Abstract
We synthesized cerium-doped cobalt-alumina (CoxCey/Al2O3) catalysts for the propane steam reforming (PSR) reaction. Adding cerium introduces oxygen vacancies, and the oxygen transfer capacity of the Ce promoter favors CO to CO2 conversion during PSR, inhibiting coke deposition and promoting hydrogen production. The best PSR activity was achieved at 700 °C using the Co0.85Ce0.15/Al2O3 catalyst, which showed 100% propane (C3H8) conversion and about 75% H2 selectivity, and 6% CO, 5% CO2, and 4% CH4 were obtained. In contrast, the H2 selectivity of the base catalyst, Co/Al2O3, is 64%. The origin of the difference in activity was the lower C3H8 gas desorption temperature of the Co0.85Ce0.15/Al2O3 catalyst compared to that of the Co/Al2O3 catalyst; thus, the PSR occurred at low temperatures. Furthermore, more CO was adsorbed on the Co0.85Ce0.15/Al2O3 catalyst, and subsequently, desorbed as CO2. The activation energy for water desorption from the Co0.85Ce0.15/Al2O3 catalyst was 266.96 kJ/mol, higher than that from Co/Al2O3. Furthermore, the water introduced during the reaction probably reacted with CO on the Co0.85Ce0.15/Al2O3 catalyst, increasing CO2 generation. Finally, we propose a mechanism involving the Co0.85Ce0.15/Al2O3 catalyst, wherein propane is reformed on CoxCey sites, forming H2, and CO, followed by the conversion of CO to CO2 by water on CeO2 sites.
1. Introduction
At the Davos World Economic Forum in January 2017, a hydrogen consortium was formed consisting of chief executive officers from major companies in Asia, Europe, and the United States of America, as well as national policy makers. At the Sustainability Innovation Forum in November 2017, the hydrogen consortium predicted that the demand for hydrogen fuel cell vehicles would increase from 10 to 15 million cars by 2030 and to 500,000 trucks by 2030. Moreover, the demand for hydrogen is expected to expand to 10 times its current level because of its wide use in other industries [1]. Thus, the era of the hydrogen economy has arrived. The increasing use of hydrogen energy will help protect the environment, although it may not represent the next-generation energy revolution. Methods for producing hydrogen from hydrocarbons include catalytic cracking [2], catalytic partial oxidation [3], carbon dioxide reforming [4], and steam catalytic reforming [5]. Without considering the CO2 separation, treatment, and storage steps, the steam catalytic reforming process has been evaluated as the most economical large-scale hydrogen production process and has already been commercialized. The economic efficiency of these processes can be evaluated based on the raw material composition of the supplied hydrocarbon gas and the final consumption and destination of the produced synthesis gas. In addition, because CO2 emission regulations are becoming stricter, the cost of CO2 disposal will exert a significant impact on the overall economic assessment. In the steam reforming (SR) process, steam is added to a hydrocarbon (here, propane steam reforming or PSR), resulting in the decomposition of the hydrocarbon, as shown in Equation (1) [6].
C3H8 + 6H2O → 3CO2 + 10H2, ΔH298 = 499 kJ/mol
The CO2/H2 ratio of the product gas is 0.3, which is lower than that of the partial oxidation process, and it is advantageous in that a larger amount of hydrogen can be obtained from a small amount of hydrocarbon. However, the PSR process has a disadvantage: It requires a high water vapor/carbon ratio of over six to prevent coke formation on the catalyst. In addition, the process consumes a large amount of energy because the process temperature is as high as 800 °C. The entire SR process is now briefly described [7] and is divided into pretreatment and posttreatment processes based on the use of a steam reformer. In the pretreatment process, a hydrogen desulfurization system (HDS) and a prereformer are installed. In the posttreatment step, there is a high-temperature shift (HTS) reactor, a low-temperature shift (LTS) reactor, a CO2 separator, and a methanation reactor. As parts of the CO2 separation device, a conventional absorption device and a pressure swing adsorption (PSA) device for increasing the separation efficiency are used. In particular, the final section of the hydrocarbon reformer system must purify the raw hydrogen. In modern plants, a PSA unit is used to achieve the final product purity. In this process, the separated carbon dioxide can be collected and used for various purposes without being disposed of in the atmosphere. In particular, carbon capture and utilization (CCU) devices are attractive, and the reduction of carbon dioxide by hydrogen (thermal reaction) or water (photoreaction) to convert it into fuel or to other high-value chemicals is one such application. However, in this paper, only the main process, steam reforming, is discussed. Cobalt [8] and nickel [9] catalysts are widely used in the PSR process. Precious metal catalysts such as Rh show low carbon deposition, but are not used for economic reasons [10]. Nickel has a sintering temperature of 590 °C, which is lower than the process temperature of the steam catalytic reaction (600–800 °C); therefore, nickel is usually dispersed and supported on a carrier such as Al2O3, KOH, MgO, or urania. The carrier is added to promote the gasification of the carbon on the catalyst surface. As the catalytic species can evaporate at high temperatures, they are added in the form of potassium alumina-silicates or calcium magnesia silicates, for example, NiO/K-Al2O3, NiO/Al2O3/CaO, and NiO/MgAl2O4 [11,12,13]. Many researchers have reported that NiC formation is suppressed by the steam reforming of catalysts loaded with promoters such as Pt, Ir, Sn, Pb, Ge, As, Sb, Bi, Ag, Cu, and Zn [14,15,16,17,18,19,20].
Previously, we have confirmed that the deposition of coke on the Co–Mn catalyst is lower than that on other catalysts and hydrogen production is close to 75% [21]. Furthermore, we have shown that, in the Co/Mn catalyst, the oxygen vacancies of the manganese oxides are well supplied with oxygen for the reforming of propane on the cobalt sites, enabling the reforming reaction to occur continuously. However, a promoter with greater oxygen transfer capability is required to confirm this effect. Therefore, in this study, cobalt was selected as a main catalyst and another metal catalyst for promoting oxygen vacancies, which was added as a catalytic promoter. However, in the PSR process, the deterioration of the catalyst because of carbon deposition on the catalyst surface is a major obstacle to commercial use. Therefore, generally, in the steam reforming process, excess steam is added to prevent carbon deposition. Carbon deposition on the catalyst is produced in the form of encapsulating coke and whisker-like coke [22]. The encapsulating coke reduces catalyst activity, and the whisker coke increases catalyst sintering, which eventually blocks the movement of the reactant gas, and thus, increases the pressure differential in the reactor. In both cases, however, these factors contribute to reduce the activity of the catalyst. On the other hand, the deposition of encapsulating coke is somewhat related to the CO generated in the steam reforming reaction [23]. Therefore, it is possible to control the encapsulating coke deposition if the amount of CO adsorbed on the catalyst surface is reduced by converting the produced CO into CO2 during the reaction. Furthermore, the water gas shift (WGS) of CO can assist in the production of hydrogen, as shown by Equation (2):
CO + H2O → CO2 + H2.
In this study, we used ceria as a co-catalyst, expecting it to play a role as an oxidation catalyst to convert CO to CO2 [24]. The aim of this study is to suppress the deterioration of the catalyst by converting the CO gas generated during the PSR reaction to CO2 by using the lattice oxygen of CexOy, which was used as a promoter to achieve higher hydrogen production over the Co-based catalyst. A catalyst loaded with 30% cobalt oxide on 70% γ-Al2O3 was used as the base catalyst, and four kinds of cerium-doped catalysts were prepared: 30Co0.97Ce0.03/70γ-Al2O3, 30Co0.85Ce0.15/70γ-Al2O3, 30Co0.79Ce0.21/70γ-Al2O3, and 30Co0.70Ce0.30/70γ-Al2O3 containing 0.1, 0.5, 0.7, and 1.0 mol.% Ce per mole of Co, respectively. The catalysts were prepared by a conventional impregnation method. The physical properties of the catalyst before and after the reaction are compared, and based on the results, a PSR mechanism is proposed.
2. Results and Discussion
2.1. Characteristics of 30CoxCey/70γ-Al2O3 Catalysts
The crystallinities and phase purities of the as-prepared 30CoxCey/70γ-Al2O3 catalysts were determined by XRD analysis, as shown in Figure 1A. The XRD pattern of the pure 30Co/70γ-Al2O3 catalyst was assigned to a cubic form of Co3O4 in the Fd-3m space group [JCPDS card no. 01-073-1701]. The main peaks observed at 2θ = 31.27° (220), 33.85° (311), 44.80° (400), 59.36° (511), and 65.24° (440) were assigned to CoAl2O4 [JCPDS card no. 00-003-0896]. These results confirm that the Co3O4 and CoAl2O4 phases co-exist in the prepared samples [25]. However, in the case of the 30CoxCey/70γ-Al2O3 catalyst, the amount of Ce in the sample was insufficient to yield diffraction peaks corresponding to CeO2, even though Ce had been added. Only the 30Co0.7Ce0.3/70r-Al2O3 catalyst with the highest amount of Ce showed a weak diffraction peak at 28.57°, corresponding to the (111) crystal face of CeO2 [JCPDS card no. 00-002-1306]. Interestingly, compared with the diffraction patterns of the 30Co/70γ-Al2O3 catalyst, the diffraction peaks of the 30CoxCey/70γ-Al2O3 catalysts shifted to higher angles with increasing Ce addition. Thus, according to Bragg’s law, the distance (d) between the lattice planes was reduced [26]. In addition, the increase in crystal size and lattice strain arise from the differences in the ionic radii of Co and Ce. The TEM images are shown in Figure 1B. The surfaces of the 30CoxCey/70γ-Al2O3 catalysts were found to have short rod-like particles of Co and Ce species on the surface of the spherical γ-Al2O3 support.

Figure 1.
XRD patterns and TEM images of the fresh 30CoxCey/70γ-Al2O3 catalysts.
The elemental ratios of the catalysts were examined using EDS/element mapping, and the results are shown in Figure 2A,B. Due to the small amount of Ce (beyond the limit of EDS analysis, which only observed a small part of the surface), the presence of Ce was only confirmed in 30Co0.79Ce0.21/70γ-Al2O3 and 30Co0.70Ce0.30/70γ-Al2O3. The amounts of Ce present in these catalysts were about 10% and 20% of the amount of Co, respectively, representing 1/2 and 1/3, respectively, of the amounts of Ce added in the synthesis step. These differences are considered to be due to the limitations of EDS analysis. However, because there was an increase in the proportion of Ce with respect to the amount added, we determined that the catalysts had been successfully formed. Elemental mapping images are shown in Figure 2B and were used to confirm the presence of trace elements contained in the 30Co0.85Ce0.15/70γ-Al2O3 catalyst. Fortunately, Ce, which did not appear in the EDS spectrum, was found to be evenly dispersed over the catalyst surface. Further, when the intensities of the colored spots corresponding to the respective elements were determined, the intensities of the spots were found to vary in proportion with the amount of the elements present in the catalyst. Thus, we concluded that the catalysts prepared in this study had been synthesized quantitatively.
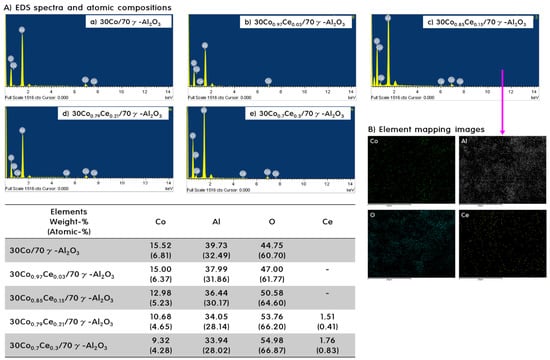
Figure 2.
EDS spectra and atomic compositions (A) of all catalysts and mapping images for elements (B) present in the 30Co0.85Ce0.15/70γ-Al2O3 catalyst.
Figure 3 shows the N2 adsorption/desorption isotherm curves obtained at 77 K for the fresh 30CoxCey/70γ-Al2O3 catalysts. Using Kelvin’s equation, the radii of pores in which capillary condensation occurs can be determined as a function of the relative pressure (P/P0). The mean pore diameter, Dp, was calculated from Dp = 4VT/S, where VT is the total volume of the pores and S is the BET surface area. According to the International Union of Pure and Applied Chemistry classification, the isotherms of all catalysts are type IV. In addition, hysteresis was observed at high relative pressures for all the catalysts, indicating the presence of bulk mesopores formed between the particles. The changes in the mean surface area and pore volume are listed in each figure. The BET specific surface areas of the 30CoxCey/70γ-Al2O3 catalysts (127–182 m2 g−1) are significantly higher than that of the 30Co/70γ-Al2O3 catalyst (96 m2 g−1). In particular, except for the 30Co0.7Ce0.3/70γ-Al2O3 catalyst, the BET specific surface area increased as the amount of added Ce increased. In the 30Co0.7Ce0.3/70γ-Al2O3 catalyst, the specific surface area was slightly reduced because of the strong connections between the large quantity of cerium oxide. In general, the specific surface area of regular particles is strongly related to the particle size, with smaller particles having a greater surface area [27]. Moreover, when the surface area is large, the catalytic active site also increases, and as a result, the catalyst performance tends to increase [28]. The surface area of the catalyst in this study seems to be more affected by the bulk pores formed by sintering particles than their particle size, meaning that there was significant space between particles in the 30CoxCey/70γ-Al2O3 catalysts; this might increase the gas adsorption sites for reaction. On the other hand, the total pore volumes and pore diameters in all catalysts showed the same trend with the surface area.

Figure 3.
N2 adsorption/desorption isotherm curves at 77 K for the fresh 30CoxCey/70γ-Al2O3 catalysts.
Many previous studies [29,30] have confirmed that the activities of catalysts containing reduced metals rather than metal oxides are better for hydrocarbon reforming. Therefore, reduced catalysts were used in this study, and H2-TPR measurements of all catalysts were performed to determine the appropriate reduction temperature. Figure 4A shows the H2-TPR profiles of the 30CoxCey/70γ-Al2O3 catalysts, where the peak area corresponds to the hydrogen uptake and the peak position corresponds to the reduction temperature of the catalyst species. In general, the reduction peak for pure Co3O4 to CoO is observed at about 250 °C, whereas the peak for CoO to Co metal occurs at about 400 °C [31]. From the XRD pattern shown in Figure 1, we confirmed that all the catalysts contained Co3O4 and CoAl2O4. Finally, the TPR profile for 30Co/70γ-Al2O3 shows a large peak at 850 °C, corresponding to the reduction of the stoichiometric cobalt aluminate species (spinel CoAl2O4) [32]. Two distinct peaks centered at 420 and 500 °C were observed, which are attributed to the two-step reduction of Co3O4 to CoO and then to Co, respectively, which interacts with the Al2O3 support [33]. However, in the catalysts substituted with Ce, Co partially escapes from the skeleton and is present outside the lattice as Co3O4. The reduction peak for this, i.e., the peak corresponding to the reduction of Co3O4 to CoO, was observed at about 200 °C. Furthermore, in the catalysts containing Ce, the reduction of Co3O4 and CoO occurs at lower temperatures, about 400 °C. In particular, the reduction of Co ions in CoAl2O4, which usually occurs at high temperatures, also shifted to a low temperature of about 700–800 °C. Thus, the addition of Ce into the framework lattice facilitates the removal of lattice oxygen, and thus, more Co is reduced. This means that the oxygen in the Co-O-Ce lattice is more easily removed than in the Co-O-Co lattice. On the other hand, in the 30Co0.7Ce0.3/70r-Al2O3 catalyst, which has the highest Ce substitution, a peak was observed in which Ce4+ was reduced to Ce3+ at about 550 °C (strictly speaking, this corresponds to the oxygen vacancy of cerium oxide). Finally, as the amount of added Ce increased, the peaks shifted to low temperature, suggesting that the reduction is affected by the synergistic effect of Co and Ce. Possibly, the increase in the strain induced by the addition of Ce improves the reduction of the catalyst by reducing the strength of the metal–oxygen bond [34]. Based on the TPR profiles, all the catalysts were pretreated with hydrogen at 700 °C before PSR, and the XRD patterns of the catalysts after reduction are shown in Figure 4B. Metallic Co was observed in the XRD patterns of the 30CoxCey/70γ-Al2O3 catalysts. However, the amount of metallic Co decreased with increasing Ce substitution, and many peaks corresponding to Co3O4 and CoAl2O4 remained. In addition, diffraction peaks corresponding to metallic Ce (reduced from cerium oxide) were not observed in any catalyst, but the peak at 32°, which arises from Ce2O3, was stronger only in the most substituted 30Co0.7Ce0.3/70r-Al2O3 catalyst.
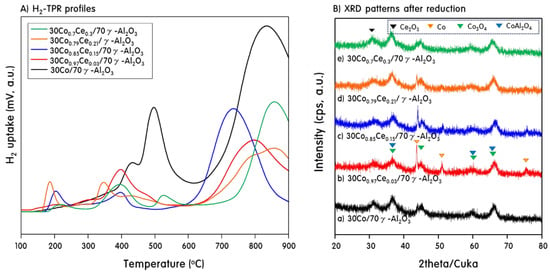
Figure 4.
H2-TPR curves of the fresh 30CoxCey/70γ-Al2O3 catalysts and XRD patterns of the reduced 30CoxCey/70γ-Al2O3 catalysts.
2.2. Gas Adsorption Ability of 30CoxCey/70γ-Al2O3 Catalysts
In general, the reactivity of a catalyst is closely related to the reactant adsorption capacity. Therefore, to investigate the adsorption capacity for C3H8 (the feed gas) and CO (the intermediate gas), C3H8-, and CO-TPD were performed, and the results are shown in Figure 5A,B, respectively. Chemisorption is the process by which adsorbed species on the catalyst surface are combined and the product is desorbed from the surface; usually, a catalyst with a high gas adsorption capacity is also highly active [35]. In the case of the 30Co/70γ-Al2O3 catalyst, propane desorption peaks were observed near 420 and 800 °C. On the other hand, most Ce-containing catalysts showed low desorption peaks at low temperatures and large desorption peaks at high temperatures. In particular, compared with the 30Co/70γ-Al2O3 catalyst, the high-temperature (800 °C) desorption peak shifted to a lower temperature (near 700 °C), and the quantity desorbed also remarkably increased. These results suggest that the addition of Ce improves the interaction between propane gas and the catalyst and more propane is adsorbed during the propane steam reforming reaction at high temperatures. On the other hand, the results for CO-TPD are shown in Figure 5B. There are three losses at 100 (even though very small), 200, and 400 °C on 30Co/70γ-Al2O3. The CO desorption usually occurs in three parts: The loss curve corresponding to the desorption of CO molecules physically adsorbed on the catalyst surface occurs at about 100–150 °C, whereas the desorption curve for CO molecules chemically adsorbed on the catalyst surface is observed at about 200–600 °C. Finally, CO gas strongly adsorbs to the lattice, and a curve corresponding to the desorption of CO2 bound to the lattice oxygen is observed at about 700–900 °C [36]. For the 30Co/70γ-Al2O3 catalyst, only the desorption of CO molecules adsorbed physically or chemically on the surface of the cobalt oxide was observed. However, when Ce was added, the CO desorption shifted to higher temperatures. Generally, higher desorption temperatures indicate stronger interactions between CO and the catalyst. Furthermore, the CO2 desorption curve was observed at about 850 °C for the 30CoxCey/70γ-Al2O3 catalysts. Other studies have also reported CO adsorption on CeO2 (2CeO2 + CO → Ce2O3 + CO2) [37]. This result suggests that the Ce-substituted catalysts can easily release oxygen from the lattice, and ultimately, form oxygen vacancies within the lattice. Additionally, the adsorption of CO on 30CoxCey/70γ-Al2O3 with increasing Ce content also increased compared to that of 30Co/70γ-Al2O3. This means that the addition of Ce favors the adsorption of CO, and thus, promotes the PSR and WGS reactions.

Figure 5.
C3H8- and CO-TPD curves for the fresh 30CoxCey/70γ-Al2O3 catalysts.
In a hydrocarbon steam reforming reaction, water vapor (H2O) is another major feed gas and is involved in the WGS reaction. The water gas shift reaction is [38] CO + H2O → CO2 + H2, ΔH = −41.1 kJ/mol. It is possible to increase the thermal stability of the catalyst by converting CO, which causes the deterioration of the catalyst, to CO2. Therefore, H2O desorption experiments were performed on both catalysts, 30Co/70γ-Al2O3 and 30Co0.85Ce0.15/70γ-Al2O3, using the TGA equipment to calculate the activation energy for water adsorption on the catalysts. Figure 6A shows the results of H2O desorption experiments, and the plot of ln(ψ/Tm2) versus 1/Tm using the Ozawa equation [39] is shown in Figure 6B. The water desorption activation energies can be calculated from the slope of the plot. The activation energies of desorption for the 30Co/70γ-Al2O3 and 30Co0.85Ce0.15/70γ-Al2O3 catalysts were calculated to be 189.73 and 266.96 kJ/mol, respectively. Therefore, it is expected that the steam added to the feed gas of the PSR reaction will be adsorbed better on the surface of Ce than that of Co. In particular, Yan Li et al. [40] reported that Ce addition not only increases the dispersion of the active metals, but also accelerates the adsorption and activation of steam, as shown by the following Equation [41]: H2O + Ce2O3 → 2CeO2 + H2. Therefore, based on the overall gas-TGA results, we suggested that Co would be activated, perhaps synergistically with Ce. Therefore, the hydrocarbon reforming reaction occurs mainly on Co sites during the PSR reaction, and the WGS reaction can be promoted, whereas water adsorption occurs mainly on Ce.

Figure 6.
TGA curves (A) for H2O-desorption and the activation energies (B) calculated from Ozawa plots for the 30Co/70γ-Al2O3 and 30Co.85Ce0.15/70γ-Al2O3 catalysts.
2.3. Propane Steam Reforming Reaction over 30CoxCey/70γ-Al2O3 Catalysts
Figure 7 shows the product distributions of all catalysts during the PSR reaction at 700 °C for 10 h. The 30Co0.85Ce0.15/70γ-Al2O3 and 30Co0.79Ce0.21/70γ-Al2O3 catalysts showed 100% propane conversion, whereas 30Co/70γ-Al2O3, 30Co0.97Ce0.03/70γ-Al2O3, and 30Co0.7Ce0.3/70γ-Al2O3 showed a propane conversion of over 90%. Here, the values for all PSR experiments are the average of the results of three runs. The products detected by GC were H2, CO, CH4, and CO2, and the product distribution ratios were different for each catalyst. During the PSR of the 30Co/70γ-Al2O3 catalyst, H2, CO, and CH4 were identified as major products and little CO2 was observed. On the other hand, the catalysts with added Ce exhibited increased propane conversion and absolute hydrogen production during the PSR, and CO2 production was greatly increased with a decreasing amount of CO produced. This can be interpreted as a result of the accelerated WGS reaction induced by Ce. Here, ceria might easily form oxygen vacancies and supply oxygen to CO, as shown by the following equation: 2CeO2 ↔ Ce2O3 + 0.5O2. Thus, the 30Co0.85Ce0.15/70γ-Al2O3 catalyst is the optimal catalyst when considering propane conversion and the product distribution.
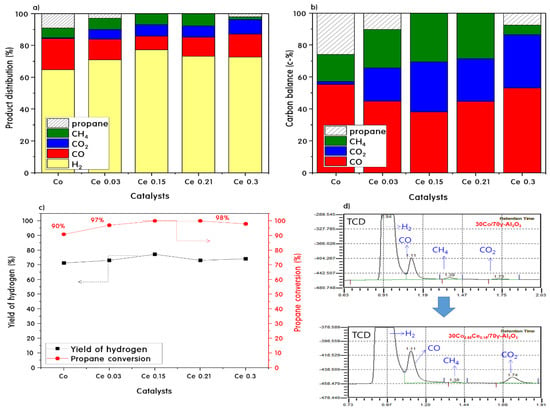
Figure 7.
Product distributions (a,b) and hydrogen yield and propane conversion (c) during propane steam reforming reaction at 700 °C after 10 h and GC signals (d).
For the best catalyst, 30Co0.85Ce0.15/70γ-Al2O3, the longevity was measured by increasing the reaction time at 700 °C to 100 h. These measurements allowed us to examine the lifetime of the catalyst. The results are shown in Figure 8. The 100% propane conversion and 78% hydrogen yield did not show any decline after 100 h. Furthermore, the amount of carbon compounds produced as intermediates or as products were constant for 100 h. This result indicates that the oxygen transport of cerium oxide in the 30Co0.85Ce0.15/70γ-Al2O3 catalyst prevents the Co from being completely reduced, and consequently, the deactivation of Co can be suppressed and the lifetime of the catalyst increased.
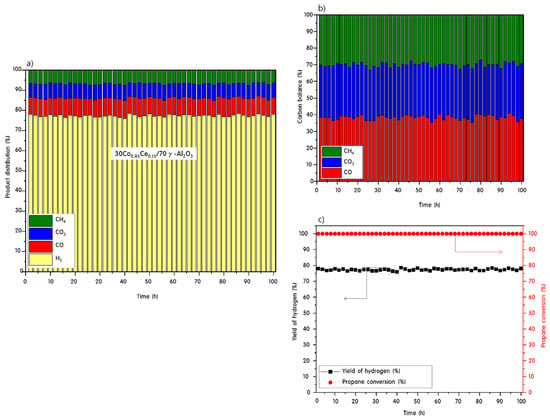
Figure 8.
Product distributions (a,b) and hydrogen yield and propane conversion (c) for the 30Co0.85Ce0.15/70γ-Al2O3 catalyst during the PSR reaction at 700 °C over 100 h.
2.4. Characteristics of 30CoxCey/70γ-Al2O3 Catalysts after Propane Steam Reforming
Figure 9 shows the TGA (and TPO) curves for coke combustion, as well as TEM images of the catalysts after PSR. We used the TEM images to determine the amount and shape of the carbon deposited on the catalyst after PSR. The TPO curve is also shown in this figure. Generally, the TPO curves are separated into three peaks at 100–200, 350, and 550 °C, respectively. Aside from the water desorption curve below 200 °C, the positions of combustion peaks on monometallic sites and bimetallic sites are related to the interaction between the metal and deposited carbon, as well as the shape of the deposited carbon particles. Usually, the coke deposited on monometallic sites burns at higher temperatures than bimetallic sites [42]. However, in this study, we could not make this generalization, i.e., the coke deposited on the Co monometallic sites on the Al2O3 support burnt at a low temperature of around 450 °C, and the peak at 580 °C was associated with a coke fraction deposited on the Co–Ce bimetallic sites. In this study, the burning of coke at higher temperatures in the Ce-substituted catalyst indicates that the shape of the formed coke particles is different. Thus, the PSR pathways on the Co metal and cobalt oxides were different, the resulting intermediates were different, and consequently, the shapes of the deposited coke particles were also different. Coke burned at a low temperature is probably an amorphous encapsulating carbon, whereas the burned coke at a high temperature probably has a crystalline whisker carbon structure such as carbon nanotubes. From the TGA analysis, the carbon deposits on the 30Co/70γ-Al2O3, 30Co0.97Ce0.03/70γ-Al2O3, 30Co0.85Ce0.15/70γ-Al2O3, 30Co0.79Ce0.21/70γ-Al2O3, and 30Co0.7Ce0.3/70γ-Al2O3 catalysts were 2.98%, 0.34%, 1.84%, 4.13%, and 5.42%, respectively. The amount of deposited coke tended to increase with increasing Ce substitution. The reason that the correlation between the amount of Ce and the amount of coke shows an opposing trend, that is, propane conversion in all the catalysts did not reach 100%. This seems to be attributed to the fact that, as the amount of Ce increased, the amount of Co participating in the substantial reforming reaction decreased, resulting in a decrease in the amount of CH4 combustion, and consequently, an increase in the carbon deposition probability. In other words, the carbon compounds, produced as intermediates, strongly interacted with the surface of the catalysts with greater Ce substitution; thus, they could not escape from the catalyst surface and accumulated. However, based on these results, we found that the addition of 15% Ce (with respect to that of Co) can suppress coke formation, as well as catalyst deactivation. To confirm this result, TEM images were obtained to determine the shape of the deposited carbon particles. The amount of carbon deposited was too small to observe particular carbon shapes such as carbon nanotubes (CNTs) or fullerenes. Nonetheless, the catalyst without Ce clearly exhibited agglomeration between metals, and the particles measuring 100 nm in diameter, before reaction, grew to micrometer size. These enormous particles inactivate the catalytic sites and the deposited carbon could not be easily burned away; thus, carbon is continuously deposited, resulting in coke formation. On the other hand, in the catalysts with Ce substitution, the needle-shaped particles before reaction did not increase in size after the reaction, but became highly dispersed. Possibly, the introduction of CexOy suppressed sintering between Co metal particles (actually CoO). This means that even if carbon is deposited on the surface of the catalyst, it could be easily burned away on the CexOy to inhibit coke formation, resulting in continuous catalyst activity.

Figure 9.
TGA and TPO curves for coke combustion and TEM images of the used 30CoxCey/70γ-Al2O3 catalysts after PSR.
Figure 10 shows the Co2p, Ce3d, Al2p, and O1s XPS spectra of two representative catalysts, 30Co/70γ-Al2O3 and 30Co0.85Ce0.15/70γ-Al2O3, before and after PSR. The Co2p spectra contain peaks corresponding to Co2+ (783.52 eV) and Co2.5+ (781.36 eV) for both catalysts before reaction [43]. However, after reaction, the peaks moved towards lower binding energies, which means the formation of reduced Co species. However, the migration was not significant for 30Co0.85Ce0.15/70γ-Al2O3. This is because Co can continue to be supplied with oxygen by the cerium oxide [44], and it would not have been perfectly reduced into Co metal after the PSR reaction. Eventually, the oxygen supply of Ce prevents sintering between the reduced Co metal particles, which slows the deterioration of the catalyst. In the O1s spectrum, various types of oxygen species were observed before PSR, but they converged to the oxygen present in the lattice after PSR, moving toward a small binding energy at 531.06 eV [45]. This means that the oxidation state of the metals present after the reaction has become simple and significant, oxygen has been lost from the lattice. This phenomenon was more significant in the catalysts containing Ce. The Al2p spectrum of the catalysts before PSR contains peaks corresponding to Al-O and Al-O-H at 74.2 and 76.8 eV, respectively [44]. After the reaction, the only Al peak in the lattice was observed at 74.1 eV. This result suggests that Al also participates in the PSR reaction to some degree, perhaps acting as an oxygen transfer medium, although the effect of Al weaker than that of cerium oxide. Based on the Ce3d5/2 peak at 885.50 eV [46], we confirmed that the Ce was present as an oxide before and after the reaction, whereas it was present as Ce4+ before the reaction. After reaction, the peak moved to a lower binding energy as a result of reduction (probably to Ce3+). Finally, the C1s spectrum is presented for analysis of the carbon content deposited after the reaction. After the reaction, two peaks corresponding to C=O and C=C were observed at 284.90 and 284.27 eV [47]. The amount of accumulated carbon was greater on the 30Co/70γ-Al2O3 catalyst than that on the 30Co0.85Ce0.15/70γ-Al2O3 catalyst. Otherwise, the peak positions for C=O and C=C shifted to the higher binding energies of 288.25 and 284.90 eV on the 30Co0.85Ce0.15/70γ-Al2O3 catalyst. As already mentioned from the TPO results, C=O corresponds to amorphous carbon and C=C is considered to correspond to a crystalline carbon such as CNT. This result means that the mechanisms on two catalysts were different depending on Ce addition.
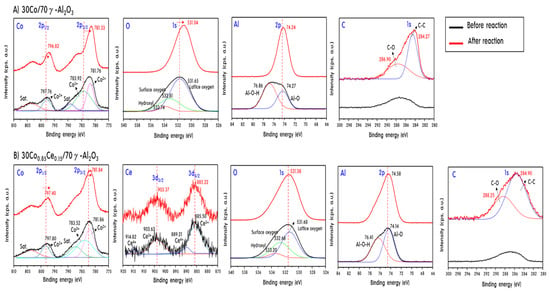
Figure 10.
XPS of the two representative catalysts, 30Co/70γ-Al2O3 (A) and 30Co.85Ce0.15/70γ-Al2O3 (B), before and after PSR.
Based on the catalytic performance for the PSR and physicochemical characteristics, Scheme 1 presents a proposed PSR mechanism for the 30Co0.85Ce0.15/70γ-Al2O3 catalyst. During reforming, the Co or CoxOy components play important roles in the dehydrogenation and thermal cracking of propane gas, transforming the propane gases to C2H4 and CH4 as an intermediate and then acetaldehyde (CH3CHO), generated by ethylene oxidation over bimetallic CoCeO [48,49]. The acetaldehyde was thermally and catalytically cracked to CH4 and CO. The CH4 and CO obtained were accompanied by other reactions, such as the CH4-steam reforming reaction and CO-WGS reaction. Based on CO- and H2O-TPD experiments, water molecules were well adsorbed on cerium oxide components and CO molecules were well adsorbed on the bimetallic CoCeO components. Finally, the oxygen vacancies were filled because of the interaction with water vapor and oxygen donated sites in cerium oxide. Meanwhile, the CO-water gas shift reaction took place predominantly on CoCeO bimetallic oxide. Based on the excellent oxygen delivery properties of Ce, the vapor (H2O) participates in the WGS reaction on the cerium oxide surface produce H2 and CO2. In conclusion, cerium oxide, as a promoter, helps to produce a high hydrogen yield in the PSR reaction when using the 30Co0.85Ce0.15/70γ-Al2O3 catalyst.
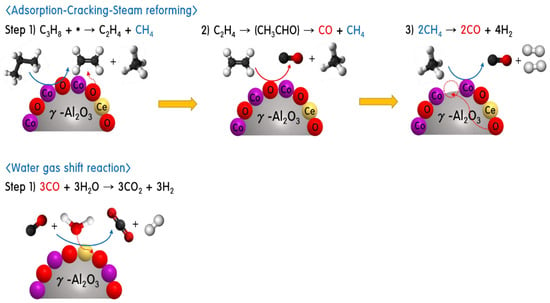
Scheme 1.
Expected PSR mechanism on the 30Co0.85Ce0.15/70γ-Al2O3 catalyst.
3. Experimantal
3.1. Preparation of 30CoxCey/70 γ-Al2O3 Catalysts
The 30CoxCey/70γ-Al2O3 catalysts were prepared using a conventional impregnation method at pH 3.0, and the obtained catalysts are denoted 30CoxCey/70γ-Al2O3 (x = 1, 0.97, 0.85, 0.79, and 0.70 and y = 0, 0.03, 0.15, 0.21, and 0.30; thus, the Ce contents were 0.1, 0.5, 0.7, and 1.0 mol.% per mole of Co) based on the added elements and ratios. Cobalt chloride (CoCl2, Daejung Chemicals & Metals Co., Ltd., Siheung, Korea) and cerium chloride (CeCl4, Daejung Chemicals & Metals Co., Ltd., Siheung, Korea) were used as metal precursors for Co and Ce. First, the appropriate amounts of CoCl2 and cerium chloride were dissolved in a 500-mL beaker containing ethanol solvent with continuous stirring. The pH values of the solutions were adjusted to 3.0 with acetic acid so that the two metals were completely dissolved, and constant stirring of the solution was carried out for about 1 h. Subsequently, 10.0 g of γ-Al2O3 powder was added to the above solution, which was stirred again for 12 h at room temperature and then evaporated at 80 °C for 2 h to remove the solvents and impurities. The obtained powders were calcined in air at 700 °C for 5 h to crystallize; finally, the five powdered catalysts were obtained: 30Co/70γ-Al2O3, 30Co0.97Ce0.03/70γ-Al2O3, 30Co0.85Ce0.15/70γ-Al2O3, 30Co0.79Ce0.21/70γ-Al2O3, and 30Co0.70Ce0.30/70γ-Al2O3. These catalysts were subjected to prehydrogen reduction at 700 °C for 2 h before use for PSR.
3.2. Characterization
X-ray diffraction (XRD, MPD, PANalytical, Almelo, The Nederland) measurements using nickel-filtered Cu Kα radiation (40 kV, 30 mA) were carried out to identify the structure and crystallinity of the synthesized 30CoxCey/70γ-Al2O3 catalysts. The catalyst particle shapes were determined by high-resolution transmission electron microscopy (TEM, H-7600, Hitachi, Tokyo, Japan) operated at 120 kV. X-ray photoelectron spectroscopy (XPS, AXIS Nova, Kratos Inc., San Diego, CA, USA) measurements for Co2p, Ce3d, Al2p, O1s, and C1s were performed using a non-monochromatic Al Kα (1486.6 eV) X-ray source. The elemental compositions of the catalysts were determined using an energy-dispersive X-ray spectroscope (EDS, EX-250, Horiba, Kyoto, Japan) operated at 15 kV. The Brunauer–Emmett–Teller (BET) surface areas of the catalysts were obtained from liquid nitrogen adsorption/desorption isotherm curves measured using a Belsorp II instrument (Belsorp II Mini, Toyonaka, Japan).
H2-temperature programmed reduction (TPR), and CO- and C3H8-TPD (temperature programmed desorption) were conducted with a BELCAT (Bel Japan Inc., Toyonaka, Japan). 0.10 g of the calcined sample was charged into the quartz tube and flushed with high-purity argon gas at 300 °C for 1 h, followed by cooling to room temperature. The catalyst for TPR was charged into the quartz tube and reduced in 5 vol% H2/Ar (ca. 30 mL min−1) from 50 to 900 °C. Wherein CO and C3H8 gases for TPD were introduced as adsorption gases (ca. 30 mL min−1) at 50 °C for 1 h. After adsorption, the system was purged with argon gas for 30 min to remove any weakly adsorbed species. The tests were initiated by heating to 900 °C at a rate of 10 °C min−1. The resulting profile was monitored using a thermal conductivity detector (TCD) and mass analyzer [Q-mass].
Thermogravimetric analysis (TGA) for coke combustion (TPO, temperature programmed oxidation) after reaction was conducted using a thermos differential and gravimetric analyzer (Shinco, Daejeon, Korea). The samples weighed 7.0–10.0 mg. The TGA data based on weight loss were collected in the 50–800 °C temperature range at a rate of 10 °C min−1 in air-flow (ca. 25 mL min−1).
The activation energies for water desorption from the catalysts were also determined using the thermogravimetric analysis apparatus. The data were collected in the range of 40–500 °C at rates of 5, 10, and 15 °C min−1 N2 flow (ca. 25 mL min−1). Before the tests, the catalysts were dried in a drying oven at 200 °C for 2 h to remove water molecules attached to the surface. After that, the samples were immediately placed in a desiccator containing a saturated solution of (NH4)2SO4 and stored for 24 h to adsorb any water.
A fixed bed-type reactor was already designed in the previous study for the PSR [21]. The catalytic activities of the 30CoxCey/70γ-Al2O3 catalysts were measured at 700 °C for 10 h at a steam-to-propane ratio of 1:6 (mol.%) with a gas hourly space velocity (GHSV) of 6000 h−1. The catalyst (1.0 g) was pelletized to a 20–24 mesh, packed in the fixed-bed quartz reactor, and then mounted vertically inside the furnace. In this study, the amount of steam was adjusted by regulating the temperature according to the partial pressure law. The reaction products during PSR were measured by an on-line gas chromatograph (GC, DS7200, Donam Company, Gwangju, Korea) equipped with a thermal conductivity detector (TCD) and flame-ionizing detector (FID). H2, CO, CO2, CH3CHO, and CH3COOH were detected with the TCD, whereas CH4, C2H4, C2H6, and C3H8, and the other products were detected with the FID. The following GC conditions were used: Carboxen-1000 column (Bruker, Billerica, MA, USA); injection temperature, 423 K; initial column temperature, 423 K; final temperature, 473 K; and detector temperatures, 423 K (TCD) and 573 K (FID). The C3H8 conversion, H2 yield, and Ci (CH4, CO2, or CO) selectivity are defined as follows:
C3H8 conversion (%) = ([C3H8]in − [C3H8]out)/[C3H8]in × 100
Hydrogen yield (%) = [H2]out/([H2]in in C3H8 + [H2]in in H2O) × 100
Ci selectivity (%) = [CO, CO2, or CH4]out/[CO2 + CO + CH4]out × 100
4. Conclusions
The purpose of this study was to improve the catalytic stability of the 30Co/70γ-Al2O3 catalyst and to promote hydrogen production by introducing Ce, which has an excellent oxygen transfer capacity. The catalytic performances of the 30CoxCey/70γ-Al2O3 catalysts were evaluated for the PSR reaction at 700 °C for 10 h. The 30Co0.85Ce0.15/70γ-Al2O3 catalyst showed 100% propane conversion, about 75% H2 selectivity, and the least carbon deposition after PSR. In particular, because the addition of Ce accelerated the WGS reaction, CO, which causes catalyst poisoning, was converted to CO2. Considering the gas adsorption capacity, propane conversion, and carbon deposition after reaction, the 30Co0.85Ce0.15/70γ-Al2O3 catalyst is the optimal Ce substitution ratio. Finally, the synergistic effect of Co and Ce in the catalyst for the PSR allowed hydrogen gas to be produced stably without catalyst deactivation after 100 h.
Author Contributions
Conceptualization, J.Y.D., M.K.; Methodology, Formal Analysis, J.Y.D.; Writing-Review & Editing, M.K., R.K.C.; Formal Analysis, N.S. and J.K.; Investigation, N.-K.P; Funding Acquisition, D.L., M.W.S.; Project Administration, H.-J.R., J.H.C.; Supervision, M.K.
Funding
This work was conducted under framework of the research and development program of the Korea Institute of Energy Research (B8-2437), and was supported by the Energy Efficiency and Resources Programs of the Korea Institute of Energy Technology Evaluation and Planning (KETEP), granted financial resources from the Ministry of Trade, Industry and Energy, Republic of Korea (20163010050080).
Conflicts of Interest
The authors declare no conflict of interest
References
- Dodds, P.E.; Staffell, L.; Hawkes, A.D.; Grunewald, P.; McDowall, W.; Ekins, P. Hydrogen and fuel cell technologies for heating: A review. Int. J. Hydrogen. Energy 2011, 40, 2065–2083. [Google Scholar] [CrossRef]
- Amin, A.M.; Croiset, E.; Epling, W. Review of methane catalytic cracking for hydrogen production. Int. J. Hydrogen. Energy 2011, 36, 2904–2935. [Google Scholar] [CrossRef]
- Enger, B.C.; Lodeng, R.; Holmen, A. A review of catalytic partial oxidation of methane to synthesis gas with emphasis on reaction mechanisms over transition metal catalysts. Appl. Catal. A 2008, 346, 1–27. [Google Scholar] [CrossRef]
- Wang, S.; Lu, G.Q.; Millar, G.J. Carbon Dioxide Reforming of Methane to Produce Synthesis Gas over Metal-Supported Catalysts: State of the Art. Energy Fuels. 1996, 10, 896–904. [Google Scholar] [CrossRef]
- Rostrup-Nielsen, J. Steam reforming of hydrocarbons. A historical perspective. Stud. Surf. Sci. Catal. 2004, 147, 121–126. [Google Scholar]
- Granger, P.; Parvulescu, V.; Kaliaguine, S.; Prellier, W. Perovskites and Related Mixed Oxides: Concepts and Applications, Band 1; John Wiley & Sons: New York, NY, USA, 2015; 1056p. [Google Scholar]
- Harshini, D.; Yoon, C.Y.; Han, J.H.; Yoon, S.P.; Nam, S.W.; Lim, T.H. Catalytic steam reforming of propane over Ni/LaAlO3 catalysts: influence of preparation methods and OSC on activity and stability. Catal. Lett. 2012, 142, 205–212. [Google Scholar] [CrossRef]
- Sohn, H.; Ozkan, U.S. Cobalt-Based Catalysts for Ethanol Steam Reforming: An Overview. Energy Fuels 2016, 30, 5309–5322. [Google Scholar] [CrossRef]
- Wu, H.; Parola, V.L.; Pantaleo, G.; Puleo, F.; Venezia, A.M.; Liotta, L.F. Ni-Based Catalysts for Low Temperature Methane Steam Reforming: Recent Results on Ni-Au and Comparison with Other Bi-Metallic Systems. Catalysts 2013, 3, 563–583. [Google Scholar] [CrossRef]
- Jakobsen, J.G.; Jakbensen, M.; Chorkendorff, I.; Sehested, J. Methane Steam Reforming Kinetics for a Rhodium-Based Catalyst. Catal. Lett. 2010, 140, 90–97. [Google Scholar] [CrossRef]
- Zhang, X.; Gong, Y.; Yu, G.; Xie, Y. Oxygen species on NiO/Al2O3 and their reactivities. J. Mol. Catal. A-Chem. 2002, 180, 293–298. [Google Scholar] [CrossRef]
- Ashok, J.; Kathiraser, Y.; Ang, M.L.; Kawi, S. Bi-functional hydrotalcite-derived NiO–CaO–Al2O3 catalysts for steam reforming of biomass and/or tar model compound at low steam-to-carbon conditions. Appl. Catal. B 2015, 172, 116–128. [Google Scholar] [CrossRef]
- Boroujerdnia, M.; Obeydavi, A. Synthesis and characterization of NiO/MgAl2O4 nanocrystals with high surface area by modified sol-gel method. Microporous Mesoporous Mater. 2016, 228, 289–296. [Google Scholar] [CrossRef]
- Avcl, A.K.; Trimm, D.L.; Aksoylu, A.E.; Onsan, A. Hydrogen production by steam reforming of n-butane over supported Ni and Pt-Ni catalysts. Appl. Catal. A. 2004, 258, 235–240. [Google Scholar]
- Ahn, K.; Choi, S.; Lee, J.; Kim, B.; Kim, J.; Kim, H. Enhanced carbon tolerance on Ni-based reforming catalyst with Ir alloying: A DFT study. Appl. Surf. Sci. 2017, 419, 678–682. [Google Scholar] [CrossRef]
- Tian, H.; Li, X.; Chen, S.; Zeng, L.; Gong, J. Role of Sn in Ni-Sn/CeO2 Catalysts for Ethanol Steam Reforming. Chin. J. Chem. 2017, 35, 651–658. [Google Scholar]
- Wang, H.; Blaylock, W.; Dam, A.H.; Lilang, S.E.; Rout, K.R.; Zhu, Y.-A.; Green, W.H.; Homen, A.; Chen, D. Steam methane reforming on a Ni-based bimetallic catalyst: density functional theory and experimental studies of the catalytic consequence of surface alloying of Ni with Ag. Catal. Sci. Technol. 2017, 7, 1713–1715. [Google Scholar] [CrossRef]
- Park, K.S.; Son, M.; Park, M.-J.; Kim, D.H.; Kim, J.H.; Park, S.H.; Choi, J.-H.; Bae, J.W. Adjusted interactions of nickel nanoparticles with cobalt-modified MgAl2O4-SiC for an enhanced catalytic stability during steam reforming of propane. Appl. Catal. A 2018, 549, 117–133. [Google Scholar] [CrossRef]
- Resini, C.; Delgado, M.C.H.; Arrighi, L.; Alemany, L.J.; Marazza, R.; Busca, G. Propene versus propane steam reforming for hydrogen production over Pd-based and Ni-based catalysts. Catal. Commun. 2005, 6, 441–445. [Google Scholar] [CrossRef]
- Anjaneyulu, C.; Costa, L.O.; Ribeiro, M.C.; Neto, R.C.; Mattos, L.V.; Venugopal, A.; Noronha, F.B. Effect of Zn addition on the performance of Ni/Al2O3 catalyst for steam reforming of ethanol. Appl. Catal. A 2016, 519, 85–98. [Google Scholar] [CrossRef]
- Lee, G.; Kim, D.; Kwak, B.S.; Kang, M. Hydrogen rich production by ethanol steam reforming reaction over Mn/Co10Si90MCM-48 catalysts. Catal. Today 2014, 232, 139–150. [Google Scholar] [CrossRef]
- Chen, A.; Yan, Y.; Elnashaie, S. Catalyst deactivation and engineering control for steam reforming of higher hydrocarbons in a novel membrane reformer. Chem. Eng. Sci. 2004, 59, 1965–1978. [Google Scholar] [CrossRef]
- Ginsburg, J.M.; Piña, J.; Solh, T.E.; Lasa, H.I. Coke Formation over a Nickel Catalyst under Methane Dry Reforming Conditions: Thermodynamic and Kinetic Models. Ind. Eng. Chem. Res. 2005, 44, 4846–4854. [Google Scholar] [CrossRef]
- Gao, F.; Tang, X.; Yi, H.; Zhao, X.; Li, C.; Li, J.; Shi, Y.; Meng, X. A Review on Selective Catalytic Reduction of NOx by NH3 over Mn–Based Catalysts at Low Temperatures: Catalysts, Mechanisms, Kinetics and DFT Calculations. Catalysts 2017, 7, 1–32. [Google Scholar] [CrossRef]
- Obata, S.; Kato, M.; Yokoyama, H.; Iwata, Y.; Kikumoto, Y.; Sakurada, O. Synthesis of nano CoAl2O4 pigment for ink-jet printing to decorate porcelain. J. Ceram. Soc. Jpn. 2011, 119, 208–213. [Google Scholar] [CrossRef]
- Liu, K.; Schmedake, T.A.; Tsu, R. A comparative study of colloidal silica spheres: Photonic crystals versus Bragg‘s law. Phys. Lett. A 2008, 372, 4517–4520. [Google Scholar] [CrossRef]
- Cepuritis, R.; Garboczi, E.J.; Ferraris, C.F.; Jacobsen, S.; Sorensen, B.E. Measurement of particle size distribution and specific surface area for crushed concrete aggregate fines. Adv. Powder Technol. 2017, 28, 706–720. [Google Scholar] [CrossRef]
- Bakar, W.A.W.A.; Ali, R.; Mohammad, N.S. The effect of noble metals on catalytic methanation reaction over supported Mn/Ni oxide based catalysts. Arab. J. Chem. 2015, 8, 632–643. [Google Scholar] [CrossRef]
- Takenaka, S.; Orita, Y.; Umebayashi, H.; Matsune, H.; Kishida, M. High resistance to carbon deposition of silica-coated Ni catalysts in propane stream reforming. Appl. Catal. A 2008, 351, 189–194. [Google Scholar] [CrossRef]
- Wang, M.; Jin, L.; Li, Y.; Lv, J.; Wei, B.; Hu, H. In-situ catalytic upgrading of coal pyrolysis tar coupled with CO2 reforming of methane over Ni-based catalysts. Fuel Process. Technol. 2018, 177, 119–128. [Google Scholar] [CrossRef]
- Cordoba, M.; Miranda, C.; Lederhos, C.; Pascual, F.; Ardila, A.; Fuentes, G.A.; Pouilloux, Y.; Ramirez, A. Catalytic performance of Co3O4 on different activated carbon supports in the benzyl alcohol oxidation. Catalysts 2017, 7, 384–396. [Google Scholar] [CrossRef]
- Petitto, S.C.; Langell, M.A. Surface composition and structure of Co3O4 (110) and the effect of impurity segregation. J. Vac. Sci. Technol. A 2004, 22, 1690–1697. [Google Scholar] [CrossRef]
- Jin, J.; Park, J.; Shon, J.K.; Kim, J.H.; Li, Z.; Park, Y.; Kim, J.M. Low temperature CO oxidation over Pd catalysts supported on highly ordered mesoporous metal oxides. Catal. Today 2012, 185, 183–190. [Google Scholar] [CrossRef]
- Ma, D.; Lu, Z.; Tang, Y.; Li, Y.; Tang, Z.; Yang, Z. Effect of lattice strain on the oxygen vacancy formation and hydrogen adsorption at CeO2 (111) surface. Phys. Lett. A 2014, 378, 2570–2575. [Google Scholar] [CrossRef]
- Kim, K.M.; Kwak, B.S.; Park, N.-K.; Lee, T.J.; Lee, S.T.; Kang, M. Effective hydrogen production from propane steam reforming over bimetallic co-doped NiFe/Al2O3 catalyst. J. Ind. Eng. Chem. 2017, 46, 324–336. [Google Scholar] [CrossRef]
- Kim, J.; Samano, E.; Koel, B.E. CO Adsorption and Reaction on Clean and Oxygen-Covered Au (211) Surfaces. J. Phys. Chem. B 2006, 110, 17512–17517. [Google Scholar] [CrossRef] [PubMed]
- Rao, G.R.; Mishra, B.G. Structural, redox and catalytic chemistry of ceria based materials. Bull. Catal. Soc. India 2003, 2, 122–134. [Google Scholar]
- Bobrova, L.; Andreev, D.; Ivanov, E.; Mezentseva, N.; Simonov, M.; Makarshin, L.; Gribovskii, L.; Sadykov, V. Water–Gas Shift Reaction over Ni/CeO2 Catalysts. Catalysts 2017, 7, 310–334. [Google Scholar] [CrossRef]
- Noisong, P.; Danvirutai, C. Kinetics and Mechanism of Thermal Dehydration of KMnPO4·H2O in a Nitrogen Atmosphere. Ind. Eng. Chem. Res. 2010, 49, 3146–3151. [Google Scholar] [CrossRef]
- Li, Y.; Wang, X.; Xie, C.; Song, C. Influence of ceria and nickel addition to alumina-supported Rh catalyst for propane steam reforming at low temperatures. Appl. Catal. A 2009, 357, 213–222. [Google Scholar] [CrossRef]
- Weerakkody, C.; Biswas, S.; Song, W.; He, J.; Wasalathanthri, N.; Dissanayake, S.; Kriz, D.A.; Dutta, B.; Sui, S.L. Controllable synthesis of mesoporous cobalt oxide for peroxide free catalytic epoxidation of alkenes under aerobic conditions. Appl. Catal. B 2018, 221, 681–690. [Google Scholar] [CrossRef]
- Pieck, C.L.; Jablonski, E.L.; Parera, J.M. Recovering of the catalytic functions of naphtha reforming catalysts by partial coke burning. Appl. Catal. 1991, 70, 19–28. [Google Scholar] [CrossRef]
- Mattos, L.V.; Rodino, E.; Resasco, D.E.; Passos, F.B.; Noronha, F.B. Partial oxidation and CO2 reforming of methane on Pt/Al2O3, Pt/ZrO2, and Pt/Ce–ZrO2 catalysts. Fuel Process. Technol. 2003, 83, 147–161. [Google Scholar] [CrossRef]
- Lu, W.; Iwasa, Y.; Ou, Y.; Jinno, D.; Kamiyama, S.; Petersen, P.M.; Ou, H. Effective optimization of surface passivation on porous silicon carbide using atomic layer deposited Al2O3. RSC Adv. 2017, 7, 8090–8097. [Google Scholar] [CrossRef]
- Deng, S.; Li, S.; Li, H.; Zhang, Yi. Oxidative dehydrogenation of ethane to ethylene with CO2 over Fe-Cr/ZrO2 catalysts. Ind. Eng. Chem. Res. 2009, 48, 7561–7566. [Google Scholar] [CrossRef]
- Xu, W.; He, H.; Yu, Y. Deactivation of a Ce/TiO2 catalyst by SO2 in the selective catalytic reduction of NO by NH3. J. Phys. Chem. C 2009, 113, 4426–4432. [Google Scholar] [CrossRef]
- Kulkarni, S.B.; Patil, U.M.; Shackery, I.; Sohn, J.S.; Lee, S.; Park, B.; Jun, S.C. High-performance supercapacitor electrode based on a polyaniline nanofibers/3D graphene framework as an efficient charge transporter. J. Mater. Chem. A 2014, 2, 498–4998. [Google Scholar] [CrossRef]
- Do, J.Y.; Lee, J.H.; Park, N.-K.; Lee, T.J.; Lee, S.T.; Kang, M. Synthesis and characterization of Ni2−xPdxMnO4/γ-Al2O3 catalysts for hydrogen production via propane steam reforming. Chem. Eng. J. 2018, 334, 1668–1678. [Google Scholar] [CrossRef]
- Jo, S.W.; Im, Y.; Do, J.Y.; Park, N.-K.; Lee, T.J.; Lee, S.T.; Cha, M.S.; Jeon, M.; Kang, M. Synergies between Ni, Co, and Mn ions in trimetallic Ni1−xCoxMnO4 catalysts for effective hydrogen production from propane steam reforming. Renew. Energy 2017, 113, 248–256. [Google Scholar] [CrossRef]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).