yl)phenoxy)hex-2-enoate (5j) (1.0 g, 2.68 mmol) and thallium (III) trifluoroacetate. The product was obtained as a yellow oil in 91% yield (0.69 g). 1H NMR (400 MHz, CDCl3) δ: 1.08 (t, J = 7.0 Hz, 3H), 1.77 (m, 2H), 2.97–2.99 (m, 2H), 3.62 (s, 3H), 4.80 (s, 1H), 7.03 (d, J = 7.0 Hz, 1H), 7.58 (dd, J = 9.1, 3.5 Hz, 1H), 7.89 (d, J = 2.1 Hz, 1H), 10.07 (s, 1H). 13C NMR (176 MHz, CDCl3) δ: 13.4, 20.4, 32.5, 50.7, 97.4, 123.9, 128.2, 128.9, 131.9, 131.8, 135.4, 153.7, 166.4, 175.8, 186.4. IR (ATR) ν (cm−1): 2953, 2930, 1705, 1690, 1632, 1600, 1456, 1433, 1373, 1265, 1230, 1187, 1113, 1045, 833. LRMS (ESI): Mass calcd. for [M + Na]+ C14H15ClO4Na: 305.1; found 305.2. Anal. Calcd. for C14H15ClO4: C, 59.48; H, 5.35; found: C, 59.56; H, 5.41.
Methyl (E)-3-(4-bromo-2-formylphenoxy)hex-2-enoate (1k). The title compound was prepared according to the general procedure (Step II) methyl (E)-3-(4-bromo-2-(1,3-dithian-2-yl)phenoxy)hex-2-enoate (5k) (1.0 g, 2.40 mmol) and thallium (III) trifluoroacetate. The product was obtained as a yellow oil in 90% yield (0.70 g). 1H NMR (400 MHz, CDCl3) δ: 1.08 (t, J = 7.3 Hz, 3H), 1.80 (dq, J = 15.0, 7.5 Hz, 1H), 2.97–3.03 (m, 2H), 4.83 (s, 1H), 3.65 (s, 3H), 7.01 (d, J = 8.6 Hz, 1H), 7.75 (dd, J = 8.7, 2.6 Hz, 1H), 8.07 (d, J = 2.4 Hz, 1H),10.09 (s, 1H). 13C NMR (101 MHz, CDCl3) δ: 13.8, 20.8, 32.9, 51.1, 97.9, 119.7, 124.6, 129.6, 131.7, 138.7, 154.6, 166.8, 176.1, 186.7. IR (ATR) ν (cm−1): 2953, 1701, 1692, 1632, 1600, 1456, 1433, 1371, 1263, 1232, 1184, 1110, 1044, 833. LRMS (ESI): Mass calcd. for [M + Na]+ C14H15BrO4Na: 349.0; found 349.1. Anal. Calcd. for C14H15BrO4: C, 51.40; H, 4.62; found: C, 51.48; H, 4.70.
Methyl (E)-3-(2-formyl-4-(trifluoromethoxy)phenoxy)hex-2-enoate (1l). The title compound was prepared according to the general procedure (Step II) methyl (E)-3-(2-(1,3-dithian-2-yl)-4-(trifluoromethoxy)phenoxy)hex-2-enoate (5l) (1.0 g, 2.37 mmol) and thallium (III) trifluoroacetate. The product was obtained as a yellow oil in 85% yield (0.67 g). 1H NMR (400 MHz, CDCl3) δ: 1.10 (t, J = 7.3 Hz, 3H), 1.81 (sxt, J = 7.5 Hz, 1H), 2.97–3.04 (m, 2H), 3.66 (s, 3H), 4.85 (s, 1H), 7.17 (d, J = 8.8 Hz, 1H), 7.50 (ddd, J = 8.9, 3.1, 0.7 Hz, 1H), 7.80 (dd, J = 2.9, 1.0 Hz, 1H), 10.13 (s, 1H), 13C NMR (101 MHz, CDCl3) δ: 13.8, 20.8, 32.9, 51.1, 98.1, 120.7, 124.4, 128.3, 129.4, 146.7–146.8 (m), 153.8, 166.7, 176.0, 186.5. IR (ATR) ν (cm−1): 2950, 1704, 1690, 1635, 1603, 1458, 1431, 1370, 1262, 1231, 1184, 1110, 1044, 831. LRMS (ESI): Mass calcd. for [M + Na]+ C15H15F3O5Na: 355.1; found 355.1. Anal. Calcd. for C15H15F3O5: C, 54.22; H, 4.55; found: C, 54.30; H, 4.67.
Methyl (E)-3-(4-fluoro-2-formylphenoxy)hex-2-enoate (1m). The title compound was prepared according to the general procedure (Step II) methyl (E)-3-(2-(1,3-dithian-2-yl)-4-fluorophenoxy)hex-2-enoate (5m) (1.0 g, 2.80 mmol) and thallium (III) trifluoroacetate. The product was obtained as a colorless oil in 94% yield (0.70 g). 1H NMR (400 MHz, CDCl3) δ: 1.09 (t, J = 8.0 Hz, 3H), 1.78–1.84 (m, 2H), 2.98–3.02 (m, 2H), 3.64 (s, 3H), 4.79 (s, 1H), 7.10 (dd, J = 12.0, 8.0 Hz, 1H), 7.34–7.39 (m, 1H), 7.62 (dd, J = 8.0, 4.0 H, 1H), 10.08 (d, J = 4.0 Hz, 1H). 13C NMR (101 MHz, CDCl3) δ: 13.8, 20.8, 32.9, 51.1, 97.5, 114.7 (d, J = 23.1 Hz), 123.0 (d, J = 25.2 Hz), 124.7 (d, J = 8.0 Hz), 129.6 (d, J = 6.0 Hz), 151.6 (d, J = 2.0 Hz), 160.2 (d, J = 248.5 Hz), 166.8, 176.6, 186.9 (d, J = 2.0 Hz). IR (ATR) ν (cm−1): 2954, 1700, 1693, 1636, 1606, 1454, 1429, 1371, 1262, 1231, 1184, 1112, 1046, 833. LRMS (ESI): Mass calcd. for [M + Na]+ C14H15FO4Na: 289.1; found 289.1. Anal. Calcd. for C14H15FO4: C, 63.15; H, 5.68; found: C, 63.21; H, 5.79.
Methyl (E)-3-((1-formylnaphthalen-2-yl)oxy)-5-phenylpent-2-enoate (1n). The title compound was prepared according to the general procedure (Step II) methyl (E)-3-((1-(1,3-dithian-2-yl)naphthalen-2-yl)oxy)-5-phenylpent-2-enoate (5n) (1.0 g, 2.22 mmol) and thallium (III) trifluoroacetate. The product was obtained as a yellow oil in 85% yield (0.68 g). 1H NMR (400 MHz, CDCl3) δ: 3.12–3.19 (m, 2H), 3.38–3.45 (m, 2H), 3.64 (s, 3H), 4.88 (s, 1H), 7.02 (d, J = 9.0 Hz, 1H), 7.23–7.33 (m, 1H), 7.34–7.44 (m, 4H), 7.59 (ddd, J = 8.1, 7.0, 1.2 Hz, 1H), 7.71 (ddd, J = 8.6, 7.0, 1.3 Hz, 1H), 7.88 (d, J = 8.1 Hz, 1H), 8.09 (d, J = 8.8 Hz, 1H), 9.27 (dd, J = 8.8, 0.7 Hz, 1H), 10.44 (s, 1H). 13C NMR (101 MHz, CDCl3) δ: 32.9, 33.4, 51.1, 98.8, 120.8, 121.7, 125.6, 126.4, 126.8, 128.4, 128.5, 128.6, 129.9, 131.0, 131.6, 137.3, 140.4, 157.9, 166.8, 175.2, 190.6. IR (ATR) ν (cm−1): 2951, 1703, 1690, 1635, 1604, 1456, 1429, 1371, 1262, 1231, 1184, 1110, 1048, 835. LRMS (ESI): Mass calcd. for [M + Na]+ C23H20O4Na: 383.1; found 383.2. Anal. Calcd. for C23H20O4: C, 76.65; H, 5.59; found: C, 76.76; H, 5.70.
Methyl (E)-3-((1-formylnaphthalen-2-yl)oxy)hex-2-enoate (1o). The title compound was prepared according to the general procedure (Step II) methyl (E)-3-((1-(1,3-dithian-2-yl)naphthalen-2-yl)oxy)hex-2-enoate (5o) (1.0 g, 2.57 mmol) and thallium (III) trifluoroacetate. The product was obtained as a yellow oil in 90% yield (0.69 g). 1H NMR (400 MHz, CDCl3) δ: 1.13 (t, J = 7.5 Hz, 3H), 1.86 (sxt, J = 7.5 Hz, 2H), 3.05 (ddd, J = 8.5, 6.1, 1.4 Hz, 2H), 3.63 (s, 3H), 4.88 (s, 1H), 7.19 (d, J = 8.8 Hz, 1H), 7.60 (ddd, J = 8.1, 6.9, 1.1 Hz, 1H), 7.72 (ddd, J = 8.6, 7.0, 1.3 Hz, 1H), 7.89 (d, J = 8.1 Hz, 1H), 8.13 (d, J = 8.8 Hz, 1H), 9.28 (dd, J = 8.6, 1.0 Hz, 1H), 10.57 (s, 1H). 13C NMR (101 MHz, CDCl3) δ: 13.9, 20.8, 33.0, 51.1, 98.6, 120.8, 125.6, 126.8, 128.4, 130.0, 131.0, 131.5, 137.4, 158.2, 167.1, 176.2, 190.7. IR (ATR) ν (cm−1): 2947, 1715, 1694, 1638, 1600, 1456, 1429, 1374, 1263, 1231, 1184, 1111, 1045, 833. LRMS (ESI): Mass calcd. for [M + Na]+ C18H18O4Na: 321.1; found 321.2. Anal. Calcd. for C18H18O4: C, 72.47; H, 6.08; found: C, 72.55; H, 6.18.
Methyl (E)-3-((1-formylnaphthalen-2-yl)oxy)but-2-enoate (1p). The title compound was prepared according to the general procedure (Step II) methyl (E)-3-((1-(1,3-dithian-2-yl)naphthalen-2-yl)oxy)hex-2-enoate (5p) (1.0 g, 2.77 mmol) and thallium (III) trifluoroacetate. The product was obtained as a yellow oil in 93% yield (0.70 g). 1H NMR (700 MHz, CDCl3) δ: 2.61 (s, 3H), 3.61 (s, 3H), 4.90 (s, 1H), 7.19 (d, J = 7.0 Hz, 1H), 7.56–7.59 (m, 1H), 7.68–7.71 (m, 1H), 7.87 (dd, J = 7.7, 1.4 Hz, 1H), 8.1 (d, J = 9.1 Hz, 1H), 9.24 (m, 1H), 10.56 (s, 1H). 13C NMR (101 MHz, CDCl3) δ: 18.1, 51.1, 98.8, 120.9, 121.6, 125.6, 126.8, 128.4, 129.9, 131.1, 131.6, 137.3, 157.8, 167.2, 172.5, 190.5. IR (ATR) ν (cm−1): 2942, 1713, 1690, 1631, 1604, 1456, 1429, 1374, 1263, 1231, 1184, 1112, 1044, 833. LRMS (ESI): Mass calcd. for [M + Na]+ C16H14O4Na: 293.1; found 293.2. Anal. Calcd. for C16H14O4: C, 71.10; H, 5.22; found: C, 71.17; H, 5.31.
3.2. General Procedure for Enantioselective Intramolecular Stetter Reaction Using Triazolium Salts O and L
A flame dried round bottom flask was charged with triazolium salt O (19.0 mg, 20 mol%) and 1.0 mL of cyclohexane. To this solution, KHMDS (0.5M in toluene 80 μL, 0.04 mmol, 20 mol%) was added via syringe and the solution was allowed to stir at ambient temperature for 20 min. A solution of the substrate (0.20 mmol) in 1.0 mL of cyclohexane was added. The resulting solution was allowed to stir at ambient temperature and monitored by TLC. The reaction mixture was placed directly onto a silica gel column and eluted with a suitable solution of hexane and ethyl acetate (80:20). Evaporation of solvent allowed analytically pure product.
A flame dried round bottom flask was charged with triazolium salt L (28.8 mg, 20 mol%) and 1.0 mL of cyclohexane. To this solution, potassium tert-butoxide (1.0 M in THF 40 μL, 0.04 mmol, 20 mol%)was added via syringe and the solution was allowed to stir at ambient temperature for 20 min. A solution of the substrate (0.20 mmol) in 1.0 mL of cyclohexane was added. The resulting solution was allowed to stir at ambient temperature and monitored by TLC. The reaction mixture was placed directly onto a silica gel column and eluted with a suitable solution of hexane and ethyl acetate (80:20). Evaporation of solvent allowed analytically pure product.
Methyl (R)-2-(3-oxo-2-propyl-2,3-dihydrobenzofuran-2-yl)acetate (2a). Yellow oil (98% yield, 97% ee). HPLC (Phenomenex Lux Celluose-1, hexanes/2-propanol 90:10, v = 0.7 mL/min, λ = 254 nm) t (major) = 14.0 min, t (minor) = 12.6 min. [α] = −19.2 (c 1.0, CH2Cl2).1H NMR (400 MHz, CDCl3) δ: 0.87 (t, J = 8.0 Hz, 3H), 1.14–1.23 (m, 1H), 1.30–1.37 (m, 1H), 1.84 (m, 2H), 2.96 (d, J = 16.0 Hz, 1H), 3.03 (d, J = 16.0 Hz, 1H), 3.53 (s, 3H), 7.09–7.11 (m, 2H), 7.59–7.64 (m, 1H), 7.69–7.72 (m, 1H). 13C NMR (101 MHz, CDCl3) δ: 14.0, 16.2, 38.7, 40.6, 51.7, 88.8, 112.9, 121.8, 124.1, 137.7, 169.0, 171.5, 202.7. IR (ATR) ν (cm−1): 2961, 2936, 2875, 1720, 1602, 1462, 1436, 1268, 1211, 1171, 1120, 993, 820. LRMS (ESI): Mass calcd. for [M + Na]+ C14H16O4Na: 271.1; found 271.1. Anal. Calcd. for C14H16O4: C, 67.73; H, 6.50; found: C, 67.80; H, 6.61.
Methyl (S)-2-(3-oxo-2-propyl-2,3-dihydrobenzofuran-2-yl)acetate (2a’). Yellow oil (98% yield, 97% ee). HPLC (Phenomenex Lux Celluose-1, hexanes/2-propanol 90:10, v = 0.7 mL/min, λ = 254 nm) t (major) = 12.7 min, t (minor) = 14.2 min. [α] = +19.4 (c 1.0, CH2Cl2).
Benzyl (R)-2-(3-oxo-2-propyl-2,3-dihydrobenzofuran-2-yl)acetate (2b). Yellow oil (95% yield, 96% ee). HPLC (Phenomenex Lux Celluose-1, hexanes/2-propanol 95:5 v = 0.7 mL/min, λ = 254 nm) t (major) = 19.1 min, t (minor) = 21.1 min. [α] = −1.4 (c 1.0, CH2Cl2). 1H NMR (400 MHz, CDCl3) δ: 0.86 (t, J = 8.0 Hz, 1H), 1.18–1.20 (m, 1H), 1.31–1.35 (m, 1H), 1.80–1.85 (m, 2H), 3.01 (d, J = 16.0 Hz, 1H), 3.09 (d, J = 16.0 Hz, 1H), 4.96 (s, 1H), 7.03–7.08 (m, 2H), 7.15–7.18 (m, 2H), 7.28–7.31 (m, 3H), 7.56–7.60 (m, 1H), 7.63 (dd, J = 8.0, 4.0 Hz, 1H). 13C NMR (101 MHz, CDCl3) δ: 14.0, 16.2, 38.9, 40.8, 66.7, 88.8, 112.9, 121.7, 121.8, 124.2, 128.2, 128.3 (2C), 128.5 (2C), 135.2, 137.7, 168.5, 171.5, 202.7. IR (ATR) ν (cm−1): 2953, 2935, 2874, 1726, 1482, 1437, 1250, 1213, 1163, 1123, 1010, 836. LRMS (ESI): Mass calcd. for [M + Na]+ C20H20O4Na: 347.1; found 347.2. Anal. Calcd. for C20H20O4: C, 74.06; H, 6.22; found: C, 74.12; H, 6.34.
Benzyl (S)-2-(3-oxo-2-propyl-2,3-dihydrobenzofuran-2-yl)acetate (2b’). Yellow oil (98% yield, 97% ee). HPLC (Phenomenex Lux Celluose-1, hexanes/2-propanol 90:10, v = 0.7 mL/min, λ = 254 nm) t (major) = 14.7 min, t (minor) = 13.2 min. [α] = +1.5 (c 1.0, CH2Cl2).
Methyl (R)-2-(2-methyl-3-oxo-2,3-dihydrobenzofuran-2-yl)acetate (2c). Yellow oil (96% yield, 96% ee). HPLC (Phenomenex Lux Celluose-1, hexanes/2-propanol 90:10, v = 0.7 mL/min, λ = 254 nm) t (major) = 15.6 min, t (minor) = 14.0 min. [α] = −12.6 (c 1.0, CH2Cl2). 1H NMR (400 MHz, CDCl3) δ: 2.96 (d, J = 16.0 Hz, 1H), 3.05 (d, J = 16.0 Hz, 1H), 3.56 (s, 3H), 7.09–7.13 (m, 2H), 7.61–7.65 (m, 1H), 7.71–7.74 (m, 1H). 13C NMR (101 MHz, CDCl3) δ: 22.3, 41.2, 51.8, 86.3, 113.3, 120.4, 121.9, 124.6, 137.8, 169.1, 170.9, 202.6. IR (ATR) ν (cm−1): 2958, 2940, 2870, 1722, 1601, 1473, 1434, 1262, 1210, 1163, 1123, 1011, 836. LRMS (ESI): Mass calcd. for [M + Na]+ C12H12O4Na: 243.1; found 243.1. Anal. Calcd. for C12H12O4: C, 65.45; H, 5.49; found: C, 65.52; H, 5.56.
Methyl (S)-2-(2-methyl-3-oxo-2,3-dihydrobenzofuran-2-yl)acetate (2c’). Yellow oil (98% yield, <99% ee). HPLC (Phenomenex Lux Celluose-1, hexanes/2-propanol 90:10, v = 0.7 mL/min, λ = 254 nm) t (major) = 14.2 min, t (minor) = 15.6 min. [α] = +13.4 (c 1.0, CH2Cl2).
Ethyl (R)-2-(2-ethyl-3-oxo-2,3-dihydrobenzofuran-2-yl)acetate (2d). Yellow oil (96% yield, 96% ee). HPLC (Phenomenex Lux Cellulose-1, hexanes/2-propanol 98:2, v = 0.7 mL/min, λ = 254 nm) t (major) = 23.5 min, t (minor) = 26.0 min. [α] = −19.5 (c 1.0, CH2Cl2). 1H NMR (400 MHz, CDCl3) δ: 0.85 (t, J = 7.5 Hz, 3H), 0.97 (t, J = 7.2 Hz, 3H), 1.89 (q, J = 7.3 Hz, 2H), 2.99 (dd, J = 16.1, 16.1 Hz, 2H), 3.95 (m, 2H), 7.04–7.14 (m, 2H), 7.61 (ddd, J = 8.4, 7.2, 1.5 Hz, 1H), 7.69 (ddd, J = 7.6, 1.5, 0.7 Hz, 1H). 13C NMR (101 MHz, CDCl3) δ: 7.3, 13.7, 29.9, 40.7, 60.8, 89.1, 112.9, 121.7, 122.0, 124.1, 137.7, 168.6, 171.7, 202.8. IR (ATR) ν (cm−1): 2952, 2930, 2874, 1720, 1482, 1434, 1256, 1211, 1168, 1120, 1012, 833. LRMS (ESI): Mass calcd. for [M + Na]+ C14H16O4Na: 271.1; found 271.1. Anal. Calcd. for C14H16O4: C, 67.73; H, 6.50; found: C, 67.80; H, 6.61.
Ethyl (S)-2-(2-ethyl-3-oxo-2,3-dihydrobenzofuran-2-yl)acetate (2d’). Yellow oil (98% yield, 91% ee). HPLC (Phenomenex Lux Amylose-1, hexanes/2-propanol 90:10, v = 0.7 mL/min, λ = 254 nm) t (major) = 10.0 min, t (minor) = 9.5 min. [α] = +18.8 (c 1.0, CH2Cl2).
Methyl (R)-2-(3-oxo-2-pentyl-2,3-dihydrobenzofuran-2-yl)acetate (2e). Yellow oil (95% yield, 98% ee). HPLC (Phenomenex Lux Cellulose-1, hexanes/2-propanol 90:10, v = 0.7 mL/min, λ = 254 nm) t (major) = 14.0 min, t (minor) = 11.6 min. [α] = −27.8 (c 1.0, CH2Cl2). 1H NMR (400 MHz, CDCl3) δ: 0.83 (t, J = 7.1 Hz, 3H), 0.88–1.03 (m, 2H), 1.19–1.25 (m, 4H), 1.80–1.87 (m, 2H), 2.97 (d, J = 16.4 Hz, 1H), 3.00 (d, J = 16.4 Hz, 1H), 3.52 (s, 3H), 7.06–7.11 (m, 2H), 7.61 (ddd, J = 8.4, 7.2, 1.5 Hz, 1H), 7.69 (dd, J = 7.9, 1.6 Hz, 1H). 13C NMR (101 MHz, CDCl3) δ: 13.9, 22.2, 22.4, 31.7, 36.5, 40.6, 51.8, 88.8, 112.9, 121.8, 124.2, 137.7, 169.1, 171.6, 202.8. IR (ATR) ν (cm−1): 2950, 2934, 2870, 1725, 1480, 1438, 1257, 1209, 1171, 1122, 1012, 833. LRMS (ESI): Mass calcd. for [M + Na]+ C16H20O4Na: 299.1; found 299.2. Anal. Calcd. for C16H20O4: C, 69.55; H, 7.30; found: C, 69.64; H, 7.38.
Methyl (S)-2-(3-oxo-2-pentyl-2,3-dihydrobenzofuran-2-yl)acetate (2e’). Yellow oil (95% yield, 98% ee). HPLC (Phenomenex Lux Cellulose-1, hexanes/2-propanol 90:10, v = 0.7 mL/min, λ = 254 nm) t (major) = 11.6 min, t (minor) = 14.6 min. [α] = +27.2 (c 1.0, CH2Cl2).
Methyl (R)-2-(2-hexyl-3-oxo-2,3-dihydrobenzofuran-2-yl)acetate (2f). Yellow oil (93% yield, 98% ee). HPLC (Phenomenex Lux Cellulose-1, hexanes/2-propanol 90:10, v = 0.7 mL/min, λ = 254 nm) t (major) = 13.7 min, t (minor) = 10.9 min. [α] = −28.3 (c 1.0, CH2Cl2). 1H NMR (700 MHz, CDCl3) δ: 0.86 (t, J = 7.0 Hz, 3H), 1.17–1.26 (m, 7H), 1.27–1.32 (m, 2H), 1.82–1.90 (m, 2H), 2.97 (d, J = 14.0 Hz, 1H), 3.02 (d, J = 14.0 Hz, 1H), 3.54 (s, 3H), 7.11 (m, 2H), 7.62–7.64 (m, 1H), 7.69 (dd, J = 7.0, 2.0 Hz, 1H). 13C NMR (176 MHz, CDCl3) δ: 14.9, 23.4, 23.6, 30.2, 32.4, 37.6, 41.6, 52.7, 89.8, 113.9, 122.7, 122.8, 125.2, 138.7, 170.1, 172.6, 203.8. IR (ATR) ν (cm−1): 2958, 2940, 2870, 1722, 1601, 1473, 1434, 1262, 1210, 1163, 1123, 1011, 836. LRMS (ESI): Mass calcd. for [M + Na]+ C17H22O4Na: 313.1; found 313.2. Anal. Calcd. for C17H22O4: C, 70.32; H, 7.64; found: C, 70.39; H, 7.72.
Methyl (S)-2-(2-hexyl-3-oxo-2,3-dihydrobenzofuran-2-yl)acetate (2f’). Yellow oil (93% yield, 98% ee). HPLC (Phenomenex Lux Cellulose-1, hexanes/2-propanol 90:10, v = 0.7 mL/min, λ = 254 nm) t (major) = 10.6 min, t (minor) = 13.6 min. [α] = +28.3 (c 1.0, CH2Cl2).
Methyl (R)-2-(2-(cyclohexylmethyl)-3-oxo-2,3-dihydrobenzofuran-2-yl)acetate (2g). Yellow oil (91% yield, 98% ee). HPLC (Phenomenex Lux Cellulose-1, hexanes/2-propanol 90:10, v = 0.7 mL/min, λ = 254 nm) t (major) = 15.3 min, t (minor) = 12.2 min. [α] = −19.2 (c 1.0, CH2Cl2). 1H NMR (400 MHz, CDCl3) δ: 0.87–1.01 (m, 3H), 1.04–1.17 (m, 4H), 1.24–1.36 (m, 3H), 1.67 (dd, J = 14.7, 6.8 Hz, 3H), 1.79 (dd, J = 14.7, 5.1 Hz, 1H), 3.00 (s, 2H), 3.50 (s, 3H), 7.07–7.12 (m, 2H), 7.61 (ddd, J = 8.4, 7.2, 1.5 Hz, 1H), 7.71 (dd, J = 8.1, 1.2 Hz, 1H). 13C NMR (101 MHz, CDCl3) δ: 26.0, 26.1, 33.1, 34.2, 34.7, 40.8, 43.6, 51.7, 89.1, 113.0, 121.8, 124.2, 137.7, 169.1, 171.3, 203.0. IR (ATR) ν (cm−1): 2952, 2944, 2876, 1709, 1607, 1466, 1430, 1267, 1212, 1155, 1123, 1006, 834. LRMS (ESI): Mass calcd. for [M + Na]+ C18H22O4Na: 325.1; found 325.2. Anal. Calcd. for C18H22O4: C, 66.45; H, 6.82; found: C, 66.54; H, 6.88.
Methyl (S)-2-(2-(cyclohexylmethyl)-3-oxo-2,3-dihydrobenzofuran-2-yl)acetate (2g’). Yellow oil (95% yield, <99% ee). HPLC (Phenomenex Lux Cellulose-1, hexanes/2-propanol 90:10, v = 0.7 mL/min, λ = 254 nm) t (major) = 12.0 min, t (minor) = 14.6 min. [α] = +20.2 (c 1.0, CH2Cl2).
Methyl (R)-2-(3-oxo-2-phenethyl-2,3-dihydrobenzofuran-2-yl)acetate (2h). Yellow oil (92% yield, 98% ee). HPLC (Phenomenex Lux Cellulose-1, hexanes/2-propanol 90:10, v = 0.7 mL/min, λ = 254 nm) t (major) = 18.4 min, t (minor) = 15.2 min. [α] = −38.0 (c 1.0, CH2Cl2). 1H NMR (400 MHz, CDCl3) δ: 2.19 (ddd, J = 11.3, 6.1, 2.9 Hz, 2H), 2.47 (ddd, J = 13.4, 11.0, 6.4 Hz, 1H), 2.63 (ddd, J = 13.4, 11.0, 6.3 Hz, 1H), 2.98–3.10 (m, 2H), 3.55 (s, 3H), 7.09–7.21 (m, 5H), 7.22–7.28 (m, 2H), 7.64 (ddd, J = 8.5, 7.2, 1.5 Hz, 1H), 7.73 (ddd, J = 7.6, 1.5, 0.7 Hz, 1H). 13C NMR (101 MHz, CDCl3) δ: 29.2, 38.3, 40.7, 51.8, 88.4, 113.1, 121.7, 122.0, 124.2, 126.2, 128.3 (2C), 128.4 (2C), 137.9, 140.6, 169.0, 171.6, 202.3. IR (ATR) ν (cm−1): 2953, 2939, 2870, 1701, 1604, 1459, 1433, 1271, 1215, 1148, 1119, 1012, 834. LRMS (ESI): Mass calcd. for [M + Na]+ C19H18O4Na: 333.1; found 333.1. Anal. Calcd. for C19H18O4: C, 73.53; H, 5.85; found: C, 73.60; H, 5.94.
Methyl (S)-2-(3-oxo-2-phenethyl-2,3-dihydrobenzofuran-2-yl)acetate (2h’). Yellow oil (92% yield, 98% ee). HPLC (Phenomenex Lux Cellulose-1, hexanes/2-propanol 90:10, v = 0.7 mL/min, λ = 254 nm) t (major) = 15.6 min, t (minor) = 18.6 min. [α] = +38.4 (c 1.0, CH2Cl2).
Methyl (R)-2-(6-methyl-2-methyl-3-oxo-2,3-dihydrobenzofuran-2-yl)acetate (2i). Yellow oil (94% yield, 94% ee). HPLC (Phenomenex Lux Cellulose-1, hexanes/2-propanol 90:10, v = 0.7 mL/min, λ = 254 nm) t (major) = 23.3 min, t (minor) = 20.3 min. [α] = −1.6 (c 1.0, CH2Cl2). 1H NMR (700 MHz, CDCl3) δ: 1.44 (s, 3H), 2.34 (s, 3H), 2.90 (d, J = 14.0 Hz, 1H), 3.00 (d, J = 14.0 Hz, 1H), 3.53 (s, 3H), 6.96 (d, J = 7.0 Hz, 1H), 7.40 (d, J = 2.1 Hz, 1H), 7.47 (s, 3H). 13C NMR (101 MHz, CDCl3) δ: 20.2, 22.0, 40.8, 51.4, 86.0, 112.5, 119.9, 123.6, 131.1, 138.7, 168.8, 169.0, 202.3. IR (ATR) ν (cm−1): 2956, 2941, 2869, 1715, 1609, 1462, 1433, 1270, 1212, 1155, 1123, 1006, 833. LRMS (ESI): Mass calcd. for [M + Na]+ C13H14O5Na: 273.1; found 273.2. Anal. Calcd. for C13H14O5: C, 62.39; H, 5.64; found: C, 62.47; H, 5.70.
Methyl (S)-2-(6-methyl-2-methyl-3-oxo-2,3-dihydrobenzofuran-2-yl)acetate (2i’). Yellow oil (96% yield, 93% ee). HPLC (Phenomenex Lux Cellulose-1, hexanes/2-propanol 90:10, v = 0.7 mL/min, λ = 254 nm) t (major) = 20.2 min, t (minor) = 23.5 min. [α] = +1.7 (c 1.0, CH2Cl2).
Methyl (R)-2-(5-chloro-3-oxo-2-propyl-2,3-dihydrobenzofuran-2-yl)acetate (2j). Yellow oil (95% yield, 98% ee). HPLC (Phenomenex Lux Cellulose-1, hexanes/2-propanol 90:10, v = 0.7 mL/min, λ = 254 nm) t (major) = 9.5 min, t (minor) = 7.6 min. [α] = −24.8 (c 1.0, CH2Cl2). 1H NMR (700 MHz, CDCl3) δ: 0.89 (t, J = 7.0 Hz, 3H), 1.20 (m, 1H), 1.34 (m, 1H), 1.81 (dtd, J = 17.6, 13.9, 5.6 Hz, 2H), 3.00 (d, J = 14.0 Hz, 1H), 3.06 (d, J = 14.0 Hz, 1H) 3.56 (s, 3H), 7.02 (d, J = 7.0 Hz, 1H), 7.56 (dd, J = 8.4, 2.1 Hz, 1H), 7.67 (d, J = 1.4 Hz, 1H). 13C NMR (101 MHz, CDCl3) δ: 15.0, 17.2, 39.8, 41.6, 52.8, 90.9, 115.2, 124.0, 124.5, 128.2, 138.4, 170.0, 170.8, 202.6. IR (ATR) ν (cm−1): 2961, 2878, 1722, 1620, 1482, 1247, 1210, 1155, 1123, 1009, 829. LRMS (ESI): Mass calcd. for [M + Na]+ C14H15ClO4Na: 305.1; found 305.2. Anal. Calcd. for C14H15ClO4: C, 59.48; H, 5.35; found: C, 59.55; H, 5.43.
Methyl (S)-2-(5-chloro-3-oxo-2-propyl-2,3-dihydrobenzofuran-2-yl)acetate (2j’). Yellow oil (94% yield, 98% ee). HPLC (Phenomenex Lux Cellulose-1, hexanes/2-propanol 90:10, v = 0.7 mL/min, λ = 254 nm) t (major) = 7.6 min, t (minor) = 9.6 min. [α] = +25.6 (c 1.0, CH2Cl2).
Methyl (R)-2-(5-bromo-3-oxo-2-propyl-2,3-dihydrobenzofuran-2-yl)acetate (2k). Yellow oil (90% yield, 93% ee). HPLC (Phenomenex Lux Cellulose-1, hexanes/2-propanol 90:10, v = 0.7 mL/min, λ = 254 nm) t (major) = 13.0 min, t (minor) = 9.5 min. [α] = −28.3 (c 1.0, CH2Cl2). 1H NMR (400 MHz, CDCl3) δ: 0.87 (t, J = 7.3 Hz, 3H), 1.10–1.24 (m, 1H), 1.26–1.38 (m, 1H), 1.81 (dtd, J = 17.6, 13.9, 5.6 Hz, 2H), 2.99 (d, J = 16.6 Hz, 1H), 3.04 (d, J = 16.6 Hz, 1H), 3.54 (s, 3H), 7.00 (d, J = 8.8 Hz, 1H), 7.68 (dd, J = 8.8, 2.2 Hz, 1H), 7.81 (dd, J = 2.2, 0.5 Hz, 1H). 13C NMR (101 MHz, CDCl3) δ: 14.01, 16.22, 38.80, 40.63, 51.89, 89.80, 114.30, 114.65, 123.63, 126.69, 140.15, 168.99, 170.24, 201.38. IR (ATR) ν (cm−1): 2955, 2870, 1715, 1622, 1478, 1244, 1211, 1156, 1123, 1009, 831. LRMS (ESI): Mass calcd. for [M + Na]+ C14H15BrO4Na: 349.0; found 349.1. Anal. Calcd. for C14H15BrO4: C, 51.40; H, 4.62; found: C, 51.49; H, 4.70.
Methyl (S)-2-(5-bromo-3-oxo-2-propyl-2,3-dihydrobenzofuran-2-yl)acetate (2k’). Yellow oil (90% yield, 94% ee). HPLC (Phenomenex Lux Cellulose-1, hexanes/2-propanol 90:10, v = 0.7 mL/min, λ = 254 nm) t (major) = 9.5 min, t (minor) = 12.7 min. [α] = +28.7 (c 1.0, CH2Cl2).
Methyl (R)-2-(3-oxo-2-propyl-5-(trifluoromethoxy)-2,3-dihydrobenzofuran-2-yl)acetate (2l). Yellow oil (94% yield, 97% ee). HPLC (Phenomenex Lux Cellulose-1, hexanes/2-propanol 90:10, v = 0.7 mL/min, λ = 254 nm) t (major) = 9.5 min, t (minor) = 10.7 min. [α] = −17.3 (c 1.0, CH2Cl2). 1H NMR (400 MHz, CDCl3) δ: 0.88 (t, J = 7.2 Hz, 1H), 1.17–1.24 (m, 1H), 1.31–1.39 (m, 1H), 1.78–1.87 (m, 2H), 2.99 (d, J = 16.8 Hz, 1H), 3.07 (d, J = 16.8 Hz, 1H), 3.55 (s, 3H), 7.11 (d, J = 8.8 Hz, 1H), 7.46 (dd, J = 9.2, 2.8 Hz, 1H), 7.54 (m, 1H). 13C NMR (101 MHz, CDCl3) δ: 14.0, 16.2, 38.7, 40.5, 51.8, 90.3, 114.0, 116.3, 120.5 (d, J = 257.6 Hz), 122.4, 130.9, 143.5 (d, J = 2.0 Hz), 169.2 (d, J = 52.3 Hz), 201.8. IR (ATR) ν (cm−1): 2961, 2878, 1722, 1620, 1482, 1247, 1210, 1195, 1155, 1116, 1009, 829. LRMS (ESI): Mass calcd. for [M + Na]+ C15H15F3O5Na: 355.1; found 355.2. Anal. Calcd. for C15H15F3O5: C, 54.22; H, 4.55; found: C, 54.31; H, 4.65.
Methyl (S)-2-(3-oxo-2-propyl-5-(trifluoromethoxy)-2,3-dihydrobenzofuran-2-yl)acetate (2l’). Yellow oil (92% yield, 85% ee). HPLC (Phenomenex Lux Cellulose-1, hexanes/2-propanol 90:10, v = 0.7 mL/min, λ = 254 nm) t (major) = 10.7 min, t (minor) = 9.6 min. [α] = +15.9 (c 1.0, CH2Cl2).
Methyl (R)-2-(5-fluoro-3-oxo-2-propyl-2,3-dihydrobenzofuran-2-yl)acetate (2m). Yellow oil (97% yield, 86% ee). HPLC (Phenomenex Lux Cellulose-1, hexanes/2-propanol 90:10, v = 0.7 mL/min, λ = 254 nm) t (major) = 12.2 min, t (minor) = 9.5 min. [α] = −33.9 (c 1.0, CH2Cl2). 1H NMR (400 MHz, CDCl3) δ: 0.87 (t, J = 7.3 Hz, 3H), 1.11–1.26 (m, 1H), 1.28–1.38 (m, 1 H), 1.81 (dtd, J = 17.3, 13.0, 5.6 Hz, 2H), 2.99 (d, J = 16.6 Hz, 1H), 3.03 (d, J = 16.6 Hz, 1H), 3.54 (s, 3H), 7.05 (dd, J = 9.7, 3.5 Hz, 1H), 7.31–7.37 (m, 2H). 13C NMR (101 MHz, CDCl3) δ: 14.0, 16.2, 38.8, 40.7, 51.8, 90.0, 109.2 (d, J = 23.1 Hz), 113.9 (d, J = 8.1 Hz), 122.3 (d, J = 7.0 Hz), 125.2 (d, J = 26.1 Hz), 156.4, 158.8, 168.2 (d, J = 134.8 Hz), 202.5. IR (ATR) ν (cm−1): 2953, 2935, 1726, 1482, 1437, 1250, 1213, 1163, 1123, 1084, 1009, 837. LRMS (ESI): Mass calcd. for [M + Na]+ C14H15FO4Na: 289.1; found 289.1. Anal. Calcd. for C14H15FO4: C, 58.13; H, 5.23; found: C, 58.20; H, 5.33.
Methyl (S)-2-(5-fluoro-3-oxo-2-propyl-2,3-dihydrobenzofuran-2-yl)acetate (2m’). Yellow oil (95% yield, 96% ee). HPLC (Phenomenex Lux Cellulose-1, hexanes/2-propanol 90:10, v = 0.7 mL/min, λ = 254 nm) t (major) = 9.6 min, t (minor) = 12.0 min. [α] = +36.5 (c 1.0, CH2Cl2).
Methyl (R)-2-(1-oxo-2-phenethyl-1,2-dihydronaphtho [2,1-b]furan-2-yl)acetate (2n). Yellow oil (94% yield, 28% ee). HPLC (Phenomenex Lux Cellulose-1, hexanes/2-propanol 90:10, v = 0.7 mL/min, λ = 254 nm) t (major) = 28.2 min, t (minor) = 28.0 min. [α] = −4.3 (c 1.0, CH2Cl2). 1H NMR (400 MHz, CDCl3) δ: 2.26–2.35 (m, 2H), 2.48–2.56 (m, 1H), 2.61–2.68 (m, 1H), 3.04 (d, J = 16.0 Hz, 1H), 3.10 (d, J = 16.0 Hz, 1H), 3.55 (s, 3H), 7.11–7.14 (m, 2H), 7.15–7.18 (m, 1H), 7.22–7.26 (m, 2H), 7.30 (d, J = 8.0 Hz, 1H), 7.51 (ddd, J = 8.0, 6.8, 1.2 Hz, 1H), 7.70 (ddd, J = 8.4, 7.2, 1.6 Hz, 1H), 7.87 (d, J = 8.4 Hz, 1H), 8.12 (d, J = 8.8 Hz, 1H), 8.80–8.82 (m, 1H). 13C NMR (101 MHz, CDCl3) δ: 29.2, 38.2, 40.5, 51.8, 89.4, 113.6, 113.7, 123.2, 125.4, 126.1, 128.3 (2C), 128.4 (2C), 128.5, 129.2, 129.3, 129.8, 140.0, 140.6, 169.0, 174.3, 201.6. IR (ATR) ν (cm−1): 3081, 3060, 3023, 2948, 1713, 1683, 1585, 1509, 1430, 1366, 1234, 1207, 1163, 1151, 1117, 1073, 1061, 1044, 1002, 1482, 1437, 1250, 1213, 1163, 1123, 1084, 1009, 837. LRMS (ESI): Mass calcd. for [M + Na]+ C23H20O4Na: 383.1; found 383.2. Anal. Calcd. for C23H20O4: C, 76.65; H, 5.59; found: C, 76.77; H, 5.68.
Methyl (S)-2-(1-oxo-2-phenethyl-1,2-dihydronaphtho[2,1-b]furan-2-yl)acetate (2n’). Yellow oil (90% yield, 50% ee). HPLC (Phenomenex Lux Amylose-1, hexanes/2-propanol 90:10, v = 0.7 mL/min, λ = 254 nm) t (major) = 19.2 min, t (minor) = 26.8 min. [α] = +9.2 (c 1.0, CH2Cl2).
Methyl (R)-2-(1-oxo-2-propyl-1,2-dihydronaphtho[2,1-b]furan-2-yl)acetate (2o). Yellow oil (94% yield, 50% ee). HPLC (Phenomenex Lux Cellulose-1, hexanes/2-propanol 90:10, v = 0.7 mL/min, λ = 254 nm) t (major) = 22.0 min, t (minor) = 15.8 min. [α] = −7.7 (c 1.0, CH2Cl2). 1H NMR (400 MHz, CDCl3) δ: 0.85 (t, J = 7.0 Hz, 3H), 1.88–1.96 (m, 2H), 2.99 (d, J = 16.0 Hz, 1H), 3.04 (d, J = 16.0 Hz, 1H), 3.51 (s, 3H), 7.24 (d, J = 9.0 Hz, 1H), 7.47 (m, 1H), 7.66 (m, 1H), 7.81 (d, J = 8.0 Hz, 1H), 8.06 (d, J = 9.0 Hz, 1H), 8.75 (d, J = 8.0 Hz, 1H). 13C NMR (101 MHz, CDCl3) δ: 14.0, 16.3, 22.7, 29.7, 38.6, 40.5, 51.8, 89.9, 113.6, 123.2, 125.2, 128.5, 129.2, 129.7, 139.7, 169.1, 174.2, 202.0. IR (ATR) ν (cm−1): 2949, 2928, 1734, 1688, 1630, 1578, 1515, 1437, 1359, 1288, 1211, 1163, 1151, 1123, 1076, 991, 912, 837. LRMS (ESI): Mass calcd. for [M + Na]+ C18H18O4Na: 321.1; found 321.2. Anal. Calcd. for C18H18O4: C, 72.47; H, 6.08; found: C, 72.55; H, 6.17.
Methyl (S)-2-(1-oxo-2-propyl-1,2-dihydronaphtho[2,1-b]furan-2-yl)acetate (2o’). Yellow oil (94% yield, 32% ee). HPLC (Phenomenex Lux Cellulose-1, hexanes/2-propanol 90:10, v = 0.7 mL/min, λ = 254 nm) t (major) = 22.0 min, t (minor) = 15.8 min. [α] = +5.7 (c 1.0, CH2Cl2).
Methyl (R)-2-(2-methyl-1-oxo-1,2-dihydronaphtho[2,1-b]furan-2-yl)acetate (2p). Yellow oil (98% yield, 32% ee). HPLC (Phenomenex Lux Cellulose-1, hexanes/2-propanol 90:10, v = 0.7 mL/min, λ = 254 nm) t (major) = 18.8 min, t (minor) = 24.0 min. [α] = −6.2 (c 1.0, CH2Cl2). 1H NMR (400 MHz, CDCl3) δ: 1.59 (s, 3H), 2.99 (d, J = 16.0 Hz, 1H), 3.06 (d, J = 16.0 Hz, 1H), 3.57 (s, 3H), 7.25 (d, J = 9.2 Hz, 1H), 7.45 (m, 1H), 7.68 (m, 1H), 7.85 (d, J = 8.0 Hz, 1H), 8.09 (d, J = 9.2 Hz, 1H). 13C NMR (101 MHz, CDCl3) δ: 22.3, 41.0, 51.8, 87.3, 112.1, 113.9, 123.2, 125.3, 128.5, 129.3, 129.5, 129.8, 139.8, 169.1, 173.6, 202.0. IR (ATR) ν (cm−1): 2940, 2924, 1730, 1680, 1634, 1580, 1510, 1430, 1355, 1285, 1209, 1163, 1151, 1128, 1070, 991, 912, 837. LRMS (ESI): Mass calcd. for [M + Na]+ C16H14O4Na: 293.1; found 293.2. Anal. Calcd. for C16H14O4: C, 71.10; H, 5.22; found: C, 71.18; H, 5.31.
Methyl (S)-2-(2-methyl-1-oxo-1,2-dihydronaphtho[2,1-b]furan-2-yl)acetate (2p’). Yellow oil (98% yield, 3% ee). HPLC (Phenomenex Lux Cellulose-1, hexanes/2-propanol 90:10, v = 0.7 mL/min, λ = 254 nm) t (major) = 18.8 min, t (minor) = 24.0 min. [α] = +1.8 (c 1.0, CH2Cl2).
4. Conclusions
In conclusion, camphor-derived L and pinene-derived O triazolium salts as N-heterocyclic carbene precursors have been found to be efficient for the enantioselective intramolecular Stetter reaction with varied β,β-substituted Michael acceptors. In general, with 20 mol% of the NHC catalysts L or O, the desired 2,2-disubstituted coumaranone and naphthofuranone derivatives bearing fully-substituted quaternary stereogenic centers could be obtained with excellent yields of up to 99% ee. Through a simple change of the terpene-derived triazolium salts, NHCs L and O promote very high but opposite enantiofacial selectivity. Further applications of these terpene-derived triazolium salts in other asymmetric reactions are currently underway.
Supplementary Materials
The following are available online at http://www.mdpi.com/2073-4344/9/2/192/s1.
Funding
This project was financed by the National Science Center (Poland) as part of the SONATA BIS program (UMO-2016/22/E/ST5/00469).
Conflicts of Interest
The author declares no conflict of interest.
References
- Schreiber, S.L. Target-oriented and diversity-oriented organic synthesis in drug discovery. Science 2000, 287, 1964–1969. [Google Scholar] [CrossRef] [PubMed]
- Galloway, W.R.J.D.; Isidro-Llobet, A.; Spring, D.R. Diversity-oriented synthesis as a tool for the discovery of novel biologically active small molecules. Nat. Commun. 2010, 1, 80. [Google Scholar] [CrossRef] [PubMed]
- Ge, H.M.; Zhu, C.H.; Shi, D.H.; Zhang, L.D.; Xie, D.Q.; Yang, J.; Ng, S.W.; Tan, R.X. Hopeahainol A: An acetylcholinesterase inhibitor from Hopea hainanensis. Chem. A Eur. J. 2008, 14, 376–381. [Google Scholar] [CrossRef] [PubMed]
- Wada, S.I.; Hitomi, T.; Tokuda, H.; Tanaka, R. Anti-tumor-initiating effects of spiro-biflavonoids from Abies sachalinensis. Chem. Biodivers. 2010, 7, 2303–2308. [Google Scholar] [CrossRef] [PubMed]
- Fedorova, T.E.; Ivanova, S.Z.; Fedorov, S.V.; Babkin, V.A. Larisinol, a new spirobiflavonoid from Larix gmelinii bark. Chem. Nat. Compd. 2007, 43, 208–209. [Google Scholar] [CrossRef]
- Bassarello, C.; Bifulco, G.; Montoro, P.; Skhirtladze, A.; Kemertelidze, E.; Pizza, C.; Piacente, S. Gloriosaols A and B, two novel phenolics from Yucca gloriosa: Structural characterization and configurational assignment by a combined NMR-quantum mechanical strategy. Tetrahedron 2007, 63, 148–154. [Google Scholar] [CrossRef]
- Kwon, Y.-J.; Sohn, M.-J.; Zheng, C.-J.; Kim, W.-G. Fumimycin benzofuranone peptide deformylase inhibitor w unusual skeleton. Org. Lett. 2007, 9, 2449–2451. [Google Scholar] [CrossRef] [PubMed]
- Kwon, Y.J.; Sohn, M.J.; Zheng, C.J.; Kim, W.G. Fumimycin: A peptide deformylase inhibitor with an unusual skeleton produced by Aspergillus fumisynnematus. Org. Lett. 2007, 9, 2449–2451. [Google Scholar] [CrossRef] [PubMed]
- Son, J.K.; Jung, S.J.; Jung, J.H.; Fang, Z.; Lee, C.S.; Seo, C.S.; Moon, D.C.; Min, B.S.; Kim, M.R.; Woo, M.H. Anticancer Constituents from the Roots of Rubia cordifolia L. Chem. Pharm. Bull. (Tokyo) 2008, 56, 213–216. [Google Scholar] [CrossRef] [PubMed]
- Harrowven, D.C.; Lucas, M.C.; Howes, P.D. The synthesis of a natural product family: From debromoisolaurinterol to the aplysins. Tetrahedron 2001, 57, 791–804. [Google Scholar] [CrossRef]
- Lardic, M.; Patry, C.; Duflos, M.; Guillon, J.; Massip, S.; Cruzalegui, F.; Edmonds, T.; Giraudet, S.; Marini, L.; Leonce, S. Synthesis and primary cytotoxicity evaluation of arylmethylenenaphthofuranones derivatives. J. Enzym. Inhib. Med. Chem. 2006, 21, 313–325. [Google Scholar] [CrossRef] [PubMed]
- Braun, M.; Hessamian-Alinejad, A.; De Lacroix, B.F.; Alvarez, B.H.; Fischer, G. Novel spiroannulated 3-benzofuranones. Synthesis and inhibition of the human peptidyl prolyl cis/trans isomerase Pin1. Molecules 2008, 13, 995–1003. [Google Scholar] [CrossRef] [PubMed]
- Adediran, S.A.; Cabaret, D.; Drouillat, B.; Pratt, R.F.; Wakselman, M. The synthesis and evaluation of benzofuranones as β-lactamase substrates. Bioorg. Med. Chem. 2001, 9, 1175–1183. [Google Scholar] [CrossRef]
- Frenette, M.; MacLean, P.D.; Barclay, L.R.C.; Scaiano, J.C. Radically different antioxidants: Thermally generated carbon-centered radicals as chain-breaking antioxidants. J. Am. Chem. Soc. 2006, 128, 16432–16433. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Lampkins, A.J.; Baker, M.B.; Sumpter, B.G.; Huang, J.; Abboud, K.A.; Castellano, R.K. Benzotrifuranone: Synthesis, structure, and access to polycyclic heteroaromatics. Org. Lett. 2009, 11, 4314–4317. [Google Scholar] [CrossRef] [PubMed]
- Sato, S.; Okusa, N.; Ogawa, A.; Ikenoue, T.; Seki, T.; Tsuji, T. Identification and preliminary SAR studies of (+)-geodin as a glucose uptake stimulator for rat adipocytes. J. Antibiot. (Tokyo) 2005, 58, 583–589. [Google Scholar] [CrossRef] [PubMed]
- Gentles, J.C. Experimental ringworm in guinea pigs: Oral treatment with griseofulvin. Nature 1958, 182, 476–477. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Yang, G.; Meng, Q.; Liu, J.; Yang, S. Linobiflavonoid inhibits human lung adenocarcinoma A549 cells: Effect on tubulin protein. Mol. Biol. Rep. 2013, 6019–6025. [Google Scholar] [CrossRef]
- Katoh, T.; Ohmori, O. Studies toward the total synthesis of Sch 202596, an antagonist of the galanin receptor subtype GalR1: Synthesis of geodin, the spirocoumaranone subunit of Sch 202596. Tetrahedron Lett. 2000, 41, 465–469. [Google Scholar] [CrossRef]
- Trost, B.M.; Jiang, C. Catalytic enantioselective construction of all-carbon quaternary stereocenters. Synthesis 2006, 3, 369–396. [Google Scholar] [CrossRef]
- Chen, Z.S.; Huang, X.Y.; Chen, L.H.; Gao, J.M.; Ji, K. Rh(II)/Pd(0) Dual Catalysis: Regiodivergent Transformations of Alkylic Oxonium Ylides. ACS Catal. 2017, 7, 7902–7907. [Google Scholar] [CrossRef]
- Kuppusamy, R.; Gandeepan, P.; Cheng, C.H. RhIII-Catalyzed [4 + 1] Annulations of 2-Hydroxy- and 2-Aminobenzaldehydes with Allenes: A Simple Method toward 3-Coumaranones and 3-Indolinones. Org. Lett. 2015, 17, 3846–3849. [Google Scholar] [CrossRef] [PubMed]
- Petersen, A.B.; Rønnest, M.H.; Larsen, T.O.; Clausen, M.H. The chemistry of griseofulvin. Chem. Rev. 2014, 114, 12088–12107. [Google Scholar] [CrossRef] [PubMed]
- Enders, D.; Niemeier, O.; Henseler, A. Organocatalysis by N-heterocyclic carbenes. Chem. Rev. 2007, 107, 5606–5655. [Google Scholar] [CrossRef] [PubMed]
- Bugaut, X.; Glorius, F. Organocatalytic umpolung: N-heterocyclic carbenes and beyond. Chem. Soc. Rev. 2012, 41, 3511–3522. [Google Scholar] [CrossRef] [PubMed]
- Flanigan, D.M.; Romanov-Michailidis, F.; White, N.A.; Rovis, T. Organocatalytic Reactions Enabled by N-Heterocyclic Carbenes. Chem. Rev. 2015, 115, 9307–9387. [Google Scholar] [CrossRef]
- Ryan, S.J.; Candish, L.; Lupton, D.W. Acyl anion free n-heterocyclic carbene organocatalysis. Chem. Soc. Rev. 2013, 42, 4906–4917. [Google Scholar] [CrossRef]
- Douglas, J.; Churchill, G.; Smith, A.D. NHCs in asymmetric organocatalysis: Recent advances in azolium enolate generation and reactivity. Synthesis 2012, 44, 2295–2309. [Google Scholar] [CrossRef]
- Dzieszkowski, K.; Rafiński, Z. N-Heterocyclic Carbene Catalysis under Oxidizing Conditions. Catalysts 2018, 8, 549. [Google Scholar] [CrossRef]
- Breslow, R. Rapid Deuterium Exchange in Thiazolium Salts. J. Am. Chem. Soc. 1957, 79, 1762–1763. [Google Scholar] [CrossRef]
- Breslow, R. On the Mechanism of Thiamine Action. IV. Evidence from Studies on Model Systems. J. Am. Chem. Soc. 1958, 80, 3719–3726. [Google Scholar] [CrossRef]
- Berkessel, A.; Elfert, S.; Yatham, V.R.; Neudörfl, J.M.; Schlörer, N.E.; Teles, J.H. Umpolung by N-heterocyclic carbenes: Generation and reactivity of the elusive 2,2-diamino enols (breslow intermediates). Angew. Chem. Int. Ed. 2012, 59, 12370–12374. [Google Scholar] [CrossRef] [PubMed]
- De Alaniz, J.R.; Rovis, T. A highly enantio- and diastereoselective catalytic intramolecular Stetter reaction. J. Am. Chem. Soc. 2005, 127, 6284–6289. [Google Scholar] [CrossRef] [PubMed]
- Kerr, M.S.; Rovis, T. Enantioselective synthesis of quaternary stereocenters via a catalytic asymmetric stetter reaction. J. Am. Chem. Soc. 2004, 126, 8876–8877. [Google Scholar] [CrossRef] [PubMed]
- Moore, J.L.; Kerr, M.S.; Rovis, T. Enantioselective formation of quaternary stereocenters using the catalytic intramolecular Stetter reaction. Tetrahedron 2006, 62, 11477–11482. [Google Scholar] [CrossRef]
- Jousseaume, T.; Wurz, N.E.; Glorius, F. Highly enantioselective synthesis of α-amino acid derivatives by an NHC-catalyzed intermolecular stetter reaction. Angew. Chem. Int. Ed. 2011, 50, 1410–1414. [Google Scholar] [CrossRef]
- Dirocco, D.A.; Noey, E.L.; Houk, K.N.; Rovis, T. Catalytic asymmetric intermolecular stetter reactions of enolizable aldehydes with nitrostyrenes: Computational study provides insight into the success of the catalyst. Angew. Chem. Int. Ed. 2012, 51, 2391–2394. [Google Scholar] [CrossRef]
- Garapati, V.K.R.; Gravel, M. Oxazolium Salts as Organocatalysts for the Umpolung of Aldehydes. Org. Lett. 2018, 20, 6372–6375. [Google Scholar] [CrossRef]
- Zhao, M.; Liu, J.L.; Liu, H.F.; Chen, J.; Zhou, L. Construction of Bisbenzopyrone via N-Heterocyclic Carbene Catalyzed Intramolecular Hydroacylation-Stetter Reaction Cascade. Org. Lett. 2018, 20, 2676–2679. [Google Scholar] [CrossRef]
- Ragno, D.; Di Carmine, G.; Brandolese, A.; Bortolini, O.; Giovannini, P.P.; Massi, A. Immobilization of Privileged Triazolium Carbene Catalyst for Batch and Flow Stereoselective Umpolung Processes. ACS Catal. 2017, 7, 6365–6375. [Google Scholar] [CrossRef]
- He, J.; Zheng, J.; Liu, J.; She, X.; Pan, X. N-heterocyclic carbene catalyzed nucleophilic substitution reaction for construction of benzopyrones and benzofuranones. Org. Lett. 2006, 8, 4637–4640. [Google Scholar] [CrossRef] [PubMed]
- Padmanaban, M.; Biju, A.T.; Glorius, F. Efficient Synthesis of Benzofuranones: N-Heterocyclic Carbene (NHC)/Base-Catalyzed Hydroacylation–Stetter–Rearrangement Cascade. Org. Lett. 2011, 20, 5624–5627. [Google Scholar] [CrossRef] [PubMed]
- Filloux, C.M.; Lathrop, S.P.; Rovis, T. Multicatalytic, asymmetric Michael/Stetter reaction of salicylaldehydes and activated alkynes. Proc. Natl. Acad. Sci. USA 2010, 107, 20666–20671. [Google Scholar] [CrossRef] [PubMed]
- Rafiński, Z.; Kozakiewicz, A.; Rafińska, K. (−)-β-pinene-derived N-heterocyclic carbenes: Application to highly enantioselective intramolecular Stetter reaction. ACS Catal. 2014, 4, 1404–1408. [Google Scholar] [CrossRef]
- Rafiński, Z.; Kozakiewicz, A.; Rafińska, K. Highly efficient synthesis of spirocyclic (1R)-camphor-derived triazolium salts: Application in the catalytic asymmetric benzoin condensation. Tetrahedron 2014, 70, 5739–5745. [Google Scholar] [CrossRef]
- Rafiński, Z.; Kozakiewicz, A. Enantioselective Synthesis of Chromanones Bearing Quaternary Substituted Stereocenters Catalyzed by (1R)-Camphor-Derived N-Heterocyclic Carbenes. J. Org. Chem. 2015, 80, 7468–7476. [Google Scholar] [CrossRef]
- Rafiński, Z. Enantioselective benzoin condensation catalyzed by spirocyclic terpene-based N-heterocyclic carbenes. Tetrahedron 2016, 72, 1860–1867. [Google Scholar] [CrossRef]
- Rafiński, Z. Novel (−)-β-Pinene-Derived Triazolium Salts: Synthesis and Application in the Asymmetric Stetter Reaction. ChemCatChem 2016, 8, 2599–2603. [Google Scholar] [CrossRef]
- Collett, C.J.; Massey, R.S.; Taylor, J.E.; Maguire, O.R.; O’Donoghue, A.M.C.; Smith, A.D. Rate and equilibrium constants for the addition of N-heterocyclic carbenes into benzaldehydes: A remarkable 2-substituent effect. Angew. Chem. Int. Ed. 2015, 54, 6887–6892. [Google Scholar] [CrossRef]
- Collett, C.J.; Massey, R.S.; Maguire, O.R.; Batsanov, A.S.; O’donoghue, A.M.C.; Smith, A.D. Mechanistic insights into the triazolylidene-catalysed Stetter and benzoin reactions: Role of the N-aryl substituent. Chem. Sci. 2013, 4, 1514–1522. [Google Scholar] [CrossRef]
- Mahatthananchai, J.; Bode, J.W. The effect of the N-mesityl group in NHC-catalyzed reactions. Chem. Sci. 2012. [Google Scholar] [CrossRef] [PubMed]
- Moore, J.L.; Silvestri, A.P.; De Alaniz, J.R.; Dirocco, D.A.; Rovis, T. Mechanistic investigation of the enantioselective intramolecular stetter reaction: Proton transfer is the first irreversible step. Org. Lett. 2011, 13, 1742–1745. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).