Solution X-Ray Absorption Spectroscopy (XAS) for Analysis of Catalytically Active Species in Reactions with Ethylene by Homogeneous (Imido)vanadium(V) Complexes—Al Cocatalyst Systems
Abstract
1. Introduction
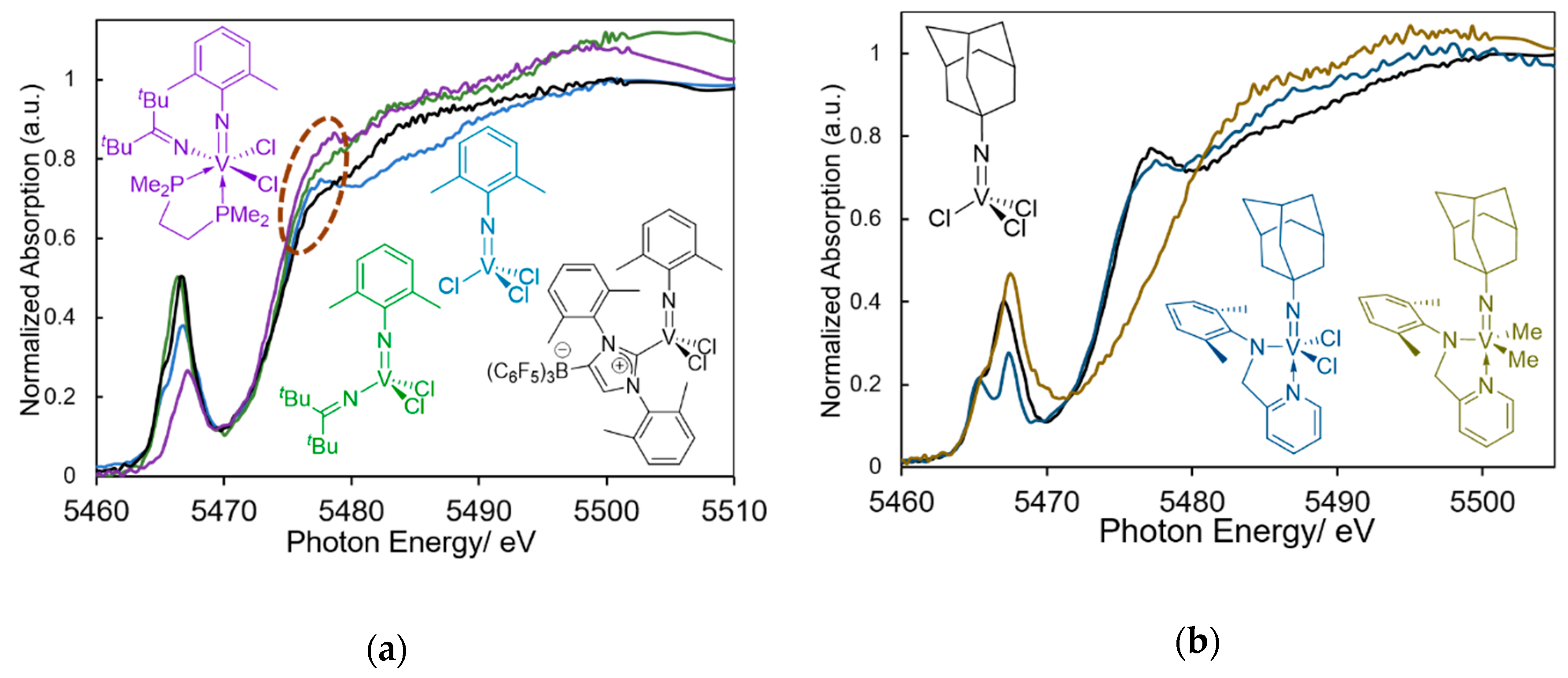
2. V-K Edge XANES (X-ray Absorption Near Edge Structure) and EXAFS (Extended X-ray Absorption Fine Structure) Spectra of Vanadium Complexes: Effect of Oxidation State, Geometries toward the Pre-Edge Peak Intensity, and the Edge Absorption in the XANES Spectra
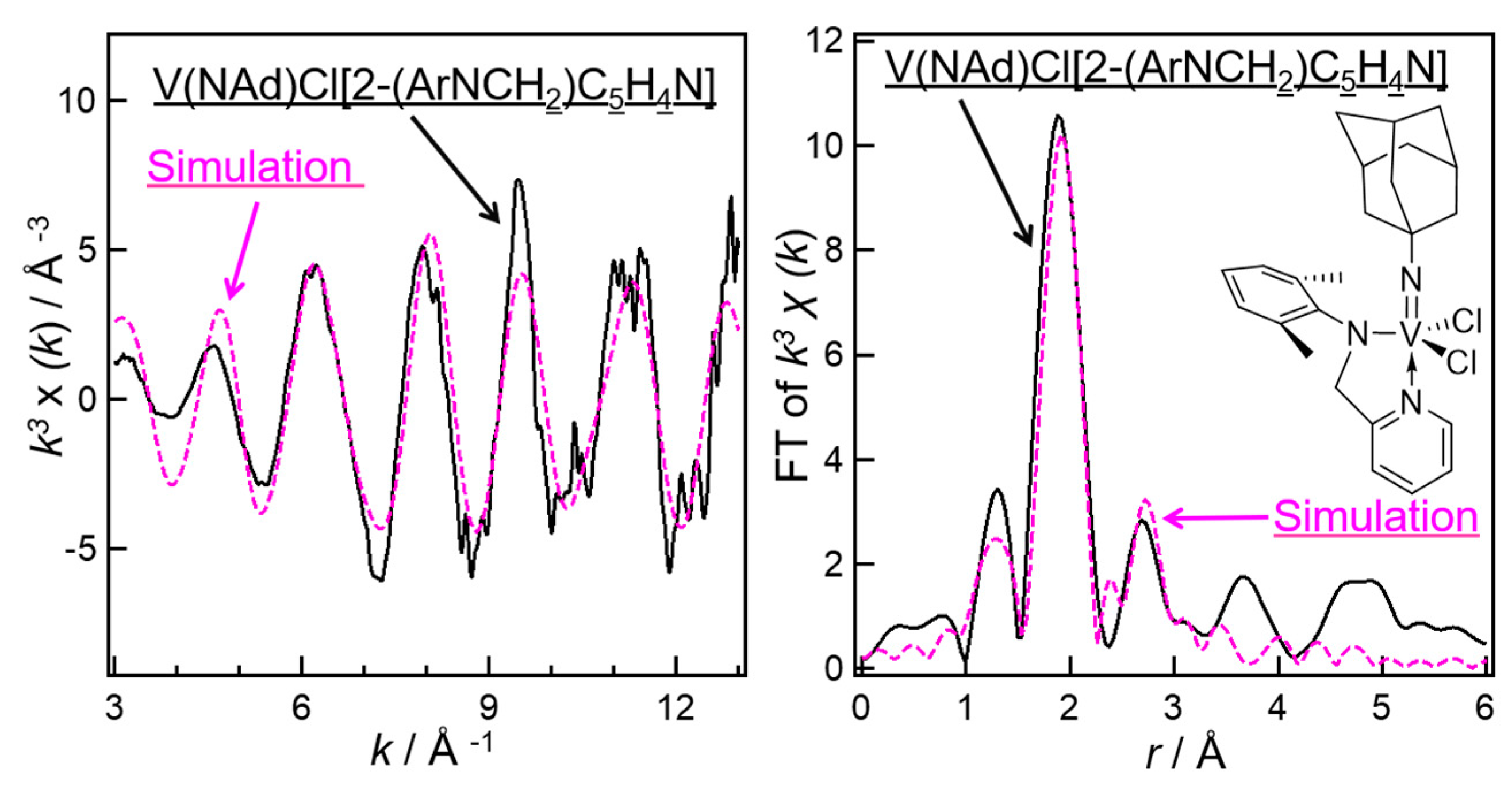
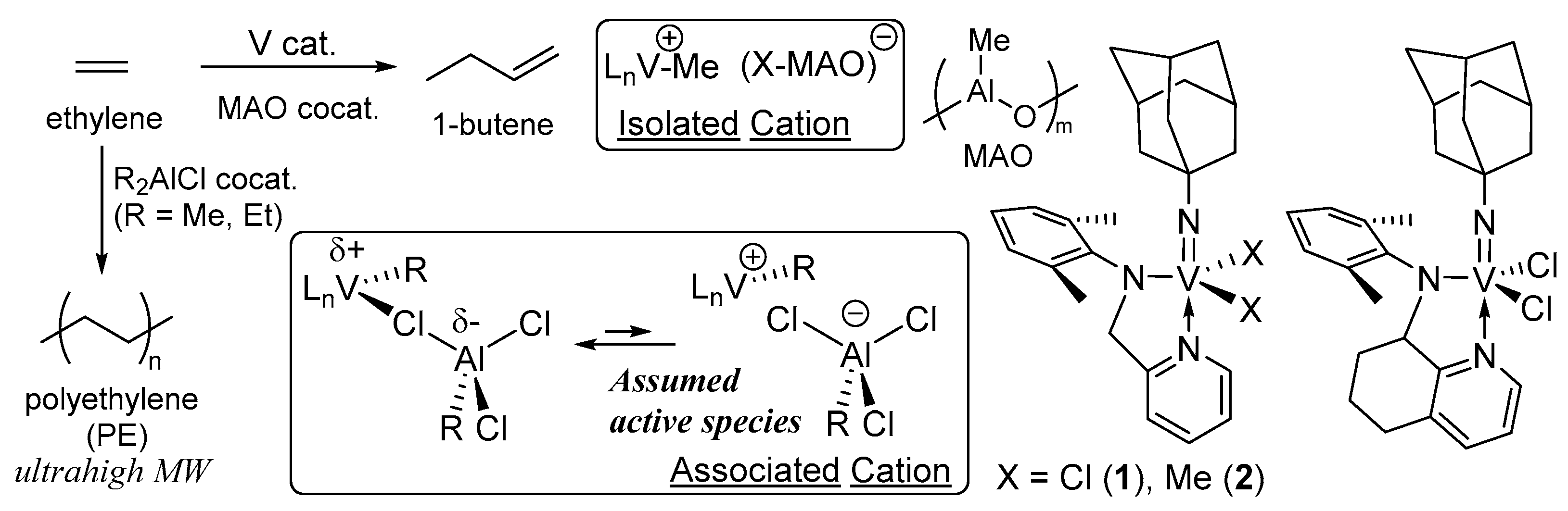
3. Solution XANES Analysis for Exploring Oxidation State of the Catalytically Active Species in Ethylene Dimerization/Polymerization Using V(NAd)X2[2-(ArNCH2)C5H4N] (X = Cl, Me)—Al Cocatalyst Systems
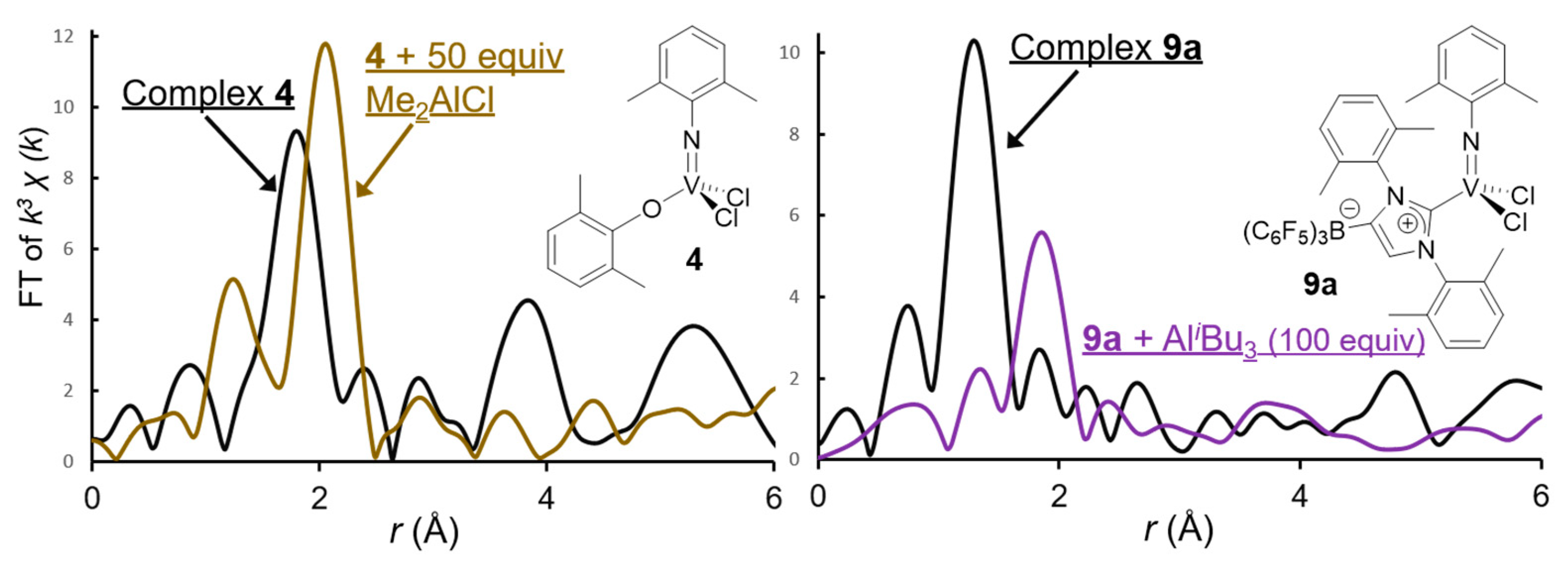
4. Solution XANES Analysis for Exploring the Oxidation State of Catalytically Active Species in Ethylene Polymerization Using (Imido)vanadium(V) Dichloride Complex Catalysts Containing Anionic Ancillary Donor Ligands in the Presence of Al Cocatalysts
5. Concluding Remarks
Funding
Acknowledgments
Conflicts of Interest
References
- Cornils, B.; Hermann, W.A.; Beller, M.; Paciello, R. Applied Homogeneous Catalysis with Organometallic Compounds; Wiley-VCH: Weinheim, Germany, 2017. [Google Scholar]
- Crabtree, R.H. The Organometallic Chemistry of the Transition Metals, 6th ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2014. [Google Scholar]
- Ananikov, V.P. Understanding Organometallic Reaction Mechanisms and Catalysis; Wiley-VCH: Weinheim, Germany, 2015. [Google Scholar]
- Pregosin, P.S. NMR in Organometallic Chemistry; Wiley-VCH: Weinheim, Germany, 2012. [Google Scholar]
- Talsi, E.; Bryliakov, K. Application of EPR and NMR Spectroscopy in Homogeneous Catalysis; CRC Press, Taylor & Francis: Boca Raton, FL, USA, 2017. [Google Scholar]
- Goswami, M.; Chirila, A.; Rebreyend, C.; de Bruin, B. EPR Spectroscopy as a tool in homogeneous catalysis research. Top. Catal. 2015, 58, 719–750. [Google Scholar] [CrossRef]
- Thomas, J.M.; Sankar, G. The role of XAFS in the in situ and ex situ elucidation of active sites in designed solid catalysts. J. Synchrotron Radiat. 2001, 8, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Meitzner, G. In-Situ Spectroscopy in Heterogeneous Catalysis; Wiley-VCH: Weinheim, Germany, 2002; pp. 179–194. [Google Scholar]
- Dent, A.J. Development of time-resolved XAFS instrumentation for quick EXAFS and energy-dispersive EXAFS measurements on catalyst systems. Top. Catal. 2002, 18, 27–35. [Google Scholar] [CrossRef]
- Thomas, J.M.; Catlow, C.R.A.; Sankar, G. Determining the structure of active sites, transition states and intermediates in heterogeneously catalysed reactions. Chem. Commun. 2002, 24, 2921–2925. [Google Scholar] [CrossRef] [PubMed]
- Koningsberger, D.C.; Ramaker, D.E. Handbook of Heterogeneous Catalysis, 2nd ed.; Wiley-VCH: Weinheim, Germany, 2008. [Google Scholar]
- Bare, S.R.; Ressler, T. Characterization of catalysts in reactive atmospheres by X–ray absorption spectroscopy. Adv. Catal. 2009, 52, 339–465. [Google Scholar]
- Iwasawa, Y.; Asakura, K.; Tada, M. (Eds.) XAFS Techniques for Catalysts, Nanomaterials, and Surfaces; Springer: Basel, Switzerland, 2017. [Google Scholar]
- Linehan, J.C.; Balasubramanian, M.; Fulton, J.L. Homogeneous Catalysis: From Metal Atoms to Small Clusters. In XAFS Techniques for Catalysts, Nanomaterials, and Surfaces; Iwasawa, Y., Asakura, K., Tada, M., Eds.; Springer: Basel, Switzerland, 2017; pp. 431–450. [Google Scholar]
- Bartlett, S.A.; Moulin, J.; Tromp, M.; Reid, G.; Dent, A.J.; Cibin, G.; McGuinness, D.S.; Evans, J. Activation of [CrCl3{R-SN(H)S-R}] catalysts for selective trimerization of ethene: A freeze-quench Cr K-edge XAFS study. ACS Catal. 2014, 4, 4201–4204. [Google Scholar] [CrossRef]
- Takaya, H.; Nakajima, S.; Nakagawa, N.; Isozaki, K.; Iwamoto, T.; Imayoshi, R.; Gower, N.J.; Adak, L.; Hatakeyama, T.; Honma, T.; et al. Investigation of Organoiron Catalysis in Kumada–Tamao–Corriu-Type Cross-Coupling Reaction Assisted by Solution-Phase X-ray Absorption Spectroscopy. Bull. Chem. Soc. Jpn. 2015, 88, 410–418. [Google Scholar] [CrossRef]
- Agata, R.; Takaya, H.; Matsuda, H.; Nakatani, N.; Takeuchi, K.; Iwamoto, T.; Hatakeyama, T.; Nakamura, N. Iron-Catalyzed Cross Coupling of Aryl Chlorides with Alkyl Grignard Reagents: Synthetic Scope and FeII/FeIV Mechanism Supported by X-ray Absorption Spectroscopy and Density Functional Theory Calculations. Bull. Chem. Soc. Jpn. 2019, 92, 381–390. [Google Scholar] [CrossRef]
- Hirano, M.; Sano, K.; Kanazawa, Y.; Komine, N.; Maeno, Z.; Mitsudome, T.; Takaya, H. Mechanistic Insights on Pd/Cu-Catalyzed Dehydrogenative Coupling of Dimethyl Phthalate. ACS Catal. 2018, 8, 5827–5841. [Google Scholar] [CrossRef]
- Nomura, K.; Mitsudome, T.; Igarashi, A.; Nagai, G.; Tsutsumi, K.; Ina, T.; Omiya, T.; Takaya, H.; Yamazoe, S. Synthesis of (adamantylimido)vanadium(V) dimethyl complex containing (2-amilidomethyl)pyridine ligand and selected reactions: Exploring the oxidation state of the catalytically active species in ethylene dimerization. Organometallics 2017, 36, 530–542. [Google Scholar] [CrossRef]
- Nagai, G.; Mitsudome, T.; Tsutsumi, K.; Sueki, S.; Ina, T.; Tamm, M.; Nomura, K. Effect of Al cocatalyst in ethylene and ethylene/norbornene (co)polymerization by (imido)vanadium bichloride complexes containing anionic N-heterocyclic carbenes having weakly coordinating borate moiety. J. Jpn. Pet. Inst. 2017, 60, 256–262. [Google Scholar] [CrossRef]
- Nomura, K.; Tsutsumi, K.; Nagai, G.; Omiya, T.; Ina, T.; Yamazoe, S.; Mitsudome, T. Solution XAS analysis of various (imido)vanadium(V) dichloride complexes containing monodentate anionic ancillary donor ligands: Effect of aluminium cocatalyst in ethylene/norbornene (co)polymerization. J. Jpn. Pet. Inst. 2018, 61, 282–287. [Google Scholar] [CrossRef]
- Nomura, K.; Oshima, M.; Mitsudome, T.; Harakawa, H.; Hao, P.; Tsutsumi, K.; Nagai, G.; Ina, T.; Takaya, H.; Sun, W.-H.; et al. Synthesis, structural analysis of (imido)vanadium dichloride complexes containing 2- (2′-benz-imidazolyl) pyridine ligands: Effect of Al cocatalyst for efficient ethylene (co)polymerization. ACS Omega 2017, 2, 8660–8673. [Google Scholar] [CrossRef] [PubMed]
- Nomura, K.; Nagai, G.; Mitsudome, T.; Tamm, M.; Yamazoe, S. XAS Analysis for reactions of (arylimido)vanadium(V) dichloride complexes containing anionic NHC that contains weakly coordinating B(C6F5)3 moiety (WCA-NHC) or phenoxide ligands with Al alkyls: A potential ethylene polymerization catalyst with WCA-NHC ligand. ACS Omega 2019, 4, 18833–18845. [Google Scholar]
- Kuboki, M.; Nomura, K. (Arylimido)niobium(V) complexes containing 2-pyridylmethylanilido ligand as catalyst precursors for ethylene dimerization that proceeds via cationic Nb(V) species. Organometallics 2019, 38, 1544–1559. [Google Scholar] [CrossRef]
- Nomura, K.; Mitsudome, T.; Tsutsumi, K.; Yamazoe, S. Solution XAS analysis for exploring the active species in homogeneous vanadium complex catalysis. J. Phys. Soc. Jpn. 2018, 87, 061014. [Google Scholar] [CrossRef]
- Nomura, K.; Mitsudome, T.; Yamazoe, S. Direct observation of catalytically active species in reaction solution by X-ray absorption spectroscopy (XAS). Jpn. J. Appl. Phys. 2019, 58, 100502. [Google Scholar] [CrossRef]
- Hagen, H.; Boersma, J.; van Koten, G. Homogeneous vanadium-based catalysts for the Ziegler–Natta polymerization of α-olefins. Chem. Soc. Rev. 2002, 31, 357–364. [Google Scholar] [CrossRef]
- Gambarotta, S. Vanadium-based Ziegler–Natta: Challenges, promises, problems. Coord. Chem. Rev. 2003, 237, 229–243. [Google Scholar] [CrossRef]
- Nomura, K. New Developments in Catalysis Research; Bevy, L.P., Ed.; NOVA Science Publishers: New York, NY, USA, 2005; pp. 199–217. [Google Scholar]
- Redshaw, C. Vanadium procatalysts bearing chelating aryloxides: Structure–activity trends in ethylene polymerization. Dalton Trans. 2010, 39, 5595–5604. [Google Scholar] [CrossRef]
- Nomura, K.; Zhang, S. Design of vanadium complex catalysts for precise olefin polymerization. Chem. Rev. 2011, 111, 2342–2362. [Google Scholar] [CrossRef] [PubMed]
- Nomura, K.; Hou, X. Olefin Polymerization by Vanadium Complex Catalysts. In Handbook of Transition Metal Polymerization Catalysts, 2nd ed.; Hoff, R., Ed.; Wiley: Hoboken, NJ, USA, 2018; pp. 313–338. [Google Scholar]
- Carrick, W.L. Mechanism of ethylene polymerization with vanadium catalysts. J. Am. Chem. Soc. 1958, 80, 6455–6456. [Google Scholar] [CrossRef]
- Carrick, W.L.; Kluiber, R.W.; Bonner, E.F.; Wartman, L.H.; Rugg, F.M.; Smyth, J.J. Transition metal catalysts. I. Ethylene polymerization with a soluble catalyst formed from an aluminum halide, tetraphenyltin and a vanadium Halide. J. Am. Chem. Soc. 1960, 82, 3883–3887. [Google Scholar] [CrossRef]
- Phillips, G.W.; Carrick, W.L. Transition metal catalysts VIII. The role of oxygen in ethylene polymerizations with the AlBr3-VXn-Sn(C6H5)4 catalyst. J. Polym. Sci. 1962, 59, 401–412. [Google Scholar] [CrossRef]
- Junghanns, E.; Gumboldt, O.; Bier, G. Polymerisation von äthylen und propylen zu amorphen copolymerisaten mit katalysatoren aus vanadiumoxychlorid und aluminiumhalogenalkylen. Makromol. Chem. 1962, 58, 18–42. [Google Scholar] [CrossRef]
- Natta, G.; Mazzanti, G.; Valvassori, A.; Sartori, G.; Fiumani, D. Ethylene–propylene copolymerization in the presence of catalysts prepared from vanadium triacetylacetonate. J. Polym. Sci. 1961, 51, 411–427. [Google Scholar] [CrossRef]
- Christman, D.L.; Keim, G.I. Reactivities of nonconjugated dienes used in preparation of terpolymers in homogeneous systems. Macromolecules 1968, 1, 358–363. [Google Scholar] [CrossRef]
- Christman, D.L. Preparation of polyethylene in solution. J. Polym. Sci. Part A-1 1972, 10, 471. [Google Scholar] [CrossRef]
- Natta, G.; Zambelli, A.; Lanzi, G.; Pasquon, I.; Mognaschi, E.R.; Segre, A.L.; Centola, P. Polymerization of propylene to syndiotactic polymer. Part. I: Valence of active vanadium in the catalytic systems. Makromol. Chem. 1965, 81, 161–172. [Google Scholar] [CrossRef]
- Zambelli, A.; Pasquon, I.; Signorini, R.; Natta, G. Polymerization of propylene to syndiotactic polymer. III. Behaviour of the catalyst system VCl4–Al(C2H5)2Cl in the presence of lewis bases. Makromol. Chem. 1968, 112, 160–182. [Google Scholar] [CrossRef]
- Lehr, M.H. The active oxidation state of vanadium in soluble monoolefin polymerization catalysts. Macromolecules 1968, 1, 178–184. [Google Scholar] [CrossRef]
- Lehr, M.H.; Carman, C.J. Electron spin resonance evidence of inactive V(III) precursor to catalytically active V(III) in vanadium tetrachloride Ziegler catalysts. Macromolecules 1969, 2, 217–219. [Google Scholar] [CrossRef]
- Pioneering, E.S.R. Study for monitoring the catalytically active species (VCl4—Et2AlCl catalyst system). Macromolecules 1969, 2, 217. [Google Scholar]
- Bigmore, H.B.; Zuideveld, M.A.; Kowalczyk, R.M.; Cowley, A.R.; Kranenburg, M.; McInnes, E.J.L.; Mountford, P. Synthesis, structures, and olefin polymerization capability of vanadium(4+) imido compounds with fac-N3 donor ligands. Inorg. Chem. 2006, 45, 6411–6423. [Google Scholar] [CrossRef]
- Soshnikov, I.E.; Semikolenova, N.V.; Shubin, A.A.; Bryliakov, K.P.; Zakharov, V.A.; Redshaw, C.; Talsi, E.P. EPR Monitoring of vanadium(IV) species formed upon activation of vanadium(V) polyphenolate precatalysts with AlR2Cl and AlR2Cl/ethyltrichloroacetate (R = Me, Et). Organometallics 2009, 28, 6714–6720. [Google Scholar] [CrossRef]
- Soshnikov, I.E.; Semikolenova, N.V.; Bryliakov, K.P.; Zakharov, V.A.; Redshaw, C.; Talsi, E.P. An EPR study of the vanadium species formed upon interaction of vanadyl N and C-capped tris(phenolate) complexes with AlEt3 and AlEt2Cl. J. Mol. Catal. A 2009, 303, 23–29. [Google Scholar] [CrossRef]
- Soshnikov, I.E.; Semikolenova, N.V.; Bryliakov, K.P.; Shubin, A.A.; Zakharov, V.A.; Redshaw, C.; Talsi, E.P. An EPR study of the V(IV) species formed upon activation of a vanadyl phenoxyimine polymerization catalyst with AlR3 and AlR2Cl (R = Me, Et). Macromol. Chem. Phys. 2009, 210, 542–548. [Google Scholar] [CrossRef]
- Asakura, H.; Shishido, T.; Yamazoe, S.; Teramura, K.; Tanaka, T. Structural analysis of group V, VI, and VII metal compounds by XAFS. J. Phys. Chem. C 2011, 115, 23653–23663. [Google Scholar] [CrossRef]
- Yamamoto, T. What is the origin of pre-edge peaks in K-edge XANES spectra of 3d transition metals: Electric dipole or quadrupole? Adv. X-Ray Chem. Anal. Jpn. 2007, 38, 45–65. [Google Scholar]
- Yamamoto, T. Assignment of pre-edge peaks in K-edge x-ray absorption spectra of 3d transition metal compounds: Electric dipole or quadrupole? X-Ray Spectrom. 2008, 37, 572–584. [Google Scholar] [CrossRef]
- Wong, J.; Lytle, F.W.; Messmer, R.P.; Maylotte, D.H. K-edge absorption spectra of selected vanadium compounds. Phys. Rev. B 1984, 30, 5596–5610. [Google Scholar] [CrossRef]
- Zhang, W.; Nomura, K. Synthesis of (1-adamantylimido)vanadium(V) complexes containing aryloxo, ketimide ligands: Effect of ligand substituents in olefin insertion/metathesis polymerization. Inorg. Chem. 2008, 47, 6482–6492. [Google Scholar] [CrossRef]
- Buijink, J.-K.F.; Teubin, J.H.; Kooijman, H.; Spek, A.L. Synthesis, molecular structure, and reactivity of a half-sandwich vanadium(III) imido complex: The first vanadium(V) alkylidene. Organometallics 1994, 13, 2922–2924. [Google Scholar] [CrossRef]
- Yamada, J.; Fujiki, M.; Nomura, K. Synthesisof various (arylimido)vanadium(V)-methyl complexes containing ketimide ligands and reactions with alcohols, thiols, borates: Implications for unique reactivity toward alcohols. Organometallics 2007, 26, 2579–2588. [Google Scholar] [CrossRef]
- Nomura, K.; Sagara, A.; Imanishi, Y. Olefin polymerization and ring-opening metathesis polymerization of norbornene by (arylimido)(aryloxo)vanadium(V) complexes of the type VX2(NAr)(Oar′). Remarkable effect of aluminum cocatalyst for the coordination and insertion and ring-opening metathesis polymerization. Macromolecules 2002, 35, 1583–1590. [Google Scholar]
- Yi, J.; Nakatani, N.; Nomura, K.; Hada, M. Time-dependent DFT study of K-edge spectra for vanadium and titanium complexes: Effects of chloride ligands on pre-edge features. Submitted for publication. [CrossRef]
- Igarashi, A.; Kolychev, E.L.; Tamm, M.; Nomura, K. Synthesis of (imido)vanadium(V) dichloride complexes containing anionic N-heterocyclic carbenes that contain a weakly coordinating borate moiety: New MAO-free ethylene polymerization catalysts. Organometallics 2016, 35, 1778–1784. [Google Scholar] [CrossRef]
- Yamada, J.; Nomura, K. A vanadium(V) alkylidene complex exhibiting remarkable catalytic activity for ring-opening metathesis polymerization (ROMP). Organometallics 2005, 24, 2248–2250. [Google Scholar] [CrossRef]
- Zhang, S.; Nomura, K. Highly efficient dimerization of ethylene by (imido)vanadium complexes containing (2-anilidomethyl)pyridine ligands: Notable ligand effect toward activity and selectivity. J. Am. Chem. Soc. 2010, 132, 4960–4965. [Google Scholar] [CrossRef]
- Peuckert, M.; Keim, W. A new nickel complex for the oligomerization of ethylene. Organometallics 1983, 2, 594–597. [Google Scholar] [CrossRef]
- Keim, W. Nickel: An element with wide application in industrial homogeneous catalysis. Angew. Chem. Int. Ed. Engl. 1990, 29, 235–244. [Google Scholar] [CrossRef]
- Keim, W. Oligomerization of ethylene to α-olefins: Discovery and development of the Shell Higher Olefin Process (SHOP). Angew. Chem. Int. Ed. 2013, 52, 12492–12496. [Google Scholar] [CrossRef]
- Pillai, S.M.; Ravindranathan, M.; Sivaram, S. Dimerization of ethylene and propylene catalyzed by transition-metal complexes. Chem. Rev. 1986, 86, 353–399. [Google Scholar] [CrossRef]
- Skupinska, J. Oligomerization of α-olefins to higher oligomers. Chem. Rev. 1991, 91, 613–648. [Google Scholar] [CrossRef]
- Speiser, F.; Braunstein, P.; Saussine, L. Catalytic ethylene dimerization and oligomerization: Recent developments with nickel complexes containing P, N-chelating ligands. Acc. Chem. Res. 2005, 38, 784–793. [Google Scholar] [CrossRef]
- Belov, G.P.; Matkovsky, P.E. Processes for the production of higher linear α-olefins. Pet. Chem. 2010, 50, 283–289. [Google Scholar] [CrossRef]
- Mahdaviani, S.H.; Parvari, M.; Soudbar, D. Ethylene dimerization by a homogeneous Ti-based three component catalyst system: Process evaluation and optimization of parametric performance. Proc. Eng. 2012, 42, 616–622. [Google Scholar] [CrossRef][Green Version]
- McGuinness, D.S. Olefin oligomerization via metallacycles: Dimerization, trimerization, tetramerization, and beyond. Chem. Rev. 2011, 111, 2321–2341. [Google Scholar] [CrossRef]
- Agapie, T. Selective ethylene oligomerization: Recent advances in chromium catalysis and mechanistic investigations. Coord. Chem. Rev. 2011, 255, 861–880. [Google Scholar] [CrossRef]
- Van Leeuwen, P.W.N.M.; Clément1, N.D.; Tschan, M.J.-L. New processes for the selective production of 1-octene. Coord. Chem. Rev. 2011, 255, 1499–1517. [Google Scholar] [CrossRef]
- Zhang, W.; Sun, W.-H.; Redshaw, C. Tailoring iron complexes for ethylene oligomerization and/or polymerization. Dalton Trans. 2013, 42, 8988–8997. [Google Scholar] [CrossRef]
- Wang, S.; Sun, W.-H.; Redshaw, C. Recent progress on nickel-based systems for ethylene oligo-/polymerization catalysis. J. Organomet. Chem. 2014, 751, 717–741. [Google Scholar] [CrossRef]
- Boudier, A.; Breuil, P.-A.R.; Magna, L.; Olivier-Bourbigou, H.; Braunstein, P. Ethylene oligomerization using iron complexes: Beyond the discovery of bis(imino)pyridine ligands. Chem. Commun. 2014, 50, 1398–1407. [Google Scholar] [CrossRef]
- Small, B.L. Discovery and development of pyridine-bis(imine) and related catalysts for olefin polymerization and oligomerization. Acc. Chem. Res. 2015, 48, 2599–2611. [Google Scholar] [CrossRef]
- Wang, Z.; Solan, G.A.; Zhang, W.; Sun, W.-H. Carbocyclic-fused N, N, N-pincer ligands as ring-strain adjustable supports for iron and cobalt catalysts in ethylene oligo-/polymerization. Coord. Chem. Rev. 2018, 363, 92–108. [Google Scholar] [CrossRef]
- Bryliakov, K.P.; Antonov, A.A. Recent progress of transition metal based catalysts for the selective dimerization of ethylene. J. Organomet. Chem. 2018, 867, 55–61. [Google Scholar] [CrossRef]
- Reagan, W.K. Process for Olefin Polymerization; EP 0417477; Phillips Petroleum Company: Bartlesville, OK, USA, 1991. [Google Scholar]
- Suzuki, Y.; Kinoshita, S.; Shibahara, A.; Ishii, S.; Kawamura, K.; Inoue, Y.; Fujita, T. Trimerization of ethylene to 1-hexene with titanium complexes bearing phenoxy-imine ligands with pendant donors combined with MAO. Organometallics 2010, 29, 2394–2396. [Google Scholar] [CrossRef]
- Tang, X.-Y.; Igarashi, A.; Sun, W.-H.; Inagaki, A.; Liu, J.; Zhang, W.; Li, Y.-S.; Nomura, K. Synthesis of (imido)vanadium(V) complexes containing 8-(2,6-dimethylanilide)-5,6,7-trihydroquinoline ligands: Highly active catalyst precursors for ethylene dimerization. Organometallics 2014, 33, 1053–1060. [Google Scholar] [CrossRef]
- Igarashi, A.; Zhang, S.; Nomura, K. Ethylene dimerization/polymerization catalyzed by (adamantylimido)vanadium(V) complexes containing (2-anilidomethyl)pyridine ligands: Factors affecting the ethylene reactivity. Organometallics 2012, 31, 3575–3581. [Google Scholar] [CrossRef]
- Wang, W.; Nomura, K. Remarkable effects of aluminum cocatalyst and comonomer in ethylene copolymerizations catalyzed by (arylimido)(aryloxo)vanadium complexes: Efficient synthesis of high molecular weight ethylene/norbornene copolymer. Macromolecules 2005, 38, 5905–5913. [Google Scholar] [CrossRef]
- Wang, W.; Nomura, K. Notable effects of aluminum alkyls and solvents for highly efficient ethylene (co)polymerizations catalyzed by (arylimido)(aryloxo)vanadium complexes. Adv. Synth. Catal. 2006, 348, 743–750. [Google Scholar] [CrossRef]
- Nomura, K.; Bahuleyan, B.K.; Zhang, S.; Sharma, P.M.V.; Katao, S.; Igarashi, A.; Inagaki, A.; Tamm, M. Synthesis and structural analysis of (imido)vanadium(V) dichloride complexes containing iminoimidazolide and iminoimidazolidide ligands, and their use as catalyst precursors for ethylene (co)polymerization. Inorg. Chem. 2014, 53, 607–623. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Katao, S.; Sun, W.-H.; Nomura, K. Synthesis of (arylimido)vanadium(V) complexes containing (2-anilidomethyl)pyridine ligands and their use as the catalyst precurosors for olefin polymerization. Organometallics 2009, 28, 5925–5933. [Google Scholar] [CrossRef]
- Nomura, K.; Izawa, I.; Yi, J.; Nakatani, N.; Aoki, H.; Ina, T.; Mitsudome, T.; Tomotsu, N.; Yamazoe, S. Solution XAS analysis for exploring active species in syndiospecific styrene polymerization and 1-hexene polymerization using half-titanocene—MAO catalysts: Significant changes in the oxidation state in the presence of styrene. Organometallics 2019, 38, 4497–4507. [Google Scholar] [CrossRef]





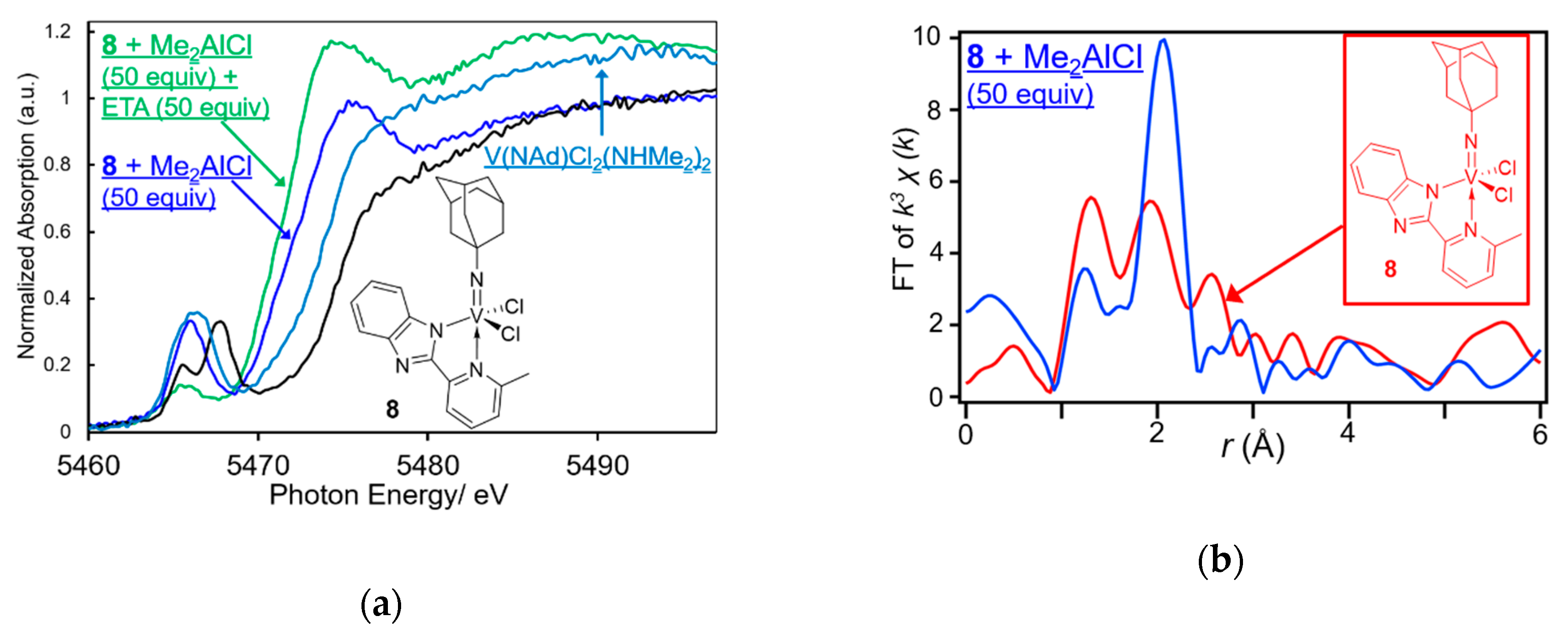
| Cat. | Al Cocat. | Al/V | Oligomer | PE | |||
|---|---|---|---|---|---|---|---|
| (μmol) | Molar Ratio | Activity b | TOF c/h−1 (s−1) | C4’ d/% | Activity b | Mηe × 10−6 | |
| 1 (0.5) | MAO | 500 | 50,100 | 1,830,000 (508) | 92.5 | -- | -- |
| 1 (0.1) | MAO | 1500 | 76,500 | 2,730,000 (758) | 97.0 | -- | -- |
| 1 (5.0) | Me2AlCl | 200 | trace | -- | -- | 704 | 5.92 |
| 1 (5.0) | Et2AlCl | 100 | trace | -- | -- | 137 | 6.76 |
| 2 (0.5) | MAO | 500 | 98,800 | 3,460,000 (961) | 91.0 | -- | -- |
| 2 (0.5) | MAO | 1500 | 82,300 | 2,880,000 (800) | 87.9 | -- | -- |
| 3 (0.1) | MAO | 3000 | 205,400 | 7,190,000 (2000) | 97.7 | -- | -- |
| 3 (0.1) | MAO | 4000 | 274,000 | 9,600,000 (2670) | 97.7 | -- | -- |
| Run | Cat. (μmol) | Al Cocat. | Al/V b | ETA c | Temp./°C | Time/min | Activity d |
|---|---|---|---|---|---|---|---|
| 1 | 4 (1.0) | MAO | 2500 | -- | 25 | 10 | 2930 |
| 2 | 4 (0.05) | Me2AlCl | 5000 | -- | 0 | 10 | 27,500 |
| 3 | 4 (0.05) | Et2AlCl | 5000 | -- | 0 | 10 | 11,700 |
| 4 | 4 (0.05) | Et2AlCl | 5000 | -- | 0 | 10 | 11,400 |
| 5 | 4 (0.05) | Et2AlCl | 5000 | 10 | 0 | 10 | 1080 |
| 6 | 4 (0.05) | iBu2AlCl | 5000 | -- | 0 | 10 | 52,000 |
| 7 | 4 (1.0) | Et2Al(OEt) | 500 | -- | 0 | 10 | trace |
| 8 | 4 (1.0) | iBu3Al | 500 | -- | 0 | 10 | trace |
| 9 | 5 (2.0) | MAO | 1000 | -- | 25 | 10 | 507 |
| 10 | 5 (0.04) | Et2AlCl | 2000 | -- | 0 | 10 | 38,300 |
| 11 | 5 (0.04) | Et2AlCl | 2000 | -- | 25 | 10 | 7650 |
| 12 | 6 (2.0) | MAO | 1000 | -- | 25 | 10 | 627 |
| 13 | 6 (0.04) | Et2AlCl | 1000 | -- | 0 | 10 | 32,000 |
| 14 | 6 (0.04) | Et2AlCl | 1000 | -- | 25 | 10 | 6000 |
| 15 | 8 (0.05) | Me2AlCl | 5000 | -- | 25 | 6 | 21,700 |
| 16 | 8 (0.05) | Me2AlCl | 10,000 | -- | 25 | 6 | 55,700 |
| 17 | 8 (0.05) | Me2AlCl | 10,000 | 50 | 25 | 6 | 46,700 |
| 18 | 8 (0.05) | Me2AlCl | 10,000 | 100 | 25 | 6 | 82,000 |
| Run | Cat. (μmol) | Al Cocat. | Al/V b | Temp./°C | Activity c | Mnd × 10−4 | Mw/Mnd |
|---|---|---|---|---|---|---|---|
| 19 | 9a (1.0) | MAO | 500 | 25 | 1580 | 1.53 | 1.93 |
| 20 | 9b (0.2) | MAO | 2000 | 25 | 19,900 | ||
| 21 | 9b (0.2) | MAO | 3000 | 25 | 19,500 | 9.46 | 1.56 |
| 22 | 9a (0.2) | Et3Al | 25 | 0 | 954 | 1.31 | 1.89 |
| 23 | 9a (0.2) | iBu3Al | 25 | 0 | 7430 | 1.43 | 1.72 |
| 24 | 9a (0.2) | iBu3Al | 50 | 0 | 6360 | 2.32 | 1.42 |
| 25 | 9a (0.2) | iBu3Al | 50 | 25 | 11,000 | 1.80 | 1.76 |
| 26 | 9a (0.2) | Et2AlCl | 2500 | 0 | 1970 | insoluble | |
| 27 | 9b (0.1) | iBu3Al | 600 | 25 | 22,400 | ||
| 28 | 9b (0.02) | iBu3Al | 2000 | 25 | 47,100 | ||
| 29 | 9b (0.02) | iBu3Al | 2500 | 25 | 66,000 | 13.7 | 2.35 |
| Complex 4 | 4 + Me2AlCl | Complex 9a | 9a + AliBu3 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Atom | C.N. | r (Å) | C.N. | r (Å) | Atom | C.N. | r (Å) | C.N. | r (Å) |
| N(O) | 2.4 ± 0.3 | 1.80 ± 0.05 | 1.3 ± 0.2 | 1.64 ± 0.04 | N(C) | 2.1 ± 0.2 | 1.62 ± 0.03 | 0.8 ± 0.3 | 1.66 ± 0.17 |
| Cl | 1.9 ± 0.2 | 2.18 ± 0.03 | 2.0 ± 0.2 | 2.45 ± 0.03 | Cl | 1.0 ± 0.2 | 2.16 ± 0.04 | ||
| Cl | 1.0 ± 0.2 | 2.34 ± 0.05 | 1.0 ± 0.2 | 2.34 ± 0.04 | |||||
| Complex 8 | 8 + Me2AlCl | |||
|---|---|---|---|---|
| Atom | C.N. | r (Å) | C.N. | r (Å) |
| N | 1.7(2) | 1.683(5) | 0.9(3) | 1.64(2) |
| N | 1.2(8) | 2.290(42) | ||
| Cl | 1.6(2) | 2.293(3) | 2.6(1) | 2.455(7) |
© 2019 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nomura, K. Solution X-Ray Absorption Spectroscopy (XAS) for Analysis of Catalytically Active Species in Reactions with Ethylene by Homogeneous (Imido)vanadium(V) Complexes—Al Cocatalyst Systems. Catalysts 2019, 9, 1016. https://doi.org/10.3390/catal9121016
Nomura K. Solution X-Ray Absorption Spectroscopy (XAS) for Analysis of Catalytically Active Species in Reactions with Ethylene by Homogeneous (Imido)vanadium(V) Complexes—Al Cocatalyst Systems. Catalysts. 2019; 9(12):1016. https://doi.org/10.3390/catal9121016
Chicago/Turabian StyleNomura, Kotohiro. 2019. "Solution X-Ray Absorption Spectroscopy (XAS) for Analysis of Catalytically Active Species in Reactions with Ethylene by Homogeneous (Imido)vanadium(V) Complexes—Al Cocatalyst Systems" Catalysts 9, no. 12: 1016. https://doi.org/10.3390/catal9121016
APA StyleNomura, K. (2019). Solution X-Ray Absorption Spectroscopy (XAS) for Analysis of Catalytically Active Species in Reactions with Ethylene by Homogeneous (Imido)vanadium(V) Complexes—Al Cocatalyst Systems. Catalysts, 9(12), 1016. https://doi.org/10.3390/catal9121016





