Abstract
Tandem isomerization–hydroformylation of oleonitrile (an 18-carbon nitrile with a remote internal (9-)C=C bond) has been studied using Rh-bisphosphite catalyst systems, targeting formation of the linear aldehyde. The best compromise between regioselectivity (l/b = 58:42) and chemoselectivity (60%) was obtained at 120 °C and 10 bar CO/H2 (1:1) with a catalyst based on Biphephos at a 0.5 mol % catalyst load and a low ligand excess (2 equiv. versus Rh). These values stand among the better reported ones for the tandem isomerization–hydroformylation of long chain olefins with a single-component catalyst system.
1. Introduction
Major improvements have been achieved in olefin hydroformylation since its discovery in 1938 by Otto Roelen [1]. The synthesis of linear aldehydes is of high interest for industrial purposes, and very high regioselectivities (l/b > 99:1) have been achieved from terminal alkenes with rhodium catalysts using bulky bisphosphine (e.g., Bisbi [2] and Naphos [3]) and bisphosphite (e.g., Biphephos [4]) ligands. However, the selective formation of linear aldehydes is much more challenging when starting from internal alkene as, in this case, preliminary isomerization of the C=C bond is necessary to bring it to the terminal position before performing the hydroformylation process itself. Coupling isomerization and hydroformylation reactions in the same process refers to so-called tandem reactions. Several challenges are met in this tandem process: (i) internal alkenes are thermodynamically more stable than terminal ones, which are therefore (dynamically) formed in minutes amounts [5,6]; (ii) hydroformylation and isomerization are competitive reactions, and hence various (undesired) branched aldehydes can be obtained; and (iii) rather facile competitive hydrogenation of all kinds of C=C bonds often plagues the chemoselectivity of the reaction. Rhodium-catalyzed tandem isomerization–hydroformylation of middle-chain alkenes (<10 C) has been widely studied over the last two decades, generally with bulky ligands [7,8]. Regioselectivities in the range l/b = 95:5–98:2 were obtained with 2-alkenes (e.g., 2-hexene, 2-octene) [9,10,11,12,13,14], while with 4-octene regioselectivities up to l/b = 89:11 were observed [15,16]. On the other hand, the hydroformylation of long-chain alkenes (>10 C), with a C=C bond in a deep internal position (>5 C away), remains poorly documented. While the hydroformylation of this kind of substrate without specific regioselectivities targeted (i.e., via the hydroformylation of both internal and terminal C=C bonds) is quite common [17,18,19,20,21], the more challenging tandem isomerization–hydroformylation for the synthesis of terminal aldehydes remains less-studied. In 2005, Behr and coworkers performed the tandem isomerization–hydroformylation of methyl oleate with a Rh–Biphephos catalyst leading to a 26% maximum yield in linear aldehydes (70% conversion, l/b = 40:60) [22]. In 2015, Pandey and Chikkali explored the hydroformylation of the same substrate with a Rh-bisphosphite catalyst, reaching regioselectivities of up to l/b = 75:25 but only at 32% conversion (48 h, 1 mol % Rh) as experiments with >90% conversion resulted in l/b < 5:95, yet in all cases with unspecified chemoselectivity [23]. The authors also reported the tandem isomerization–hydroformylation of cashew nut shell liquid that contains a fatty mono-olefin (13 C) with C=C in the middle chain position capped by a phenol ring [24]. In 2013, Nozaki and coworkers combined tandem isomerization–hydroformylation of methyl oleate with hydrogenation of the resulting aldehydes in alcohols for a high final l/b = 81:19 and yet 29% of direct alkene hydrogenation [25]. Very recently, Behr and coworkers developed an efficient combination of Pd and Rh catalysts to convert methyl oleate into the corresponding linear aldehydes: HPdBr(P(tBu)3)2 catalyzes the dynamic isomerization of the C=C bond and a Rh–Biphephos catalyst converts the terminal olefin into aldehydes with a l/b of up to 91:9 [26,27]. It is noteworthy that, in all of the above examples, harsh conditions as well as a long reaction time favor the desired isomerization–hydroformylation and also all side reactions, especially the hydrogenation of C=C bond(s) and the direct hydroformylation of internal alkenes.
In line with our previous reports on the hydroformylation of 10-undecenitrile [28,29], we here report on the tandem isomerization–hydroformylation of oleonitrile (1, an 18 C-nitrile with a remote internal (9-)C=C bond) using simple Rh-bisphosphite catalyst systems (Scheme 1).
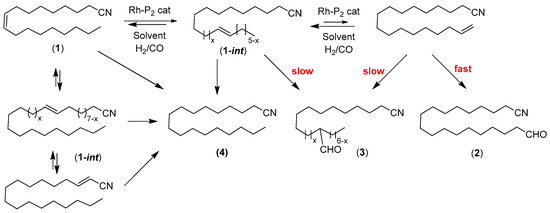
Scheme 1.
Rh-catalyzed tandem isomerization–hydroformylation of oleonitrile (1).
2. Results and Discussion
The tandem isomerization–hydroformylation of 1 by Rh(acac)(CO)2-bisphosphite systems (A very minor amount (ca. 0.8%) of terminal alkenes and of hydrogenated product 4 (ca. 8%) are present in the starting material, as observed by 1H NMR; see Appendix A section) leads to the corresponding linear aldehyde (19-oxononadecanenitrile, 2), a mixture of branched aldehydes collectively referred to as 3 (15 branched aldehydes can be considered from 2-formyloctadecanenitrile to 17-methyl-18-oxooctadecanenitrile), along with the hydrogenation product (4) and all internal alkene isomers (1-int) that originate from isomerization of the starting product (Note that oleonitrile (1) and isomers (1-int) formed during the course of the reaction cannot be readily differentiated by GLC or 1H NMR analysis. Hence, the reported amount 1 + 1-int stands for all alkene in solution and conv. 1 is a minimal value.). The process is quite challenging, since isomerization in the “opposite” direction shall also occur and lead to the formation of a thermodynamically stable α,β-unsaturated nitrile, which may be considered as a “dead-end”, or a bias toward poorer chemoselectivities due to the easier propensity of the latter compound to undergo hydrogenation. Reduction products of aldehydes (i.e., alcohols) were never observed under the reaction conditions investigated.
Preliminary experiments were aimed at determining the optimal solvent, amount of catalyst, and ligand-to-Rh ratio for an efficient conversion of oleonitrile into aldehydes; they were performed at 120 °C and 20 bar CO/H2 (1:1). Toluene, 1,4-dioxane (Table 1, entries 1 and 2), and even acetonitrile (results essentially similar to those in 1,4-dioxane, entry 2), some of the solvents mainly reported in the hydroformylation of long-chain olefins and compatible with our reaction conditions, were compared. The minimal differences observed indicated that those solvents can be used indifferently. Toluene was used in the rest of the study, but it was checked that the overall trends remain also valuable for MeCN.

Table 1.
Tandem isomerization–hydroformylation of oleonitrile by the Rh(acac)(CO)2–Biphephos system. 1
Aldehydes were obtained as the major products (62–90% in the final mixture) in experiments carried until nearly full conversion of 1 at [1]0/[Rh] = 200. At lower catalyst load (i.e., [1]0/[Rh] = 2000, entry 3), a dramatic decrease in conversion and, to a lesser extent, in chemoselectivity was observed. Under those demanding conditions, there is an accumulation of internal olefins (3), as isomerization (to form in particular the terminal olefin) is a slow process. Also, the worse [2 + 3]/[4] ratio observed under these conditions may be diagnostic of a catalyst decay, e.g., by the formation of Rh nanoparticles, that are more active for hydrogenation. Hence, working at a relatively high catalyst loading ([1]0/[Rh] = 200) appears compulsory to selectively form aldehydes. On the other hand, we were able to decrease the ligand-to-metal ratio down to [L]/[Rh] = 2 (Table 1, entry 4), which showed only a slight difference with experiments conducted with 10 equiv. of ligand (essentially in terms of aldehyde regioselectivity). This can be explained by the fact that, at this relatively high catalyst loading/concentration, 2 equiv. of Biphephos are sufficient to ensure the coordination of all Rh centers (The use of a larger ligand excess (10–20 equiv. Biphephos versus Rh) was demonstrated to be compulsory when working at a low catalyst load/concentration ([1]0/[Rh] = 20,000) in Rh-catalyzed hydroformylation of 10-undecenitrile [28,29]). This limited (optimized) amount of ligand is also prone to favor the catalytic activity, since there is limited competition between substrate and ligand for coordination onto rhodium. Taking advantage of this trend, subsequent experiments were realized at [L]/[Rh] = 2. In all of the cases, among aldehydes, branched aldehydes were predominant (l/b = 11:89–21:79), evidencing that improvement of regioselectivity remains the most challenging objective.
As a matter of fact, screening of the temperature and pressure clearly highlighted the difficulty to simultaneously improve the chemo- and regioselectivity (Table 2). A temperature increase favors regioselectivity (by fastening the dynamic equilibrium between terminal and internal olefins, and eventually the hydroformylation of the terminal olefin), but leads to a more prevalent hydrogenation of the substrate. Lower pressures (10 and 5 bar, entries 6 and 7) favor the regioselectivity. This last observation could be explained by a more favorable competitive coordination between the olefin and CO. A good compromise between chemo- (60%) and regioselectivity (l/b = 58:42) could be obtained at 10 bar and 120 °C with >90% conversion (entry 6). To our knowledge, this represents the best results described in the literature for the tandem isomerization–hydroformylation of this kind of long-chain olefin (i.e., with a C=C bond in a deep internal position (>5 C away)) at full conversion using a single rhodium catalyst.

Table 2.
Variation of temperature and pressure in the tandem isomerization–hydroformylation of oleonitrile by the Rh(acac)(CO)2–Biphephos system in toluene (A reaction conducted in MeCN at [1]0/[Rh] = 200, [Biphephos]/[Rh] = 2, [1]0 = 0.1 mol L−1, T = 120 °C, p = 5 bar CO/H2 (1:1) over 91 h afforded 100% conversion (1 + 1-int < 0.1 mol %), 70 mol % of 2 + 3 with l/b = 42:58, and 30 mol % of 4 (HF = 81%); compare with entry 7). 1
To tackle the substrate hydrogenation, one of the most significant issues in tandem isomerization–hydroformylation [22,25], increasing the amount of carbon monoxide regarding the amount of hydrogen (i.e., CO/H2 = 4:1) was investigated (Table 3). At 10 bar overall pressure (entry 10), as expected, a significant improvement in favor of hydroformylation was noted. However, the improvement in the chemoselectivity goes hand-in-hand with a decrease in regioselectivity. A more limited impact of this parameter was observed at 20 bar (entry 11), evidencing subtle effects between overall and relative pressures. An experiment conducted at CO/H2 = 4:1 and 140 °C confirmed the drop of chemoselectivity at high temperature (entry 12).

Table 3.
Tandem isomerization–hydroformylation of oleonitrile by the Rh(acac)(CO)2–Biphephos system at a CO/H2 ratio = 4. 1
The A4N3 bisphosphite ligand (Figure 1) was also used in combination with Rh(acac)(CO)2 (Table 4). This ligand was efficiently used by Nozaki et al. in tandem isomerization–hydroformylation of olefins, in particular of methyl oleate [25]. This ligand was also used in 10-undecenitrile hydroformylation by our group, proving a strong ability to favor the formation of linear aldehydes [30]. Xantphos, another well-known ligand that favors high regioselectivity for the linear aldehyde, was also tested [31,32]. In the tandem isomerization–hydroformylation of 1, the A4N3 ligand induced an interesting regioselectivity (l/b = 64:36); however, hydrogenation was much more important (HF = 49% only), and the activity of this catalytic system was lower than the respective performance of the Biphephos-based system (entries 13 and 14). On the other hand, the Xantphos ligand (Figure 1; used under slightly different conditions) presented a poor regioselectivity (entry 15).
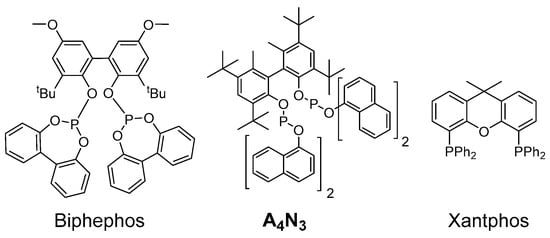
Figure 1.
Ligands used in the tandem isomerization–hydroformylation of oleonitrile.

Table 4.
Comparison of Rh–Biphephos, Rh–A4N3, and Rh–Xantphos catalytic systems. 1
3. Materials and Methods
3.1. General Considerations
Toluene was purified over alumina columns using a MBraun system (M. Braun Inertgas-Systeme GmbH, Garching, Germany). Acetonitrile and 1,4-dioxane were dried over CaH2, distilled, and stored in a Schlenk flask under an inert atmosphere and over molecular sieves. All reactions involving Rh catalysts were performed under an inert atmosphere (argon) using standard Schlenk techniques. Rh(acac)(CO)2 was generously provided by Umicore or purchased from Strem Chemicals and stored under air. Biphephos was purchased from Aldrich (St. Louis, MO, USA) and Strem Chemicals (Newburyport, MA, USA) and stored in the glove box. The A4N3 ligand was purchased from MCAT and used as received. Oleonitrile was supplied by Arkema (Colombes, France) (contains 8 mol % of 4). It was used after elution through a short alumina column and vacuum distillation (Kügelrohr distillation) at 170 °C under 0.03 mmHg. 1H and 13C NMR spectra were recorded on Bruker AC-300 and AM-400 spectrometers (Bruker, Wissembourg, France). 1H and 13C chemical shifts were determined using residual signals of the deuterated solvents and were calibrated versus SiMe4.
3.2. General Procedure for Hydroformylation Reactions with Oleonitrile
In a typical experiment, the desired amount of Rh(acac)(CO)2 (as a 1.0 g L−1 in toluene solution, 0.9 mg, 3.5 μmol) was added on Biphephos (5.5 mg, 7.0 μmol at [L]/[Rh] = 2, 27.5 mg, 35 μmol at [L]/[Rh] = 10 (weighted in the glove box)) in a Schlenk flask. A solution of oleonitrile (190 mg, 0.72 mmol) in the desired solvent (7 mL) was added onto the resulting mixture. The solution was transferred under argon into a 90 mL or 45 mL stainless-steel autoclave under argon equipped with a magnetic stir bar. The reactor was sealed, charged with CO/H2 at the desired pressure at room temperature, and then heated with silicon oil set at the desired temperature. After the appropriate reaction time, the reactor was cooled to room temperature and vented to atmospheric pressure. The solution was analyzed by NMR analysis (after evaporation of solvent).
3.3. 1H NMR Characterization
The detailed information is shown in Appendix A section.
Oleonitrile (1): 1H NMR (CDCl3, 400 MHz, 298 K): δ = 5.33 (m, 2H, CH=CH2), 2.31 (t, J = 8 Hz, 2H, CH2CN), 2.01 (m, 4H, CH2CH=CH), 1.64 (m, 2H, CH2CH2CN), 1.43 (m, 2H CH2CH2CH2CN), 1.35–1.20 (m, 18H, CH2 chain), 0.87 (t, J = 8 Hz, 3H, CH3CH2) ppm.
Hydroformylation products (2) and (3) derived from Oleonitrile: Typical signals for these aldehydes were observed in 1H NMR (CDCl3, 400 MHz, 298 K) at δ = 9.71 (t, J = 2 Hz, 1H) ppm for linear aldehyde (2) and at δ = 9.62–9.50 (d, J = 2 Hz, 1H) ppm for the branched aldehydes (3). The aldehyde signals were also evidenced in 13C{1H} NMR (CDCl3, 100 MHz, 298 K): the linear aldehyde appeared at δ = 202.7 ppm; all branched aldehydes were found in the range δ = 200.14–205.46 ppm.
Hydrogenation product (4) derived from Oleonitrile: Typical signals for this product were observed in 1H NMR (CDCl3, 400 MHz, 298 K) at δ = 0.84 (t, J = 6.6 Hz, 3H, CH3–CH2) ppm.
4. Conclusions
A combination of Rh(acac)(CO)2 and Biphephos promotes the tandem isomerization–hydroformylation of the long-chain fatty oleonitrile. The possibility to obtain linear aldehydes starting from this substrate was confirmed, highlighting the influence of the overall and relative pressures and of temperature. The best regioselectivities obtained were always accompanied by relatively low chemoselectivities (plagued by significant hydrogenation) or low yields. The optimal “compromise” found at 10 bar and 120 °C allowed us to reach 60% chemoselectivity and l/b = 58:42, a result that ranks among the better ones reported in the hydroformylation of methyl oleate or oleonitrile with a single Rh-biphosphite system [22,23,24,25].
Acknowledgments
This work was supported by the French National Research Agency, ANR project MEMCHEM (14-CE06-0022-02), and Arkema Co. We thank Umicore Co. (Angelino Doppiu) for a generous gift of Rh(acac)(CO)2.
Author Contributions
Lucas Le Goanvic and Jean-François Carpentier conceived and designed the experiments; Lucas Le Goanvic performed the experiments (Jérémy Ternel performed experiments involving Xantphos ligand); Lucas Le Goanvic and J Jean-François Carpentier analyzed the data; Jean-Luc Couturier and Jean-Luc Dubois contributed reagents/materials and analysis; Lucas Le Goanvic and Jean-François Carpentier wrote the paper.
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A
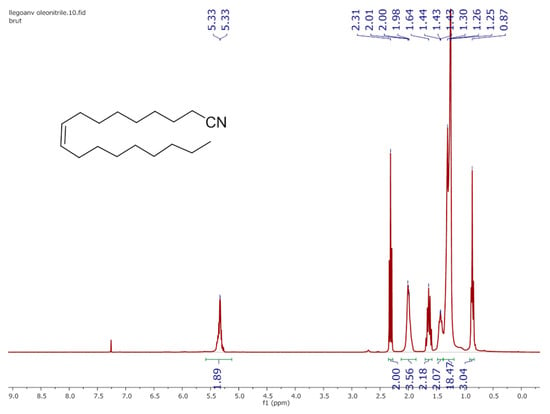
Figure A1.
1H NMR spectrum (400 MHz, CDCl3, 298 K) of starting oleonitrile material furnished by Arkema Company.
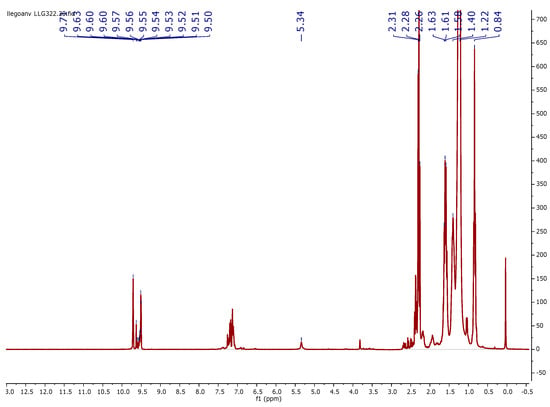
Figure A2.
1H NMR spectrum (400 MHz, CDCl3, 298 K) of a typical final mixture (Table 2, entry 8). Signal of oleonitrile and formed internal olefins (1 + 1-int) are found at δ 5.34 ppm. Linear aldehyde signal (2) was found at δ 9.71 ppm; signals for all branched aldehydes (3) are found at 9.50–9.63 ppm. The signal of the hydrogenated product (4) was found at δ 0.84 ppm.
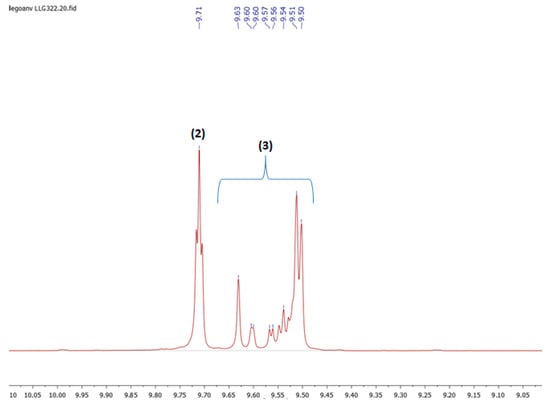
Figure A3.
Detail of the aldehyde region of the 1H NMR spectrum (400 MHz, CDCl3, 298 K) of a typical final mixture in the hydroformylation of oleonitrile (Table 2, entry 8).
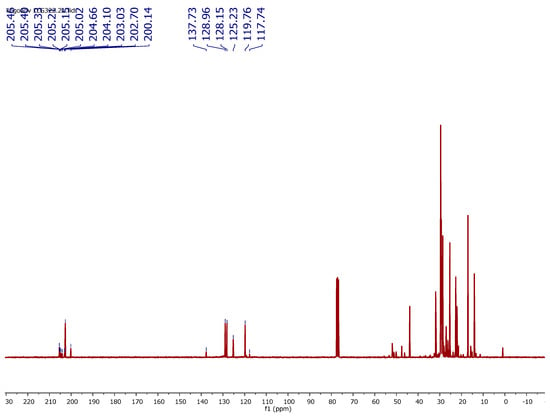
Figure A4.
13C NMR spectrum (100 MHz, CDCl3, 298 K) of a typical final mixture (Table 2, entry 8). Signal of oleonitrile and formed internal olefins (1 + 1-int) are found at δ 117–137 ppm. Aldehyde signals (2 + 3) were found at δ 200–205 ppm.
References
- Roelen, O. To Chemische Verwertungsgesellschaft Oberhausen m.b.H. German Patent DE 849548, 1938/1952; US Patent 2327066, 1943. Chem. Abstr. 1944, 38, 3631. [Google Scholar]
- Casey, C.P.; Paulsen, E.L.; Beuttenmueller, E.W.; Proft, B.R.; Petrovich, L.M.; Matter, B.A.; Powell, D.R. Electron withdrawing substituents on equatorial and apical phosphines have opposite effects on the regioselectivity of rhodium catalyzed hydroformylation. J. Am. Chem. Soc. 1997, 119, 11817–11825. [Google Scholar] [CrossRef]
- Klein, H.; Jackstell, R.; Wiese, K.-D.; Borgmann, C.; Beller, M. Highly selective catalyst systems for the hydroformylation of internal olefins to linear aldehydes. Angew. Chem. Int. Ed. 2001, 40, 3408–3411. [Google Scholar] [CrossRef]
- Van Rooy, A.; Kamer, P.C.J.; Van Leeuwen, P.W.N.M.; Goubitz, K.; Fraanje, J.; Veldman, N.; Spek, A.L. Bulky diphosphite modified rhodium catalyst; hydroformylation and characterization. Organometallics 1996, 15, 835–847. [Google Scholar] [CrossRef]
- Ohls, E.; Stein, M. The thermochemistry of long chain olefin isomers during hydroformylation. New J. Chem. 2017, 41, 7347–7355. [Google Scholar] [CrossRef]
- Roesle, P.; Durr, C.J.; Moller, H.M.; Cavallo, L.; Caporaso, L.; Mecking, S. Mechanistic features of isomerizing alkoxycarbonylation of methyl oleate. J. Am. Chem. Soc. 2012, 134, 17696–17703. [Google Scholar] [CrossRef] [PubMed]
- Vilches-Herrera, M.; Domke, L.; Börner, A. Isomerization-hydroformylation tandem reactions. ACS Catal. 2014, 4, 1706–1724. [Google Scholar] [CrossRef]
- Goldbach, V.; Roesle, P.; Mecking, S. Catalytic isomerizing ω-functionalization of fatty acids. ACS Catal. 2015, 5, 5951–5972. [Google Scholar] [CrossRef]
- Billig, E.; Abatjoglou, A.G.; Bryant, D.R.; Union Carbide Corporation. European Patent 213639. Chem. Abstr. 1987, 107, 7392. [Google Scholar]
- Billig, E.; Abatjoglou, A.G.; Bryant, D.R.; Union Carbide Corporation. European Patent 214622. Chem. Abstr. 1987, 107, 25126. [Google Scholar]
- Billig, E.; Abatjoglou, A.G.; Bryant, D.R.; Union Carbide Corporation. US Patent 4769498. Chem. Abstr. 1989, 111, 117287. [Google Scholar]
- Yan, Y.; Zhang, X.; Zhang, X. A tetraphosphorus ligand for highly regioselective isomerization-hydroformylation of internal olefins. J. Am. Chem. Soc. 2006, 128, 16058–16061. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Chie, Y.; Guan, Z.; Zhang, X. Highly regioselective isomerization—Hydroformylation of internal olefins to linear aldehyde using Rh complexes with tetraphosphorus ligands. Org. Lett. 2008, 10, 3469–3472. [Google Scholar] [CrossRef] [PubMed]
- Sémeril, D.; Matt, D.; Toupet, L. Highly regioselective hydroformylation with hemispherical chelators. Chem. Eur. J. 2008, 14, 7144–7155. [Google Scholar] [CrossRef] [PubMed]
- Behr, A.; Obst, D.; Schulte, C.; Schosser, T. Highly selective tandem isomerization-hydroformylation reaction of trans-4-octene to n-nonanal with rhodium-Biphephos catalysis. J. Mol. Catal. A Chem. 2003, 206, 179–184. [Google Scholar] [CrossRef]
- Behr, A.; Obst, D.; Schulte, C. Kinetik der isomerisierenden Hydroformylierung von trans-4-Octen. Chem. Ing. Tech. 2004, 76, 904–910. [Google Scholar] [CrossRef]
- Vanbésien, T.; Monflier, E.; Hapiot, F. Hydroformylation of vegetable oils: More than 50 years of technical innovation, successful research, and development. Eur. J. Lipid Sci. Technol. 2016, 118, 26–35. [Google Scholar] [CrossRef]
- Muilwijk, K.F.; Kamer, P.C.J.; Van Leeuwen, P.W.N.M. A bulky phosphite-modified rhodium catalyst for the hydroformylation of unsaturated fatty acid esters. J. Am. Oil Chem. Soc. 1997, 74, 223–228. [Google Scholar] [CrossRef]
- Kandanarachchi, P.; Guo, A.; Petrovic, Z. The hydroformylation of vegetable oils and model compounds by ligand modified rhodium catalysis. J. Mol. Catal. A Chem. 2002, 184, 65–71. [Google Scholar] [CrossRef]
- Benetskiy, E.; Lühr, S.; Vilches-Herrera, M.; Selent, D.; Jiao, H.; Domke, L.; Dyballa, K.; Franke, R.; Börner, A. Rhodium-catalyzed non-isomerizing hydroformylation of methyl oleate applying lactame-based phosphoramidite ligands. ACS Catal. 2014, 4, 2130–2136. [Google Scholar] [CrossRef]
- Frankel, E.N.; Metlin, S.; Rohwedder, W.K.; Wender, I. Hydroformylation of fatty unsaturated esters. J. Am. Oil Chem. Soc. 1969, 46, 133–138. [Google Scholar] [CrossRef]
- Behr, A.; Obst, D.; Westfechtel, A. Isomerizing hydroformylation of fatty acid esters: Formation of ω-aldehydes. Eur. J. Lipid Sci. Technol. 2005, 107, 213–219. [Google Scholar] [CrossRef]
- Pandey, S.; Chikkali, S.H. Highly regioselective isomerizing hydroformylation of long-chain internal olefins catalyzed by a rhodium bis(phosphite) complex. ChemCatChem 2015, 7, 3468–3471. [Google Scholar] [CrossRef]
- Pandey, S.; Shinde, D.R.; Chikkali, S.H. Isomerizing hydroformylation of cashew nut shell liquid. ChemCatChem 2017, 9, 3997–4004. [Google Scholar] [CrossRef]
- Yuki, Y.; Takahashi, K.; Tanaka, Y.; Nozaki, K. Tandem isomerization/hydroformylation/hydrogenation of internal alkenes to n-alcohols using Rh/Ru dual- or ternary-catalyst systems. J. Am. Chem. Soc. 2013, 135, 17393–17400. [Google Scholar] [CrossRef] [PubMed]
- Gaide, T.; Bianga, J.; Schlipköter, K.; Behr, A.; Vorholt, A.J. Linear selective isomerization/hydroformylation of unsaturated fatty acid methyl esters: A bimetallic approach. ACS Catal. 2017, 7, 4163–4171. [Google Scholar] [CrossRef]
- Furst, M.R.L.; Korkmaz, V.; Gaide, T.; Seidensticker, T.; Behr, A.; Vorholt, A.J. Tandem reductive hydroformylation of castor oil derived substrates and catalyst recycling by selective product crystallisation. ChemCatChem 2017, 9, 4319–4323. [Google Scholar] [CrossRef]
- Ternel, J.; Couturier, J.-L.; Dubois, J.-L.; Carpentier, J.-F. Rhodium-catalyzed tandem isomerization/hydroformylation of the bio-sourced 10-Undecenenitrile: Selective and productive catalysts for production of polyamide-12 precursor. Adv. Synth. Catal. 2013, 355, 3191–3204. [Google Scholar] [CrossRef]
- Ternel, J.; Couturier, J.-L.; Dubois, J.-L.; Carpentier, J.-F. Rhodium versus Iridium catalysts in the controlled tandem hydroformylation-isomerization of functionalized unsaturated fatty substrates. ChemCatChem 2015, 7, 513–520. [Google Scholar] [CrossRef]
- Le Goanvic, L.; Couturier, J.-L.; Dubois, J.-L.; Carpentier, J.-F. Ruthenium-catalyzed hydroformylation of the functional unsaturated fatty nitrile 10-undecenitrile. J. Mol. Catal. A Chem. 2016, 417, 116–121. [Google Scholar] [CrossRef]
- Van der Veen, L.A.; Kamer, P.C.J.; Van Leeuwen, P.W.N.M. Hydroformylation of internal olefins to linear aldehydes with novel rhodium catalysts. Angew. Chem. Int. Ed. 1999, 38, 336–338. [Google Scholar] [CrossRef]
- Van Leeuwen, P.W.N.M.; Kamer, P.C.J. Featuring Xantphos. Catal. Sci. Technol. 2018. [Google Scholar] [CrossRef]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).