Abstract
Cu-containing CHA type (Cu-CHA) zeolites have been widely investigated owing to their excellent low-temperature activity and high hydrothermal stability in selective catalytic reduction of NOx with NH3 (NH3-SCR). Herein, a series of Cu-SAPO-44 zeolites were prepared by one-pot method with dual-amine templates and the subsequent ion exchange (IE) with NH4NO3. The effect of NH4NO3 treatment on Cu species was investigated by X-ray powder diffraction (XRD), N2 adsorption-desorption isotherm, inductively coupled plasma (ICP); field-emission scanning electron microscope (FE-SEM), high-resolution transmission electron microscope (HRTEM), X-ray absorption fine structure (XAFS), and H2-temperature-programmed reduction (H2-TPR). The results indicated that—besides the main SAPO-44 structure—the CuO phase was detected by XRD in original samples. After IE with NH4NO3, the Cu contents decreased greatly from ICP analysis. The removal of CuO agglomerations and the presence of highly dispersed CuO nanoparticles (~2.36 nm) were confirmed by SEM, TEM and H2-TPR. Furthermore, a significant increase in the proportion of isolated Cu2+ was derived from XAFS. As a result, the activity at higher temperature (≥350 °C) was improved a lot.
1. Introduction
Aqueous ion exchange (IE) is the most widely used method in preparation of small pore Cu-chabazite (Cu-CHA) zeolites [1,2,3]. Multiple IE procedures are time consuming; therefore, one-pot synthesis methods were hence developed [4,5,6,7,8,9]. For instance, Ren et al. pioneered the synthesis of Cu-SSZ-13 using Cu2+-tetraethylenepentamine (Cu-TEPA) complex as structure-directing agent (SDA), with Cu loading in the range 0–10 wt.% [10]. Soon after, Cu-SAPO-34 zeolites with controllable Cu-loadings were synthesized by one-pot method using Cu-TEPA and low-cost SDAs [6,11,12,13]. More recently, another Cu-CHA zeolite, Cu-SAPO-44, was also reported [14]. In contrast to IE method, one-pot method could directly introduce Cu2+ ions into zeolites frameworks, and thus achieve high Cu loading and high dispersion of Cu species, leading to excellent NH3-SCR activity [15].
The downside, however, is that one-pot synthesis method tend to introduce excessive Cu species into CHA zeolites, which is detrimental to the hydrothermal stability of Cu-CHA catalysts [5]. IE with NH4NO3 was discovered as an efficient approach to achieve moderate Cu-loadings in one-pot synthesis of Cu-CHA zeolites by removing the excessive Cu species [10,16,17]. Xie et al. reported that one-time IE with NH4NO3 was completely sufficient to eliminate excess Cu species from the SSZ-13 structure and re-disperse the remaining Cu2+ ions [15]. Guo et al. demonstrated that Cu2+ ions migrated from large cages to more stable locations in six-membered rings of CHA structure after IE with NH4NO3 [17]. Nevertheless, there still lacks direct observation on the structural change of Cu species from one-pot synthesized Cu-CHA zeolites.
Herein, a series of Cu-SAPO-44 zeolites were fabricated through one-pot method with the dual-amine templates (denoted as Cux-SAPO-44) followed with ion exchange with NH4NO3 solution (denoted as Cux-SAPO-44-IE). The effects of IE with NH4NO3 on the textural and physical-chemical properties of Cu-SAPO-44, as well as the nature and location of active Cu species, were investigated. Interestingly, IE with NH4NO3 removed excessive CuO agglomerations, leading to a high proportion of isolated Cu2+ ions responsible for the high SCR activity.
2. Results and Discussion
2.1. XRD
Figure 1 displays XRD patterns of Cu-SAPO-44 zeolites before and after IE with NH4NO3. The diffraction peaks for all samples are in accordance with those of pure SAPO-44 reported in our previous work [18], confirming the CHA structure [14,19]. However, the CuO phase was present in Cux-SAPO-44. Interestingly, the Cux-SAPO-44-IE samples show only diffraction patterns of SAPO-44, suggesting that IE with NH4NO3 can remove the excess CuO species and simultaneously retain the SAPO-44 structure.
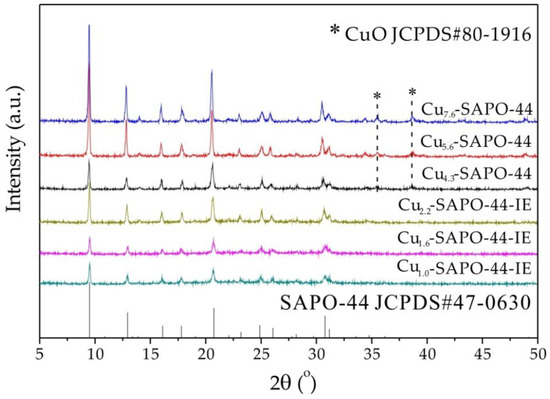
Figure 1.
XRD patterns of Cux-SAPO-44 and Cux-SAPO-44-IE.
2.2. ICP
The compositions of all samples were analyzed by ICP and listed in Table 1. Although the Si/Al ratios do not change much after NH4NO3 treatment, the Cu contents decrease evidently after IE with NH4NO3, in accordance with above XRD results.

Table 1.
ICP results for Cux-SAPO-44 and Cux-SAPO-44-IE.
2.3. Catalytic Activity
SCR performances of Cux-SAPO-44 and Cux-SAPO-44-IE are shown in Figure 2. The Cux-SAPO-44 samples show 90% NOx conversion between 200 and 350 °C as well as high N2 selectivity. However, NOx conversions decrease rapidly when the reaction temperatures are above 300 °C, due to the over-oxidation of NH3 in the presence of CuO [15,20,21]. Notably, Cux-SAPO-44-IE samples exhibit improved NOx conversion at high temperatures, reaching above 90% at 200–450 °C with nearly 100% N2 selectivity. This is consistent with the fact that the excess CuO species promote the oxidation of NH3 [10,15,17]. To gain more information about the beneficial role of IE, further characterization was performed taking the control sample (Cu5.6-SAPO-44) and the best sample (Cu1.6-SAPO-44-IE) as examples.
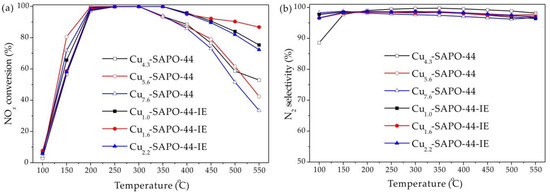
Figure 2.
NO conversion (a) and N2 selectivity (b) for Cux-SAPO-44 and Cux-SAPO-44-IE as a function of temperature. Reaction conditions: [O2] = 5.3 vol.%, [NO] = [NH3] = 500 ppm, balance He, total flow rate = 300 mL/min, GHSV = 100,000 h−1.
Based on the practical consideration, NH3-SCR performance of the Cu1.6-SAPO-44-IE catalyst was also evaluated as 5 vol.% H2O contained in feeding gas under the GHSV of 100,000 h−1, and the result was shown in Figure 3. Unsurprisingly, a decrease in NOx conversion was observed at low temperatures (<250 °C), which could be ascribed to the competitive adsorption of H2O with NH3. In contrast, NOx conversion at high temperatures (>400 °C) was promoted, most likely due to the inhibition effect of H2O on the unselective catalytic oxidation of NH3 [22].
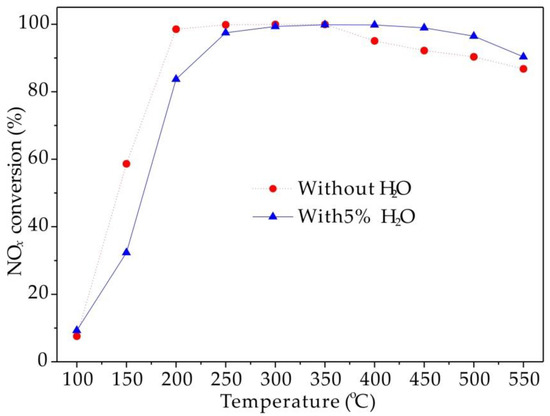
Figure 3.
Effect of H2O on NH3-SCR performance of Cu1.6-SAPO-44-IE.
2.4. N2 Adsorption/Desorption
The N2 adsorption/desorption isotherms of Cu5.6-SAPO-44 and Cu1.6-SAPO-44-IE exhibit the typical microporosity (Figure 4), similar to the pure SAPO-44 [18]. As a result, their BET surface areas are comparable. Furthermore, the pore volume of Cu1.6-SAPO-44-IE slightly increased in comparison with Cu5.6-SAPO-44 (Table 2), suggesting the CuO species that block the pores in CHA zeolite have been partially removed, as detected by ICP (Table 1).
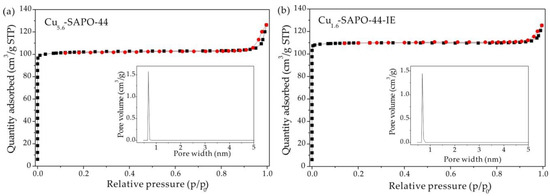
Figure 4.
N2 adsorption/desorption isotherms and pore diameter distribution for (a) Cu5.6-SAPO-44 and (b) Cu1.6-SAPO-44-IE.

Table 2.
Textural properties and XAFS fitting results for Cu5.6-SAPO-44 and Cu1.6-SAPO-44-IE.
2.5. Electron Micrograph
SEM and TEM were conducted for Cu5.6-SAPO-44 and Cu1.6-SAPO-44-IE (Figure 5). It is notable that both Cu5.6-SAPO-44 (Figure 5a,b) and Cu1.6-SAPO-44-IE (Figure 5d,e) show the cubic-like rhombohedral morphology, similar to those reported in literature [19]. According to EDS elemental mappings, some large Cu-containing agglomerations are observed in Cu5.6-SAPO-44 that can be ascribed to the aggregated CuO nanoparticles (Figure 5c), owing to its high Cu content. As reported by He et al., the excess Cu species in Cu-SSZ-13 zeolites could be effectively removed by ion exchange using NH4NO3 solution [15]. Accordingly, large CuO particles can be scoured into CuO nanocrystals and re-dispersed as Cu2+ ions during this ion exchange process. In this work, after IE with NH4NO3, highly dispersed Cu-containing species were observed over the entire zeolite as for Cu1.6-SAPO-44-IE (Figure 5f), which can be attributed to the remaining CuO nanoparticles and Cu2+ ions at the framework ion-exchange sites. In order to clarify the highly dispersed Cu-containing species after IE with NH4NO3, Cu1.6-SAPO-44-IE was further characterized by TEM (Figure 5g–i). The well-dispersed nanocrystals were observed in Cu1.6-SAPO-44-IE (Figure 5g), which were proved to be CuO nanoparticles by identifying the characteristic spacings of 2.27 and 1.87 Å for the (2 0 0) and the (-2 0 2) lattice planes of monoclinic CuO (Figure 5h). A particle count taken from many TEM images, obtained from different regions of the sample, confirmed the presence of monodispersed CuO nanoparticles with a mean diameter of 2.36 nm anchored on the surface of Cu1.6-SAPO-44-IE (Figure 5i). Combined with ICP data, the results confirm that IE with NH4NO3 can not only remove the excessive Cu species (in the form of CuO agglomerations) but also improve the dispersion of the remaining CuO nanoparticles. This result is consistent with the observation from other researchers that CuO aggregates decrease the SCR performance at high temperature range as a result of parasitic NH3 oxidation [15,23].
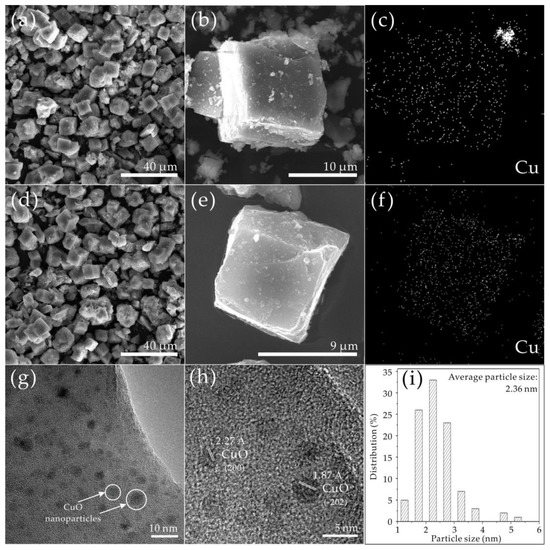
Figure 5.
Electron micrograph of Cu5.6-SAPO-44 and Cu1.6-SAPO-44-IE. FESEM images for Cu5.6-SAPO-44 (a,b) and Cu1.6-SAPO-44-IE (d,e), Cu mapping for Cu5.6-SAPO-44 (c) and Cu1.6-SAPO-44-IE (f); TEM image (g), HRTEM image (h) and particle size distribution of CuO nanoparticles (i) of Cu1.6-SAPO-44-IE.
2.6. H2-TPR
H2-TPR was also performed on Cu5.6-SAPO-44 and Cu1.6-SAPO-44-IE, and the results were shown in Figure 6. For Cu5.6-SAPO-44 (a), the obvious reduction peak centered at ~300 °C could be ascribed to the reduction of CuO (>3 nm) to Cu0 [24]. However, this peak disappeared in Cu1.6-SAPO-44-IE (b), and a broad reduction peak ranging from 160 to 750 °C emerged, suggesting the removal of large agglomerated CuO particles and the existence of highly dispersed Cu2+ and CuO microcrystals after NH4NO3 treatment, in accordance with the results of XRD and SEM.
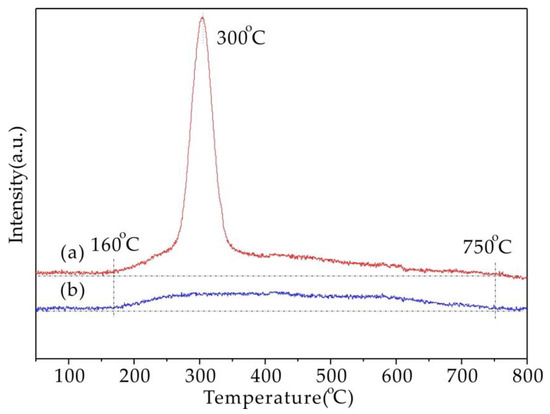
Figure 6.
H2-TPR profiles of Cu5.6-SAPO-44 (a) and Cu1.6-SAPO-44-IE (b).
2.7. XAFS
Individual Cu species were studied by XAFS in combination with linear combination fitting. Figure 7a shows the X-ray absorption near edge structure (XANES) spectra of Cu K-edge for Cu5.6-SAPO-44, Cu1.6-SAPO-44-IE, and reference samples. The XANES spectrum of Cu5.6-SAPO-44 resembled that of CuO, while that for Cu1.6-SAPO-44-IE is similar to that of CuSO4. The corresponding Fourier-transformed k3-weighted Cu K-edge patterns give more direct evidence (Figure 7b). In the case of Cu5.6-SAPO-44, two characteristic peaks at approximately 2.6 and 3.0 Å were detected, which are attributed to the neighboring Cu atoms (Cu-O-Cu) in CuO [25]. For Cu1.6-SAPO-44-IE, the peak at ~1.5 Å assigned to the Cu-O scatterings is similar with that of CuSO4, confirming the predominance of isolated mononuclear Cu2+ species [26]. In addition, weak peaks at ~2.5 Å for Cu1.6-SAPO-44-IE were distinguished, indicating the coexistence of few CuO nanoparticles. The relative amount of Cu2+ and CuO could be obtained from the intense analysis of XANES spectra using linear combination fitting (LCF) [27]. For Cu5.6-SAPO-44, the majority of Cu species are CuO nanoparticles, while the Cu2+ ions are dominant in Cu1.6-SAPO-44-IE (Figure 8). Thus, the isolated Cu2+ proportion was significantly improved via IE.
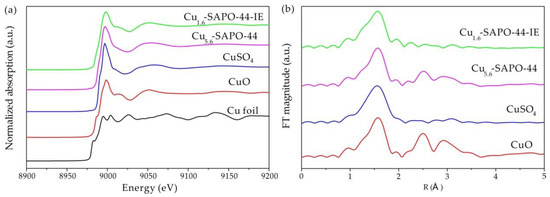
Figure 7.
XANES (a), and FT-EXAFS (b) of Cu K-edge spectra for Cu5.6-SAPO-44, Cu1.6-SAPO-44-IE and the references.
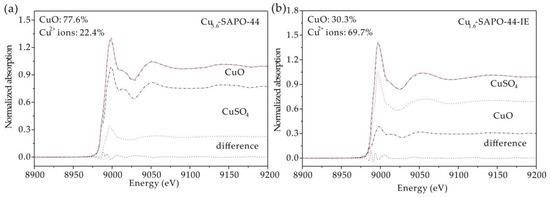
Figure 8.
Linear combination fitting (LCF) of XANES spectra for (a) Cu5.6-SAPO-44, (b) Cu1.6-SAPO-44-IE.
Consequently, the enhanced high-temperatures activity is attributed to the removal of CuO agglomerations and the relocation of the Cu2+ ions for Cux-SAPO-44-IE [10,17]. However, Cu1.6-SAPO-44-IE still exhibited lower activity at high temperature (350–550 °C) compared with the traditional ion-exchanged counterpart with a comparative Cu content that contains only isolated Cu2+ ions [28].
3. Materials and Methods
3.1. Catalyst Preparation
In preparation of Cu-SAPO-44, the gel has a molar ratio of 0.14–0.22 Cu:0.44 TEPA:0.6 SiO2:0.8 Al2O3:1 P2O5:40 H2O:2 N,N,N′,N′-tetramethyl-1,6-hexanediamine (TMHD). CuSO4 solution (20 wt.%) and TEPA were mixed under vigorous stirring at room temperature to get a homogeneous sol After that, phosphoric acid (H3PO4, 85 wt.% aqueous solution), pseudo-boehmite (Al2O3, 78 wt.%), colloidal silica sol (30 wt.% suspension in water), TMHD template and deionized water were added to the Cu-TEPA complex solution under vigorous stirring. Then, the resulting viscous gel was aged with stirring at room temperature over 12 h, and further hydrothermally heated statically in a Teflon-lined steel autoclave at 200 °C for 96 h. The product was obtained by centrifugal, washed several times with deionized water, and dried at 100 °C for 12 h. Cux-SAPO-44 zeolites were obtained by calcination at 550 °C for 6 h in air to remove the structure-directing agent, where “x” represents the Cu content in the catalyst determined by ICP analysis.
In this study, the as-prepared Cu-CHA zeolite samples, have high Cu contents of 4.3, 5.6, and 7.6 wt.% respectively. Then unroasted Cux-SAPO-44 (1 g) zeolites were conducted ion exchange with 1.0 M NH4NO3 solution at 80 °C for 8 h to remove the abundant Cu, and dried Cux-SAPO-44-IE (0.9 g) can be obtained. Finally, the dried samples were calcined at 550 °C for 6 h [17] to remove structure-directing agent, and obtained Cux-SAPO-44-IE, samples with Cu contents of 1.0, 1.6, and 2.2 wt.% (corresponding original x = 4.3, 5.6, and 7.6 wt.%, respectively).
3.2. Catalyst Characterizations
X-ray diffraction (XRD) was used to obtained the information about the crystalline structure of the catalysts, and equipped with Cu Kα radiation (λ = 1.5418 Å). The BET surface area and pore size distribution of Cu5.6-SAPO-44 and Cu1.6-SAPO-44-IE were determined using BET measurements (Micromeritics ASAP 2020, Norcross, GA, USA) after dehydration of the catalysts at 300 °C for 9 h under vacuum. ICP-atomic emission spectrometry (ICP-AES) was carried out to determine the elemental contents of samples by using a PerkinElmer Optima 2100DV (Waltham, MA, USA). FE-SEM equipped with energy dispersive spectroscopy (EDS) was performed on a Hitachi SU-70 microscope (Tokyo, Japan). HRTEM was conducted on a JEOL JEM-2010 (Tokyo, Japan) and a FEI Tecnai G2 F20 transmission electron microscope operating at 200 kV. H2-TPR experiments were performed on a chemisorption analyzer (XianQuan, TP-5000, TianJin, China). The samples (50 mg) were pretreated with pure O2 flow at 500 °C for 30 min, and cooled down to the room temperature in the presence of O2. Subsequently, the samples were reduced under a flow of 5 vol.% H2/N2 (50 mL/min) and then was heated to 800 °C with the rate of 10 °C/min. XAFS spectra were measured for the Cu K-edge at 1W1B beamline of Beijing synchrotron radiation facility (BSRF, Beijing, China) in the transmission mode and fluorescence mode at room temperature. XAFS raw data were analyzed using IFEFFIT software package [29].
3.3. Catalyst Activity Measurements
The steady state NH3-SCR activity tests of Cu-SAPO-44 catalysts (~120 mg, 40–60 meshes) were performed at atmospheric pressure in a fixed-bed quartz tube reactor (6.0 mm i.d.). The gas consisting of 500 ppm NO, 500 ppm NH3, 5.3 vol. % O2, 5 vol. % H2O (when used) and balance, He. The total gas flow rate was 300 mL/min, corresponding to a gas hourly space velocity (GHSV) of 100,000 h−1. The measurement was carried out in the temperature range 100–550 °C. The NO and NO2 concentrations of the reactor inlet/outlet were monitored by a chemiluminiscence NOx analyzer (42i-HL, Thermo, Waltham, MA, USA). In addition, N2O and NH3 were detected by quadrupole mass spectrometer (MS, OmniStar 200, Balzers, Switzerland) with a m/z of 44 for N2O, and 17 for NH3. During the test, each temperature point is stable for at least 30 min. The catalytic activity and N2 selectivity were calculated according to the following equations:
4. Conclusions
Cu-SAPO-44 zeolites were synthesized using a one-pot approach with dual-amine templates. Furthermore—although the total content of Cu was decreased—the CuO dispersion and isolated Cu2+ proportion were significantly improved via the subsequent IE with NH4NO3. The SCR activity was thus promoted at higher temperature. Cu1.6-SAPO-44-IE shows >90% NOx conversion in the temperature range 200–500 °C.
Author Contributions
Conceptualization, N.Z., Y.X. and Z.Z.; data curation, N.Z. and Y.X.; formal analysis, N.Z., Y.X., Q.L., and Z.Z.; funding acquisition, Y.X. and Z.Z.; investigation, N.Z., X.M., Y.Q., L.Z. and Y.X.; methodology, N.Z., L.Z. and Y.X; writing–original draft, N.Z. and Y.X.; writing–review and editing, Z.Z.
Funding
This research was funded by National Natural Science Foundation of China (Grant No. 21906063 and 21876061) and Key Technology R&D Program of Shandong Province (Grant No. 2019GSF109042).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kwak, J.H.; Tonkyn, R.G.; Kim, D.H.; Szanyi, J.; Peden, C.H.F. Excellent activity and selectivity of Cu-SSZ-13 in the selective catalytic reduction of NOx with NH3. J. Catal. 2010, 275, 187–190. [Google Scholar] [CrossRef]
- Gao, F.; Walter, E.D.; Washton, N.M.; Szanyi, J.; Peden, C.H.F. Synthesis and evaluation of Cu-SAPO-34 catalysts for ammonia selective catalytic reduction. 1. Aqueous solution ion exchange. ACS Catal. 2013, 3, 2083–2093. [Google Scholar] [CrossRef]
- Fickel, D.W.; D’Addio, E.; Lauterbach, J.A.; Lobo, R.F. The ammonia selective catalytic reduction activity of copper-exchanged small-pore zeolites. Appl. Catal. B Environ. 2011, 102, 441–448. [Google Scholar] [CrossRef]
- Wang, J.H.; Zhao, H.W.; Haller, G.; Li, Y.D. Recent advances in the selective catalytic reduction of NOx with NH3 on Cu-chabazite catalysts. Appl. Catal. B Environ. 2017, 202, 346–354. [Google Scholar] [CrossRef]
- Beale, A.M.; Gao, F.; Lezcano-Gonzalez, I.; Peden, C.H.F.; Szanyi, J. Recent advances in automotive catalysis for NOx emission control by small-pore microporous materials. Chem. Soc. Rev. 2015, 44, 7371–7405. [Google Scholar] [CrossRef] [PubMed]
- Turrina, A.; Eschenroeder, E.C.V.; Bode, B.E.; Collier, J.E.; Apperley, D.C.; Cox, P.A.; Casci, J.L.; Wright, P.A. Understanding the structure directing action of copper-polyamine complexes in the direct synthesis of Cu-SAPO-34 and Cu-SAPO-18 catalysts for the selective catalytic reduction of NO with NH3. Microporous Mesoporous Mater. 2015, 215, 154–167. [Google Scholar] [CrossRef]
- Xin, Y.; Li, Q.; Zhang, Z.L. Zeolitic materials for deNOx selective catalytic reduction. ChemCatChem 2018, 10, 29–41. [Google Scholar] [CrossRef]
- Shan, W.P.; Song, H. Catalysts for the selective catalytic reduction of NOx with NH3 at low temperature. Catal. Sci. Technol. 2015, 5, 4280–4288. [Google Scholar] [CrossRef]
- Liu, F.D.; Yu, Y.B.; He, H. Environmentally-benign catalysts for the selective catalytic reduction of NOx from diesel engines: Structure-activity relationship and reaction mechanism aspects. Chem. Commun. 2014, 50, 8445–8463. [Google Scholar] [CrossRef]
- Ren, L.M.; Zhu, L.F.; Yang, C.G.; Chen, Y.M.; Sun, Q.; Zhang, H.Y.; Li, C.J.; Nawaz, F.; Meng, X.J.; Xiao, F.S. Designed copper-amine complex as an efficient template for one-pot synthesis of Cu-SSZ-13 zeolite with excellent activity for selective catalytic reduction of NOx by NH3. Chem. Commun. 2011, 47, 9789–9791. [Google Scholar] [CrossRef]
- Martínez-Franco, R.; Moliner, M.; Franch, C.; Kustov, A.; Corma, A. Rational direct synthesis methodology of very active and hydrothermally stable Cu-SAPO-34 molecular sieves for the SCR of NOx. Appl. Catal. B Environ. 2012, 127, 273–280. [Google Scholar] [CrossRef]
- Deka, U.; Lezcano-Gonzalez, I.; Warrender, S.J.; Picone, A.L.; Wright, P.A.; Weckhuysen, B.M.; Beale, A.M. Changing active sites in Cu-CHA catalysts: deNOx selectivity as a function of the preparation method. Microporous Mesoporous Mater. 2013, 166, 144–152. [Google Scholar] [CrossRef]
- Gao, F.; Walter, E.D.; Washton, N.M.; Szanyi, J.; Peden, C.H.F. Synthesis and evaluation of Cu/SAPO-34 catalysts for NH3-SCR 2: Solid-state ion exchange and one-pot synthesis. Appl. Catal. B Environ. 2015, 162, 501–514. [Google Scholar] [CrossRef]
- Xin, Y.; Zhang, N.N.; Wang, X.; Li, Q.; Ma, X.C.; Qi, Y.X.; Zheng, L.R.; Anderson, J.A.; Zhang, Z.L. Efficient synthesis of the Cu-SAPO-44 zeolite with excellent activity for selective catalytic reduction of NOx by NH3. Catal. Today 2019, 332, 35–41. [Google Scholar] [CrossRef]
- Xie, L.J.; Liu, F.D.; Ren, L.M.; Shi, X.L.; Xiao, F.S.; He, H. Excellent performance of one-pot synthesized Cu-SSZ-13 catalyst for the selective catalytic reduction of NOx with NH3. Environ. Sci. Technol. 2014, 48, 566–572. [Google Scholar] [CrossRef] [PubMed]
- Ren, L.M.; Zhang, Y.B.; Zeng, S.J.; Zhu, L.F.; Sun, Q.; Zhang, H.Y.; Yang, C.G.; Meng, X.J.; Yang, X.G.; Xiao, F.S. Design and synthesis of a catalytically active Cu-SSZ-13 zeolite from a copper-amine complex template. Chin. J. Catal. 2012, 33, 92–105. [Google Scholar] [CrossRef]
- Guo, Q.; Fan, F.; Ligthart, D.A.J.M.; Li, G.; Feng, Z.; Hensen, E.J.M.; Li, C. Effect of the nature and location of copper species on the catalytic nitric oxide selective catalytic reduction performance of the copper/SSZ-13 zeolite. ChemCatChem 2014, 6, 634–639. [Google Scholar] [CrossRef]
- Li, H.; Xin, Y.; Wang, X.; Zhou, Y.H.; Li, Q.; Zhang, Z.L. A novel dual-template method for synthesis of SAPO-44 zeolite. RSC Adv. 2016, 6, 35910–35913. [Google Scholar] [CrossRef]
- Xin, Y.; Wang, X.; Li, Q.; Ma, X.C.; Qi, Y.X.; Zheng, L.R.; Anderson, J.A.; Zhang, Z.L. The potential of Cu-SAPO-44 in selective catalytic reduction of NOx with NH3. ChemCatChem 2016, 8, 3740–3745. [Google Scholar] [CrossRef]
- Xue, J.J.; Wang, X.Q.; Qi, G.; Wang, J.; Shen, M.Q.; Li, W. Characterization of copper species over Cu/SAPO-34 in selective catalytic reduction of NOx with ammonia: Relationships between active Cu sites and de-NOx performance at low temperature. J. Catal. 2013, 297, 56–64. [Google Scholar] [CrossRef]
- Wang, L.; Li, W.; Qi, G.; Weng, D. Location and nature of Cu species in Cu/SAPO-34 for selective catalytic reduction of NO with NH3. J. Catal. 2012, 289, 21–29. [Google Scholar] [CrossRef]
- Li, J.; Chang, H.; Ma, L.; Hao, J.; Yang, R.T. Low-temperature selective catalytic reduction of NOx with NH3 over metal oxide and zeolite catalysts-A review. Catal. Today 2011, 175, 147–156. [Google Scholar] [CrossRef]
- Gao, F.; Walter, E.D.; Karp, E.M.; Luo, J.; Tonkyn, R.G.; Kwak, J.H.; Szanyi, J.; Peden, C.H.F. Structure-activity relationships in NH3-SCR over Cu-SSZ-13 as probed by reaction kinetics and EPR studies. J. Catal. 2013, 300, 20–29. [Google Scholar] [CrossRef]
- Richter, M.; Fait, M.J.G.; Eckelt, R.; Schneider, M.; Radnik, J.; Heidemann, D.; Fricke, R. Gas-phase carbonylation of methanol to dimethyl carbonate on chloride-free Cu-precipitated zeolite Y at normal pressure. J. Catal. 2007, 245, 11–24. [Google Scholar] [CrossRef]
- Yamashita, H.; Matsuoka, M.; Tsuji, K.; Shioya, Y.; Anpo, M.; Che, M. In-situ XAFS, photoluminescence, and IR investigations of copper ions included within various kinds of zeolites. Structure of Cu(I) ions and their interaction with CO molecules. J. Phys. Chem. 1996, 100, 397–402. [Google Scholar] [CrossRef]
- Korhonen, S.T.; Fickel, D.W.; Lobo, R.F.; Weckhuysen, B.M.; Beale, A.M. Isolated Cu2+ ions: Active sites for selective catalytic reduction of NO. Chem. Commun. 2011, 47, 800–802. [Google Scholar] [CrossRef] [PubMed]
- Gaur, A.; Shrivastava, B.D.; Joshi, S.K. Copper K-edge XANES of Cu(I) and Cu(II) oxide mixtures. J. Phys. Conf. Ser. 2009, 190, 012084. [Google Scholar] [CrossRef]
- Peng, Z.L. The Optimization on in Situ Synthesis Conditions of Cu-SSZ-13 Denitration Catalyst; Taiyuan University of Technology: Taiyuan, China, 2015. [Google Scholar]
- Newville, M. IFEFFIT: Interactive XAFS analysis and FEFF fitting. J. Synchrotron Radiat. 2001, 8, 322–324. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).