Abstract
A transition-metal-free strategy for the synthesis of 2,3-dihydrobenzothiazin-4-one derivatives has been established. Using HI as the catalyst, various 2-substituted, N-substituted compounds and variations on the benzamide ring were synthesized in excellent yields via the reaction between 2-mercaptobenzamides and aldehydes. In addition, an easy experimental procedure, broad substrate scope, and excellent functional group tolerance demonstrate that this catalytic protocol provides a valuable complementary approach for accessing 2,3-dihydrobenzothiazin-4-one derivatives.
1. Introduction
2,3-Dihydrobenzothiazin-4-ones, as important sulfur- and nitrogen-containing heterocyclic compounds, have been widely explored in synthetic and pharmaceutical chemistry [1,2,3]. Some derivatives have shown antimalarial, antitumor, and COX-2 inhibitory activities [4,5,6] (Figure 1).
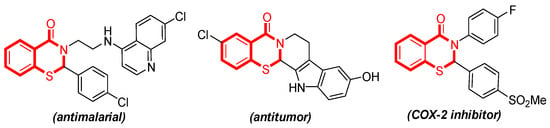
Figure 1.
Some selected important 2,3-dihydrobenzothiazin-4-ones.
Over the past few decades, considerable efforts have been dedicated to the development of efficient strategies for constructing 2,3-dihydrobenzothiazin-4-ones [7,8,9,10,11]. Among these methods, transition-metal-free approaches have garnered significant attention as potentially more sustainable alternatives to metal-catalyzed processes [12,13,14,15]. In recent years, several novel transition-metal-free strategies have been reported. In 2017, the Shi group reported the synthesis of 2,3-dihydrobenzothiazin-4-ones from 2-mercaptobenzamides and propiolate derivatives in the presence of K3PO4 [16]. This methodology is limited to the preparation of 2,3-dihydrobenzothiazin-4-ones bearing carbonyl or ester groups (Scheme 1a). In addition, the Zhou group employed triphenylphosphine (Ph3P) as a key additive in the synthesis of 2,3-dihydrobenzothiazin-4-ones from benzodithiol-3-ones (Scheme 1b) [17,18,19]. Meanwhile, our group has also developed a series of 2,3-dihydrobenzothiazin-4-ones through the selective cyclization of 2-alkylthiobenzamides and the ring-expansion of benzoisothiazol-3-ones [20,21]. However, these approaches required the use of excess amounts of strong acids and additives (Scheme 1c). Therefore, the development of novel catalytic strategies for the synthesis of 2,3-dihydrobenzothiazin-4-ones would constitute a highly significant contribution to the field of synthetic chemistry.
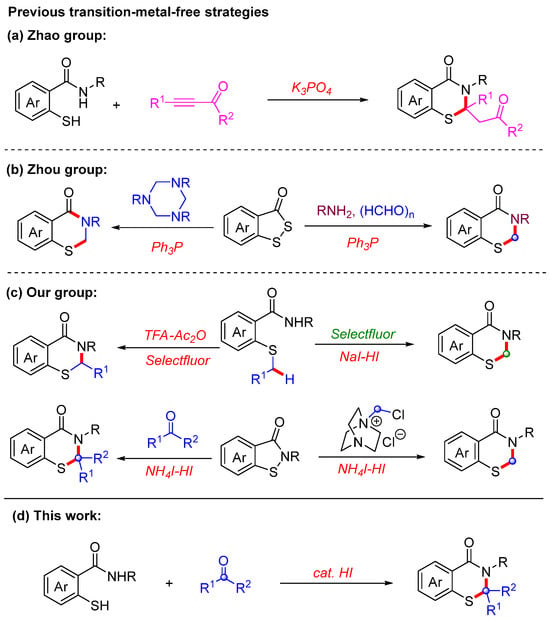
Scheme 1.
Recently reported metal-free strategies for the synthesis of 2,3-dihydrobenzothiazin-4-one derivatives.
In recent years, acid-catalyzed cyclization strategies have been widely applied to the synthesis of various heterocyclic frameworks, such as dihydroquinazolines [22], dihydropyrimidines [23], and thiazolidines/oxazolidines [24,25], where strong acids facilitate carbonyl activation and intramolecular nucleophilic attack. These precedents highlight the general utility of acid catalysis in heterocycle construction, but methods targeting 2,3-dihydrobenzothiazin-4-ones remain limited [26]. In our continuing investigations into sulfur chemistry and based on our previous work [21,27,28,29,30,31,32,33,34], we herein report a novel HI-catalyzed synthesis of 2,3-dihydrobenzothiazin-4-one derivatives using 2-mercaptobenzamides and aldehydes or ketones as starting materials (Scheme 1d).
2. Results and Discussion
The investigation commenced with the reaction of 2-mercapto-N-methylbenzamide (1a), benzaldehyde (2a), and an acid catalyst for the synthesis of 3-methyl-2-phenyl-2,3-dihydro-4H-benzo[e][1,3]thiazin-4-one (3a) in an organic solvent at 100 °C for 6 h. (Scheme 2). To our delight, the use of hydroiodic acid (HI) as the acid catalyst and acetonitrile (MeCN) as the solvent afforded the desired product 3a in 96% yield (Table 1, entry 1). A subsequent solvent screening demonstrated that MeCN is the optimal solvent, with DCM and toluene (PhMe) also serving as viable alternatives (Table 1, entries 2–7). Further evaluation of the HI catalyst loading indicated that increasing the amount to 10 mol% did not improve the yield of 3a, whereas reducing the loading to 2.5 mol% still provided the desired product 3a in 90% yield (Table 1, entries 8–9). When alternative acid catalysts, such as HBr, HCl, and HOAc, were examined, HI was confirmed to be the optimal catalyst. In contrast, HCl and HOAc failed to promote the formation of product 3a (Table 1, entries 10–12). Additionally, altering the amount of benzaldehyde (2a) did not result in a significant enhancement in the yield of 3a (Table 1, entries 13–14). Furthermore, the investigation of reaction temperatures indicated that 100 °C is the optimal temperature for this reaction (Table 1, entries 15–16). Finally, in the absence of the HI catalyst, no desired product 3a was observed (Table 1, entry 17).
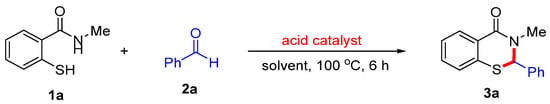
Scheme 2.
The reaction of 2-mercapto-N-methylbenzamide (1a) and benzaldehyde (2a) for the synthesis of 3-methyl-2-phenyl-2,3-dihydro-4H-benzo[e][1,3]thiazin-4-one (3a).

Table 1.
Optimization of reaction conditions for 3-methyl-2-phenyl-2,3-dihydro-4H-benzo[e][1,3]thiazin-4-one (3a) a.
With the optimized reaction conditions established, the reaction of 2-mercapto-N-methylbenzamide with various aldehydes was initially explored (Scheme 3). As we expected, a range of aryl aldehydes afforded the desired products 3a–j in excellent yields. Both electron-donating (MeO, Me, and t-Bu) and electron-withdrawing (F, Cl, Br, and CF3) groups located at the para-, meta-, or ortho-positions on the aryl ring were well tolerated under the optimized conditions. Notably, halogen groups can be further transformed into other valuable functionalities. Both 1-naphthaldehyde and 2-naphthaldehyde yielded the corresponding products 3k and 3l in 93% and 95% yields, respectively. Furthermore, the heteroaryl aldehyde 5-methylfuran-2-carbaldehyde afforded the desired product 3m in 87% yield. In addition to (hetero)aryl aldehydes, different aliphatic aldehydes were also suitable substrates, furnishing the desired products 3n–o in moderate to excellent yields. Finally, the use of cyclohexanone afforded product 3p in moderate yield in the presence of excess HI.
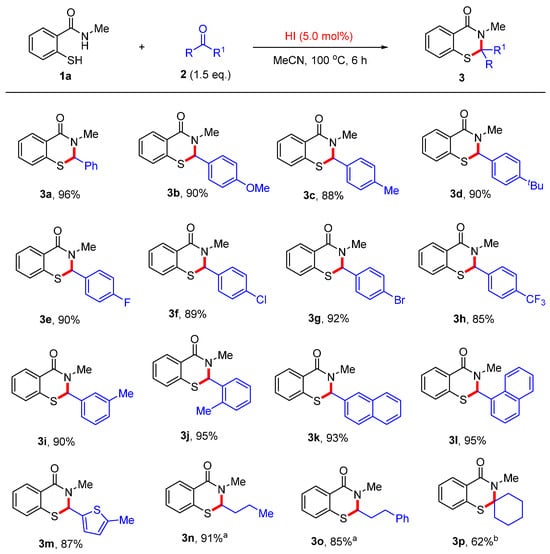
Scheme 3.
Scope of aldehydes and ketones. Reaction conditions: 1a (0.4 mmol), 2 (0.6 mmol), HI (0.02 mmol, 55 wt.% in H2O), MeCN (4.0 mL), 100 °C, 6 h. Isolated yields. a HI (0.4 mmol). b HI (1.2 mmol).
Next, the substrate scope of 2-mercaptobenzamides was also explored (Scheme 4). In the presence of HI (5.0 mol%), a variety of N-alkyl-substituted substrates, including ethyl, n-propyl, n-butyl, and benzyl groups, afforded the desired products 3q–t in excellent yields. Other alkyl groups, such as cyclopropyl and cyclohexyl, afforded the desired products 3u–v in good yields using 1.0 equivalent of HI. Moreover, the NH2-amide substrate also provided the desired product 3w in 68%. It should be noted that various N-aryl-substituted substrates afforded the corresponding products 3x–ac in good yields using a catalytic amount of HI. Next, substituents on 2-mercaptobenzamides, such as 4-chloro and 5-methyl groups, were well-tolerated, leading to the corresponding products 3ad–ae in good yields. Moreover, N-(4-methoxyphenyl)-2-mercaptobenzamide reacted with cyclohexanone to afford the desired product 3af in 55% yield when excess HI was used.
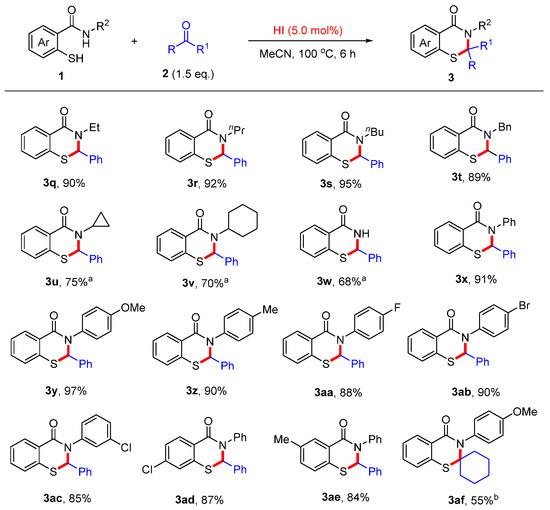
Scheme 4.
Scope of 2-mercaptobenzamides. Reaction conditions: 1a (0.4 mmol), 2 (0.6 mmol), HI (0.02 mmol, 55 wt.% in H2O), MeCN (4.0 mL), 100 °C, 6 h. Isolated yields. a HI (0.4 mmol). b HI (1.2 mmol).
To demonstrate the synthetic applicability of our novel approach, the gram-scale reaction was carried out to synthesize 3-methyl-2-phenyl-2,3-dihydro-4H-benzo[e][1,3]thiazin-4-one (Scheme 5). After adjusting the solvent volume and reaction times, the desired product 3a was obtained in 85% yield by flash chromatography, and the purity of the product was confirmed by NMR analysis.
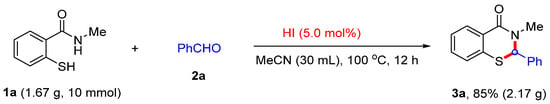
Scheme 5.
The gram-scale synthesis for 3-methyl-2-phenyl-2,3-dihydro-4H-benzo[e][1,3]thiazin-4-one (3a).
In order to investigate the reaction mechanism, control experiments were conducted (Scheme 6). In the presence of HI (5.0 mol%) or HI (1.0 eq.), the corresponding disulfide 4a was not detected by NMR analysis. These findings suggest that the disulfide 4a may not be involved in this reaction.
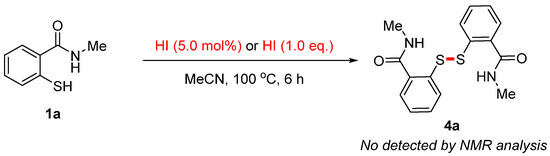
Scheme 6.
Control experiments.
Based on previous work and control experiments, the proposed mechanism is illustrated in Scheme 7 [21,35,36,37,38]. The initial protonation of benzaldehyde by HI facilitates nucleophilic attack by 2-mercaptobenzamide 1a to form the hemithioacetal intermediate A. Next, intermediate A undergoes protonation by HI to generate intermediate B, which subsequently undergoes dehydration to yield the corresponding intermediate C. Finally, an intramolecular cyclization followed by deprotonation yields product 3a and regenerates HI.
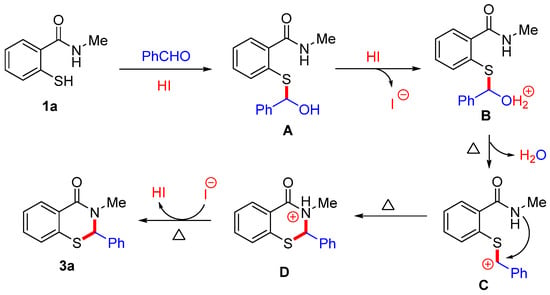
Scheme 7.
The plausible reaction mechanism.
3. Materials and Methods
3.1. General Information
All the solvents and commercially available reagents were purchased and used directly. Thin layer chromatography (TLC) was performed on EMD precoated plates (silica gel 60 F254, Art 5715, Yantai Jiangyou Silica gel Development Co., Ltd., Yantai, China) and visualized by fluorescence quenching under UV light. Column chromatography was performed on EMD Silica Gel 60 (200–300 Mesh, Shanghai Titan Technology Co., Ltd., Shanghai, China) using a forced flow of 0.5–1.0 bar. The 1H and 13C NMR spectra were obtained on Bruker AVANCE III–300 and 400 spectrometers (Bruker Corporation, Billerica, MA, USA). 1H NMR data were reported as chemical shift (δ ppm), multiplicity, coupling constant (Hz), and integration. 13C NMR data were reported in terms of chemical shift (δ ppm), multiplicity, and coupling constant (Hz). Mass (HRMS) analysis was conducted using the Agilent 6200 Accurate-Mass TOF LC/MS system (Agilent Technologies Co., Ltd., Santa Clara, CA, USA), with electrospray ionization (ESI). The melting points were measured by X4-A microscopic melting point apparatus (Shanghai INESA Physico-Optical Instrument Co., Ltd., Shanghai, China).
2-Mercaptobenzamides (1a–n), aldehydes and ketones (2) were purchased from Adamas-beta® (Shanghai, China), Energy-chemical (Shanghai, China), BLDpharm (Shanghai, China), J&K@ (Shanghai, China), Chemieliva (Chongqing, China), TCI (Shanghai, China), J&K@ (Shanghai, China), Enamine (Shanghai, China), Aurora Fine Chemicals (San Diego, CA, USA), Atomax Chemicals (Shenzhen, China), SAGECHEM (Hangzhou, China) or Sigma-Aldrich (Shanghai, China). 2-Mercaptobenzamides (1o–p) were prepared from commercial methyl 4-chloro-2-mercaptobenzoate or methyl 5-methyl-2-mercaptobenzoate (2.0 mmol) and phenylamine (4.8 mmol) in DCM with reflux for 12 h according to the reported procedure [39].
3.2. Optimization of the Reaction Conditions for Product 3a
A 30 mL Schlenk tube was charged with 2-mercapto-N-methylbenzamide (1a, 0.4 mmol), benzaldehyde (2a, 0.4–0.8 mmol), catalyst (2.5–10.0 mol%) and commercial-grade MeCN (4.0 mL). The tube was sealed and the reaction was then stirred vigorously at 100 °C for 6 h. After cooling to room temperature, the reaction mixture was then concentrated in vacuo. The residue was purified by flash chromatography on silica gel to yield the desired product 3a.
Caution! All reactions must be conducted in a well-ventilated fume hood. The use of appropriate personal protective equipment (PPE) is essential during chemical handling and manipulations. A blast shield should be placed in front of reactions performed in MeCN with concentrated HI in a sealed Schlenk tube at 100 °C.
3.3. Synthetic Procedures for the Synthesis of Products 3
A 30 mL Schlenk tube was charged with 2-mercaptobenzamides (1, 0.4 mmol), aldehydes or ketones (2, 0.6 mmol), HI (55 wt.% in H2O, 5.0 mol%, 0.02 mmol, 2.7 μL) and commercial-grade MeCN (4.0 mL). The tube was sealed and the reaction was then stirred vigorously at 100 °C for 6 h. After cooling to room temperature, the reaction mixture was then concentrated in vacuo. The residue was purified by flash chromatography on silica gel to yield the desired products 3.
3-Methyl-2-phenyl-2,3-dihydro-4H-benzo[e][1,3]thiazin-4-one (3a). White solid, 98.0 mg, yield: 96% (known compound [40]). 1H NMR (300 MHz, CDCl3) δ 8.16 (dd, J = 7.5, 1.8 Hz, 1H), 7.30–7.18 (m, 7H), 7.09 (dd, J = 7.6, 1.4 Hz, 1H), 5.64 (s, 1H), 3.24 (s, 3H). 13C NMR (75 MHz, CDCl3) δ 164.26, 138.42, 132.98, 132.03, 130.02, 128.83, 128.65, 128.45, 127.34, 126.24, 126.22, 63.81, 36.03.
2-(4-Methoxyphenyl)-3-methyl-2,3-dihydro-4H-benzo[e][1,3]thiazin-4-one (3b). White solid, 102.7 mg, yield: 90% (known compound [11]). 1H NMR (300 MHz, CDCl3) δ 8.07 (dd, J = 7.8, 1.6 Hz, 1H), 7.22–7.06 (m, 4H), 7.01 (dd, J = 7.4, 1.6 Hz, 1H), 6.71–6.66 (m, 2H), 5.54 (s, 1H), 3.63 (s, 3H), 3.12 (s, 3H). 13C NMR (75 MHz, CDCl3) δ 164.27, 159.64, 133.30, 131.99, 130.17, 130.01, 128.82, 127.59, 127.36, 126.13, 114.00, 63.55, 55.26, 35.75.
3-Methyl-2-(p-tolyl)-2,3-dihydro-4H-benzo[e][1,3]thiazin-4-one (3c). White solid, 94.8 mg, yield: 88%, m.p.: 96–97 °C. 1H NMR (300 MHz, CDCl3) δ 8.08 (dd, J = 7.7, 1.7 Hz, 1H), 7.23–7.09 (m, 2H), 7.07–6.96 (m, 5H), 5.54 (s, 1H), 3.14 (s, 3H), 2.18 (s, 3H). 13C NMR (75 MHz, CDCl3) δ 164.31, 138.38, 135.35, 133.16, 131.99, 130.01, 129.35, 128.83, 127.35, 126.19, 126.16, 63.71, 35.92, 21.05. HRMS (ESI, m/z): calcd for C16H15NNaOS [M + Na]+, 292.0767; found, 292.0769.
2-(4-(Tert-butyl)phenyl)-3-methyl-2,3-dihydro-4H-benzo[e][1,3]thiazin-4-one (3d). White solid, 112.0 mg, yield: 90%, m.p.: 100–101 °C. 1H NMR (300 MHz, CDCl3) δ 8.10 (dd, J = 7.8, 1.6 Hz, 1H), 7.22–7.07 (m, 6H), 7.01 (dd, J = 7.9, 1.1 Hz, 1H), 5.55 (s, 1H), 3.14 (s, 3H), 1.17 (s, 9H). 13C NMR (75 MHz, CDCl3) δ 164.21, 151.52, 135.35, 133.30, 131.94, 130.05, 128.79, 127.30, 126.09, 125.98, 125.64, 63.62, 35.95, 34.55, 31.22. HRMS (ESI, m/z): calcd for C19H21NNaOS [M + Na]+, 334.1236; found, 334.1237.
2-(4-Fluorophenyl)-3-methyl-2,3-dihydro-4H-benzo[e][1,3]thiazin-4-one (3e). White solid, 98.5 mg, yield: 90%, m.p.: 109–110 °C. 1H NMR (300 MHz, CDCl3) δ 8.08 (dd, J = 7.6, 1.7 Hz, 1H), 7.25–7.11 (m, 4H), 7.02 (dd, J = 7.6, 1.4 Hz, 1H), 6.90–6.82 (m, 2H), 5.55 (s, 1H), 3.17 (s, 3H). 13C NMR (75 MHz, CDCl3) δ 164.11, 162.54 (d, J = 248.0 Hz), 134.27 (d, J = 3.2 Hz), 132.73, 132.14, 130.05, 128.75, 127.99 (d, J = 8.4 Hz), 127.41, 126.36, 115.62 (d, J = 21.8 Hz), 63.22, 36.02. HRMS (ESI, m/z): calcd. for C15H13FNOS [M + H]+: 274.0696, found: 274.0698.
2-(4-Chlorophenyl)-3-methyl-2,3-dihydro-4H-benzo[e][1,3]thiazin-4-one (3f). White solid, 103.2 mg, yield: 89%, m.p.: 82–83 °C. 1H NMR (300 MHz, CDCl3) δ 8.07 (dd, J = 7.7, 1.7 Hz, 1H), 7.23–7.07 (m, 6H), 7.00 (dd, J = 7.5, 1.5 Hz, 1H), 5.52 (s, 1H), 3.17 (s, 3H). 13C NMR (75 MHz, CDCl3) δ 164.06, 137.13, 134.29, 132.54, 132.19, 130.04, 128.81, 128.73, 127.54, 127.44, 126.43, 63.19, 36.15. HRMS (ESI, m/z): calcd. for C15H13ClNOS [M + H]+: 290.0401, found: 290.0402.
2-(4-Bromophenyl)-3-methyl-2,3-dihydro-4H-benzo[e][1,3]thiazin-4-one (3g). White solid, 123.0 mg, yield: 92%, m.p.: 150–151 °C. 1H NMR (300 MHz, CDCl3) δ 8.07 (dd, J = 7.7, 1.7 Hz, 1H), 7.32–7.27 (m, 2H), 7.24–7.12 (m, 2H), 7.06–6.99 (m, 3H), 5.50 (s, 1H), 3.18 (s, 3H).13C NMR (75 MHz, CDCl3) δ 164.05, 137.69, 132.49, 132.20, 131.77, 130.05, 128.73, 127.85, 127.44, 126.45, 122.46, 63.26, 36.17. HRMS (ESI, m/z): calcd. for C15H13BrNOS [M + H]+: 333.9896, found: 333.9896.
3-Methyl-2-(4-(trifluoromethyl)phenyl)-2,3-dihydro-4H-benzo[e][1,3]thiazin-4-one (3h). Colorless oil, 110.0 mg, yield: 85%. 1H NMR (300 MHz, CDCl3) δ 8.22–8.11 (m, 1H), 7.52 (d, J = 8.2 Hz, 2H), 7.38–7.22 (m, 4H), 7.09 (dd, J = 7.4, 1.6 Hz, 1H), 5.65 (s, 1H), 3.29 (s, 3H). 13C NMR (75 MHz, CDCl3) δ 164.00, 142.72, 132.30, 132.16, 130.59 (q, J = 32.5 Hz), 130.10, 128.69, 127.45, 126.58, 126.49, 125.67 (q, J = 3.8 Hz), 123.76 (q, J = 272.3 Hz), 63.21, 36.31. HRMS (ESI, m/z): calcd. for C16H13F3NOS [M + H]+: 324.0664, found: 324.0660.
3-Methyl-2-(m-tolyl)-2,3-dihydro-4H-benzo[e][1,3]thiazin-4-one (3i). White solid, 97.0 mg, yield: 90%, m.p.: 96–97 °C. 1H NMR (300 MHz, CDCl3) δ 8.18–8.15 (m, 1H), 7.29–7.00 (m, 7H), 5.60 (s, 1H), 3.22 (s, 3H), 2.27 (s, 3H).13C NMR (75 MHz, CDCl3) δ 164.27, 138.47, 138.33, 133.12, 132.00, 130.00, 129.30, 128.82, 128.52, 127.30, 126.98, 126.17, 123.33, 63.80, 35.96, 21.46. HRMS (ESI, m/z): calcd for C16H15NNaOS [M + Na]+, 292.0767; found, 292.0767.
3-Methyl-2-(o-tolyl)-2,3-dihydro-4H-benzo[e][1,3]thiazin-4-one (3j). Colorless oil, 102.3 mg, yield: 95%. 1H NMR (300 MHz, CDCl3) δ 8.15–8.12 (m, 1H), 7.20–7.04 (m, 4H), 6.98–6.91 (m, 3H), 5.68 (s, 1H), 3.04 (s, 3H), 2.34 (s, 3H). 13C NMR (75 MHz, CDCl3) δ 164.74, 135.29, 135.00, 132.90, 132.06, 131.45, 130.05, 128.69, 128.55, 127.43, 126.27, 126.23, 125.33, 60.93, 35.67, 19.49. HRMS (ESI, m/z): calcd for C16H15NNaOS [M + Na]+, 292.0767; found, 292.0765.
3-Methyl-2-(naphthalen-2-yl)-2,3-dihydro-4H-benzo[e][1,3]thiazin-4-one (3k). Colorless oil, 113.6 mg, yield: 93%. 1H NMR (300 MHz, CDCl3) δ 8.22–8.19 (m, 1H), 7.77–7.69 (m, 3H), 7.53 (d, J = 1.9 Hz, 1H), 7.46–7.39 (m, 3H), 7.24–7.14 (m, 2H), 7.05–7.02 (m, 1H), 5.75 (s, 1H), 3.30 (s, 3H). 13C NMR (75 MHz, CDCl3) δ 164.36, 135.41, 133.01, 132.80, 132.71, 132.11, 130.04, 128.89, 128.85, 128.18, 127.60, 127.43, 126.69, 126.67, 126.32, 124.94, 124.39, 63.95, 36.22. HRMS (ESI, m/z): calcd. for C19H16NOS [M + H]+: 306.0947, found: 306.0945.
3-Methyl-2-(naphthalen-1-yl)-2,3-dihydro-4H-benzo[e][1,3]thiazin-4-one (3l). White solid, 116.0 mg, yield: 95%, m.p.: 169–170 °C. 1H NMR (300 MHz, CDCl3) δ 8.29–8.24 (m, 1H), 8.05 (d, J = 8.4 Hz, 1H), 7.88 (d, J = 8.0 Hz, 1H), 7.77 (d, J = 8.2 Hz, 1H), 7.65–7.59 (m, 1H), 7.57–7.51 (m, 1H), 7.30–7.17 (m, 4H), 7.01–6.96 (m, 1H), 6.39 (s, 1H), 3.22 (s, 3H). 13C NMR (75 MHz, CDCl3) δ 164.90, 134.27, 133.36, 132.08, 131.87, 130.23, 129.63, 129.56, 129.35, 128.29, 127.54, 126.69, 126.27, 126.10, 125.00, 123.93, 122.79, 61.03, 35.93. HRMS (ESI, m/z): calcd. for C19H16NOS [M + H]+: 306.0947, found: 306.0950.
3-Methyl-2-(5-methylthiophen-2-yl)-2,3-dihydro-4H-benzo[e][1,3]thiazin-4-one (3m). White solid, 95.7 mg, yield: 87%, m.p.: 91–92 °C. 1H NMR (300 MHz, CDCl3) δ 8.05 (dd, J = 7.7, 1.6 Hz, 1H), 7.27–7.08 (m, 3H), 6.37–6.35 (m, 1H), 6.36 (dt, J = 3.6, 1.2 Hz, 1H), 5.67 (s, 1H), 3.20 (s, 3H), 2.24 (s, 3H). 13C NMR (75 MHz, CDCl3) δ 163.57, 140.66, 140.03, 133.25, 132.10, 130.14, 128.74, 127.60, 126.34, 126.19, 124.54, 60.56, 35.90, 15.34. HRMS (ESI, m/z): calcd. for C14H14NOS2 [M + H]+: 276.0511, found: 276.0511.
3-Methyl-2-propyl-2,3-dihydro-4H-benzo[e][1,3]thiazin-4-one (3n). Colorless oil, 80.5 mg, yield: 91%. 1H NMR (300 MHz, CDCl3) δ 8.11–8.07 (m, 1H), 7.39–7.22 (m, 3H), 4.33 (dd, J = 8.9, 5.9 Hz, 1H), 3.25 (s, 3H), 1.93–1.77 (m, 2H), 1.56–1.28 (m, 2H), 0.88 (t, J = 7.4 Hz, 3H). 13C NMR (75 MHz, CDCl3) δ 163.30, 133.87, 131.89, 130.01, 128.78, 127.83, 125.93, 62.87, 36.52, 36.16, 20.01, 13.34. HRMS (ESI, m/z): calcd for C12H15NNaOS [M + Na]+, 244.0767; found, 244.0767.
3-Methyl-2-phenethyl-2,3-dihydro-4H-benzo[e][1,3]thiazin-4-one (3o). Colorless oil, 96.3 mg, yield: 85%. 1H NMR (300 MHz, CDCl3) δ 8.10 (dd, J = 8.1, 1.5 Hz, 1H), 7.40–7.34 (m, 1H), 7.30–7.11 (m, 7H), 4.24 (dd, J = 8.7, 6.0 Hz, 1H), 3.16 (s, 3H), 2.85–2.62 (m, 2H), 2.24–2.15 (m, 2H). 13C NMR (75 MHz, CDCl3) δ 163.10, 139.92, 133.49, 131.97, 130.14, 128.89, 128.69, 128.33, 127.90, 126.41, 126.13, 62.14, 35.88, 35.24, 32.56. HRMS (ESI, m/z): calcd. for C17H18NOS [M + H]+: 284.1104, found: 284.1106.
3-Methylspiro[benzo[e][1,3]thiazine-2,1′-cyclohexan]-4(3H)-one (3p). White solid, 61.3 mg, yield: 62%, m.p.: 92–93 °C. 1H NMR (300 MHz, CDCl3) δ 8.11 (dd, J = 8.2, 1.6 Hz, 1H), 7.38–7.32 (m, 1H), 7.28–7.21 (m, 2H), 3.22 (s, 3H), 2.18–2.11 (m, 2H), 1.94–1.59 (m, 8H). 13C NMR (75 MHz, CDCl3) δ 164.72, 133.48, 131.71, 130.39, 128.91, 127.30, 125.78, 69.31, 34.90, 27.94, 25.24, 22.83. HRMS (ESI, m/z): calcd for C14H17NNaOS [M + Na]+, 270.0923; found, 270.0925.
3-Ethyl-2-phenyl-2,3-dihydro-4H-benzo[e][1,3]thiazin-4-one (3q). White solid, 97.1 mg, yield: 90% (known compound [11]). 1H NMR (300 MHz, CDCl3) δ 8.16–7.94 (m, 1H), 7.21–7.10 (m, 7H), 6.99 (dd, J = 7.6, 1.5 Hz, 1H), 5.61 (s, 1H), 4.17–4.06 (m, 1H), 3.22–3.10 (m, 1H), 1.20 (t, J = 7.2 Hz, 3H). 13C NMR (75 MHz, CDCl3) δ 163.71, 139.25, 132.81, 131.92, 129.91, 129.25, 128.46, 128.25, 127.44, 126.29, 126.24, 61.55, 43.56, 13.68.
2-Phenyl-3-propyl-2,3-dihydro-4H-benzo[e][1,3]thiazin-4-one (3r). Colorless oil, 104.2 mg, yield: 92% (known compound [41]). 1H NMR (300 MHz, CDCl3) δ 8.16–8.13 (m, 1H), 7.28–7.17 (m, 7H), 7.07 (dd, J = 7.7, 1.4 Hz, 1H), 5.65 (s, 1H), 4.29–4.20 (m, 1H), 3.04–2.95 (m, 1H), 1.80–1.65 (m, 2H), 0.99 (t, J = 7.4 Hz, 3H). 13C NMR (75 MHz, CDCl3) δ 163.94, 139.15, 132.78, 131.90, 129.94, 129.29, 128.44, 128.19, 127.45, 126.27, 126.20, 61.91, 50.43, 21.68, 11.44.
3-Butyl-2-phenyl-2,3-dihydro-4H-benzo[e][1,3]thiazin-4-one (3s). Colorless oil, 113.0 mg, yield: 95% (known compound [20]). 1H NMR (300 MHz, CDCl3) δ 8.14 (dd, J = 7.6, 1.8 Hz, 1H), 7.28–7.16 (m, 7H), 7.06 (dd, J = 7.4, 1.6 Hz, 1H), 5.66 (s, 1H), 4.34–4.24 (m, 1H), 3.06–2.97 (m, 1H), 1.74–1.64 (m, 2H), 1.48–1.35 (m, 2H), 0.93 (t, J = 7.3 Hz, 3H). 13C NMR (75 MHz, CDCl3) δ 163.91, 139.12, 132.78, 131.91, 129.93, 129.26, 128.45, 128.20, 127.44, 126.28, 126.21, 61.83, 48.55, 30.51, 20.21, 13.86.
3-Benzyl-2-phenyl-2,3-dihydro-4H-benzo[e][1,3]thiazin-4-one (3t). Colorless oil, 118.0 mg, yield: 89% (known compound [41]). 1H NMR (300 MHz, CDCl3) δ 8.22 (dd, J = 7.4, 2.4 Hz, 1H), 7.35–7.21 (m, 12H), 7.05 (d, J = 7.4 Hz, 1H), 5.87 (d, J = 15.2 Hz, 1H), 5.57 (s, 1H), 3.91 (d, J = 15.3 Hz, 1H). 13C NMR (75 MHz, CDCl3) δ 164.22, 138.65, 136.58, 133.03, 132.25, 130.26, 128.84, 128.60, 128.36, 128.04, 127.81, 127.56, 126.31, 126.26, 60.67, 50.68.
3-Cyclopropyl-2-phenyl-2,3-dihydro-4H-benzo[e][1,3]thiazin-4-one (3u). Colorless oil, 84.5 mg, yield: 75%. 1H NMR (300 MHz, CDCl3) δ 8.11 (dd, J = 7.8, 1.7 Hz, 1H), 7.22–7.09 (m, 7H), 6.99 (dd, J = 7.5, 1.5 Hz, 1H), 5.69 (s, 1H), 2.91–2.84 (m, 1H), 1.05–0.98 (m, 1H), 0.85–0.72 (m, 3H). 13C NMR (75 MHz, CDCl3) δ 165.50, 139.76, 132.71, 132.17, 129.96, 128.91, 128.48, 128.09, 127.38, 126.08, 126.03, 62.95, 32.01, 9.39, 7.09. HRMS (ESI, m/z): calcd. for C17H16NOS [M + H]+: 282.0947, found: 282.0946.
3-Cyclohexyl-2-phenyl-2,3-dihydro-4H-benzo[e][1,3]thiazin-4-one (3v). White solid, 90.5 mg, yield: 70%, m.p.: 165–166 °C. 1H NMR (300 MHz, CDCl3) δ 8.15–8.12 (m, 1H), 7.29–7.13 (m, 7H), 7.04–7.01 (m, 1H), 5.80 (s, 1H), 4.81–4.80 (m, 1H), 2.02–1.97 (m, 1H), 1.89–1.82 (m, 1H), 1.74–1.34 (m, 6H), 1.15–1.01 (m, 2H). 13C NMR (75 MHz, CDCl3) δ 163.79, 140.84, 132.43, 131.74, 130.17, 129.98, 128.02, 127.72, 127.57, 126.32, 126.20, 57.07, 54.82, 31.62, 30.11, 25.82, 25.68, 25.44. HRMS (ESI, m/z): calcd. for C20H22NOS [M + H]+: 324.1417, found: 324.1416.
2-Phenyl-2,3-dihydro-4H-benzo[e][1,3]thiazin-4-one (3w). White solid, 65.6 mg, yield: 68% (known compound [21]). 1H NMR (300 MHz, DMSO-d6) δ 9.06 (d, J = 3.8 Hz, 1H), 8.01–7.98 (m, 1H), 7.50–7.27 (m, 8H), 6.14 (d, J = 3.8 Hz, 1H). 13C NMR (75 MHz, DMSO-d6) δ 164.82, 139.13, 135.94, 132.86, 129.84, 129.03, 128.94, 128.91, 127.65, 127.39, 126.49, 57.81.
2,3-Diphenyl-2,3-dihydro-4H-benzo[e][1,3]thiazin-4-one (3x). White solid, 115.6 mg, yield: 91% (known compound [42]). 1H NMR (300 MHz, CDCl3) δ 8.21 (dd, J = 7.7, 1.7 Hz, 1H), 7.43–7.21 (m, 12H), 7.15 (dd, J = 7.6, 1.4 Hz, 1H), 6.04 (s, 1H). 13C NMR (75 MHz, CDCl3) δ 163.84, 142.51, 139.46, 133.28, 132.41, 130.43, 129.52, 129.22, 128.47, 128.28, 127.69, 127.19, 126.60, 126.39, 125.91, 65.27.
3-(4-Methoxyphenyl)-2-phenyl-2,3-dihydro-4H-benzo[e][1,3]thiazin-4-one (3y). White solid, 134.8 mg, yield: 97% (known compound [21]). 1H NMR (300 MHz, CDCl3) δ 8.20 (dd, J = 7.7, 1.7 Hz, 1H), 7.40–7.37 (m, 2H), 7.32–7.21 (m, 7H), 7.14 (dd, J = 7.6, 1.4 Hz, 1H), 6.89–6.84 (m, 2H), 5.97 (s, 1H), 3.78 (s, 3H). 13C NMR (75 MHz, CDCl3) δ 164.01, 158.46, 139.49, 135.37, 133.27, 132.32, 130.39, 129.53, 128.46, 128.27, 127.65, 127.33, 126.57, 126.36, 114.45, 65.36, 55.49.
2-Phenyl-3-(p-tolyl)-2,3-dihydro-4H-benzo[e][1,3]thiazin-4-one (3z). White solid, 119.2 mg, yield: 90% (known compound [42]). 1H NMR (300 MHz, CDCl3) δ 8.19 (dd, J = 7.7, 1.7 Hz, 1H), 7.40–7.37 (m, 2H), 7.29–7.17 (m, 7H), 7.14–7.09 (m, 3H), 5.98 (s, 1H), 2.30 (s, 3H). 13C NMR (75 MHz, CDCl3) δ 163.88, 140.00, 139.59, 137.10, 133.30, 132.35, 130.41, 129.85, 129.59, 128.46, 128.26, 127.68, 126.61, 126.36, 125.80, 65.29, 21.14.
3-(4-Fluorophenyl)-2-phenyl-2,3-dihydro-4H-benzo[e][1,3]thiazin-4-one (3aa). White solid, 118.0 mg, yield: 88% (known compound [42]). 1H NMR (300 MHz, CDCl3) δ 8.20 (dd, J = 7.8, 1.7 Hz, 1H), 7.39–7.35 (m, 2H), 7.33–7.22 (m, 7H), 7.15 (dd, J = 7.6, 1.4 Hz, 1H), 7.06–7.00 (m, 2H), 5.98 (s, 1H). 13C NMR (75 MHz, CDCl3) δ 163.97, 161.27 (d, J = 247.3 Hz), 139.11, 138.33 (d, J = 3.3 Hz), 133.31, 132.52, 130.44, 129.30, 128.57, 128.44, 127.92 (d, J = 8.5 Hz), 127.70, 126.57, 126.46, 116.08 (d, J = 22.7 Hz), 65.33.
3-(4-Bromophenyl)-2-phenyl-2,3-dihydro-4H-benzo[e][1,3]thiazin-4-one (3ab). White solid, 142.7 mg, yield: 90% (known compound [42]). 1H NMR (300 MHz, CDCl3) δ 8.18 (dd, J = 7.7, 1.7 Hz, 1H), 7.46–7.42 (m, 2H), 7.39–7.12 (m, 10H), 5.99 (s, 1H). 13C NMR (75 MHz, CDCl3) δ 163.76, 141.38, 139.03, 133.27, 132.64, 132.29, 130.47, 129.20, 128.61, 128.49, 127.75, 127.59, 126.55, 126.49, 120.63, 65.12.
3-(3-Chlorophenyl)-2-phenyl-2,3-dihydro-4H-benzo[e][1,3]thiazin-4-one (3ac). White solid, 119.5 mg, yield: 85%, m.p.: 126–127 °C. 1H NMR (300 MHz, CDCl3) δ 8.19 (dd, J = 7.8, 1.6 Hz, 1H), 7.41–7.35 (m, 3H), 7.33–7.14 (m, 9H), 6.02 (s, 1H). 13C NMR (75 MHz, CDCl3) δ 163.77, 143.40, 138.94, 134.62, 133.29, 132.64, 130.49, 130.12, 129.20, 128.59, 128.47, 127.74, 127.38, 126.55, 126.49, 126.38, 124.07, 65.23. HRMS (ESI, m/z): calcd. for C20H15ClNOS [M + H]+: 352.0557, found: 352.0563.
7-Chloro-2,3-diphenyl-2,3-dihydro-4H-benzo[e][1,3]thiazin-4-one (3ad). White solid, 122.4 mg, yield: 87%, m.p.: 131–132 °C. 1H NMR (300 MHz, CDCl3) δ 8.06 (d, J = 8.4 Hz, 1H), 7.34–7.17 (m, 10H), 7.14–7.08 (m, 2H), 5.97 (s, 1H). 13C NMR (75 MHz, CDCl3) δ 163.15, 142.25, 139.05, 138.57, 135.19, 131.82, 129.29, 128.60, 128.50, 127.88, 127.35, 127.29, 126.81, 126.57, 125.78, 65.39. HRMS (ESI, m/z): calcd. for C20H15ClNOS [M + H]+: 352.0557, found: 352.0549.
6-Methyl-2,3-diphenyl-2,3-dihydro-4H-benzo[e][1,3]thiazin-4-one (3ae). White solid, 111.3 mg, yield: 84%, m.p.: 165–166 °C. 1H NMR (400 MHz, CDCl3) δ 8.01 (d, J = 2.2 Hz, 1H), 7.42–7.40 (m, 2H), 7.37–7.31 (m, 4H), 7.27–7.21 (m, 4H), 7.12 (d, J = 7.9 Hz, 1H), 7.03 (d, J = 7.9 Hz, 1H), 6.00 (s, 1H), 2.32 (s, 3H). 13C NMR (101 MHz, CDCl3) δ 164.02, 142.63, 139.62, 136.35, 133.44, 130.80, 129.66, 129.26, 129.21, 128.44, 128.20, 127.59, 127.13, 126.60, 125.88, 65.27, 21.07. HRMS (ESI, m/z): calcd. for C21H18NOS [M + H]+: 332.1104, found: 332.1105.
3-(4-Methoxyphenyl)spiro[benzo[e][1,3]thiazine-2,1′-cyclohexan]-4(3H)-one (3af). White solid, 74.7 mg, yield: 55%, m.p.: 169–170 °C. 1H NMR (300 MHz, CDCl3) δ 8.12 (dd, J = 7.8, 1.5 Hz, 1H), 7.38 (dd, J = 7.5, 1.5 Hz, 1H), 7.32–7.24 (m, 2H), 7.17–7.14 (m, 2H), 6.96–6.93 (m, 2H), 3.83 (s, 3H), 2.19–2.14 (m, 2H), 1.78–1.52 (m, 8H). 13C NMR (75 MHz, CDCl3) δ 165.04, 159.16, 134.20, 132.03, 131.46, 130.81, 130.62, 129.28, 127.55, 125.95, 114.47, 70.48, 55.47, 36.98, 25.04, 22.98. HRMS (ESI, m/z): calcd. for C20H22NO2S [M + H]+: 340.1366, found: 340.1370.
3.4. Synthetic Procedure for Gram-Scale Reaction
A 150 mL Schlenk tube was charged with 2-mercapto-N-methylbenzamide (1a, 10 mmol, 1.67 g), benzaldehyde (2a, 15 mmol, 1.59 g), HI (55 wt.% in H2O, 0.5 mmol, 67.5 μL) and commercial-grade MeCN (30.0 mL). The tube was sealed and the reaction was then stirred vigorously at 100 °C for 12 h. After cooling to room temperature, the reaction mixture was then concentrated in vacuo. The residue was purified by flash chromatography on silica gel to yield the desired product 3a (2.17 g, 85%).
3.5. Control Experiments
A 30 mL Schlenk tube was charged with 2-mercapto-N-methylbenzamide (1a, 0.4 mmol), HI (55% in water, 0.02 mmol, 2.7 μL) or HI (55 wt.% in H2O, 0.4 mmol, 54.0 μL) and commercial-grade MeCN (4.0 mL). The tube was sealed and the reaction was then stirred vigorously at 100 °C for 6.0 h. After that, the reaction mixture was concentrated in vacuo. The desired product 4a was not detected by NMR analysis.
4. Conclusions
In summary, we have developed a HI-catalyzed method for the synthesis of 2,3-dihydrobenzothiazin-4-ones from 2-mercaptobenzamides and aldehydes. A wide range of 2-substituted and N-substituted products, along with variations on the benzamide ring, have been obtained in excellent yields. The gram-scale reaction demonstrates the practical applicability and synthetic utility of this novel approach. Notably, this catalytic methodology provides a valuable and complementary strategy for the preparation of biologically active 2,3-dihydrobenzothiazin-4-one derivatives.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/catal16010068/s1, Figure S1: 2-Mercaptobenzamides; Figure S2: Aldehydes and ketones; Figures S3–S66: 1H and 13C NMR Spectra.
Author Contributions
Synthesis and characterization, Y.H. and L.P.; data curation, Y.H. and L.P.; writing—original draft preparation, B.L. and K.Y.; writing—review and editing, B.L. and K.Y.; funding acquisition, Y.H., B.L., L.P. and K.Y. All authors have read and agreed to the published version of the manuscript.
Funding
Y.H. and B.L. are grateful for financial support from the Nanjing University of Science and Technology. K.Y. and L.P. are grateful for financial support from the Changzhou University.
Data Availability Statement
Data are contained within the article and Supplementary Materials.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Berwaldt, G.A.; Gouvea, D.P.; da Silva, D.S.; das Neves, A.M.; Soares, M.S.P.; Azambuja, J.H.; Siqueira, G.M.; Spanevello, R.M.; Cunico, W. Synthesis and biological evaluation of benzothiazin-4-ones: A possible new class of acetylcholinesterase inhibitors. J. Enzyme Inhib. Med. Chem. 2019, 34, 197–203. [Google Scholar] [CrossRef]
- Shimizu, M.; Yamanaka, M.; Ando, W.; Shimada, S.; Konakahara, T.; Sakai, N. Efficient synthesis of 2-alkylidene-4H-3,1-benzoxathiin-4-ones and determination of their double bond configuration. Heterocycles 2014, 89, 981–993. [Google Scholar] [CrossRef]
- Ewies, E.F.; El-Hag, F. Synthesis, reactions, and antimicrobial evaluations of new benzo[e][1,3]thiazine derivatives. J. Heterocycl. Chem. 2020, 57, 163–172. [Google Scholar]
- Solomon, V.R.; Haq, W.; Srivastava, K.; Puri, S.K.; Katti, S.B. Synthesis and antimalarial activity of side chain modified 4-aminoquinoline derivatives. J. Med. Chem. 2007, 50, 394–398. [Google Scholar] [CrossRef]
- Wang, S.; Fang, K.; Dong, G.; Chen, S.; Liu, N.; Miao, Z.; Yao, J.; Li, J.; Zhang, W.; Sheng, C. Scaffold diversity inspired by the natural product evodiamine: Discovery of highly potent and multitargeting antitumor agents. J. Med. Chem. 2015, 58, 6678–6696. [Google Scholar] [CrossRef]
- Zarghi, A.; Zebardast, T.; Daraie, B.; Hedayati, M. Design and synthesis of new 1,3-benzthiazinan-4-one derivatives as selective cyclooxygenase (COX-2) inhibitors. Bioorg. Med. Chem. 2009, 17, 5369–5373. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Yuan, D.; Yang, K.; Li, B. Recent advances in the synthesis of benzo[d]isothiazol-3(2H)-one and benzo[e][1,3]thiazin-4-one derivatives. Molecules 2025, 30, 2099. [Google Scholar] [CrossRef]
- Nosova, E.V.; Lipunova, G.N.; Charushin, V.N.; Chupakhin, O.N. Synthesis and biological activity of 2-amino- and 2-aryl (heteryl)substituted 1,3-benzothiazin-4-ones. Mini-Rev. Med. Chem. 2019, 19, 999–1014. [Google Scholar] [CrossRef] [PubMed]
- Xiong, J.; Zhong, G.; Liu, Y. Domino reactions initiated by copper-catalyzed aryl–I bond thiolation for the switchable synthesis of 2,3-dihydrobenzothiazinones and benzoisothiazolones. Adv. Synth. Catal. 2019, 361, 550–555. [Google Scholar] [CrossRef]
- Yang, K.; Niu, B.; Ma, Z.; Wang, H.; Lawrence, B.; Ge, H. Silver-promoted site-selective intramolecular cyclization of 2-methylthiobenzamide through α-C(sp3)–H functionalization. J. Org. Chem. 2019, 84, 14045–14052. [Google Scholar] [CrossRef]
- Geng, H.J.; Gao, N.; Li, Y.; Zhang, W.; Su, X.; Guo, C. Design, synthesis and antifungal activity of 2-aryl-2,3-dihydro-4-one derivative. J. Shenyang Pharm. Univ. 2012, 29, 834–840. [Google Scholar]
- Li, M.Y.; Gu, A.; Li, J.; Liu, Y. Advanced green synthesis: Solvent-free and catalyst-free reaction. Green Synth. Catal. 2025, 6, 36–66. [Google Scholar] [CrossRef]
- Qin, G.; Wang, R.; Cheng, Z.; Zhang, Y.; Wang, B.; Xia, Y.; Jin, W.; Liu, C. Electrooxidative trifunctionalization of alkenes with N-chlorosuccinimide and ArSSAr/ArSH to α,β-dichloride arylsulfoxides. Green Synth. Catal. 2024, 5, 131–135. [Google Scholar] [CrossRef]
- Kim, Y.; Li, C.J. Perspectives on green synthesis and catalysis. Green Synth. Catal. 2020, 1, 1–11. [Google Scholar] [CrossRef]
- Cao, C.K.; Tretyakov, E.; Chen, C. Transition-metal-free trifluoromethylthiolation-acylation of arynes by insertion into the C–S bonds. Green Synth. Catal. 2021, 2, 62–65. [Google Scholar] [CrossRef]
- Wang, H.H.; Shi, T.; Gao, W.W.; Zhang, H.H.; Wang, Y.Q.; Li, J.F.; Hou, Y.S.; Chen, J.H.; Peng, X.; Wang, Z. Double 1,4-addition of (thio)salicylamides/thiosalicylic acids with propiolate derivatives: A direct, general synthesis of diverse heterocyclic scaffolds. Org. Biomol. Chem. 2017, 15, 8013–8017. [Google Scholar] [CrossRef]
- Zhang, G.; Wan, H.; Dong, N.; Zhu, A.; Zhou, Y.; Song, Q. Metal-free three-component tandem cyclization for modular synthesis of 2,3-dihydrobenzothiazin4-ones. Org. Chem. Front. 2024, 11, 2021–2026. [Google Scholar] [CrossRef]
- Zhang, B.; He, S.; Dong, N.; Zhu, A.; Duan, H.; Wang, D.; Zhou, Y. Substituent-controlled divergent cyclization reactions of benzo[c][1,2]dithiol-3-ones and hexahydro-1,3,5-triazines. Org. Chem. Front. 2024, 11, 3302–3307. [Google Scholar] [CrossRef]
- Liu, X.; Lv, W.; Dong, J.; Liu, Z.; Hu, F.; Zhou, C.; Zhou, Y. Synthesis of benzo[e][1,3]thiazin-4-ones via PPh3-promoted cyclization of benzo[c][1,2]dithiol-3-ones and amidines. Adv. Synth. Catal. 2024, 366, 1978–1982. [Google Scholar] [CrossRef]
- Dai, S.; Yang, K.; Luo, Y.; Xu, Z.; Li, Z.; Li, Z.Y.; Li, B.; Sun, X. Metal-free and Selectfluor-mediated diverse transformations of 2-alkylthiobenzamides to access 2,3-dihydrobenzothiazin-4-ones, benzoisothiazol-3-ones and 2-alkylthiobenzonitriles. Org. Chem. Front. 2022, 9, 4016–4022. [Google Scholar] [CrossRef]
- Yang, K.; Li, Q.; Luo, Y.; Yuan, D.; Qi, C.; Li, Z.; Li, B.; Sun, X. Transition-metal-free skeletal editing of benzoisothiazol-3-ones to 2,3-dihydrobenzothiazin-4-ones via single-carbon insertion. Org. Chem. Front. 2025, 12, 478–484. [Google Scholar] [CrossRef]
- Marinhoa, E.; Proença, F.M. Acid catalyzed synthesis of 2-(2-aminophenyl)quinazoline-4-amine and reaction with aromatic aldehydes. RSC Adv. 2016, 6, 6138–6143. [Google Scholar] [CrossRef]
- Kappe, C.O. Recent advances in the Biginelli dihydropyrimidine synthesis. New tricks from an old dog. Acc. Chem. Res. 2000, 33, 879–888. [Google Scholar] [CrossRef]
- Kondo, T.; Yoshimura, T.; Yuanjun, D.; Kimura, Y.; Yamada, H.; Toshimitsu, A. Simple, selective, and practical synthesis of 2-substituted 4(3H)-quinazolinones by Yb(OTf)3-catalyzed condensation of 2-aminobenzamide with carboxamides. Heterocycles 2015, 90, 857–865. [Google Scholar] [CrossRef]
- Sahiba, N.; Sethiya, A.; Soni, J.; Agarwal, D.K.; Agarwal, S. Saturated five-membered thiazolidines and their derivatives: From synthesis to biological applications. Topics Curr. Chem. 2020, 378, 34. [Google Scholar] [CrossRef]
- Moreau, R.C.; Delacoux, E. 3-Alkyl-2-aryl-4-oxodihydro-l,3-benzothiazines. Bull. Soc. Chim. Fr. 1962, 502–505. [Google Scholar]
- Yang, K.; Li, Q.; Li, Z.; Sun, X. Transition-metal-free C–S bond cleavage and transformation of organosulfur compounds. Chem. Commun. 2023, 59, 5343. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Hu, Q.; Yang, K.; Elsaid, M.; Liu, C.; Ge, H. Recent advances in direct α-C(sp3)–H bond functionalization of thioethers. Green Synth. Catal. 2022, 3, 203–211. [Google Scholar] [CrossRef]
- Feng, X.Q.; Wang, H.C.; Li, Z.; Tang, L.; Sun, X.; Yang, K. Transition-metal-catalyzed remote C−H functionalization of thioethers. RSC Adv. 2022, 12, 10835–10845. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Dai, S.; Li, Z.; Li, Y.Z.; Sun, X. Amide-assisted α-C(sp3)–H acyloxyation of organic sulfides to access α-acyloxy sulfides. Org. Chem. Front. 2021, 8, 4974–4979. [Google Scholar] [CrossRef]
- Yang, K.; Luo, Y.; Hu, Q.; Song, M.; Liu, J.; Li, Z.; Li, B.; Sun, X. Selective C(sp3)–S bond cleavage of thioethers to build up unsymmetrical disulfides. J. Org. Chem. 2023, 88, 13699–13711. [Google Scholar] [CrossRef]
- Yang, K.; Hu, Q.; Li, Q.; Liu, J.; Li, Z.Y.; Li, B.; Sun, X. Metal-free and NBS-mediated direct thiol-disulfide exchange reaction to access unsymmetrical disulfides. Eur. J. Org. Chem. 2023, 26, e202300394. [Google Scholar] [CrossRef]
- Song, M.; Hu, Q.; Li, Z.Y.; Sun, X.; Yang, K. NFSI-catalyzed S−S bond exchange reaction for the synthesis of unsymmetrical disulfides. Chin. Chem. Lett. 2022, 33, 4269–4272. [Google Scholar] [CrossRef]
- Yang, K.; Li, Y.; Song, M.; Dai, S.; Li, Z.Y.; Sun, X. Metal-free direct C(sp3)–H functionalization of 2-alkylthiobenzoic acid to access 1,3-benzooxathiin-4-one. Chin. Chem. Lett. 2021, 32, 146–149. [Google Scholar] [CrossRef]
- Nyitrai, J.; Fetter, J.; Hornyak, G.; Zauer, K.; Lempert, K.; Koczka, I.; Sagi, I.; Rethati, C. The synthesis of (3,4,5,6-tetrahydro-4-oxo-2H-1,3-thiazin-2-yl)alkanoic acids, their derivatives, and some related compounds. Tetrahedron 1978, 34, 1031–1035. [Google Scholar] [CrossRef]
- Joseph, J.T.; Elmore, J.D.; Wong, J.L. Comparative sulfhydryl reaction pathways of chlorooxirane and chloroacetaldehyde. J. Org. Chem. 1990, 55, 471–474. [Google Scholar] [CrossRef]
- Han, M.Y.; Lin, J.; Li, W.; Luan, W.Y.; Mai, P.L.; Zhang, Y. Catalyst-free nucleophilic addition reactions of silyl glyoxylates in water. Green Chem. 2018, 20, 1228–1232. [Google Scholar] [CrossRef]
- Yuan, D.; Huang, Y.; Tang, L.; Yang, K. Metal-free C(sp3)–S bond cleavage of thioethers to selectively access aryl aldehydes and dithioacetals. Chemistry 2025, 7, 89. [Google Scholar] [CrossRef]
- Wang, Z.; Kuninobu, Y.; Kanai, M. Copper-catalyzed intramolecular N–S bond formation by oxidative dehydrogenative cyclization. J. Org. Chem. 2013, 78, 7337–7342. [Google Scholar] [CrossRef]
- Unsworth, W.P.; Kitsiou, C.; Taylor, R.J.K. Direct imine acylation: Rapid access to diverse heterocyclic scaffolds. Org. Lett. 2013, 15, 258–261. [Google Scholar] [CrossRef]
- Guo, C.; Su, X.; Sun, L.; Luo, W.; Gao, N.; Zhang, X.; Yu, S.; Cong, L.; Tian, Y.; Zhang, W.; et al. Preparation of 2-aryl-2,3-Dihydro-4H-1,3-Benzothiazin-4-one Derivatives Useful as Fungicides. CN Patent 102367239, 7 March 2012. [Google Scholar]
- Azizi, N.; Farzaneh, F.; Habibnejad, N. Recyclable magnetic camphor sulfonic acid: A reliable and highly efficient ionic organocatalyst for benzothiazin-4-one synthesis in green media. Catal. Lett. 2022, 152, 3146–3157. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license.