Immobilization of an Endo-β-N-acetylglucosaminidase for the Release of Bioactive N-glycans
Abstract
1. Introduction
2. Results and Discussion
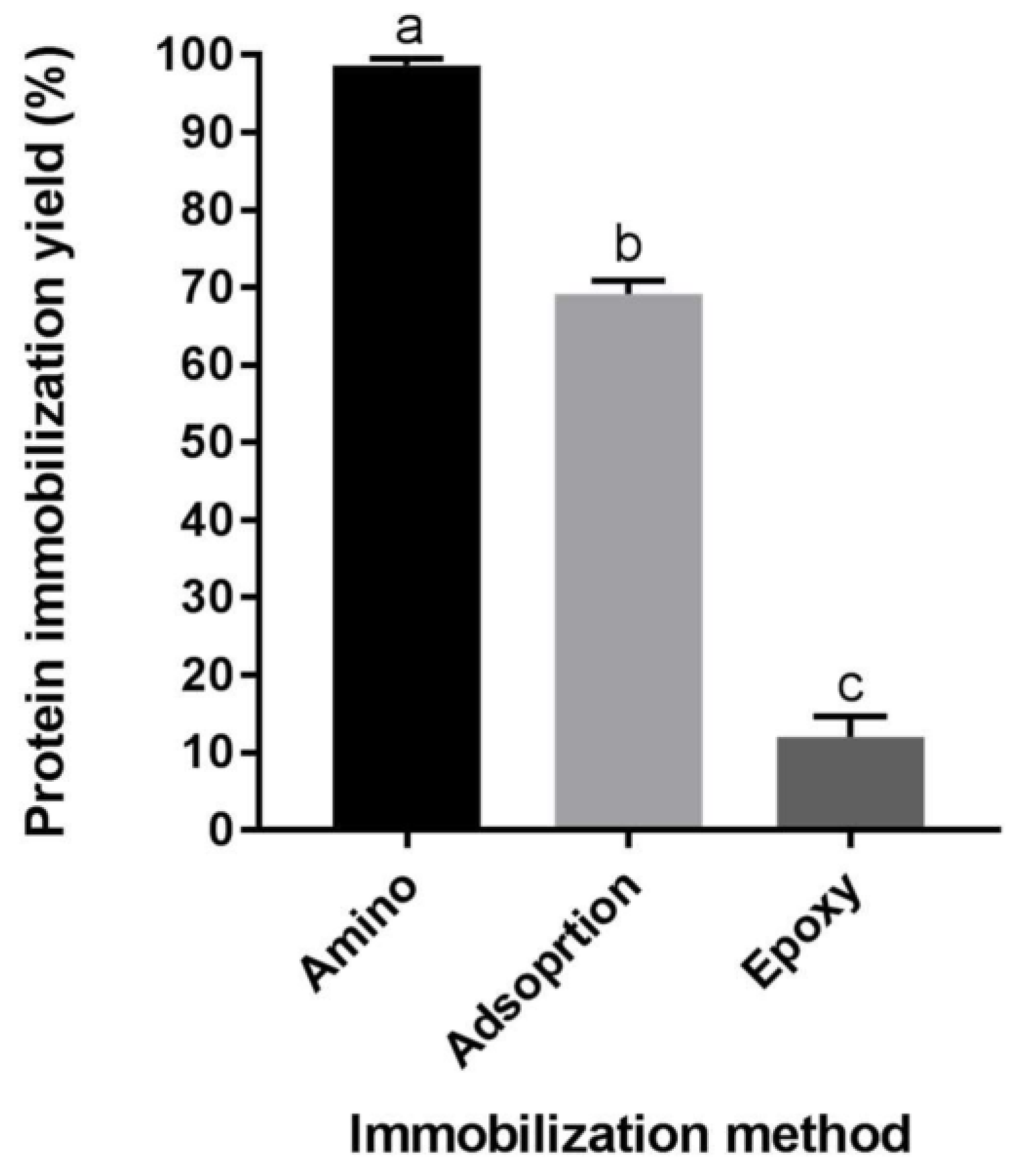
2.1. Protein Immobilization Yield
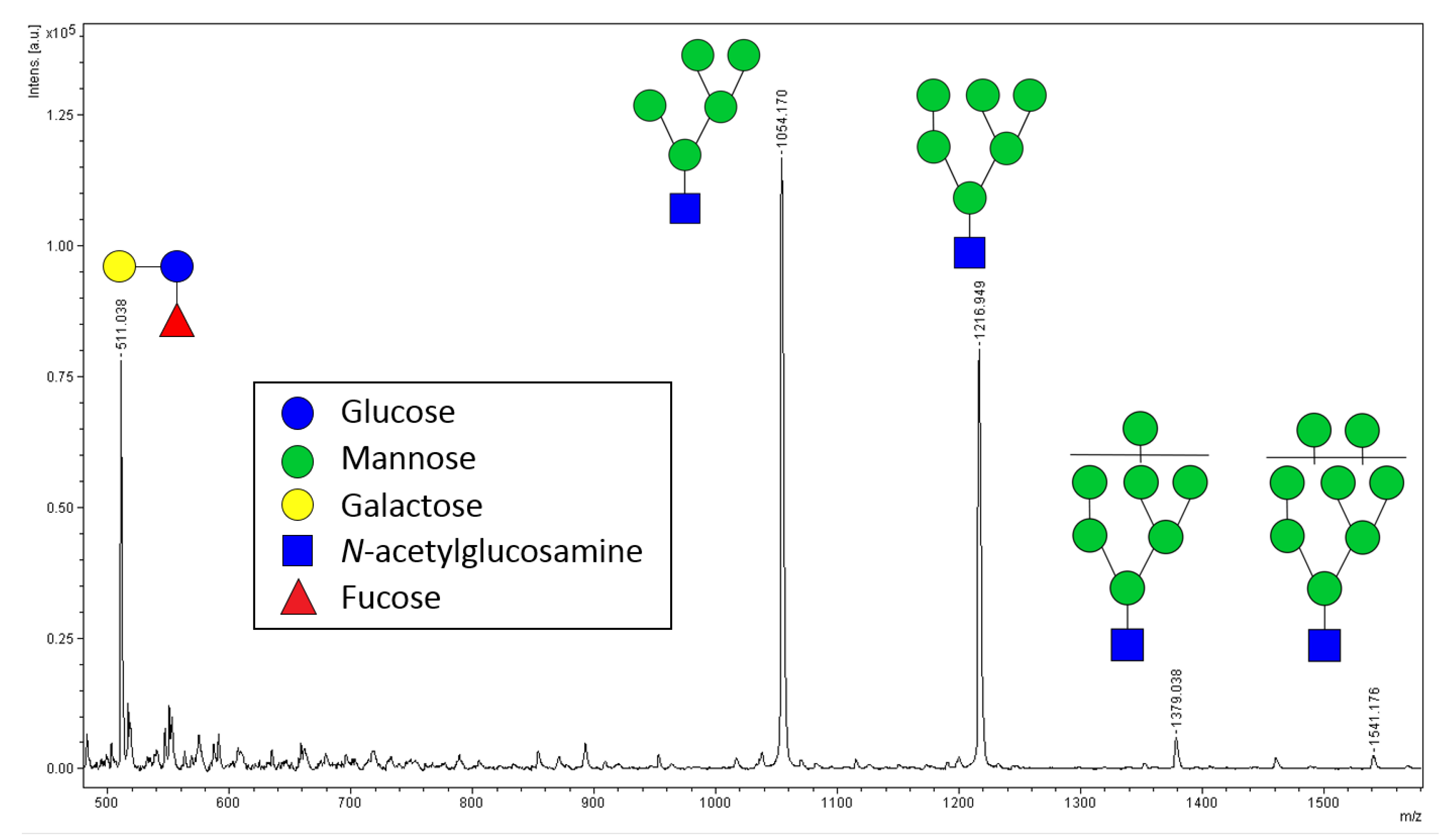
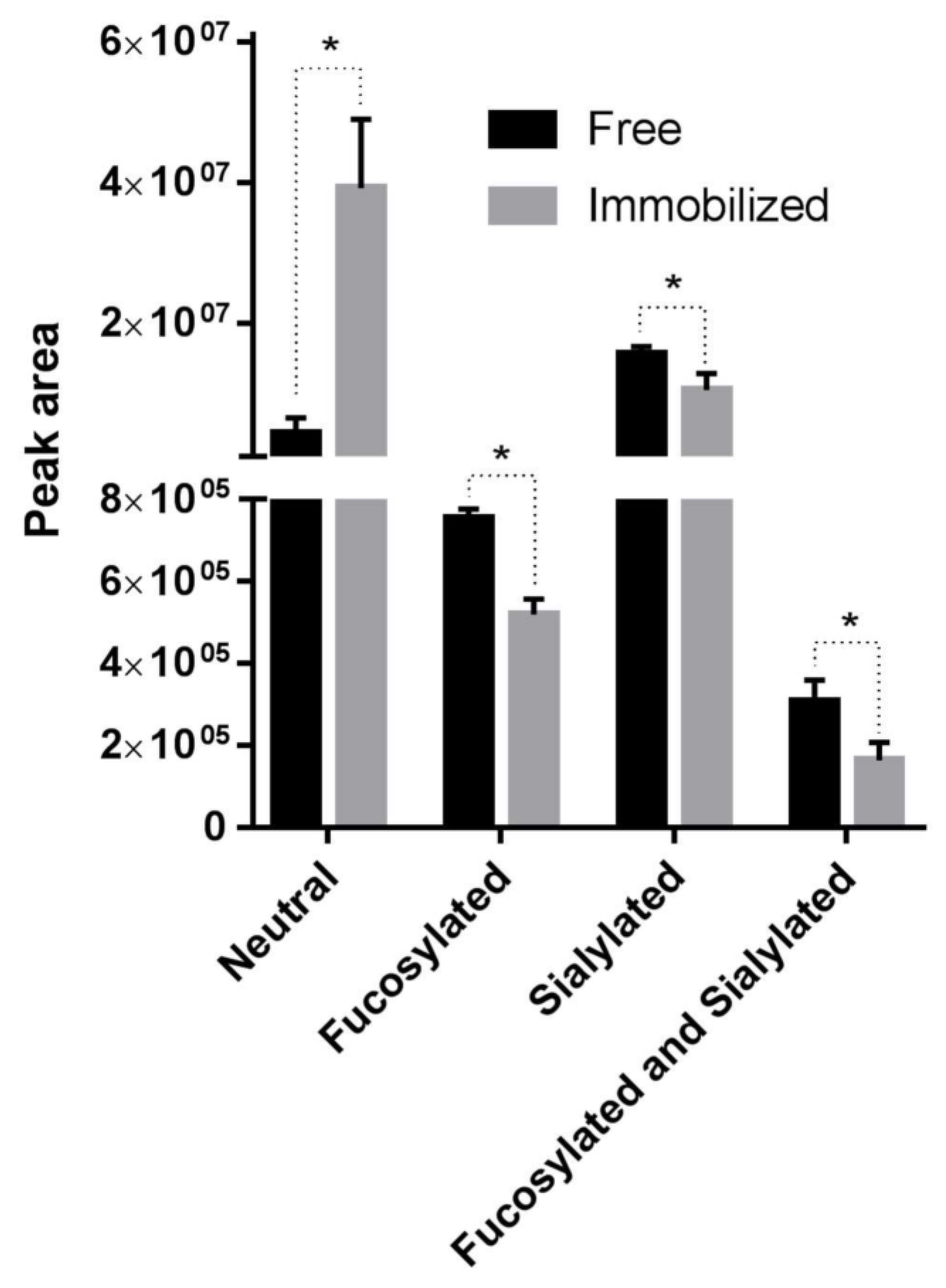
2.2. Relative Quantification of N-glycans by MALDI-ToF MS
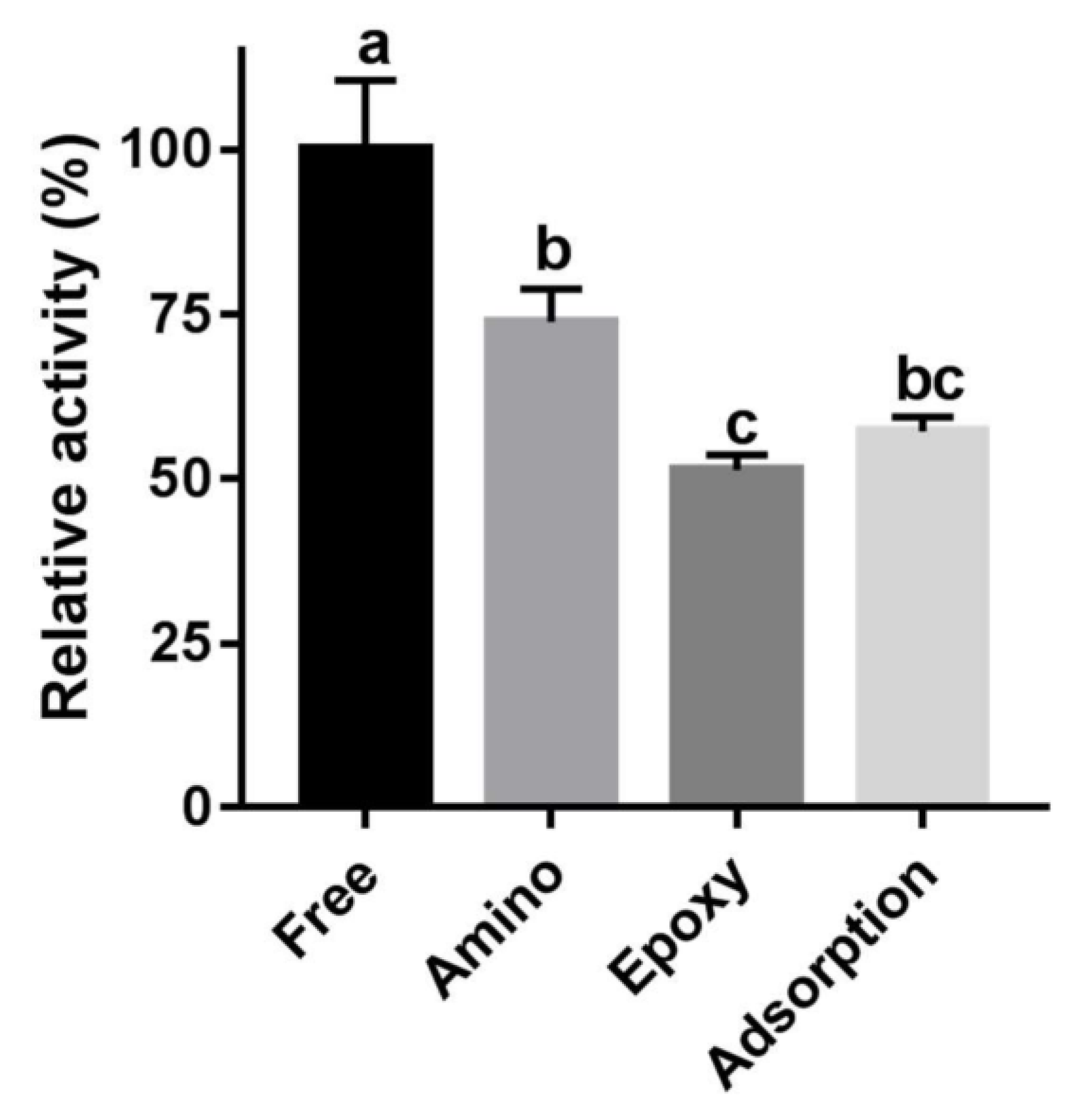
2.3. Comparing Immobilized Enzyme Activities
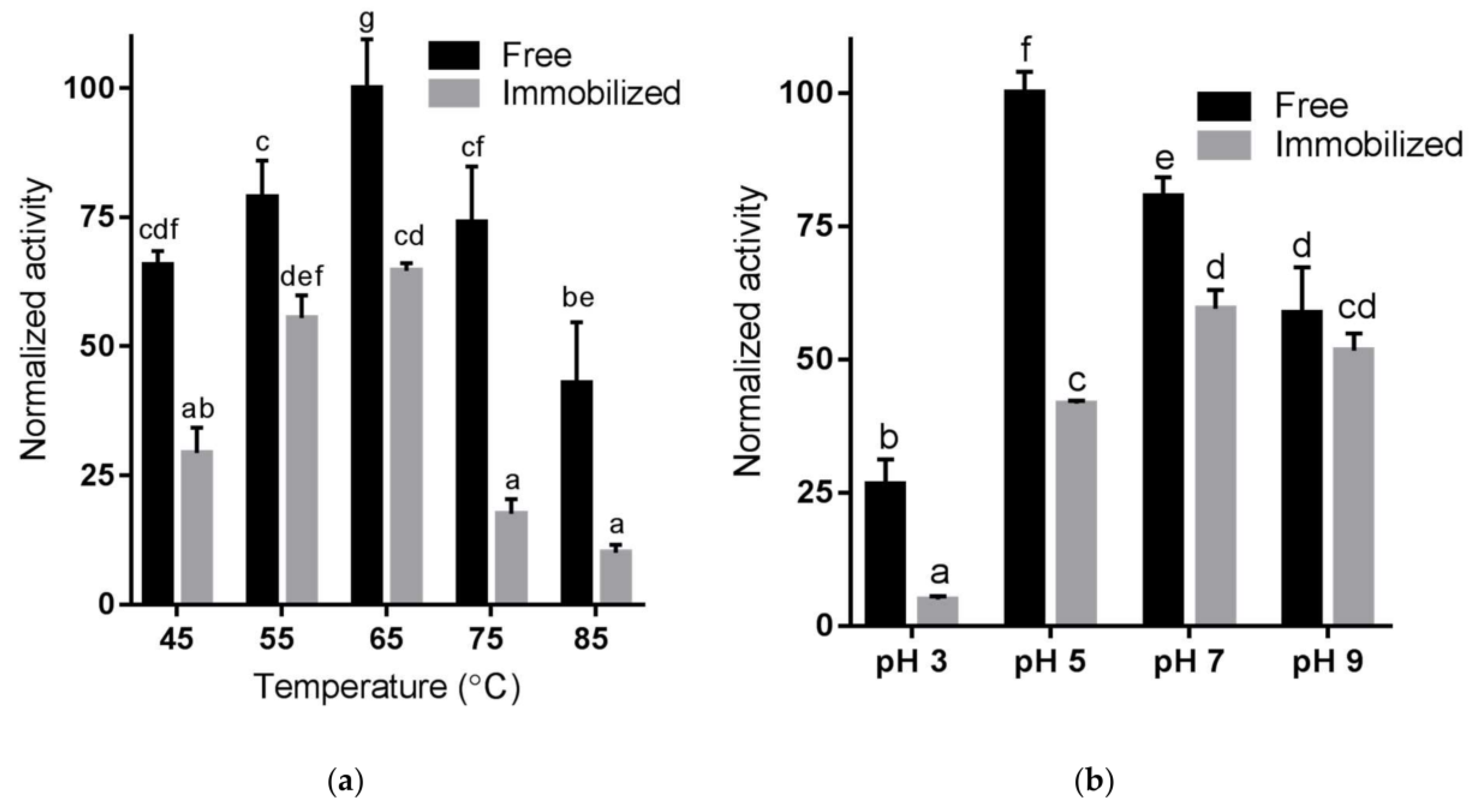
2.4. Temperature and pH Sensitivity of Immobilized Enzyme Using RNase B
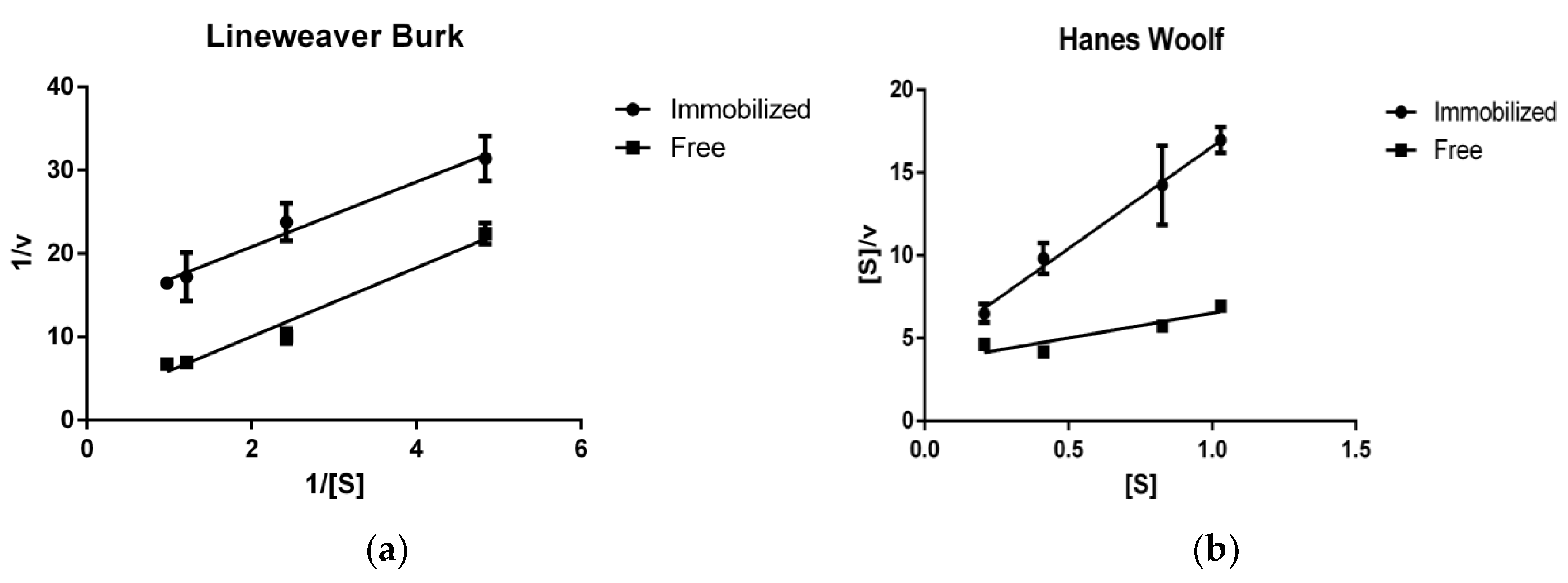
2.5. Enzyme Kinetics Using RNase B
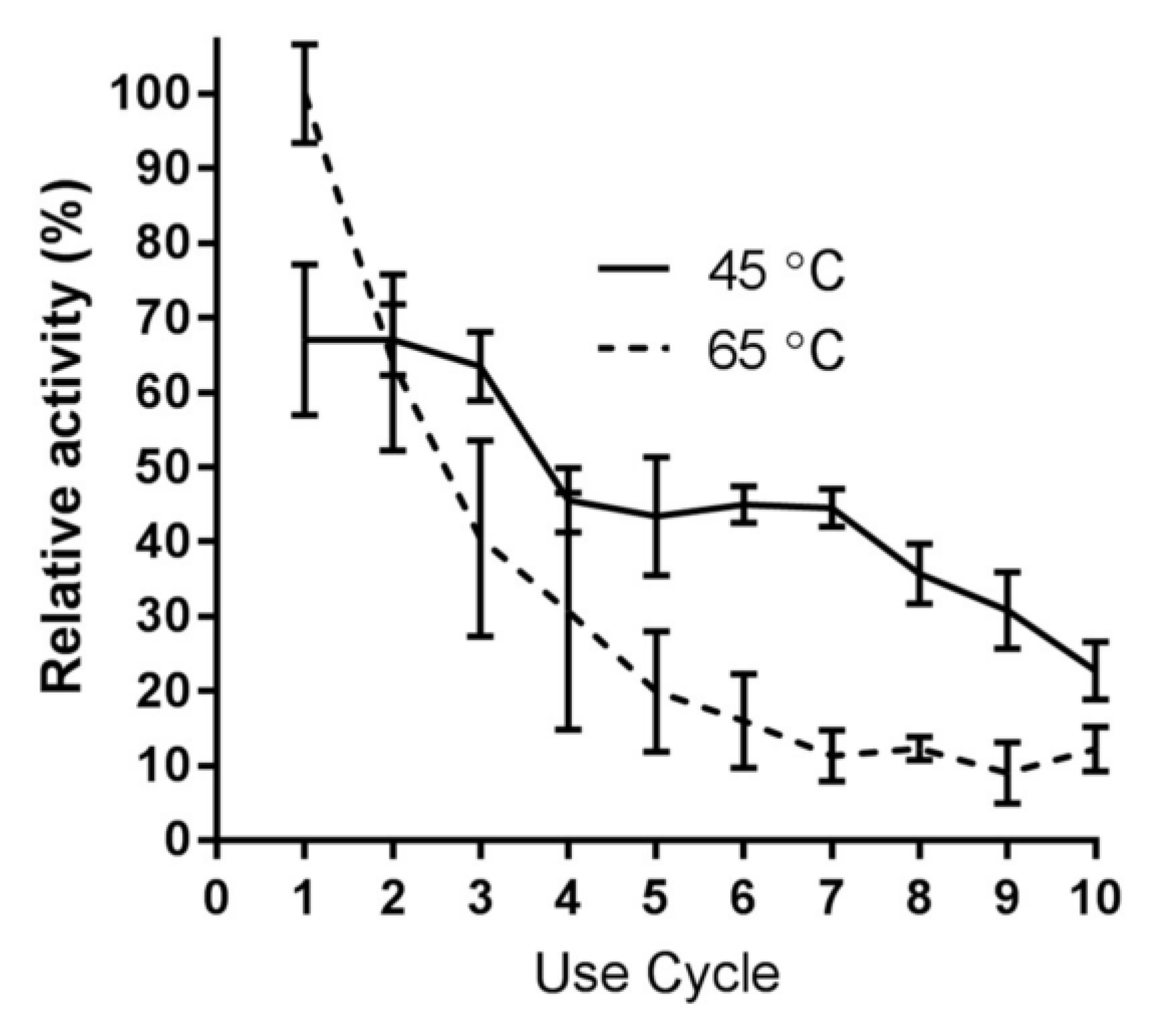
2.6. Immobilized Enzyme Reusability with RNase B
2.7. Release of N-glycans from Bovine whey Proteins
3. Materials and Methods
3.1. Enzyme Production
3.2. Enzyme Immobilization
3.3. Evaluating Protein Immobilization Yield
3.4. N-glycan Analysis by MALDI-ToF Mass Spectrometry
3.5. Preliminary Comparison of Immobilization Methods Efficiency Using Ribonuclease (RNase) B
3.6. Effect of Immobilization on Enzyme Resilience to pH and Thermal Changes
3.7. Determination of Kinetic Parameters of Enzyme Using RNase B
3.8. Evaluating Reusability of Immobilized Endo BI-1 Using RNase B
3.9. Release of N-glycans from Bovine Colostrum whey Proteins by Free and Immobilized Endo BI-1
3.10. N-glycan Analysis by Mass Spectrometry Nano-LC-Chip Q ToF
4. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Lafite, P.; Daniellou, R. Rare and unusual glycosylation of peptides and proteins. Nat. Prod. Rep. 2012, 29, 729–738. [Google Scholar] [CrossRef] [PubMed]
- Montreuil, J. Primary Structure of Glycoprotein Glycans Basis for the Molecular Biology of Glycoproteins. In Advances in Carbohydrate Chemistry and Biochemistry; Elsevier: Cambridge, MA, USA, 1980; Volume 37, pp. 157–223. ISBN 978-0-12-007237-8. [Google Scholar]
- Zivkovic, A.M.; German, J.B.; Lebrilla, C.B.; Mills, D.A. Human milk glycobiome and its impact on the infant gastrointestinal microbiota. Proc. Natl. Acad. Sci. USA 2011, 108, 4653–4658. [Google Scholar] [CrossRef] [PubMed]
- Thurl, S.; Munzert, M.; Henker, J.; Boehm, G.; Müller-Werner, B.; Jelinek, J.; Stahl, B. Variation of human milk oligosaccharides in relation to milk groups and lactational periods. Br. J. Nutr. 2010, 104, 1261–1271. [Google Scholar] [CrossRef] [PubMed]
- Helenius, A.; Aebi, M. Intracellular Functions of N-Linked Glycans. Science 2001, 291, 2364–2369. [Google Scholar] [CrossRef] [PubMed]
- Karav, S.; Cohen, J.L.; Barile, D.; de Moura Bell, J.M.L.N. Recent advances in immobilization strategies for glycosidases. Biotechnol. Prog. 2017, 33, 104–112. [Google Scholar] [CrossRef] [PubMed]
- Karav, S.; Parc, A.L.; de Moura Bell, J.M.L.N; Frese, S.A.; Kirmiz, N.; Block, D.E.; Barile, D.; Mills, D.A. Oligosaccharides Released from Milk Glycoproteins Are Selective Growth Substrates for Infant-Associated Bifidobacteria. Appl. Environ. Microbiol. 2016, 82, 3622–3630. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.L.; Du, Y.M.; Wang, W.; Conway, L.P.; Cai, Z.P.; Voglmeir, J.; Liu, L. Comparison of the bifidogenic activity of human and bovine milk N-glycome. J. Funct. Foods 2017, 33, 40–51. [Google Scholar] [CrossRef]
- Garrido, D.; Nwosu, C.; Ruiz-Moyano, S.; Aldredge, D.; German, J.B.; Lebrilla, C.B.; Mills, D.A. Endo-β-N-acetylglucosaminidases from Infant Gut-associated Bifidobacteria Release Complex N-glycans from Human Milk Glycoproteins. Mol. Cell. Proteom. 2012, 11, 775–785. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.-L.; Wang, W.; Du, Y.-M.; Wu, H.; Yu, X.-B.; Ye, K.-P.; Li, C.-B.; Jung, Y.-S.; Qian, Y.-J.; Voglmeir, J.; et al. Comparison of anti-pathogenic activities of the human and bovine milk N-glycome: Fucosylation is a key factor. Food Chem. 2017, 235, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Blaschek, K.M.; Wendorff, W.L.; Rankin, S.A. Survey of Salty and Sweet Whey Composition from Various Cheese Plants in Wisconsin. J. Dairy Sci. 2007, 90, 2029–2034. [Google Scholar] [CrossRef] [PubMed]
- Walstra, P.; Walstra, P.; Wouters, J.T.M.; Geurts, T.J. Dairy Science and Technology, 2nd ed.; CRC Press: Boca Raton, Fl, USA, 2010; ISBN 978-1-4200-2801-0. [Google Scholar]
- USDA. Dairy Products 2016 Summary; USDA National Agricultural Statistics Service, United States Department of Agriculture: Washington, DC, USA, 2017.
- Cao, L.; van Langen, L.; Sheldon, R.A. Immobilised enzymes: Carrier-bound or carrier-free? Curr. Opin. Biotechnol. 2003, 14, 387–394. [Google Scholar] [CrossRef]
- Cao, L. Immobilised enzymes: science or art? Curr. Opin. Chem. Biol. 2005, 9, 217–226. [Google Scholar] [CrossRef] [PubMed]
- DiCosimo, R.; McAuliffe, J.; Poulose, A.J.; Bohlmann, G. Industrial use of immobilized enzymes. Chem. Soc. Rev. 2013, 42, 6437–6474. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, P. Enzymes in Food Processing: A Condensed Overview on Strategies for Better Biocatalysts. Enzyme Res. 2010, 2010, 862537. [Google Scholar] [CrossRef] [PubMed]
- Alloue, W.A.M.; Destain, J.; Medjoub, T.E.; Ghalfi, H.; Kabran, P.; Thonart, P. Comparison of Yarrowia lipolytica Lipase Immobilization Yield of Entrapment, Adsorption, and Covalent Bond Techniques. Appl. Biochem. Biotechnol. 2008, 150, 51–63. [Google Scholar] [CrossRef] [PubMed]
- Mateo, C.; Grazú, V.; Pessela, B.C.C.; Montes, T.; Palomo, J.M.; Torres, R.; López-Gallego, F.; Fernández-Lafuente, R.; Guisán, J.M. Advances in the design of new epoxy supports for enzyme immobilization–stabilization. Biochem. Soc. Trans. 2007, 35, 1593–1601. [Google Scholar] [CrossRef] [PubMed]
- Karav, S.; Le Parc, A.; de Moura Bell, J.M.L.N.; Rouquié, C.; Mills, D.A.; Barile, D.; Block, D.E. Others Kinetic characterization of a novel endo-β-N-acetylglucosaminidase on concentrated bovine colostrum whey to release bioactive glycans. Enzyme Microb. Technol. 2015, 77, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Masuko, T.; Minami, A.; Iwasaki, N.; Majima, T.; Nishimura, S.-I.; Lee, Y.C. Carbohydrate analysis by a phenol–sulfuric acid method in microplate format. Anal. Biochem. 2005, 339, 69–72. [Google Scholar] [CrossRef] [PubMed]
- Scott, T.A., Jr.; Melvin, E.H. Determination of dextran with anthrone. Anal. Chem. 1953, 25, 1656–1661. [Google Scholar] [CrossRef]
- Pfenninger, A.; Karas, M.; Finke, B.; Stahl, B.; Sawatzki, G. Mass Spectrometric Investigations of Human Milk Oligosaccharides. In Bioactive Components of Human Milk; Advances in Experimental Medicine and Biology; Springer: Boston, MA, USA, 2001; pp. 279–284. ISBN 978-1-4613-5521-2. [Google Scholar]
- Nishimura, S.-I.; Niikura, K.; Kurogochi, M.; Matsushita, T.; Fumoto, M.; Hinou, H.; Kamitani, R.; Nakagawa, H.; Deguchi, K.; Miura, N.; et al. High-Throughput Protein Glycomics: Combined Use of Chemoselective Glycoblotting and MALDI-TOF/TOF Mass Spectrometry. Angew. Chem. Int. Ed. 2005, 44, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Bucknall, M.; Fung, K.Y.C.; Duncan, M.W. Practical quantitative biomedical applications of MALDI-TOF mass spectrometry. J. Am. Soc. Mass Spectrom. 2002, 13, 1015–1027. [Google Scholar] [CrossRef]
- Bothner, B.; Chavez, R.; Wei, J.; Strupp, C.; Phung, Q.; Schneemann, A.; Siuzdak, G. Monitoring Enzyme Catalysis with Mass Spectrometry. J. Biol. Chem. 2000, 275, 13455–13459. [Google Scholar] [CrossRef] [PubMed]
- Leboeuf, E.; Immerzeel, P.; Gibon, Y.; Steup, M.; Pauly, M. High-throughput functional assessment of polysaccharide-active enzymes using matrix-assisted laser desorption/ionization–time-of-flight mass spectrometry as exemplified on plant cell wall polysaccharides. Anal. Biochem. 2008, 373, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.-J.; Tholey, A.; Heinzle, E. Application of automated matrix-assisted laser desorption/ionization time-of-flight mass spectrometry for the measurement of enzyme activities. Rapid Commun. Mass Spectrom. 2001, 15, 1327–1333. [Google Scholar] [CrossRef] [PubMed]
- Mechref, Y.; Novotny, M.V.; Krishnan, C. Structural Characterization of Oligosaccharides Using Maldi-TOF/TOF Tandem Mass Spectrometry. Anal. Chem. 2003, 75, 4895–4903. [Google Scholar] [CrossRef] [PubMed]
- Gaur, R.; Pant, H.; Jain, R.; Khare, S.K. Galacto-oligosaccharide synthesis by immobilized Aspergillus oryzae β-galactosidase. Food Chem. 2006, 97, 426–430. [Google Scholar] [CrossRef]
- Wong, D.E.; Senecal, K.J.; Goddard, J.M. Immobilization of chymotrypsin on hierarchical nylon 6,6 nanofiber improves enzyme performance. Colloids Surf. B Biointerfaces 2017, 154, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Rehman, H.U.; Aman, A.; Silipo, A.; Qader, S.A.U.; Molinaro, A.; Ansari, A. Degradation of complex carbohydrate: Immobilization of pectinase from Bacillus licheniformis KIBGE-IB21 using calcium alginate as a support. Food Chem. 2013, 139, 1081–1086. [Google Scholar] [CrossRef] [PubMed]
- Karav, S.; de Moura Bell, J.M.L.N.; Parc, A.L.; Liu, Y.; Mills, D.A.; Block, D.E.; Barile, D. Characterizing the release of bioactive N-glycans from dairy products by a novel endo-β-N-acetylglucosaminidase. Biotechnol. Prog. 2015, 31, 1331–1339. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Teoh, W.Y.; Gooding, J.J.; Selomulya, C.; Amal, R. Functionalization Strategies for Protease Immobilization on Magnetic Nanoparticles. Adv. Funct. Mater. 2010, 20, 1767–1777. [Google Scholar] [CrossRef]
- Iyer, P.V.; Ananthanarayan, L. Enzyme stability and stabilization—Aqueous and non-aqueous environment. Process Biochem. 2008, 43, 1019–1032. [Google Scholar] [CrossRef]
- Busto, M.D.; García-Tramontín, K.E.; Ortega, N.; Perez-Mateos, M. Preparation and properties of an immobilized pectinlyase for the treatment of fruit juices. Bioresour. Technol. 2006, 97, 1477–1483. [Google Scholar] [CrossRef] [PubMed]
- Demirel, G.; Özçetin, G.; Şahin, F.; Tümtürk, H.; Aksoy, S.; Hasirci, N. Semi-interpenetrating polymer networks (IPNs) for entrapment of glucose isomerase. React. Funct. Polym. 2006, 66, 389–394. [Google Scholar] [CrossRef]
- Wei, L.; Zhang, W.; Lu, H.; Yang, P. Immobilization of enzyme on detonation nanodiamond for highly efficient proteolysis. Talanta 2010, 80, 1298–1304. [Google Scholar] [CrossRef] [PubMed]
- Klein, M.P.; Nunes, M.R.; Rodrigues, R.C.; Benvenutti, E.V.; Costa, T.M.H.; Hertz, P.F.; Ninow, J.L. Effect of the Support Size on the Properties of β-Galactosidase Immobilized on Chitosan: Advantages and Disadvantages of Macro and Nanoparticles. Biomacromolecules 2012, 13, 2456–2464. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, S.; Vohra, A.; Lakhanpal, A.; Gupta, R. Immobilization of Commercial Pectinase (Polygalacturonase) on Celite and Its Application in Juice Clarification. J. Food Process. Preserv. 2015, 6, 2135–2141. [Google Scholar] [CrossRef]
- Parc, A.L.; Karav, S.; de Moura Bell, J.M.L.N.; Frese, S.A.; Liu, Y.; Mills, D.A.; Block, D.E.; Barile, D. A novel endo-β-N-acetylglucosaminidase releases specific N-glycans depending on different reaction conditions. Biotechnol. Prog. 2015, 31, 1323–1330. [Google Scholar] [CrossRef] [PubMed]
- Axelsson, A.; Zacchi, G. Economic evaluation of the hydrolysis of lactose using immobilized β-galactosidase. Appl. Biochem. Biotechnol. 1990, 24, 679–693. [Google Scholar] [CrossRef]
- Nwosu, C.C.; Aldredge, D.L.; Lee, H.; Lerno, L.A.; Zivkovic, A.M.; German, J.B.; Lebrilla, C.B. Comparison of the Human and Bovine Milk N-glycome via High-Performance Microfluidic Chip Liquid Chromatography and Tandem Mass Spectrometry. J. Proteome Res. 2012, 11, 2912–2924. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Orlando, R. Kinetics of N-Glycan Release from Human Immunoglobulin G (IgG) by PNGase F: All Glycans Are Not Created Equal. J. Biomol. Tech. JBT 2017. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, H.; Matsumura, M.; Veliky, I.A. Diffusion characteristics of substrates in Ca-alginate gel beads. Biotechnol. Bioeng. 1984, 26, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Manjon, A.; Iborra, J.L.; Lozano, P.; Canovas, M. A practical experiment on enzyme immobilization and characterization of the immobilized derivatives. Biochem. Mol. Biol. Educ. 1995, 23, 213–216. [Google Scholar] [CrossRef]
- Park, E.; Yang, H.; Kim, Y.; Kim, J. Analysis of oligosaccharides in beer using MALDI-TOF-MS. Food Chem. 2012, 134, 1658–1664. [Google Scholar] [CrossRef] [PubMed]
- Lineweaver, H.; Burk, D. The determination of enzyme dissociation constants. J. Am. Chem. Soc. 1934, 56, 658–666. [Google Scholar] [CrossRef]
- Hanes, C.S. Studies on plant amylases: The effect of starch concentration upon the velocity of hydrolysis by the amylase of germinated barley. Biochem. J. 1932, 26, 1406–1421. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J.L.; Barile, D.; Liu, Y.; de Moura Bell, J.M.L.N. Role of pH in the recovery of bovine milk oligosaccharides from colostrum whey permeate by nanofiltration. Int. Dairy J. 2017, 66, 68–75. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cohen, J.L.; Karav, S.; Barile, D.; De Moura Bell, J.M.L.N. Immobilization of an Endo-β-N-acetylglucosaminidase for the Release of Bioactive N-glycans. Catalysts 2018, 8, 278. https://doi.org/10.3390/catal8070278
Cohen JL, Karav S, Barile D, De Moura Bell JMLN. Immobilization of an Endo-β-N-acetylglucosaminidase for the Release of Bioactive N-glycans. Catalysts. 2018; 8(7):278. https://doi.org/10.3390/catal8070278
Chicago/Turabian StyleCohen, Joshua L., Sercan Karav, Daniela Barile, and Juliana M. L. N. De Moura Bell. 2018. "Immobilization of an Endo-β-N-acetylglucosaminidase for the Release of Bioactive N-glycans" Catalysts 8, no. 7: 278. https://doi.org/10.3390/catal8070278
APA StyleCohen, J. L., Karav, S., Barile, D., & De Moura Bell, J. M. L. N. (2018). Immobilization of an Endo-β-N-acetylglucosaminidase for the Release of Bioactive N-glycans. Catalysts, 8(7), 278. https://doi.org/10.3390/catal8070278







