Abstract
A cascade strategy for the catalytic valorization of aqueous solutions of levulinic acid as well as of γ-valerolactone to 2-methyltetrahydrofuran or to monoalcohols, 2-butanol and 2-pentanol, has been studied and optimized. Only commercial catalytic systems have been employed, adopting sustainable reaction conditions. For the first time, the combined use of ruthenium and rhenium catalysts supported on carbon, with niobium phosphate as acid co-catalyst, has been claimed for the hydrogenation of γ-valerolactone and levulinic acid, addressing the selectivity to 2-methyltetrahydrofuran. On the other hand, the use of zeolite HY with commercial Ru/C catalyst favors the selective production of 2-butanol, starting again from γ-valerolactone and levulinic acid, with selectivities up to 80 and 70 mol %, respectively. Both levulinic acid and γ-valerolactone hydrogenation reactions have been optimized, investigating the effect of the main reaction parameters, to properly tune the catalytic performances towards the desired products. The proper choice of both the catalytic system and the reaction conditions can smartly switch the process towards the selective production of 2-methyltetrahydrofuran or monoalcohols. The catalytic system [Ru/C + zeolite HY] at 200 °C and 3 MPa H2 is able to completely convert both γ-valerolactone and levulinic acid, with overall yields to monoalcohols of 100 mol % and 88.8 mol %, respectively.
1. Introduction
Nowadays, the development of clean energy sources is a principal benchmark and renewable biomasses represent ideal starting materials for this purpose. In fact, as a stable and independent alternative to fossil fuels, biomass has emerged as a potentially inexhaustible resource for the production of energy, transportation fuels and chemicals [1,2]. Biomass resources can be advantageously converted into interesting platform chemicals, such as 2-furaldehyde [3,4], 5-hydroxymethyl-2-furaldehyde [5,6,7], levulinic acid (LA) [8,9] and γ-valerolactone (GVL) [10,11]. Focusing on the last two mentioned bio-chemicals, LA is a versatile bio-product, being considered as one of the United States Department of Energy’s (DOE’s) top 12 bio-derived feedstocks. It can be used as a solvent, antifreeze, food flavoring agent and it can be employed for the synthesis of pharmaceuticals, plasticizers, biofuels and many other high-value chemicals [9,12]. Instead, GVL is one of the most important products deriving from LA hydrogenation, currently exploited for the production of both energy and carbon-based consumer products [13,14,15,16,17]. In this sense, it is a renewable and safe-to-store potential biofuel employed as replacement of ethanol in gasoline/ethanol blends [18,19]. Furthermore, it can be used for the production of promising food additives [13], solvents [20] and it is an intermediate for the synthesis of many other fine-chemicals such as valeric biofuels, butene, 4-hydroxypentanol, 2-methyltetrahydrofuran and acrylic polymers [12,21,22,23]. In the literature, multiple interesting pathways for the production of LA and GVL have been reported [24,25,26]. In this context, the possibility of upgrading these already valuable chemical intermediates into other classes of more added-value compounds, such as 2-methyltetrahydrofuran (2-MeTHF) and 2-butanol (2-BuOH), represents a smart challenging possibility. Regarding the first one, it is a versatile aprotic and hydrophobic organic solvent, which is non-toxic and non-ozone depleting in nature [27] and a promising biofuel [28], being identified as a key component for P-series fuels [29]. On the other hand, 2-BuOH is mainly used for the synthesis of methylethylketone, but very recently it has found other applications both as solvent and fuel additive, thanks to its high octane number [30]. Regarding the possible synthesis of 2-MeTHF, some processes use furfural as the key intermediate, whereas some methods adopt LA. 2-MeTHF synthesis starting from furfural has been investigated more in-depth [31,32,33,34]. Concerning the other possible route, the presence of 2-MeTHF was reported for the first time as a by-product of the LA hydrogenation with CuCr2O3 [35]. Afterward, many works have been published on this topic, which have partially clarified the reaction mechanism, and significantly improved the catalytic performances for the synthesis of 2-MeTHF [36,37,38,39,40,41,42,43,44,45]. Two reaction mechanisms have been proposed for 2-MeTHF synthesis from LA, the first one occurring by LA dehydration via angelicalactone (AGL) (path A, Scheme 1), whereas the second one by LA hydrogenation to 4-hydroxypentanoic acid (HPA) (path B, Scheme 1), both reported in Scheme 1.
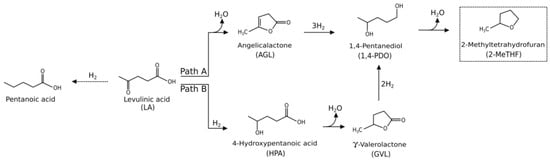
Scheme 1.
Reaction pathways for levulinic acid (LA) conversion to 2-methyltetrahydrofuran (2-MeTHF).
Pathway A was proposed by Thomas and Barile [46] and, more recently, Al-Shaal et al. [47] have demonstrated that the presence of AGL as starting material was beneficial for the one-pot conversion to 2-MeTHF, adopting a commercial Ru/C catalyst. However, the AGL path and its positive role for the 2-MeTHF production was proposed under solvent-free conditions [47], whilst the hydrogenation path (path B) in water medium occurs by a different mechanism through HPA intermediate, which has been extensively ascertained and demonstrated [48,49]. LA catalytic hydrogenation may give different reaction products, depending on the kind of the adopted catalyst and experimental conditions (path B, Scheme 1). The reaction proceeds by LA reduction to HPA, which readily dehydrates to GVL. The hydrogenation of GVL into 1,4-pentanediol (1,4-PDO) is reported to be strongly inhibited by water [42], thus highlighting evident difficulties to perform this reaction in water already at this backward step and, even with greater difficulty, for the final 1,4-PDO dehydration to 2-MeTHF [45]. Main by-products of this reaction include pentanoic acid and 1-pentanol. In greater detail, LA transformation into GVL, 1,4-PDO, and 2-MeTHF consists of consecutive series of hydrogenation/dehydration paths, both occurring via the formation of well-defined but short-lived intermediates [38], as shown in Scheme 2.
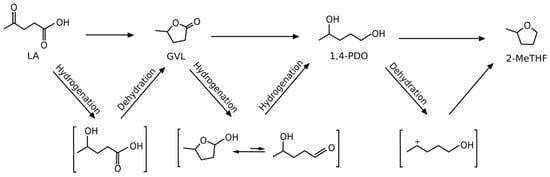
Scheme 2.
LA hydrogenation and dehydration reactions to give γ-valerolactone (GVL), 1,4-pentanediol (1,4-PDO) and 2-MeTHF.
In the above scheme, the metal-catalyzed addition of H2 to the LA keto group forms a hydroxyl acid that undergoes intramolecular etherification to give GVL. Further hydrogenation of the C=O bond leads to the cyclic hemiacetal, which is in equilibrium with the open hydroxyl-aldehyde form. The remaining carbonyl group is hydrogenated to afford 1,4-PDO. Acid-catalyzed dehydration leads to cyclization of the diol by intramolecular etherification to give 2-MeTHF. A possible further reaction is the dehydration/hydrogenation of 1,4-PDO to the monoalcohols 1- or 2-pentanol. Most of these steps are reversible, which afford an additional challenge for the selectivity control. According to this mechanism, in the field of homogeneous catalysis, interesting results were reported by Mehdi et al. [37], which adopted homogeneous catalysts based on ruthenium phosphine compounds for the LA and GVL conversion to 2-MeTHF. In the case of LA, starting from Ru(acac)3, NH4PF6 and PBu3, LA was quantitatively converted to 2-MeTHF at a temperature of 200 °C, 8.2 MPa of hydrogen pressure and extended reaction times (about 46 h). Geilen et al. [38] reported that a homogeneous Ru-based molecular catalyst system bearing expensive phosphine ligands in combination with ionic and/or acid additives can selectively convert bio-derived LA into 1,4-PDO or 2-MeTHF, via formation of GVL intermediate. The authors reported the best 2-MeTHF yield of 92 mol %, employing Ru(acac)3 as the metal precursor, triphos as the ligand, and the ionic liquid 1-butyl-2-(4-sulfobutyl)imidazolium-p-toluenesulfonate as an additive, working at 160 °C for 18 h, with 10 MPa H2. Despite the promising 2-MeTHF yield, significant drawbacks for this catalytic system remain, e.g. its non-reusability, the necessity of special handling of metal complexes, the high cost for the catalyst preparation, as well as the tedious work-up procedures. Heterogeneous catalysts represent the best choice, and many interesting works have been published in this topic, such as the research of Elliot and Frye [36], which performed the hydrogenation of a 10 wt % LA aqueous solution, at a temperature range of 200–250 °C, with 10 MPa H2. Working in water, the yields to 2-MeTHF were very scarce, adopting different hydrogenation catalysts, such as 5% Ni/C-5% Re/C, 50% Ni/C, 50% Ni/C-5% Re/C and 50% Ni/C-7.5% Re/C. However, under the same reaction conditions, but using 1,4-dioxane instead of water as solvent, when a 50% Ni/C-7.5% Re/C catalyst was employed, the reaction rate was significantly improved, as well as the yield to 2-MeTHF which, however, reached the maximum value of 15 mol %, after 6 h of reaction. LA hydrogenation with 5%Re/C - 5%Pd/C improved significantly the 2-MeTHF yield, up to 90 mol %. Heterogeneous copper catalysts have been extensively used for LA conversion to 2-MeTHF [39,40,50]. In particular, Upare et al. [39] investigated the vapor-phase conversion of LA to 2-MeTHF in 1,4-dioxane, employing properly synthesized nanocomposite Cu/SiO2 catalysts. The best result was claimed with an 80% Cu/SiO2 catalyst, obtaining in this case a maximum 2-MeTHF yield of 64 mol %, working at 265 °C for 100 h on stream, with 2.5 MPa H2. Regarding the effect of the copper loading, the authors found that a lower one favored the selective hydrocyclization of LA to GVL, while a higher one led to further hydrogenation to 2-MeTHF and 1-pentanol, via GVL formation. The process was further improved by promoting the catalyst with Ni, e.g., Cu(72%)-Ni(8%)/SiO2, thus achieving the best 2-MeTHF yield of 89 mol %, working at 265 °C for 300 h on steam, with 2.5 MPa H2. Du et al. [40] investigated the catalytic performances of a series of synthesised copper-zirconia catalysts for the hydrogenolysis of GVL in ethanol at 200 °C with 6 MPa H2, finding that a yield to 2-MeTHF of 6 mol % was produced when the reaction was performed at 220 °C. A further increase of the temperature up to 240 °C led to an increase in the 2-MeTHF yield up to about 13 mol %. In the attempt to improve the 2-MeTHF yield, subsequent runs were focused on the same reaction at 240 °C over a series of 30% Cu/ZrO2 catalysts with modified acidic properties of the catalyst surface obtained by calcination in air at different temperatures (300–700 °C), for 4 h. In the best case, a remarkable 2-MeTHF yield was improved up to 91 mol %, after 6 h of reaction. The authors proposed that the surface acidic sites in the Cu-based catalysts play a key role in the hydrogenolysis mechanism of lactones and the authors concluded that a synergistic cooperation between dispersed Cu and the acid sites of the catalyst surface was essential to facilitate the direct reduction of the carbonyl group in the GVL molecule or accelerate the subsequent dehydration of intermediate 1,4-PDO to give 2-MeTHF. Al-Shaal et al. [42] used Ru/C as the only catalyst, under solvent-free conditions, for the one-pot LA conversion into 2-MeTHF, achieving the complete conversion at 190 °C and 10 MPa H2, with a maximum 2-MeTHF yield of 61 mol %. Upare et al. [44] used Ru over graphene oxide for the study of LA conversion in 1,4-dioxane, producing a stable 48 mol % 2-MeTHF yield, operating at 265 °C and 2.5 MPa H2. Mizugaki et al. [43] used a Pt-Mo supported on acidic supports for the conversion of LA to 2-MeTHF. The most active synthesised catalyst was Pt(3.9%)-Mo(0.13%)/H-β zeolite, which allowed the maximum 2-MeTHF yield of 86 mol %, working in water at 130 °C for 24 h, with 5 MPa H2. Obregón et al. [51] studied the one-pot LA conversion to 2-MeTHF, with Ni/Al2O3, Cu/Al2O3 and Ni-Cu/Al2O3 catalysts in green solvents, such as water and biomass-derived alcohols, such as EtOH, 1-BuOH, and 2-PrOH. The best catalytic performances were achieved with bimetallic Ni-Cu/Al2O3 catalysts, achieving the maximum 2-MeTHF yield of 56 mol %, working in 2-PrOH, at 250 °C for 5 h, with 7 MPa H2. Lastly, in a subsequent work, Obregón et al. [45] reported the complete LA conversion, with a maximum 2-MeTHF yield of 80 mol %, adopting a Ni-Cu/Al2O3 catalyst, in 2-PrOH as solvent, after 20 h at 250 °C and 4 MPa H2.
Regarding 2-BuOH, at the moment its traditional industrial production is carried out through the acid-catalyzed hydration of 1- or 2-butene, both obtained from the C4 fraction arising from steam cracking of oil-derived hydrocarbons [52]. In recent years, the possibility of obtaining 2-BuOH from renewable resources has been evaluated. It is noteworthy that Shabaker et al. [53] patented the catalytic decarboxylation of LA to methyl ethyl ketone, which was subsequently hydrogenated to 2-BuOH, employing copper faujasite as decarboxylation catalyst, and nickel or ruthenium as hydrogenation one. Al-Shaal et al. [42] reported a total LA conversion, under solvent-free conditions, with a selectivity to 2-BuOH close to 40 mol %, adopting a 5% Ru/C catalyst, and working at 190 °C and for 24 h, with 10 MPa H2. Regarding the related reaction mechanism, under solvent-free conditions, 2-BuOH is predominantly formed via subsequent hydrogenation/decarbonylation reactions of the GVL [42], as shown in Scheme 3.
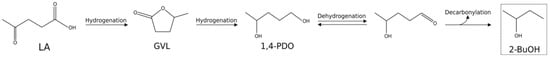
Scheme 3.
Reaction pathway for the 2-butanol (2-BuOH) synthesis starting from GVL under solvent-free conditions.
Obregón et al. [45] reported the GVL hydrogenation, achieving a maximum 2-BuOH yield of 37.3 mol %, working in 2-PrOH, at 250 °C and 4 MPa H2. Lastly, Lv et al. [54] have recently used nanoporous Ru for LA hydrogenation in water, reporting the combined production of both 2-BuOH and 2-Pentanol (2-PeOH), the latter derived mainly from 1,4-PDO hydrogenation, with a total [2-BuOH + 2-PeOH] yield of 78.8 mol %, working at 140 °C for 20 h, with 6 MPa H2. Instead, when the same catalyst was employed at 100 °C and 6 MPa H2 for 15 h, a maximum 74.6 mol % yield to 1,4-PDO was reached.
In this work, an alternative and green approach has been studied, which combines hydrogenation-decarboxylation of LA or of GVL to selectively give [2-BuOH + 2-PeOH] or 2-MeTHF, in the presence of commercial Ru/C and [Ru/C + Re/C] catalytic systems. The main reaction conditions, e.g., temperature, hydrogen pressure, and presence of heterogeneous acid co-catalyst (niobium phosphate or HY acid zeolite) have been investigated. This study has been carried out adopting water as the only reaction solvent and heterogeneous commercial catalytic systems, which are more economical, available, and reproducible respect to ad hoc prepared ones.
2. Results and Discussion
Starting from the previous results reached on LA hydrogenation to GVL in the presence of Ru/C and an acid co-catalyst [55], thus research began from the GVL hydrogenation, which is considered to be the most demanding step, owing to the high thermodynamic stability of GVL [49]. The effect of the addition of Re/C to commercial Ru/C catalyst has been investigated, on the basis of the promising results obtained in the literature in the presence of the bimetallic Ru-Re system employed in the hydrogenation of levulinic acid and succinic acid where the introduction of Re amount improves the selectivity to 1,4-PDO [56], an intermediate towards 2-MeTHF. Moreover, the addition of an acid co-catalyst to Ru and [Ru/C + Re/C] systems has been considered because it is known that this acid component is able to activate not only the lactone ring opening, but also the internal dehydration of 1,4-PDO to 2-MeTHF [57,58,59]. For this purpose, in the first part of this work, niobium phosphate (NBP) has been selected as co-catalyst, thanks to its well-known acidic properties, which are preserved also in an aqueous medium, even at high temperatures [7,60]. This is an amorphous solid with strong Brønsted and medium–strong Lewis acid sites located at the surface, due to the presence of coordinatively unsaturated Nb5+ species. This acidity has been already exploited for the successful dehydration of saccharides to 5-hydroxymethyl-2-furaldehyde [7,61,62], and therefore its use for the 2-MeTHF synthesis appeared really promising. The results of this first group of experiments are reported in Table 1.

Table 1.
Hydrogenation of γ-valerolactone (GVL) with Ru/C, Re/C catalysts, and niobium phosphate (NBP) as acid co-catalyst. Reaction conditions: GVL: 1.68 g; Ru: 2 mg (GVL/Ru: 847.63 mol/mol); H2O: 40 mL; PH2: 9.0 MPa.
The negligible activity ascertained in the experiment carried out in the presence of only Ru/C (run 1, Table 1) respect to that of the runs with [Ru/C + NBP] (runs 2–5, Table 1) confirm that the presence of the acid NBP co-catalyst is essential for GVL conversion, 2-MeTHF, and 2-BuOH being the main reaction products, together with lower amounts of 1,4-PDO and 2-PeOH, in agreement with the literature data [42]. Thus, the acid character of the co-catalyst favors the interaction with the carboxylic group of the lactone, enhancing in this way its ring opening and subsequent conversion [58,63,64,65]. In the first run carried out in the presence of [Ru/C + NBP] (run 2, Table 1), a moderate GVL conversion and a well-balanced selectivity to both 2-MeTHF and 2-BuOH were ascertained, which could be selectively tuned to one of these products, by a further optimization of the reaction parameters, in particular reaction time and temperature. First, the effect of the reaction time was investigated, keeping constant the reaction temperature, but adopting a longer reaction time (run 3, Table 1), and it was found that this parameter has certainly a positive, albeit modest, effect on the 2-MeTHF selectivity, whilst 2-BuOH has rapidly formed already at the first stages of the reaction. On the other hand, the selectivities to 2-PeOH and 1,4-PDO progressively increase during the progress of the reaction but long reaction times are required, in agreement with other previous investigations [37,38].
The effect of temperature on 2-MeTHF and 2-BuOH formation was also investigated, adopting a reaction time of 6 h, which resulted a good compromise between the catalytic activity and the simultaneous selectivity to 2-MeTHF and 2-BuOH. For this purpose, hydrogenation runs were carried out, at lower (180 °C), and at higher (210 °C) temperature (runs 4 and 5, respectively, Table 1). As a result, GVL conversion was improved with the temperature increase, being clearly unsatisfactory for the experiment at 180 °C (run 4, Table 1). Instead, regarding the selectivity parameter, it was found that a lower reaction temperature favored the selectivity to 2-MeTHF, whereas a higher one has favored the formation of both 2-BuOH and 2-PeOH, thus supporting that the dehydration is favored by a lower temperature [58,63,64,65].
Starting from the acquired results, the effect of the addition of a commercial rhenium-based catalyst (10% Re/C) to the catalytic system composed of Ru/C and NBP was investigated. As in the case of Ru/C, also the Re/C catalyst alone (run 6, Table 1) was not active to the GVL hydrogenation, even adopting higher temperature (210 °C) and longer reaction time (6 h). Instead, its synergistic effect with [Ru/C + NBP] improves the GVL conversion and the selectivity to 2-MeTHF and 1,4-PDO, to the detriment of 2-BuOH, already with low amounts of Re/C (compare runs 7 and 2, Table 1), revealing that rhenium plays a key synergistic role in this reaction, addressing towards the diol cyclization to 2-MeTHF [36,56]. On this basis, the amount of rhenium was increased, and the corresponding catalytic performances were evaluated (runs 8 and 9, Table 1). Taking into account the achieved results, it is possible to highlight that the increase of the rhenium amount improves the selectivity to 2-MeTHF, and 1,4-PDO, in agreement with the literature data [56], whilst the selectivity to 2-BuOH drastically decreases, proving that it is possible to address the reaction towards the desired product, by selecting the appropriate catalytic system. The further increase of the amount of Rhenium does not allow the corresponding improvement of the catalytic performances to 2-MeTHF, thus highlighting the achieved optimization, under the adopted reaction conditions. Similarly, the effect of the NBP on the catalytic performances was studied, without NBP and with 250, 500 and 1000 mg of it (runs 9–12, Table 1). From the obtained data, it is possible to note that GVL conversion was the highest when 500 mg of NBP were employed and, under the same reaction conditions, also the selectivity to 2-MeTHF was the highest (64.9 mol %). On the other hand, the amount of NBP does not affect the selectivity to 2-BuOH and 2-PeOH, thus confirming that the overall catalytic system favors the dehydration path. For this reason, in order to obtain 2-MeTHF, the amount of 500 mg of NBP was selected for the subsequent investigation.
With the aim of adopting milder reaction conditions, in terms of hydrogen pressure and temperature, an increase of the ruthenium amount from 2 up to 10 mg was studied and the obtained results are summarized in Table 2.

Table 2.
GVL hydrogenation reactions in the presence of different catalytic systems: effect of hydrogen pressure, temperature, and presence of an acid co-catalyst (NBP or HY zeolite). Reaction conditions: GVL: 2.52 g; H2O: 40 mL; Ru: 10 mg (GVL/Ru: 254.29 mol/mol); Re: 20 mg (GVL/Re: 234.25 mol/mol), when present; NBP: 500 mg, when present; HY zeolite: 500 mg, when present; time: 3 h.
Adopting the catalytic system [Ru/C + NBP], the increased amount of Ru allows us to reach almost complete GVL conversion (run 13, Table 2), and comparison between runs 13 and 14 confirms the possibility of working at milder reaction conditions, in terms of hydrogen pressure, still achieving a satisfactory GVL conversion together with a high selectivity to 2-BuOH. On the basis of the obtained results (run 13, Table 2 and runs 2–5, Table 1), in the presence of 10 mg of Ru, in order to obtain 2-MeTHF, it is necessary to decrease the reaction temperature and the hydrogen pressure. In fact, when the reaction was carried out at a lower temperature, 180 °C (run 15, Table 2), a lower GVL conversion was achieved but, after 3 h of reaction, the selectivities to 2-MeTHF and 1,4-PDO were higher than those at 200 °C, thus remarking that the dehydration reaction is favored by a lower temperature, as previously stated. Again, the synergistic effect of [Ru/C + Re/C + NBP] was considered (run 16, Table 2), thus confirming that the co-presence of rhenium was beneficial for both GVL conversion and 2-MeTHF selectivity: in fact, the 2-MeTHF selectivity increases after 3 h from 9.7 mol %, in the absence of Re, up to 37.8 mol %, in its presence. In this set of hydrogenation runs, also the effect of the addition of a zeolite HY as acid co-catalyst, instead of NBP, on GVL hydrogenation was investigated, in the presence of Ru or [Ru/C + Re/C] catalytic systems. This acid catalyst is well-known for the strong Brønsted acidity, due to the bridging Si–-(OH)-–Al sites, generated by the presence of aluminium inside the silicate framework and the balancing proton [66]. In addition, working in aqueous medium at high temperatures, the zeolite catalytic systems, in particular those with low Si/Al ratio, are able to maintain their stability [67], despite new acid sites are formed, as the result of Si–-O–-Si hydrolysis, ion exchange with charge compensating aluminum cations, and/or dissociative adsorption of water on framework independent alumina [67]. Preliminary runs in the presence of different amounts of HY zeolite were carried out with the [Ru/C + Re/C] catalytic system in order to compare the HY zeolite with NBP at the same total acidity level under the best reaction conditions evidenced in Table 1 (Ru: 2 mg; Re: 20 mg; GVL: 1.68 g, 200 °C, 9.0 MPa H2, 3h). On the basis of these results, which have shown that HY zeolite resulted more selective towards [2-BuOH+2-PeOH] than NBP, the right amount of HY zeolite able to maximize the selectivity to [2-BuOH + 2-PeOH] was investigated. Under the same employed reaction conditions (Ru: 2 mg; Re: 20 mg; GVL: 1.68 g, 200 °C, 9.0 MPa H2, 3h), a HY zeolite amount of 500 mg was found to be the best one in terms of GVL conversion and selectivity to [2-BuOH + 2-PeOH]. For this reason, in order to obtain [2-BuOH + 2-PeOH], this amount of HY zeolite was employed for the subsequent investigation. The hydrogenation run carried out in the presence of zeolite HY co-catalyst and Ru/C at 180 °C and 5.0 MPa H2 (run 17, Table 2) shows that the presence of this different acid component significantly enhances GVL conversion respect to the corresponding experiment with Ru/C and NBP (run 15, Table 2), as well as the selectivity to 2-MeTHF and 2-PeOH, to the detriment of 1,4-PDO. Further balancing the temperature and H2 pressure (200 °C, 3.0 MPa) a total conversion of GVL can be reached (run 18, Table 2) with complete selectivity to [2-BuOH + 2-PeOH]. This different behavior is due to the preferential reaction pathway, which follows the decarboxylation route, which is known to occur in the presence of faujasite zeolites [53,68], according to the Scheme 4.
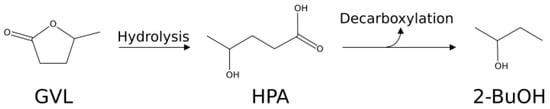
Scheme 4.
Proposed reaction pathways for the formation of 2-BuOH from GVL.
The occurred decarboxylation was confirmed by qualitative analysis of the gaseous phase, which revealed the presence of carbon dioxide as the main reaction product. It is remarkable that the reaction conditions adopted in run 18 allowed us to reach a productivity to [2-BuOH+2-PeOH] of 840 mmoles·g·Ru−1·h−1.
On the other hand, the addition of rhenium, together with zeolite HY (run 19, Table 2), favors the 2-MeTHF formation, in agreement with the previous experiments with NBP.
From all the catalytic results, it is possible to underline that the two employed metals (Ru and Re) play a synergic role in this reaction. In this regard, Di et al. [69] studied the employment of Ru/C, Re/C and bimetallic Ru-Re/C catalysts in the hydrogenation of succinic acid and γ-butyrolactone in water at 200 °C and 8.0 MPa, finding that Ru metal positively contributes to the hydrogenolysis mechanism, whereas Re metal to the hydrogenation one. A similar role of the two metals can be proposed for the present study. In particular, starting from GVL, in the presence of [Ru/C + acid co-catalyst], the Ru species can promote the conversion of GVL (assisted by the presence of the acid co-catalyst which favors the ring-opening) to 2-BuOH through the formation of the intermediate HPA, or to 1,4-PDO (through the hydrogenation), which can be converted to 2-MeTHF, in the presence of the acid co-catalyst. The conversion of GVL to 2-BuOH requires the break of C–C bond with the formation of CO2 (decarboxylation route). This hypothesis is confirmed by our experimental results: in fact, as reported in runs 2–5 in Table 1 for NBP, the main products result 2-BuOH deriving from the decarboxylation route of GVL through the break of C–C bond of the intermediate HPA, and 2-MeTHF deriving from the internal dehydration of 1,4-PDO obtained by hydrogenation. The same behavior is also observed in the presence of HY zeolite (run 17, Table 2). When Re/C is added to [Ru/C + acid co-catalyst], the second metal component (Re/C) promotes the hydrogenation of GVL through its opening-ring and hampers the decarboxylation path, thus causing the increase of the amount of 1,4-PDO, precursor of 2-MeTHF, and, as a consequence, the formation of 2-MeTHF. In fact, in the case of NBP, comparing run 2 with run 9 (Table 1), the addition of Re/C causes a decrease of 2-BuOH selectivity, from 42.2 to 11.1 mol %, thus limiting the decarboxylation route, together with an increase of 2-MeTHF and 1,4-PDO selectivities, from 35.2 to 57.5 mol %, and from 12.2 to 21.3 mol %, respectively. Also, for the runs (runs 18 and 19, Table 2) carried out in the presence of HY zeolite, it is possible to highlight the same trend: in fact, the addition of Re/C causes a decrease of 2-BuOH selectivity, from 81.3 to 47.6 mol %, and an increase of 2-MeTHF selectivity, from 0 to 35.7 mol %.
The most promising experiments for the production of 2-MeTHF and [2-BuOH + 2-PeOH] (runs 16 and 18, Table 2, respectively) were considered as references for the evaluation of the catalyst reusability. The stability of the adopted systems, [Ru/C + Re/C + NBP] and [Ru/C + HY], under the adopted reaction conditions, was verified, showing constant catalytic performances, even after five recycling tests. In addition, the ruthenium and the rhenium catalysts have shown a good resistance to leaching, as confirmed by ICP-OES analysis, which has excluded metal releases in solution.
Taking into account that GVL can be obtained by LA, with the aim of developing a direct cascade process LA to 2-MeTHF or LA to monoalcohols, occurring through the formation of the GVL intermediate but without its isolation, the [Ru/C + NBP] catalytic system was tested also for the one-pot LA conversion, investigating the effect of the reaction temperature. The results are reported in Table 3.

Table 3.
LA hydrogenation reactions carried out in the presence of [Ru/C, NBP] catalytic system, at different reaction temperatures. Reaction conditions: LA: 1.99 g; Ru: 2 mg (LA/Ru: 866.12 mol/mol); NBP: 1 g; H2O: 40 mL; PH2: 9.0 MPa; time: 3 h.
The chosen reaction conditions guarantee the almost complete LA conversion, and the reaction is very selective towards GVL, thus confirming the real possibility to build a cascade process. The selectivity to the reaction products is almost constant in the two experiments (200 and 210 °C), therefore suggesting the adoption of the lower reaction temperature. Afterward, on the basis of the obtained results, this reaction was studied in more detail, increasing the Ru amount from 2 to 10 mg, and employing different reaction conditions, in terms of temperature and hydrogen pressure. Also, in this case, the effect of the acid co-catalyst was considered. The obtained results are summarized in Table 4.

Table 4.
LA hydrogenation reactions carried out in the presence of [Ru/C + NBP (or HY zeolite)] catalytic system, at different reaction temperatures and hydrogen pressure. Reaction conditions: LA: 1.99 g; Ru: 10 mg (LA/Ru: 173.22 mol/mol); Re: 20 mg (LA/Re: 159.57 mol/mol); NBP: 500 mg, HY zeolite: 500 mg, when present; H2O: 40 mL; time: 3 h.
Working at 180 °C and 5.0 MPa of hydrogen, after 3 h of reaction, the higher amount of Ru allowed the complete LA conversion. The selectivity to 2-MeTHF has decreased from 10.1 to 3.8 mol % when NBP was replaced by zeolite HY, whilst 2-BuOH remained the main reaction product (runs 22 and 23, Table 4). On the basis of these results, taking into account that 2-BuOH derives from the decarboxylation reaction, the following study with the [Ru + HY] system was carried out decreasing the hydrogen pressure (compare runs 23, 24 and 25, Table 4). The decrease of hydrogen pressure below 3.0 MPa has evidenced a significant reduction of GVL successive reactions, and therefore this pressure value was kept for the subsequent reaction runs. When the temperature was increased from 180 to 200 °C (run 26, Table 4), an increase of selectivity to 2-BuOH and 2-PeOH was ascertained, as previously observed for the GVL conversion tests in the presence of an acid co-catalyst (runs 3 and 4, Table 1). Moreover, adopting these new optimized reaction conditions, the comparison between zeolite HY and NBP was investigated (runs 26 and 27, Table 4). The presence of zeolite HY provides a higher GVL conversion: the remaining GVL at the end of the reaction was higher for run 27 (in the presence of NBP), rather than for run 26, where the total yield to monoalcohols was 88.8 mol %, with a productivity of 510 mmoles·g·Ru−1·h−1. Up to now, the best overall [2-BuOH + 2-PeOH] yield reported in the literature amounts to 78.8 mol %, reached for LA hydrogenation in water at 140 °C and 6.0 MPa H2 in the presence of synthesized nanoporous Ruthenium [54]. Now the experimental finding confirms that the adopted acid zeolite HY enhances the subsequent hydrogenation/decarboxylation reaction towards the formation of 2-BuOH and 2-PeOH. Lastly, taking into account that the formation of 2-MeTHF is boosted in the presence of NBP, the addition of Re/C, together with Ru/C and NBP acid co-catalyst was investigated (run 28, Table 4). The result allowed a further increase of the selectivity to 2-MeTHF (compare runs 23 and 28, Table 4), thus confirming the positive role of rhenium for the 2-MeTHF production, already observed in the corresponding GVL conversion run (compare runs 15 and 16, Table 2). The reaction experiments with the highest [2-BuOH + 2-PeOH] and 2-MeTHF selectivities (runs 26 and 28, Table 4, respectively) were considered as references for performing subsequent recycling tests, as reported in Figure 1.
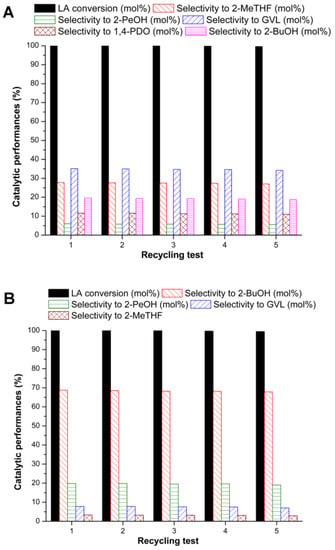
The above data confirm the good performances of the adopted [Ru/C + HY] and [Ru/C + Re/C + NBP] catalysts, even after five recycling tests. Moreover, the catalyst shows no significant leaching, thus further justifying its stability and reusability.
3. Materials and Methods
3.1. Materials
Ru/C (5 wt %) (50% moisture) was purchased from Engelhard (Iselin, NJ, United States) (average diameter: 2.0 nm), 10 wt % Re/C was purchased from Strem (average diameter: 10.4 nm) and both used as received. Levulinic acid, γ-valerolactone, 2-methyltetrahydrofuran and 2-butanol were obtained by Sigma Aldrich (St. Louis, MO, USA) and used as received. Zeolite HY (CBV 500) was purchased from Zeolyst International (Valley Forge, PA, USA) (ratio SiO2/Al2O3 mol/mol: 5.2; acidity: 1.5 mmol/g measured by NH3-TPD) [70] and it was treated at 510 °C for 2 h before its use. Instead, niobium phosphate (acidity: 0.33 mmol/g measured by an acid-base titration in water using 2-phenyl-ethylamine as the basic probe) [24] was kindly provided from CBMM Companhia Brasileira de Metalurgia e Mineracão (Araxá, Minas Gerais, Brasil), and treated at 255 °C for 6 h, under high vacuum (5 Pa) before its use [7].
3.2. Hydrogenation Reactions
Hydrogenation reactions were carried out in a stainless steel 100 mL mechanically stirred Parr 4560 autoclave, equipped with a P.I.D. controller 4843. In a typical procedure, the proper amount of the selected catalyst/catalysts, together with the acid co-catalyst (when necessary), was introduced in the reactor under an inert atmosphere. The autoclave was closed, evacuated up to 65 Pa and a solution of the starting feedstock in 40 mL of water was introduced by suction. The reactor was then pressurized with hydrogen and heated to the desired temperature, maintaining a stirring speed of 500 rpm, a value which was ascertained to assure the absence of mass transfer limitations. During the reaction, the pressure value was manually held constant at the chosen value by repeated hydrogen feeds. The course of the reaction was monitored by periodically sampling the liquid from a sampling valve and analyzing it by gas-chromatography Agilent Technologies (Santa Clara, CA, USA). Recycling experiments of the commercial catalysts were carried out in a similar manner: after removing through the sample valve the liquid reaction mixture, the autoclave containing the catalyst/catalysts was evacuated and charged again for the subsequent catalytic cycle. All experiments were carried out in duplicate and the composition of the reaction mixtures resulted reproducible to within ±5%.
3.3. Analysis of the Reaction Products
Quantitative analyses were performed with an HP 5890 gas-chromatograph equipped with an HP 3396 integrator, a flame ionization detector, and a PONA capillary column (50 m × 0.2 mm × 0.5 μm) with a stationary phase 100% dimethylpolysiloxane. The transport gas was nitrogen and the flow was 1 mL·min−1. The adopted temperature program for G.C. separation was the following: 50 °C (3 min)–20 °C·min−1–200 °C (10 min). Qualitative analyses were carried out using the gas-chromatograph Hewlett-Packard HP 6890 with an MSD HP 5973 detector, employing a G.C. column Phenonex Zebron with a stationary phase of 100% methylpolysiloxane (length of the column: 30 m, inner diameter: 0.25 mm and thickness of the stationary phase: 0.25 µm). The transport gas was helium and the flow was 1 mL·min−1. Gaseous phase was qualitatively analyzed by G.C. using an Agilent HP6890 chromatograph and a Restek Shincarbon ST column (2 m × 1 mm), and a thermal conductivity detector (TCD). The carrier gas was helium, with a constant flow rate of 15 mL min−1, and an inlet temperature of 100 °C. The adopted temperature program for the analysis of a gas volume of 100 µL was the following: 35 °C (5 min)–8 °C min−1–200 °C (5 min).
Conversion parameter was calculated on the basis of the adopted starting feedstock, e.g., GVL or LA, as follows:
GVL or LA conversion (mol %) = (converted moles of GVL or LA/starting moles of GVL or LA) × 100
Instead, the selectivity to the main reaction products, e.g., 2-MeTHF, 2-BuOH, 2-PeOH, and 1,4-PDO, was calculated always with respect to the starting feedstock, e.g., GVL or LA, as follows:
Selectivity to the product (mol %) = (obtained moles of product/converted moles of GVL or LA) × 100
Lastly, catalyst productivity to give (2-BuOH + 2-PeOH) was calculated as follows:
Productivity = millimoles (2-BuOH + 2-PeOH)/(gmetal × time (hour))
Ruthenium and Rhenium leaching was established on the reaction solution by inductively coupled plasma-optical emission spectrometry (ICP-OES), employing a Spectro-Genesis instrument (Spectro Analytical Instruments GmbH, Cleves, North Rhine-Westphalia, Germany) and using a software Smart Analyzed Vision (version 4, Spectro Analytical Instrument GmbH, Cleves, North Rhine-Westphalia, Germany)
4. Conclusions
In this research, a cascade strategy for the catalytic valorization of levulinic acid and γ-valerolactone to 2-methyltetrahydrofuran or to mono-alcohols, 2-butanol and 2-pentanol, has been studied and optimized. In the perspective of an economic, environmental and sustainable development, only commercial catalytic systems have been employed, adopting water as the only green medium. It is noteworthy our choice of employing only water whilst, in most of the best-case studies reported up to now in the literature, different organic solvents have been specially used, mainly 1,4-dioxane or alcohols, as well as solvent-free conditions, with the aim of improving the selectivity to the target products. Taking into account our greener perspective, both hydrogenation reactions have been optimized, investigating the effect of temperature, hydrogen pressure, amounts of rhenium, niobium phosphate or acid zeolite HY. The appropriate choice of the catalytic system/reaction conditions can tune this process toward to the selective production of 2-methyltetrahydrofuran or mono-alcohols. For the first time, the use of ruthenium and rhenium catalysts, both supported on carbon, together with niobium phosphate as acid co-catalyst, has been claimed in the conversion of γ-valerolactone and levulinic acid to 2-methyltetrahydrofuran, obtaining selectivities up to a maximum of about 65 and 28 mol %, respectively. On the contrary, the use of zeolite HY, together with the commercial Ru/C catalyst, favors the selective production of the mono-alcohols 2-butanol and 2-pentanol. It is remarkable that the catalytic system [Ru/C + zeolite HY] at 200 °C and 3 MPa H2 is able to completely convert both γ-valerolactone and levulinic acid, with overall yield to monoalcohols [2-butanol + 2-pentanol] of 100 mol % and 88.8 mol %, respectively. To the best of our knowledge, these yields are the best up to now reported for the synthesis of monoalcohols from these renewable starting materials.
These promising results allow us to move towards the next challenge passing from model compounds to real biomass hydrolysates as starting materials.
Author Contributions
All the authors together conceived and designed the experiments; D.L., S.F. and M.G. performed the experiments; A.M.R.G. and C.A. analyzed the data and all the authors wrote the paper.
Acknowledgments
The authors are grateful to the PRIN 2015-Project HERCULES “HEterogeneous Robust Catalysts to Upgrade Low valuE biomass Streams” (code 20153T4REF).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Shylesh, S.; Gokhale, A.A.; Ho, C.R.; Bell, A.T. Novel strategies for the production of fuels, lubricants, and chemicals from biomass. Acc. Chem. Res. 2017, 50, 2589–2597. [Google Scholar] [CrossRef] [PubMed]
- Fiorentino, G.; Ripa, M.; Ulgiati, S. Chemicals from biomass: Technological versus environmental feasibility. A review. Biofuels Bioprod. Biorefin. 2017, 11, 195–214. [Google Scholar] [CrossRef]
- Mariscal, R.; Maireles-Torres, P.; Ojeda, M.; Sádaba, I.; López Granados, M. Furfural: A renewable and versatile platform molecule for the synthesis of chemicals and fuels. Energy Environ. Sci. 2016, 9, 1144–1189. [Google Scholar] [CrossRef]
- Li, X.; Jia, P.; Wang, T. Furfural: A promising platform compound for sustainable production of C4 and C5 chemicals. ACS Catal. 2016, 6, 7621–7640. [Google Scholar] [CrossRef]
- Yu, I.K.M.; Tsang, D.C.W. Conversion of biomass to hydroxymethylfurfural: A review of catalytic systems and underlying mechanisms. Bioresour. Technol. 2017, 238, 716–732. [Google Scholar] [CrossRef] [PubMed]
- Antonetti, C.; Raspolli Galletti, A.M.; Fulignati, S.; Licursi, D. Amberlyst A-70: A surprisingly active catalyst for the MW-assisted dehydration of fructose and inulin to HMF in water. Catal. Commun. 2017, 97, 146–150. [Google Scholar] [CrossRef]
- Antonetti, C.; Melloni, M.; Licursi, D.; Fulignati, S.; Ribechini, E.; Rivas, S.; Parajó, J.C.; Cavani, F.; Raspolli Galletti, A.M. Microwave-assisted dehydration of fructose and inulin to HMF catalyzed by Niobium and Zirconium phosphate catalysts. Appl. Catal. B 2018, 206, 364–377. [Google Scholar] [CrossRef]
- Girisuta, B.; Heeres, H.J. Levulinic acid from biomass: Synthesis and applications. In Production of Platform Chemicals from Sustainable Resources; Fang, Z., Smith, R.L., Qi, X., Eds.; Springer: Singapore, 2017; Chapter 5; pp. 143–169. ISBN 978-9811041716. [Google Scholar]
- Antonetti, C.; Licursi, D.; Fulignati, S.; Valentini, G.; Raspolli Galletti, A.M. New frontiers in the catalytic synthesis of levulinic acid: From sugars to raw and waste biomass as starting feedstock. Catalysts 2016, 6, 196. [Google Scholar] [CrossRef]
- Yan, K.; Yang, Y.; Chai, J.; Lu, Y. Catalytic reactions of gamma-valerolactone: A platform to fuels and value-added chemicals. Appl. Catal. B 2015, 179, 292–304. [Google Scholar] [CrossRef]
- Zhang, Z. Synthesis of γ-Valerolactone from carbohydrates and its applications. ChemSusChem 2016, 9, 156–171. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Yao, Q.; Fu, Y. Conversion of levulinic acid and alkyl levulinates to biofuels and high-value chemicals. Green Chem. 2017, 19, 5527–5547. [Google Scholar] [CrossRef]
- Horváth, I.T.; Mehdi, H.; Fábos, V.; Boda, L.; Mika, L.T. γ-Valerolactone-A sustainable liquid for energy and carbon-based chemicals. Green Chem. 2008, 10, 238–242. [Google Scholar] [CrossRef]
- Bond, J.Q.; Alonso, D.M.; Wang, D.; West, R.M.; Dumesic, J.A. Integrated catalytic conversion of γ-valerolactone to liquid alkenes for transportation fuels. Science 2010, 327, 1110–1114. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Zeng, X.; Li, Z.; Hu, L.; Sun, Y.; Liu, S.; Lei, T.; Lin, L. Production of γ-valerolactone from lignocellulosic biomass for sustainable fuels and chemicals supply. Renew. Sustain. Energy Rev. 2014, 40, 608–620. [Google Scholar] [CrossRef]
- Upare, P.P.; Lee, J.M.; Hwang, D.W.; Halligudi, S.B.; Hwang, Y.K.; Chang, J.S. Selective hydrogenation of levulinic acid to γ-valerolactone over carbon-supported noble metal catalysts. J. Ind. Eng. Chem. 2011, 17, 287–292. [Google Scholar] [CrossRef]
- Yan, K.; Lafleur, T.; Wu, G.; Liao, J.; Ceng, C.; Xie, X. Highly selective production of value-added γ-valerolactone from biomass-derived levulinic acid using the robust Pd nanoparticles. Appl. Catal. A 2013, 468, 52–58. [Google Scholar] [CrossRef]
- Sudholt, A.; Tripathi, R.; Mayer, D.; Glaude, P.A.; Battin-Leclerc, F.; Pitsch, H. The oxidation of the novel lignocellulosic biofuel γ-valerolactone in a low pressure flame. Proc. Combust. Inst. 2017, 36, 577–585. [Google Scholar] [CrossRef]
- Lilga, M.A.; Padmaperuma, A.B.; Auberry, D.L.; Job, H.M.; Swita, M.S. Ketonization of levulinic acid and γ-valerolactone to hydrocarbon fuel precursors. Catal. Today 2018, 302, 80–86. [Google Scholar] [CrossRef]
- Fegyverneki, D.; Orha, L.; Láng, G.; Horváth, I.T. Gamma-valerolactone-based solvents. Tetrahedron 2010, 66, 1078–1081. [Google Scholar] [CrossRef]
- Yan, K.; Lafleur, T.; Wu, X.; Chai, J.; Wu, C.; Xie, X. Cascade upgrading of γ-valerolactone to biofuels. Chem. Commun. 2015, 51, 6984–6987. [Google Scholar] [CrossRef] [PubMed]
- Serrano-Ruiz, J.C.; Wang, D.; Dumesic, J.A. Catalytic upgrading of levulinic acid to 5-nonanone. Green Chem. 2010, 12, 574–577. [Google Scholar] [CrossRef]
- Wang, A.; Lu, Y.; Yi, Z.; Ejaz, A.; Hu, K.; Zhang, L.; Yan, K. Selective production of γ-valerolactone and valeric acid in one-pot bifunctional metal catalysts. ChemistrySelect 2018, 3, 1097–1101. [Google Scholar] [CrossRef]
- Raspolli Galletti, A.M.; Antonetti, C.; Ribechini, E.; Colombini, M.P.; Nassi, N.; Bonari, E. From giant reed to levulinic acid and gamma-valerolactone: A high yield catalytic route to valeric biofuels. Appl. Energy 2013, 102, 157–162. [Google Scholar] [CrossRef]
- Antonetti, C.; Bonari, E.; Licursi, D.; Nassi, N.; Raspolli Galletti, A.M. Hydrothermal conversion of giant reed to furfural and levulinic acid: Optimization of the process under microwave irradiation and investigation of distinctive agronomic parameters. Molecules 2015, 20, 21232–21253. [Google Scholar] [CrossRef] [PubMed]
- Rivas, S.; Raspolli Galletti, A.M.; Antonetti, C.; Licursi, D.; Santos, V.; Parajó, J.C. A biorefinery cascade conversion of hemicellulose-free Eucalyptus Globulus wood: Production of concentrated levulinic acid solutions for γ-Valerolactone sustainable preparation. Catalysts 2018, 8, 169. [Google Scholar] [CrossRef]
- Pace, V.; Hoyos, P.; Castoldi, L.; De María, P.D.; Alcántara, A.R. 2-Methyltetrahydrofuran (2-MeTHF): A biomass-derived solvent with broad application in organic chemistry. ChemSusChem 2012, 5, 1369–1379. [Google Scholar] [CrossRef] [PubMed]
- Climent, M.J.; Corma, A.; Iborra, S. Conversion of biomass platform molecules into fuel additives and liquid hydrocarbon fuels. Green Chem. 2014, 16, 516–547. [Google Scholar] [CrossRef]
- Kar, Y.; Deveci, H. Importance of p-series fuels for flexible-fuel vehicles (FFVs) and alternative fuels. Energy Source A Recovery Util. Environ. Eff. 2006, 28, 909–921. [Google Scholar] [CrossRef]
- Da Silva Trindade, W.R.; Dos Santos, R.G. Review on the characteristics of butanol, its production and use as fuel in internal combustion engines. Renew. Sustain. Energy Rev. 2017, 69, 642–651. [Google Scholar] [CrossRef]
- Stevens, J.G.; Bourne, R.A.; Twigg, M.V.; Poliakoff, M. Real-time product switching using a twin catalyst system for the hydrogenation of furfural in supercritical CO2. Angew. Chem. Int. Ed. 2010, 49, 8856–8859. [Google Scholar] [CrossRef] [PubMed]
- Dong, F.; Zhu, Y.; Ding, G.; Cui, J.; Li, X.; Li, Y. One-step conversion of furfural into 2-methyltetrahydrofuran under mild conditions. ChemSusChem 2015, 8, 1534–1537. [Google Scholar] [CrossRef] [PubMed]
- Chang, X.; Liu, A.F.; Cai, B.; Luo, J.Y.; Pan, H.; Huang, Y.B. Catalytic transfer hydrogenation of furfural to 2-methylfuran and 2-methyltetrahydrofuran over bimetallic copper-palladium catalysts. ChemSusChem 2016, 9, 3330–3337. [Google Scholar] [CrossRef] [PubMed]
- Yan, K.; Liao, J.; Wu, X.; Xie, X. A noble-metal free Cu-catalyst derived from hydrotalcite for highly efficient hydrogenation of biomass-derived furfural and levulinic acid. RSC Adv. 2013, 3, 3853–3856. [Google Scholar] [CrossRef]
- Christian, R.V.J.; Brown, H.D.; Hixon, R.M. Derivatives of γ-valerolactone, 1,4-pentanediol and 1,4-di-(β-cyanoethoxy)-pentane. J. Am. Chem. Soc. 1947, 69, 1961–1963. [Google Scholar] [CrossRef]
- Elliot, D.; Frye, J.G. Hydrogenated 5-Carbon Compound and Method of Making. U.S. Patent 5883266, 16 March 1999. [Google Scholar]
- Mehdi, H.; Fàbos, V.; Tuba, R.; Bodor, A.; Mika, L.T.; Horvàth, I.T. Integration of homogeneous and heterogeneous catalytic processes for a multi-step conversion of biomass: From sucrose to levulinic acid, γ-valerolactone, 1,4-pentanediol, 2-methyl-tetrahydrofuran, and alkanes. Top. Catal. 2008, 48, 49–54. [Google Scholar] [CrossRef]
- Geilen, F.M.A.; Engendahl, B.; Harwardt, A.; Marquardt, W.; Klankermayer, J.; Leitner, W. Selective and flexible transformation of biomass-derived platform chemicals by a multifunctional catalytic system. Angew. Chem. Int. Ed. 2010, 49, 5510–5514. [Google Scholar] [CrossRef] [PubMed]
- Upare, P.P.; Lee, J.M.; Hwang, Y.K.; Hwang, D.W.; Lee, J.H.; Halligudi, S.B.; Hwang, J.S.; Chang, J.S. Direct hydrocyclization of biomass-derived levulinic acid to 2-methyltetrahydrofuran over nanocomposite copper/silica catalysts. ChemSusChem 2011, 4, 1749–1752. [Google Scholar] [CrossRef] [PubMed]
- Du, X.L.; Bi, Q.Y.; Liu, Y.M.; Cao, Y.; He, H.Y.; Fan, K.N. Tunable copper-catalyzed chemoselective hydrogenolysis of biomass-derived γ-valerolactone into 1,4-pentanediol or 2-methyltetrahydrofuran. Green Chem. 2012, 14, 935–939. [Google Scholar] [CrossRef]
- Bermudez, J.M.; Menendez, J.A.; Romero, A.A.; Serrano, E.; Garcia-Martinez, J.; Luque, R. Continuous flow nanocatalysis: Reaction pathways in the conversion of levulinic acid to valuable chemicals. Green Chem. 2013, 15, 2786–2792. [Google Scholar] [CrossRef]
- Al-Shaal, M.G.; Dzierbinski, A.; Palkovits, R. Solvent-free γ-valerolactone hydrogenation to 2-methyltetrahydrofuran catalysed by Ru/C: A reaction network analysis. Green Chem. 2014, 16, 1358–1364. [Google Scholar] [CrossRef]
- Mizugaki, T.; Togo, K.; Maeno, Z.; Mitsudome, T.; Jitsukawa, K.; Kaneda, K. One-pot transformation of levulinic acid to 2-methyltetrahydrofuran catalyzed by Pt−Mo/H-β in water. ACS Sustain. Chem. Eng. 2016, 4, 682–685. [Google Scholar] [CrossRef]
- Upare, P.P.; Lee, M.; Lee, S.K.; Yoon, J.W.; Bae, J.; Hwang, D.W.; Lee, UH.; Chang, J.S.; Hwang, Y.K. Ru nanoparticles supported graphene oxide catalyst for hydrogenation of bio-based levulinic acid to cyclic ethers. Catal. Today 2016, 265, 174–183. [Google Scholar] [CrossRef]
- Obregón, I.; Gandarias, I.; Al-Shaal, M.G.; Mevissen, C.; Arias, P.L.; Palkovits, R. The role of the hydrogen source on the selective production of γ-valerolactone and 2-methyltetrahydrofuran from levulinic acid. ChemSusChem 2016, 9, 2488–2495. [Google Scholar] [CrossRef]
- Thomas, J.J.; Barile, R.G. Conversion of cellulose hydrolysis products to fuel and chemical feedstocks. Biomass Wastes 1985, 8, 1461–1494. [Google Scholar]
- Al-Shaal, M.G.; Hausoul, P.J.C.; Palkovits, R. Efficient, solvent-free hydrogenation of α-angelica lactone catalysed by Ru/C at atmospheric pressure and room temperature. Chem. Commun. 2014, 50, 10206–10209. [Google Scholar] [CrossRef] [PubMed]
- Al-Shaal, M.G.; Wright, W.R.H.; Palkovits, R. Exploring the ruthenium catalysed synthesis of γ-valerolactone in alcohols and utilisation of mild solvent-free reaction conditions. Green Chem. 2012, 14, 1260–1263. [Google Scholar] [CrossRef]
- Abdelrahman, O.A.; Heyden, A.; Bond, J.Q. Analysis of kinetics and reaction pathways in the aqueous-phase hydrogenation of levulinic acid to form γ-valerolactone over Ru/C. ACS Catal. 2014, 4, 1171–1181. [Google Scholar] [CrossRef]
- Zheng, J.; Zhu, J.; Xu, X.; Wang, W.; Li, J.; Zhao, Y.; Tang, K.; Song, Q.; Qi, X.; Kong, D.; et al. Continuous hydrogenation of ethyl levulinate to γ-valerolactone and 2-methyl tetrahydrofuran over alumina doped Cu/SiO2 catalyst: The potential of commercialization. Sci. Rep. 2016, 6, 28898–28907. [Google Scholar] [CrossRef] [PubMed]
- Obregón, I.; Gandarias, I.; Miletic´, N.; Ocio, A.; Arias, P.L. One-pot 2-methyltetrahydrofuran production from levulinic acid in green solvents using Ni-Cu/Al2O3 catalysts. ChemSusChem 2015, 8, 3483–3488. [Google Scholar] [CrossRef] [PubMed]
- Assima, G.P.; Zamboni, I.; Lavoie, J.M. Alcohol fuels: The thermochemical route. In Biofuels Production and Processing Technology; Riazi, R.M., Chiaramonti, D., Eds.; CRC Press: Boca Raton, FL, USA, 2018; Chapter 14; pp. 362–406. ISBN 978-1-4987-7893-0. [Google Scholar]
- Shabaker, J.W.; Weiner, A.; Rundell, D.N.; Sejour, H.; Goeden, G. Unit, Method, and Renewable Materials. U.S. Patent 20100281763A1, 11 November 2010. [Google Scholar]
- Lv, J.; Rong, Z.; Sun, L.; Liu, C.; Lu, A.H.; Wang, Y.; Qu, J. Catalytic conversion of biomass-derived levulinic acid into alcohols over nanoporous Ru catalyst. Catal. Sci. Technol. 2018, 8, 975–979. [Google Scholar] [CrossRef]
- Raspolli Galletti, A.M.; Antonetti, C.; De Luise, V.; Martinelli, M. A sustainable process for the production of γ-valerolactone by hydrogenation of biomass-derived levulinic acid. Green Chem. 2012, 12, 688–694. [Google Scholar] [CrossRef]
- Corbel-Demailly, L.; Ly, B.K.; Minh, D.P.; Tapin, B.; Especel, C.; Epron, F.; Cabiac, A.; Guillon, E.; le Besson, M.; Pinel, C. Heterogeneous catalytic hydrogenation of biobased levulinic and succinic acids in aqueous solutions. ChemSusChem 2013, 6, 2388–2395. [Google Scholar] [CrossRef] [PubMed]
- Buitrago-Sierra, R.; Serrano-Ruiz, J.C.; Rodríguez-Reinoso, F.; Sepúlveda-Escribano, A.; Dumesic, J.A. Ce promoted Pd-Nb catalysts for γ-valerolactone ring-opening and hydrogenation. Green Chem. 2012, 14, 3318–3324. [Google Scholar] [CrossRef]
- Luo, W.; Deka, U.; Beale, A.M.; Van Eck, E.R.H.; Bruijnincx, P.C.A.; Weckhuysen, B.M. Ruthenium-catalyzed hydrogenation of levulinic acid: Influence of the support and solvent on catalyst selectivity and stability. J. Catal. 2013, 301, 175–186. [Google Scholar] [CrossRef]
- Prati, L.; Jouve, A.; Villa, A. Production and upgrading of γ-valerolactone with bifunctional catalytic processes. In Production of Biofuels and Chemicals with Bifunctional Catalysts, 1st ed.; Fang, Z., Smith, R.L., Li, H., Eds.; Springer: Singapore, 2017; Volume 8, pp. 221–237. ISBN 978-981-10-5136-4. [Google Scholar]
- Freitas, F.A.; Licursi, D.; Lachter, E.R.; Raspolli Galletti, A.M.; Antonetti, C.; Brito, T.C.; Nascimento, R.S.V. Heterogeneous catalysis for the ketalisation of ethyl levulinate with 1,2-dodecanediol: Opening the way to a new class of bio-degradable surfactants. Catal. Commun. 2016, 73, 84–87. [Google Scholar] [CrossRef]
- Raspolli Galletti, A.M.; Carlini, C.; Sbrana, G.; Armaroli, T.; Busca, G. Selective saccharides dehydration to 5-hydroxymethyl-2-furaldehyde by heterogeneous niobium catalysts. Appl. Catal. A 1999, 183, 295–302. [Google Scholar] [CrossRef]
- Amaroli, T.; Busca, G.; Carlini, C.; Giuttari, M.; Raspolli Galletti, A.M.; Sbrana, G. Active acid sites characterization of niobium phosphate catalysts and their activity in fructose dehydration to 5-hydroxymethyl-2-furaldehyde. J. Mol. Catal. A: Chem. 2000, 151, 233–243. [Google Scholar] [CrossRef]
- Bond, J.Q.; Alonso, D.M.; West, R.M.; Dumesic, J.A. γ-Valerolactone ring-opening and decarboxylation over SiO2/Al2O3 in the presence of water. Langmuir 2010, 26, 16291–16298. [Google Scholar] [CrossRef] [PubMed]
- Kellicutt, A.B.; Salary, R.; Abdelrahman, O.A.; Bond, J.Q. An examination of the intrinsic activity and stability of various solid acids during the catalytic decarboxylation of γ-valerolactone. Catal. Sci. Technol. 2014, 4, 2267–2279. [Google Scholar] [CrossRef]
- Villa, A.; Schiavoni, M.; Chan-Thaw, C.E.; Fulvio, P.F.; Mayes, R.T.; Dai, S.; More, K.L.; Veith, G.M.; Prati, L. Acid-functionalized mesoporous carbon: An efficient support for ruthenium-catalyzed γ-valerolactone production. ChemSusChem 2015, 8, 2520–2528. [Google Scholar] [CrossRef] [PubMed]
- Busca, G. Acidity and basicity of zeolites: A fundamental approach. Microporous Mesoporous Mater. 2017, 254, 3–16. [Google Scholar] [CrossRef]
- Ravenelle, R.M.; Schüβler, F.; D’Amico, A.; Danilina, N.; Van Bokhoven, J.A.; Lercher, J.A.; Jones, C.W.; Sievers, C. Stability of zeolites in hot liquid water. J. Phys. Chem. C 2010, 114, 19582–19595. [Google Scholar] [CrossRef]
- Pine, L.A. Decarboxylation. U.S. Patent 3476803A, 4 November 1969. [Google Scholar]
- Di, X.; Li, C.; Zhang, B.; Qi, J.; Li, W.; Su, D.; Liang, C. Role of Re and Ru in Re. Ru/C bimetallic catalysts for the aqueous hydrogenation of succinic Acid. Ind. Eng. Chem. Res. 2017, 56, 4672–4683. [Google Scholar] [CrossRef]
- Van Borm, R.; Reyniers, M.F.; Martens, J.A.; Marin, G.B. Catalytic cracking of methylcyclohexane on FAU, MFI, and bimodal porous materials: Influence of acid properties and pore topology. Ind. Eng. Chem. Res. 2010, 49, 10486–10495. [Google Scholar] [CrossRef]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).