Abstract
The aqueous enzymatic extraction (AEE) of oil from Thevetia peruviana seeds was studied. The oil extraction yields obtained by treatment with cellulase, Alcalase, and Viscozyme L were compared, finding that the treatment with Alcalase at pH 6 and 50 °C gave the highest yield (63.07%), so it was selected for the optimization of the oil extraction process through a central composite design and a response surface methodology. An oil extraction yield of 78.22% was achieved under the optimum conditions (2% of Alcalase, 4 h of incubation, 250 rpm, and a solid-to-liquid ratio of 1:4.5). The predominant fatty acids in the oil obtained by both AEE and standard solvent extraction (SE) were oleic, linoleic, stearic, and palmitic acids. However, AEE produced an oil presenting lower acidity and peroxide values and greater oxidative stability, indicating that this method produces an oil with better quality. Scanning electron microscopy images of Thevetia peruviana seeds showed a structural alteration of their cell wall caused by AEE, which allowed the release of oil from this seed in the aqueous medium. The results suggest that AEE could be an excellent alternative in terms of efficiency, oil quality, and environmental friendliness for the extraction of this oil.
1. Introduction
Thevetia peruviana is a tropical evergreen shrub of the family Apocynaceae, commonly called yellow oleander, bastard oleander, tiger apple, oleander of exile, or lucky nut (Figure 1) [1]. This plant is mainly used as an ornamental plant and is native to Central and South America, especially Mexico, Brazil, and the Antilles, but it is currently also widespread on the Asian and African continents [2,3]. T. peruviana is an extremely resistant plant, with a great capacity for adaptation, so its cultivation is not complicated [4]. It grows in full sun or light shade, preferring fertile and well-drained soil. However, this plant can also grow in dry, poor, and moderately saline soils and even tolerates drought [5,6]. The plant can produce about 400–1000 fruits in a year [7], and its fruits contain three to four seeds, which have a high oil content of 60–65% [1,8,9], with oleic, palmitic, and linoleic acids being the main ones [3].

Figure 1.
Thevetia peruviana plant.
T. peruviana is toxic in all its parts, due to the presence of cardiac glycosides (Thevetin A and Thevetin B are the two major cardiac glycosides found in the seeds) [3,8]; for this reason, the use of this plant in foods has been very limited, to the point that it currently has no significant economic value [10,11,12]. However, the seed oil of T. peruviana has emerged as a promising source of oil for the production of biodiesel [2,5,13,14], since it is a renewable resource that is easy to produce, and its use does not compete with the food industry, since it is an inedible oil [12]. Unlike conventional oil crops, such as palm and soybean, whose mass cultivation has been linked to deforestation and environmental degradation [5], the possibility of growing T. peruviana in unfertile soils and in harsh climatic conditions make its use potentially interesting. This plant can potentially help to mitigate pressure on natural ecosystems and reduces the need for using high-ecological-value agricultural lands, contributing to more sustainable biofuel production and development in unfertile regions, with positive economic, social, and environmental effects [1,12]. It has been reported that approximately 3000 saplings can be planted in one hectare, from which 50–55 tons of seeds can be collected per hectare of wasteland [15,16]. The high oil content of T. peruviana not only positions it as an efficient and cost-effective source for biofuel production but also makes it attractive for industrial applications that demand large amounts of vegetable oil, such as the production of shampoos, liquid soap, alkyd resin, surfactants, and biolubricants [2,7,17].
The optimal use of oilseed resources involves the use of suitable processes for extracting oil that are not only efficient but also environmentally friendly. Traditionally, oil extraction has been carried out using mechanical methods, such as cold pressing, or using organic solvents [18]. In the case of the pressing method, although the quality of the extracted oil is high, the yield obtained is low, and the production process requires high energy and time consumption [19]. Regarding the use of solvents to extract the oil [6], such as hexane (mainly), petroleum ether, ethyl acetate, acetone, and chloroform, this generates high oil yields, but the process has several limitations, including the use of toxic solvents, high energy consumption, a prolonged extraction time, high carbon dioxide emissions, and the possible decomposition of the oil bioactive compounds during the process [20], representing risks to the environment and human health [21]. These problems have led to the emergence of the concept of green extraction, which seeks to minimize environmental impacts by reducing energy and solvent consumption and avoiding the release of volatile organic compounds [6,22]. In this context, aqueous enzymatic extraction (AEE) has emerged as a promising alternative, as it offers advantages in terms of both efficiency and sustainability.
AEE is a technique that uses specific enzymes to break down the cellular structures of oil seeds or fruits, facilitating the release of the oil in the presence of water [23]. In this method, enzymes—such as cellulases, hemicellulases, pectinases, or proteases—can be used, depending on the composition of the cell wall of each type of oilseed [21,24], in order to break down plant cell walls and release the triglycerides stored in the seeds, improving the extraction efficiency and allowing for higher oil yields at lower temperatures and pressures compared to conventional physical methods [18]. AEE has the potential to be environmentally friendly, since it uses water as the main solvent, eliminating the need for toxic organic solvents, and can be performed under milder conditions (such as at room temperature and with a neutral pH value); this enables the preservation of the bioactive compounds that are in the oils, such as antioxidants and essential fatty acids [25,26]. Thus, this method contributes to waste minimization and the efficient use of resources.
In recent years, AEE has proven to be efficient in obtaining high-quality oil from different raw materials, such as safflower [24], sacha inchi (Plukenetia volubilis L.) [27], Ricinus communis [25], mamey sapote (Pouteria sapota) [28], and Camellia sinensis [29] seeds, macauba (Acrocomia aculeata) pulp [30], rice bran [23], and tiger nut (Cyperus esculentus L.) tuber [31], among many others. However, there are no reports on obtaining oil from T. peruviana seeds through this method, perhaps due to the lack of its use as a food oil and a lack of consideration for the other likely applications of this oil and the social and environmental advantages of this plant’s exploitation.
The objective of this work was to explore AEE as a method for obtaining T. peruviana seed oils, using Alcalase (a bacterial alkaline protease produced by Bacillus licheniformis, considered one of the best enzymes for protein hydrolysis) [32,33,34], Viscozyme L (a lignocellulose-specialized multi-enzymatic complex) [27], and cellulase [35]. A central, composite experimental design coupled with a response surface methodology was used for process optimization, with the aim of increasing the yield of the AEE, and in addition, some physicochemical properties and the fatty acid composition of the oil obtained by AEE were compared with those obtained by the conventional method (hexane extraction).
2. Results and Discussion
2.1. Solvent Extraction of Oil from T. peruviana Seed
The oil content of T. peruviana seeds determined by SE was 65.60 ± 0.60% (w/w). This value is very similar to that reported in various works, in which it is mentioned that this seed has a high oil content ranging from 60–65% [5,8,17], a value that is even higher than that found in other inedible seeds considered rich in oil and relevant to biodiesel production, such as castor (45–57%) [27,36], neem (25–45%) [37,38], or Jatropha curcas (40–60%) [39] seeds. The result obtained in this determination was considered as 100% of the oil content in the T. peruviana seeds used in this work, and from these data, the yields of the enzymatic extraction process were calculated.
2.2. AEE of Oil from T. peruviana Seeds
Table 1 presents the oil extraction yields from T. peruviana seeds using AEE, evaluating cellulase, Alcalase, and Viscozyme, as well as different pH and temperatures, with the aim of identifying the enzyme and operating conditions that generate the best extraction yields. Table 1 shows that the yields obtained using the AEE range from 48.95 ± 1.05% to 66.72 ± 0.63%. It was found that from all the studied enzymes, the treatment with carbohydrases were the ones that generated the lowest extraction yields. In a reaction time of 4 h, cellulase treatment generated yields of between 49.47 and 56.06%, while with Viscozyme L, yields of between 53.67 and 59.38% were obtained. In several studies evaluating different enzymes for oil extraction, Viscozyme L was found to generate the best oil extraction yields, attributed to the presence of several carbohydrases’ activities in this cocktail, i.e., xylanase, hemicellulase, arabanase, β-glucanase, and cellulase, which have the ability to hydrolyze the different polysaccharides present in the cell walls of the seeds, facilitating the release of the oil [27,28,40]. The treatment with Alcalase generated higher extraction yields (63.07–64.19%, 4 h, pH 6, and 50–60 °C). These values could not be increased by the sequential treatment using Viscozyme + Alcalase (63.17%) and were very close to those obtained with Alcalase + Viscozyme (66.72%) (less than 3%), in which higher yields were expected both due to the mixture of enzymes and the longer reaction time (8 h). In this sense, the results obtained in this work can be attributed to the ability of proteolytic enzymes to hydrolyze oleosins, which are proteins that surround the oil body in the oilseed, decreasing its surface activity and promoting oil release [41]. Furthermore, it is important to note that the appropriate enzyme for the aqueous extraction process depends largely on the composition of the raw material used [42,43]. Our results are similar to those reported by Latif and Anwar [44] and Polmann et al. [43] in the extraction of oil from sesame seeds and pecan nut, respectively, who found that Alcalase produced the best extraction yields when evaluating different enzymes.

Table 1.
AEE oil yield using different enzymes and operational conditions.
Although a significant statistical difference was found between the extraction yields obtained with Alcalase alone and the Alcalase + Viscozyme L mixture, in terms of efficiency, Alcalase alone was a better option, since it allowed us to obtain a similar yield to that obtained with the enzyme mixture, but in half the time; for that reason, Alcalase was selected to optimize the AEE of the T. peruviana seeds. The best yields were obtained at pH 6 and temperatures of 50 and 60 °C; however, considering that there were no significant statistical differences in the yields obtained between both temperatures and given the importance of energy savings, it was decided to use the lowest temperature (50 °C) in the optimization experiments.
Optimization of Aqueous Enzymatic Extraction
The optimization of AEE for T. peruviana seed oil was performed using a central composite design (CCD) coupled with a response surface methodology (RSM). Four variables were evaluated, each at five coded levels: the enzyme amount (%) (X1), incubation time (h) (X2), agitation rate (rpm) (X3), and solid-to-liquid ratio (w/w) (X4). A total of 28 experiments were conducted, with oil yields ranging from 59.77% (run 6) to 78.76% (run 8). A statistical analysis via an ANOVA revealed the significant effects of the variables on the oil yield, and the derived second-order polynomial model demonstrated strong predictive accuracy. The second-order polynomial equation fitted to the experimental data is presented in Equation (1):
The ANOVA confirmed the significance of the model (F-value = 4.283859, p = 0.000133). The high correlation coefficient (R = 0.7706) further validated the agreement between the predicted and the experimental values, which can be also observed by the predicted values presented in Table 2 and by the residual plot in Figure 2.

Table 2.
Composite central design experiment matrix with the yields of the AEE of Thevetia peruviana seed oil.
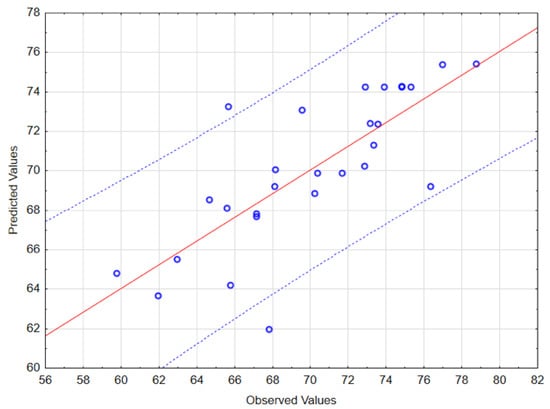
Figure 2.
Predicted vs. observed values with 95% confidence bands. The solid red line represents the ideal agreement (1:1 line), while the dotted blue lines indicate the 95% confidence interval for the predictions.
Table 3 shows the effects of different variables. Among the evaluated variables, the agitation rate (X3) exhibited the most positive linear effect (2.82, p = 0.001), which means that increasing agitation from 150 to 250 rpm enhanced the oil yield significantly. The incubation time (X2) also showed a positive linear effect (1.08, p = 0.016) indicating that longer incubation periods initially enhance the oil yield by allowing more time for the enzymatic hydrolysis of the seed cell walls. The solid-to-liquid ratio (X4) exhibited an insignificant linear effect (p = 0.21), but its quadratic term (−2.88, p = 0.0072) indicated the presence of an optimal midpoint. An excess of amount of water diluted the enzyme, whereas insufficient water increased the medium’s viscosity, hindering the suspension’s homogenization [45]. The enzyme amount (X1) had a positive linear effect (0.80, p = 0.036), but its interaction with the agitation rate (X1X3) had a negative effect (−1.29, p = 0.017), suggesting that higher enzyme amounts may require moderate agitation to achieve optimal oil extraction yields, maybe due to enzyme aggregation caused by the stirring, which can reduce the activity of the enzyme, mainly versus solid substrates, such as seeds [27]. Conversely, the incubation time–agitation rate interaction (X2X3) exhibited a positive effect (1.01, p = 0.033), suggesting that prolonged incubation combined with vigorous agitation enhances cell wall disruption.

Table 3.
Statistical analysis of central composite design.
The optimal conditions were determined using the response desirability profile. The identified optimal conditions were as follows: 2% enzyme amount, 4 h incubation time, 250 rpm agitation, and a 1:4.5 solid-to-liquid ratio. Under these conditions, the oil yield predicted by the model was 78.55%, which was experimentally validated as 78.22 ± 0.75%, confirming its reliability. The contour plots presented in Figure 3 show the relationships between the variables. The plots were generated using the variables that showed statistically significant linear effects (X1, X2, and X3). In all the graphs, the remaining variables are fixed at their optimal levels. Figure 3a,b demonstrate that the effects of the incubation time and the agitation rate were more pronounced than that of the amount of enzyme. In both cases, higher levels of these variables were required to achieve the maximum oil yields. This observation is further supported by Figure 3c, where maximum yields occurred at the +2 level for both variables, as indicated by the response desirability profile. Considering the good yields already obtained and the fact that prolonging the incubation time and increasing the agitation rate would have had a negative economic effect on the process, we decided not re-optimize the process to find the actual optimal values of both of these variables.
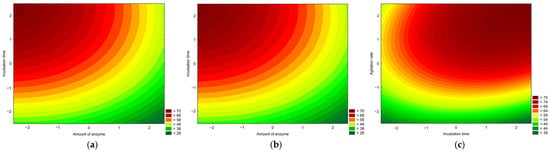
Figure 3.
Contour plots of oil yields for aqueous enzymatic extraction. (a) Amount of enzyme vs. incubation time. (b) Amount of enzyme vs. agitation rate. (c) Incubation time vs. agitation rate. In each figure, the missing variable was fixed at the optimal point.
2.3. Analysis of the Oil Obtained from T. peruviana Seed
2.3.1. Determination of Physicochemical Properties of T. peruviana Seed Oil Obtained Using AEE or SE
Some of the physicochemical properties of the T. peruviana seed oils that were obtained by AEE or SE were analyzed (Table 4). Regarding the acid value, it was found that the T. peruviana seed oil extracted using SE presented a much higher acid value (5.77 mg KOH/g oil) than that presented in the oil obtained by AEE (2.02 mg KOH/g oil). This may be due to the fact that in the SE process, the oil was exposed to a high temperature for a longer time compared to the AEE process. The high temperature during the extraction process can promote the decomposition of triacylglycerols, releasing some free fatty acids, generating higher acidity values, and leading to the spoiling of the oil [46]. Thus, it is possible to assume that AEE generated a better-quality oil than that obtained by the conventional method. In other studies, where the aqueous enzyme extraction of pumpkin [47] and melon [48] seed oils was evaluated, it was also found that the acidity value of the oil obtained by AEE was lower than that of the oil obtained by SE.

Table 4.
Physicochemical properties and antioxidant activity of Thevetia peruviana seed oils obtained by solvent extraction (SE) and aqueous enzymatic extraction (AEE).
The peroxide value is another property related to oil quality, since it represents the degree of oxidation of the oxygen-labile groups in the oil [23,49]. In this work, it was found that the peroxide value of the T. peruviana seed oil obtained by SE (150 ± 0.02 meq. O2 per kg) was much higher than that of the oil extracted by AEE (40 ± 0.01 meq. O2 per kg), which indicates that the first one had a higher degree of oxidation. Various authors explain that this result may be due to the long duration of the SE process, which includes the extraction of the oil and the evaporation of the solvent [47,50]. It has been reported that oils with high peroxide values are unstable and easily become rancid (develop an unpleasant odor); in this sense, our results suggest that T. peruviana seed oil obtained by AEE could be stored with less deterioration than the oil extracted using SE [51]. In fact, the oxidative stability of the T. peruviana seed oil obtained by AEE (11.83 ± 0.05 h) was much higher than the oil obtained by SE (4.91 ± 0.03 h). The higher oxidative stability of the oil obtained by AEE compared to that of the oil obtained by SE is probably due to the milder reaction conditions of AEE (temperature and time) [52], in comparison to SE, which reduce the oil-spoiling. Prolonged exposure to high temperatures, such as those used in SE, can deteriorate some thermosensitive antioxidant compounds present in oils, thus affecting the final quality of the oil [53,54]. Although, as will be seen later, the difference in the PUFA content between the oil obtained by AEE and SE is small and does not fully explain why the AEE oil has such a superior oxidative stability to the SE oil, there are other thermosensitive compounds such as tocopherols, polyphenols, and carotenoids, which protect the oil against oxidation, whose concentrations could have been benefited by the moderate reaction conditions of the AEE process; in addition to that, the enzymatic hydrolysis of the seed cell wall during oil extraction can facilitate a greater release of these bioactive compounds, which together resulted in the better oxidative stability of the oil obtained by AEE [45,55,56,57]. A similarly high quality of oil extracted using AEE has been obtained in the enzymatic aqueous extraction of oil from castor [58], mamey [28], pumpkin [52], and melon [53] seeds.
Regarding the iodine value, which is used to determine the unsaturation of the fatty acids contained in oils and to evaluate the stability of the oil in industrial applications [59,60], there was no significant statistically difference between the result obtained for the oil obtained by SE (39.17 ± 0.03 g I2/100 g) and that obtained by AEE (39.97 ± 0.02 g I2/100 g). On the other hand, the saponification value of the T. peruviana oil obtained by AEE (168.33 ± 0.07 mg KOH/g oil) was slightly higher than that obtained by SE (159.92 ± 0.2 mg KOH/g oil). These values are within the range reported for the saponification value of Thevetia peruviana seed oil (137.12–218.79 mg KOH/g oil) [6,7,61].
2.3.2. Fatty Acid Composition
The fatty acid composition of the T. peruviana oil obtained by AEE and SE is presented in Table 5. Ten fatty acids were found, distributed in saturated fatty acids (SFAs), monounsaturated fatty acids (MUFAs), and polyunsaturated fatty acids (PUFAs). Regarding SFAs, myristic (0.12%), palmitic (18.93–19.33%), margaric (0.12%), stearic (8.66–9.97%), arachidic (1.61–1.66%), and lignoceric (0.27%) acids were found. Among the MUFAs, palmitoleic (0.19–0.20%) and cis-eicosenoic (0.18–0.19%) acids were detected, and among the PUFAs, oleic (49.12–51.36%) and linoleic (18.09–20.41%) acids were found. For both methods (AEE and SE), the fatty acid composition was similar, with oleic, linoleic, palmitic, and stearic acids being the predominant fatty acids, indicating that AEE did not affect the lipid profile of the obtained oil (showing that there is no preferential extraction of some specific triglycerides using AEE). Furthermore, it was observed that, with respect to the SFA content, the oils obtained by AEE and SE had very close values, but with respect to unsaturated fatty acids, the oil obtained by SE (51.74%) had a higher MUFA content than the AEE (49.50%) oil, and this oil had a higher PUFA content (20.41%) than the SE (18.09%) oil, most likely due to the different operating conditions used in the extraction methods studied [60,62,63]. This may be attributed to the relatively high temperature used in SE, which can lead to the oxidation and decomposition of PUFAs; whereas the mild extraction conditions of AEE better preserved the PUFAs present in T. peruviana oil [26,64].

Table 5.
Process variables and their levels used in central composite design.
These results are consistent with the results from other works, in which the oil of this seed has been studied and the same predominant fatty acids have been found (oleic 43.72–46.94%; linoleic 15.89–31.6%; palmitic 15.45–23.28%; and stearic 6.06–10.71%) [2,3,5,6]. The difference in the reported percentages may be due to the geographical origin, watering and grown conditions, recollection season, and the genetic diversity of the seeds [25,49,60,65].
2.3.3. SEM Observation of T. peruviana Seeds
The cell wall microstructure of T. peruviana seeds before and after being subjected to extraction processes (SE and AEE) was analyzed through SEM, with the aim of understanding the extraction mechanisms of the studied methods. As can be observed in Figure 4a, the internal tissues of the untreated seed appear relatively intact, smooth, and oily. On the other hand, in Figure 4b (seeds submitted to SE), an evident change in the structure of the seed is observed, showing it to be completely porous, derived from the entry of hexane and its subsequent exit, together with the oil dissolved in it [31,66]. Finally, in Figure 4c (the seeds submitted to AEE), a change in the structure of the seed is also observed, although in a different way to that found in the SE process. In this case, the destruction of the cell wall of the seed is observed, due to the hydrolysis of the proteins present in the tissues as a result of the proteolytic activity of Alcalase [34,67,68], which facilitated the exit of the oil towards the aqueous phase. Our results are consistent with different previous studies, in which, regardless of the enzyme used, it has been widely reported that the mechanism of the enzymatic aqueous extraction of oils mainly involves the breakdown of one or more of the seed cell wall components, which promotes the release of the oil [25,69,70].
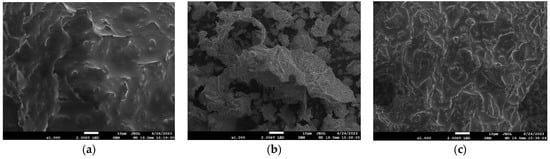
Figure 4.
The morphological alterations of the cell surface of the Thevetia peruviana seed samples, (a) without extraction, (b) with solvent extraction, and (c) with aqueous enzymatic extraction, obtained by scanning electron microscopy (SEM).
3. Materials and Methods
3.1. Materials
T. peruviana fruits (Figure 5) were collected in the city of Tuxtla Gutiérrez, Chiapas, Mexico, during May and Jun 2022. Ripe fruits were pulped, seeds were dried in sunlight, shelled, and the obtained kernels were dried in an oven (NOVATECH, HS35-ED, Avante Tecnología S.A., Mexico City, Mexico) at 70 °C for 24 h; the dried material was ground in a blender (Moulinex type 643, Groupe SEB, Selongey, France), and finally, the pulverized samples were stored in amber flasks at 4 °C until further processing. A fatty acid methyl ester mixture (FAME MIX) standard was from Supelco® (Bellefonte, PA, USA), while Viscozyme L, Alcalase, cellulase, and a Wijs reagent were obtained from Sigma Aldrich (St. Louis, MA, USA). Potassium hydroxide, hydrochloric acid, glacial acetic acid, potassium iodide, chloroform, sodium thiosulfate, hexane, ethanol, and other reagents were supplied from J.K. Baker (Center Valley, PA, USA) and were of HPLC or analytical grades.

Figure 5.
Fruit (a), seed (b), and kernels (c) of Thevetia peruviana plant.
3.2. Solvent Extraction of Oil from T. peruviana Seeds
Solvent extraction (SE) was carried out according to the Association of Official Analytical Chemists (AOAC) guidelines. First, 5 g of ground seed and 200 mL of n-hexane were placed in a Soxhlet extraction apparatus; the process was carried out at a temperature of 95 °C for 8 h. At the end of the extraction, the hexane was removed using a rotary evaporator at a reduced pressure at 50 °C. The oil obtained was dried in a drying oven at 70 °C for 24 h, and finally, the recovered oil was stored in amber flasks at 4 °C until use. The oil yield obtained by SE was considered to be 100%, for comparison with the yields obtained using AEE [71].
3.3. AEE of Oil from T. peruviana Seeds
AEE extraction was carried out according to the process reported by Díaz-Suárez et al. in the extraction of oil from seeds of Ricinus communis [27]. Briefly, 2 g of ground and dried T. peruviana seeds and 10 mL of distilled water were placed in 50 mL Erlenmeyer flasks with screw caps. The mixture was boiled for 10 min and allowed to cool to 25 °C [58]; then, the pH was adjusted to that recommended for each enzyme (Table 1), using aqueous solutions of 0.5 N HCl or NaOH. Once this was done, a mass of 2.0% (with respect to the weight of the seed) of each of the three enzymatic preparations evaluated in this work (Viscozyme L, Alcalase, or cellulase) was added; and the flasks containing the reaction mixtures were placed in an incubator at 200 rpm for 4 h. In addition, experiments were performed using the enzyme preparations Viscozyme L and Alcalase sequentially; in this case, the first enzyme (1%) was added, left to act for 4 h, and then boiled for 1 min to inactivate the enzyme, and then the second enzyme (1%) was added, leaving it to act for 4 h. During both phases of these experiments, the pH was adjusted as previously mentioned. The pH and temperatures used in these experiments were established according to what was recommended by the supplier or reported in other works [27,28,72,73,74] (Table 1). After enzymatic hydrolysis, the reaction suspension was boiled for one minute to inactivate the enzymes and then centrifuged (LW Scientific, Inc., LWS-Combo-T, Lawrenceville, GA, USA) at 4500 rpm for 30 min. The oily phase was withdrawn using a pipette and then centrifuged again (EPPENDORF, 5415c, Hamburg, Germany) at 10,000 rpm for 30 min to remove water and seed residues. The extracted oil was dried in an oven at 70 °C for 24 h and was finally weighed on an analytical balance. The oil extraction yield was calculated using Equation (2). Control seed samples were submitted to the same protocol but in the absence of enzymes.
3.4. The Optimization of the AEE of Oil from T. peruviana Seeds
The optimization of the AEE of oil from T. peruviana seeds was carried out using the enzyme that generated the best yields in the previous experiments (Section 3.3). A central composite design was used to determine the optimal conditions for the AEE, evaluating four variables, each with five levels. The maximal values of each variable were decided, considering that a further increase in the values could be economically unsatisfactory (e.g., too-long reaction times or a too-high amount of enzymes). Their coded and uncoded values are presented in Table 5.
The matrix reporting the 28 experiments performed is presented in Table 3. In each case, the yields of the oil extraction were quantified. The second-order polynomial equation, representing a quadratic regression model, used in the response surface methodology (RSM), is shown in Equation (3):
where Xi and Xj are the coded level of variables xi and xj; βi, βii, and βij are the coefficients for the linear, quadratic, and interaction effects, respectively; β0 is the constant variable; and Y is the response variable. This quadratic equation was utilized to draw the response surfaces for all the studied variables.
3.5. The Determination of Some of the Physicochemical Characteristics of the Extracted Oils
The T. peruviana oils obtained by SE or AEE were analyzed in terms of their acidity, saponification, peroxide, and iodine values, following the procedures recommended by the American Oil Chemists’ Society (AOCS) methods (AOCS, 2004). In addition, the oxidative stability of the oils was determined using a Rancimat apparatus (Metrohm CH series 679, Herisau, Switzerland) [27]. Each experiment was performed in triplicate, and the results are shown as the mean values ± SD.
The fatty acid composition of the obtained oils was determined by gas chromatography in an Agilent 7890A GC system (Agilent, Santa Clara, CA, USA) equipped with a flame ionization detector, split/splitless injector, and an autosampler, using a HP-88 column, 100 m × 0.250 mm × 0.20 μm (Agilent), as reported by Topete-Betancourt et al. [73]. Fatty acid methyl esters (FAMEs) were prepared by the transesterification of each oil sample with a methanolic solution of potassium hydroxide, and then 1 µL of each FAME sample was injected, with a split ratio of 10:1. The temperatures of the injector port and the detector were 260 °C and 280 °C, respectively. Helium was used as the mobile phase at a constant flow rate of 1 mL/min. The initial oven temperature was 100 °C and was kept constant for 4 min. The temperature was then increased to 150 °C (at a rate of 3 °C/min) and finally to 240 °C (at a rate of 4 °C/min), holding for 15 min. The identification of FAMEs in T. peruviana seed oil samples was carried out by comparing their chromatographic behavior with that of a standard, commercial FAME mixture (Supelco® 37-component FAME mixture) [75]. Each experiment was carried out in triplicate, and the results are given as mean values ± SD.
3.6. Scanning Electron Micrographs (SEM) of T. peruviana Seeds
The morphological changes in the T. peruviana seed samples after being subjected to AEE and SE, as well as the untreated seeds, were observed by analyzing the images obtained by scanning electron micrography (SEM) using a JEOL field emission scanning electron microscope system model JSM7100F (JEOL Company, Tokyo, Japan). Prior to the SEM analysis, the dried samples were pasted onto a double-sided conductive carbon adhesive tape and covered with gold under a strong vacuum [27,28].
3.7. Statistical Analysis
Statistica 13.0 (TIBCO Software Inc., Palo Alto, CA, USA) was used for the experimental design and data analysis. The model’s significance was assessed using an analysis of variance (ANOVA), while a Student’s t-test determined the significance of the regression coefficients and their corresponding probabilities (T). A Fisher’s F-test was applied to evaluate the validity of the second-order model equation. The coefficient of determination (R2) quantified the proportion of variance explained by the model. Quadratic models for each variable were represented through 2D contour plots.
4. Conclusions
In this study, the AEE extraction process was evaluated for obtaining oil from T. peruviana seeds and compared with the SE process. Among the enzymes evaluated, Alcalase was the most efficient for oil extraction, with an oil yield of 78.22% under optimal reaction conditions (2% Alcalase, 4 h of incubation, 250 rpm, and a solid-to-liquid ratio of 1:4.5). Although the yield obtained with AEE was lower than that obtained by SE, the AEE oil presented a much higher quality (lower acidity and peroxide values and a higher oxidative stability), indicating that this extraction method produces a better-quality oil than SE. Also, a higher percentage of PUFAS could be obtained using AEE. Our results suggest that AEE is an excellent alternative in terms of efficiency, oil quality, and environmental friendliness for the extraction of oil from T. peruviana seeds.
Despite the promising results, this study presents some limitations that should be addressed in future research. While the aqueous enzymatic extraction (AEE) process was optimized under laboratory conditions, its scalability for industrial applications remains to be evaluated. The oil yield obtained was lower compared to the conventional solvent extraction method, which may affect its commercial viability. In this regard, studies are needed to find alternatives to improve AEE extraction yields, for example, the use of emerging technologies that could enhance cell wall disruption, such as ultrasound, microwaves, or high-pressure processing, which could improve oil recovery when combined with enzymatic methods. Further research is also needed to evaluate enzymatically extracted oil in biodiesel production, including its conversion efficiency and fuel properties. A technoeconomic evaluation of the process is also needed, as well as studies on the stability of the oil and its bioactive compounds.
Author Contributions
Conceptualization, V.T.-P., R.F.-L. and R.C.R.; methodology, L.D.-P., J.J.V.-O. and D.C.-V.; software, R.C.R. and V.Z.; validation, R.F.-L., V.T.-P. and R.C.R.; formal analysis, L.D.-P. and D.C.-V.; investigation, A.R.-Q., J.A.S.-G., D.C.-V. and L.D.-P.; data curation, S.G.-R., J.J.V.-O., L.D.-P. and D.C.-V.; writing—original draft preparation, L.D.-P., V.T.-P., R.F.-L. and D.C.-V.; writing—review and editing, L.D.-P., V.T.-P., R.F.-L. and R.C.R.; visualization, V.T.-P., R.F.-L. and R.C.R.; supervision, V.T.-P.; funding acquisition, V.T.-P., D.C.-V. and R.F.-L. All authors have read and agreed to the published version of the manuscript.
Funding
Tacias-Pascacio and Castañeda-Valbuena give thanks for the financial support from “Instituto de Ciencia, Tecnología e Innovación” from the government of Chiapas, Mexico. Fernandez-Lafuente wants to thank the support from the Ministerio de Ciencia e Innovación and the Agencia Estatal de Investigación (Spanish Government) (PID2022-136535OB-I00). The authors gratefully recognize the Universidad Politécnica de Chiapas for the technical support in the scanning electron micrograph analysis. The help and suggestions from Ángel Berenguer (Departamento de Química Inorgánica, Universidad de Alicante) during the writing of this paper and the support of the engineers Ilse Vázquez-Camas and Dilan Hernández-Miguel (Tecnológico Nacional de México/Instituto Tecnológico de Tuxtla Gutiérrez) in the experimental part of the project are gratefully recognized.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author(s).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Sanjay Basumatary, S.B. Yellow Oleander (Thevetia peruviana) Seed Oil Biodiesel as an Alternative and Renewable Fuel for Diesel Engines: A Review. Int. J. Chemtech Res. 2015, 7, 2823–2840. [Google Scholar]
- Choudhury, N.D.; Saha, N. A Preliminary Investigation of Physicochemical, Rheological and Tribological Properties of Bio-Lubricant from Thevetia peruviana Oil. Tribol. Ind. 2022, 44, 641. [Google Scholar] [CrossRef]
- Kannan, T.K.; Mohan, S.K. Thevetia peruviana—A Potential Non-Edible Plant Source for Biodiesel Production. Int. J. Ind. Eng. 2017, 1, 1–5. [Google Scholar]
- Harari, P.A.; Banapurmath, N.R.; Yaliwal, V.S.; Khan, T.M.Y.; Soudagar, M.E.M.; Sajjan, A.M. Experimental Studies on Performance and Emission Characteristics of Reactivity Controlled Compression Ignition (RCCI) Engine Operated with Gasoline and Thevetia peruviana Biodiesel. Renew. Energy 2020, 160, 865–875. [Google Scholar] [CrossRef]
- Deka, D.C.; Basumatary, S. High Quality Biodiesel from Yellow Oleander (Thevetia peruviana) Seed Oil. Biomass Bioenergy 2011, 35, 1797–1803. [Google Scholar] [CrossRef]
- Olosho, A.I.; Nair, S.K.; Ambade, A.V.; Adekola, F.A. Eco-Friendly Extraction of Thevetia peruviana Oil: A Synergistic Approach of d-Limonene and Ultrasound Activation. ACS Agric. Sci. Technol. 2023, 3, 1014–1024. [Google Scholar] [CrossRef]
- Kelechi, I.K.; Dodo, S.I.; Bello, A.M.; Hassan, U. Production and Characterization of New Surfactant Formulated from Thevetia peruviana Seed Oil for Use in Enhanced Oil Recovery. Pet. Sci. Eng. 2024, 8, 1–6. [Google Scholar] [CrossRef]
- Oluwaniyi, O.O.; Ibiyemi, S.A. Extractability of Thevetia peruviana Glycosides with Alcohol Mixture. Afr. J. Biotechnol. 2007, 6, 2166–2170. [Google Scholar]
- Yarkasuwa, C.I.; Wilson, D.; Michael, E. Production of Biodiesel from Yellow Oleander (Thevetia peruviana) Oil and Its Biodegradability. J. Korean Chem. Soc. 2013, 57, 377–381. [Google Scholar] [CrossRef]
- Adebowale, K.O.; Adewuyi, A.; Ajulo, K.D. Examination of Fuel Properties of the Methyl Esters of Thevetia peruviana Seed Oil. Int. J. Green. Energy 2012, 9, 297–307. [Google Scholar] [CrossRef]
- Aboyeji, C.M.; Babalola, F.D. Effect of Inorganic Fertilizer Application on Growth and Yield of Thevetia peruviana (Pers) Schum.(Yellow Oleander) in the Southern Guinea Savannah of Nigeria. Agrosearch 2013, 13, 32–41. [Google Scholar] [CrossRef]
- Olosho, A.I.; Alam, M.S.; Sukumaran Nair, K.; Ambade, A.V.; Adekola, F.A. Nonedible Thevetia peruviana Oil for the Synthesis of Biobased Thermosets and Vitrimers with Tunable Mechanical Properties. ACS Appl. Polym. Mater. 2024, 6, 2695–2708. [Google Scholar] [CrossRef]
- Yadav, A.K.; Khan, M.E.; Pal, A. Biodiesel Production from Oleander (Thevetia peruviana) Oil and Its Performance Testing on a Diesel Engine. Korean J. Chem. Eng. 2017, 34, 340–345. [Google Scholar] [CrossRef]
- Arun, S.B.; Suresh, R.; Yatish, K.V. Study of Performance, Combustion and Emission Characteristics of Heterogeneous Catalyzed Yellow Oleander Biodiesel on Compression Ignition (CI) Engine. Biofuels 2020, 11, 793–800. [Google Scholar] [CrossRef]
- Sut, D.; Chutia, R.S.; Bordoloi, N.; Narzari, R.; Kataki, R. Complete Utilization of Non-Edible Oil Seeds of Cascabela thevetia through a Cascade of Approaches for Biofuel and by-Products. Bioresour. Technol. 2016, 213, 111–120. [Google Scholar] [CrossRef]
- Balusamy, T.; Marappan, R. Performance Evaluation of Direct Injection Diesel Engine with Blends of Thevetia peruviana Seed Oil and Diesel. J. Sci. Ind. Res. 2007, 66, 1035–1040. [Google Scholar]
- Usman, L.A.; Oluwaniyi, O.O.; Ibiyemi, S.A.; Muhammad, N.O.; Ameen, O.M. The Potential of Oleander (Thevetia peruviana) in African Agricultural and Industrial Development: A Case Study of Nigeria. J. Appl. Biosci. 2009, 24, 1477–1487. [Google Scholar]
- Gao, Y.; Ding, Z.; Liu, Y.; Xu, Y.-J. Aqueous Enzymatic Extraction: A Green, Environmentally Friendly and Sustainable Oil Extraction Technology. Trends Food Sci. Technol. 2024, 144, 104315. [Google Scholar] [CrossRef]
- Yang, J.; Wen, C.; Duan, Y.; Deng, Q.; Peng, D.; Zhang, H.; Ma, H. The Composition, Extraction, Analysis, Bioactivities, Bioavailability and Applications in Food System of Flaxseed (Linum usitatissimum L.) Oil: A Review. Trends Food Sci. Technol. 2021, 118, 252–260. [Google Scholar] [CrossRef]
- Hu, B.; Xi, X.; Li, H.; Qin, Y.; Li, C.; Zhang, Z.; Liu, Y.; Zhang, Q.; Liu, A.; Liu, S.; et al. A Comparison of Extraction Yield, Quality and Thermal Properties from Sapindus mukorossi Seed Oil between Microwave Assisted Extraction and Soxhlet Extraction. Ind. Crops Prod. 2021, 161, 113185. [Google Scholar] [CrossRef]
- Dunford, N.T. Enzyme-Aided Oil and Oilseed Processing: Opportunities and Challenges. Curr. Opin. Food Sci. 2022, 48, 100943. [Google Scholar] [CrossRef]
- Chemat, F.; Abert Vian, M.; Ravi, H.K.; Khadhraoui, B.; Hilali, S.; Perino, S.; Fabiano Tixier, A.-S. Review of Alternative Solvents for Green Extraction of Food and Natural Products: Panorama, Principles, Applications and Prospects. Molecules 2019, 24, 3007. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Hao, J.; Wang, Z.; Liang, D.; Wang, J.; Ma, Y.; Zhang, M. Physicochemical Properties, Fatty Acid Compositions, Bioactive Compounds, Antioxidant Activity and Thermal Behavior of Rice Bran Oil Obtained with Aqueous Enzymatic Extraction. LWT 2021, 149, 111817. [Google Scholar] [CrossRef]
- Benkirane, C.; Ben Moumen, A.; Allay, A.; Rbah, Y.; Barkaoui, M.; Serghini Caid, H.; Elamrani, A.; Mansouri, F. Investigating the Potential of Aqueous Enzymatic Extraction of Safflower (Carthamus tinctorius L.) Seed Oil: Process Optimization and Oil Characterization. Biocatal. Agric. Biotechnol. 2024, 61, 103354. [Google Scholar] [CrossRef]
- Díaz-Suárez, P.; Rosales-Quintero, A.; Fernandez-Lafuente, R.; Pola-Sánchez, E.; Hernández-Cruz, M.C.; Ovando-Chacón, S.L.; Rodrigues, R.C.; Tacias-Pascacio, V.G. Aqueous Enzymatic Extraction of Ricinus communis Seeds Oil Using Viscozyme L. Ind. Crops Prod. 2021, 170, 113811. [Google Scholar] [CrossRef]
- Wang, D.; Yuan, Y.; Xie, T.; Tang, G.; Song, G.; Li, L.; Yuan, T.; Zheng, F.; Gong, J. Ultrasound-Assisted Aqueous Enzymatic Extraction of Gardenia Fruits (Gardenia jasminoides Ellis) Oil: Optimization and Quality Evaluation. Ind. Crops Prod. 2023, 191, 116021. [Google Scholar] [CrossRef]
- Nguyen, H.C.; Vuong, D.P.; Nguyen, N.T.T.; Nguyen, N.P.; Su, C.-H.; Wang, F.-M.; Juan, H.-Y. Aqueous Enzymatic Extraction of Polyunsaturated Fatty Acid–Rich Sacha Inchi (Plukenetia volubilis L.) Seed Oil: An Eco-Friendly Approach. LWT 2020, 133, 109992. [Google Scholar] [CrossRef]
- Tacias-Pascacio, V.G.; Rosales-Quintero, A.; Rodrigues, R.C.; Castañeda-Valbuena, D.; Díaz-Suarez, P.F.; Torrestiana-Sánchez, B.; Jiménez-Gómez, E.F.; Fernandez-Lafuente, R. Aqueous Extraction of Seed Oil from Mamey Sapote (Pouteria sapota) after Viscozyme l Treatment. Catalysts 2021, 11, 748. [Google Scholar] [CrossRef]
- Peng, L.; Ye, Q.; Liu, X.; Liu, S.; Meng, X. Optimization of Aqueous Enzymatic Method for Camellia sinensis Oil Extraction and Reuse of Enzymes in the Process. J. Biosci. Bioeng. 2019, 128, 716–722. [Google Scholar] [CrossRef]
- Sorita, G.D.; Favaro, S.P.; de Sousa Rodrigues, D.; da Silva Junior, W.P.; de Oliveira Leal, W.G.; Ambrosi, A.; Di Luccio, M. Aqueous Enzymatic Extraction of Macauba (Acrocomia aculeata) Pulp Oil: A Green and Sustainable Approach for High-Quality Oil Production. Food Res. Int. 2024, 182, 114160. [Google Scholar] [CrossRef]
- Hu, B.; Li, Y.; Song, J.; Li, H.; Zhou, Q.; Li, C.; Zhang, Z.; Liu, Y.; Liu, A.; Zhang, Q.; et al. Oil Extraction from Tiger Nut (Cyperus esculentus L.) Using the Combination of Microwave-Ultrasonic Assisted Aqueous Enzymatic Method—Design, Optimization and Quality Evaluation. J. Chromatogr. A 2020, 1627, 461380. [Google Scholar] [CrossRef] [PubMed]
- Memon, A.H.; Ding, R.; Yuan, Q.; Wei, Y.; Liang, H. Facile Synthesis of Alcalase-Inorganic Hybrid Nanoflowers Used for Soy Protein Isolate Hydrolysis to Improve Its Functional Properties. Food Chem. 2019, 289, 568–574. [Google Scholar] [CrossRef] [PubMed]
- Tacias-Pascacio, V.G.; Morellon-Sterling, R.; Siar, E.H.; Tavano, O.; Berenguer-Murcia, Á.; Fernandez-Lafuente, R. Use of Alcalase in the Production of Bioactive Peptides: A Review. Int. J. Biol. Macromol. 2020, 165, 2143–2196. [Google Scholar] [CrossRef]
- de Aquino, D.S.; Roders, C.; Vessoni, A.M.; Stevanato, N.; da Silva, C. Assessment of Obtaining Sunflower Oil from Enzymatic Aqueous Extraction Using Protease Enzymes. Grasas y Aceites 2022, 73, e452. [Google Scholar] [CrossRef]
- Valsalan, A.; Sivaranjana, P.; Rajini, N.; Arumugaprabu, V. Chapter 3—Enzymes for the Recovery of Oil from Edible Seeds. In Enzymes in Oil Processing; Bhawani, S.A., Khan, A., Awang Husaini, A.A.S., Asaruddin, M.R., Eds.; Elsevier: Amsterdam, The Netherlands, 2024; pp. 33–53, ISBN 978-0-323-91154-2. [Google Scholar]
- Jain, A.; Vora, K. Quality Biodiesel Production and Engine Performance & Emission Evaluation Using Blends of Castor Biodiesel; Technical Paper; SAE: London, UK, 2021. [Google Scholar]
- Devarajan, Y.; Munuswamy, D.B.; Subbiah, G.; Vellaiyan, S.; Nagappan, B.; Varuvel, E.G.; Thangaraja, J. Inedible Oil Feedstocks for Biodiesel Production: A Review of Production Technologies and Physicochemical Properties. Sustain. Chem. Pharm. 2022, 30, 100840. [Google Scholar] [CrossRef]
- Rangabashiam, D.; Munuswamy, D.B.; Duraiswamy Balasubramanian, S.; Christopher, D. Performance, Emission, and Combustion Analysis on Diesel Engine Fueled with Blends of Neem Biodiesel/Diesel/ Additives. Energy Sources Part A Recovery Util. Environ. Eff. 2024, 46, 8059–8069. [Google Scholar] [CrossRef]
- Kavitha, K.R.; Beemkumar, N.; Rajasekar, R. Experimental Investigation of Diesel Engine Performance Fuelled with the Blends of Jatropha Curcas, Ethanol, and Diesel. Environ. Sci. Pollut. Res. 2019, 26, 8633–8639. [Google Scholar] [CrossRef]
- Latif, S.; Anwar, F. Effect of Aqueous Enzymatic Processes on Sunflower Oil Quality. J. Am. Oil Chem. Soc. 2009, 86, 393–400. [Google Scholar] [CrossRef]
- de Moura, J.M.L.N.; Campbell, K.; Mahfuz, A.; Jung, S.; Glatz, C.E.; Johnson, L. Enzyme-Assisted Aqueous Extraction of Oil and Protein from Soybeans and Cream De-Emulsification. J. Am. Oil Chem. Soc. 2008, 85, 985–995. [Google Scholar] [CrossRef]
- Liu, Z.; Gui, M.; Xu, T.; Zhang, L.; Kong, L.; Qin, L.; Zou, Z. Efficient Aqueous Enzymatic-Ultrasonication Extraction of Oil from Sapindus mukorossi Seed Kernels. Ind. Crops Prod. 2019, 134, 124–133. [Google Scholar] [CrossRef]
- Polmann, G.; Badia, V.; Frena, M.; Teixeira, G.L.; Rigo, E.; Block, J.M.; Camino Feltes, M.M. Enzyme-Assisted Aqueous Extraction Combined with Experimental Designs Allow the Obtaining of a High-Quality and Yield Pecan Nut Oil. LWT 2019, 113, 108283. [Google Scholar] [CrossRef]
- Latif, S.; Anwar, F. Aqueous Enzymatic Sesame Oil and Protein Extraction. Food Chem. 2011, 125, 679–684. [Google Scholar] [CrossRef]
- Gao, Y.; Zhou, L.; Yao, F.; Chen, F. Effects of PH on the Composition and Physical Stability of Peanut Oil Bodies from Aqueous Enzymatic Extraction. J. Chem. 2021, 2021, 2441385. [Google Scholar] [CrossRef]
- Zhang, S.; Pan, Y.-G.; Zheng, L.; Yang, Y.; Zheng, X.; Ai, B.; Xu, Z.; Sheng, Z. Application of Steam Explosion in Oil Extraction of Camellia Seed (Camellia oleifera Abel.) and Evaluation of Its Physicochemical Properties, Fatty Acid, and Antioxidant Activities. Food Sci. Nutr. 2019, 7, 1004–1016. [Google Scholar] [CrossRef]
- Zhang, G.; Li, Z.; Fu, M. Comparison of Quality and Oxidative Stability of Pumpkin Seed (Cucurbita maxima) Oil between Conventional and Enzymatic Extraction Methods. Sustainable Food Technol. 2024, 2, 1033–1040. [Google Scholar] [CrossRef]
- Zhang, G.; Li, Z.; Guo, Z.; Charalampopoulos, D. Comparative Extraction of Melon Seed (Cucumis melo L.) Oil by Conventional and Enzymatic Methods: Physicochemical Properties and Oxidative Stability. J. Agric. Food Res. 2024, 16, 101182. [Google Scholar] [CrossRef]
- Wu, H.; Shi, J.; Xue, S.; Kakuda, Y.; Wang, D.; Jiang, Y.; Ye, X.; Li, Y.; Subramanian, J. Essential Oil Extracted from Peach (Prunus persica) Kernel and Its Physicochemical and Antioxidant Properties. LWT Food Sci. Technol. 2011, 44, 2032–2039. [Google Scholar] [CrossRef]
- Dursun Capar, T.; Dedebas, T.; Yalcin, H.; Ekici, L. Extraction Method Affects Seed Oil Yield, Composition, and Antioxidant Properties of European Cranberrybush (Viburnum opulus). Ind. Crops Prod. 2021, 168, 113632. [Google Scholar] [CrossRef]
- OJEH, O.A. Effect of Refining on the Physical and Chemical Properties of Cashew kernel Oil. Int. J. Food Sci. Technol. 1981, 16, 513–517. [Google Scholar] [CrossRef]
- Yang, T.; He, R.; Xia, Q.; Cacciotti, I.; Korma, S.A.; Zhang, W.; Yi, G. Phytosterol-Enriched Camellia oleifera Abel Seed Oil Obtained by Subcritical Butane Extraction: Physicochemical Properties and Oxidative Stability. Food Chem. 2025, 472, 142791. [Google Scholar] [CrossRef]
- Mat Yusoff, M.; Gordon, M.H.; Niranjan, K. Aqueous Enzyme Assisted Oil Extraction from Oilseeds and Emulsion De-Emulsifying Methods: A Review. Trends Food Sci. Technol. 2015, 41, 60–82. [Google Scholar] [CrossRef]
- Gai, Q.-Y.; Jiao, J.; Mu, P.-S.; Wang, W.; Luo, M.; Li, C.-Y.; Zu, Y.-G.; Wei, F.-Y.; Fu, Y.-J. Microwave-Assisted Aqueous Enzymatic Extraction of Oil from Isatis indigotica Seeds and Its Evaluation of Physicochemical Properties, Fatty Acid Compositions and Antioxidant Activities. Ind. Crops Prod. 2013, 45, 303–311. [Google Scholar] [CrossRef]
- Lutterodt, H.; Slavin, M.; Whent, M.; Turner, E.; Yu, L. Fatty Acid Composition, Oxidative Stability, Antioxidant and Antiproliferative Properties of Selected Cold-Pressed Grape Seed Oils and Flours. Food Chem. 2011, 128, 391–399. [Google Scholar] [CrossRef] [PubMed]
- Aziz, T.; Qadir, R.; Anwar, F.; Naz, S.; Nazir, N.; Nabi, G.; Haiying, C.; Lin, L.; Alharbi, M.; Alasmari, A.F. Optimal Enzyme-Assisted Extraction of Phenolics from Leaves of Pongamia pinnata via Response Surface Methodology and Artificial Neural Networking. Appl. Biochem. Biotechnol. 2024, 196, 6508–6525. [Google Scholar] [CrossRef]
- Coniglio, R.O.; Díaz, G.V.; Barua, R.C.; Albertó, E.; Zapata, P.D. Enzyme-Assisted Extraction of Phenolic Compounds and Proteins from Sugarcane Bagasse Using a Low-Cost Cocktail from Auricularia fuscosuccinea. Int. J. Food Sci. Technol. 2022, 57, 1114–1121. [Google Scholar] [CrossRef]
- Liu, Q.; Li, P.; Chen, J.; Li, C.; Jiang, L.; Luo, M.; Sun, A.N. Optimization of Aqueous Enzymatic Extraction of Castor (Ricinus communis) Seeds Oil Using Response Surface Methodology. Biobased Mater. Bioenergy 2019, 13, 114–122. [Google Scholar] [CrossRef]
- Xu, Y.X.; Hanna, M.A.; Josiah, S.J. Hybrid Hazelnut Oil Characteristics and Its Potential Oleochemical Application. Ind. Crops Prod. 2007, 26, 69–76. [Google Scholar] [CrossRef]
- Liu, N.; Ren, G.; Faiza, M.; Li, D.; Cui, J.; Zhang, K.; Yao, X.; Zhao, M. Comparison of Conventional and Green Extraction Methods on Oil Yield, Physicochemical Properties, and Lipid Compositions of Pomegranate Seed Oil. J. Food Compos. Anal. 2022, 114, 104747. [Google Scholar] [CrossRef]
- Akintelu, M.T.; Amoo, I.A. Proximate Characterisation and Physicochemical Properties of Raw and Boiled Milk Bush (Thevetia peruviana) Seed. Int. J. Sci. 2016, 5, 16–21. [Google Scholar] [CrossRef][Green Version]
- Liu, Z.; Liao, H.; Wei, C.; Qi, Y.; Zou, Z. Application of an Aqueous Enzymatic–Ultrasound Cavitation Method for the Separation of Sapium sebiferum Seed Kernel Oil. Ultrason. Sonochem. 2023, 101, 106704. [Google Scholar] [CrossRef]
- Vilas-Franquesa, A.; Juan, B.; Saldo, J. Targeted Analysis of Sea Buckthorn Oil Extracted by Accelerated Solvent Extraction Technique Using Green and Conventional Solvents. LWT 2022, 164, 113643. [Google Scholar] [CrossRef]
- Xue, Z.; Wan, F.; Gao, X.; Yu, W.; Zhang, Z.; Liu, J.; Kou, X. Extraction and Evaluation of Edible Oil from Schizochytrium Sp. Using an Aqueous Enzymatic Method. Front. Agric. Sci. Eng. 2021, 8, 623–634. [Google Scholar] [CrossRef]
- Zeng, W.; Endo, Y. Lipid Characteristics of Camellia Seed Oil. J. Oleo Sci. 2019, 68, 649–658. [Google Scholar] [CrossRef] [PubMed]
- Jiao, J.; Li, Z.-G.; Gai, Q.-Y.; Li, X.-J.; Wei, F.-Y.; Fu, Y.-J.; Ma, W. Microwave-Assisted Aqueous Enzymatic Extraction of Oil from Pumpkin Seeds and Evaluation of Its Physicochemical Properties, Fatty Acid Compositions and Antioxidant Activities. Food Chem. 2014, 147, 17–24. [Google Scholar] [CrossRef]
- Rovaris, Â.A.; Dias, C.O.; da Cunha, I.P.; Scaff, R.M.C.; de Francisco, A.; Petkowicz, C.L.O.; Amante, E.R. Chemical Composition of Solid Waste and Effect of Enzymatic Oil Extraction on the Microstructure of Soybean (Glycine max). Ind. Crops Prod. 2012, 36, 405–414. [Google Scholar] [CrossRef]
- Chen, Z.; Shen, R.; Xie, J.; Zeng, Y.; Wang, K.; Zhao, L.; Liu, X.; Hu, Z. Multi-Frequency Ultrasonic-Assisted Enzymatic Extraction of Coconut Paring Oil from Coconut by-Products: Impact on the Yield, Physicochemical Properties, and Emulsion Stability. Ultrason. Sonochem. 2024, 109, 106996. [Google Scholar] [CrossRef]
- Solomakou, N.; Drosaki, A.M.; Christaki, S.; Kaderides, K.; Mourtzinos, I.; Goula, A.M. Valorization of Peach (Prunus persica L.) Peels and Seeds Using Ultrasound and Enzymatic Methods. Chem. Eng. Process. Process Intensif. 2024, 206, 110072. [Google Scholar] [CrossRef]
- Guo, X.; Wu, B.; Jiang, Y.; Zhang, Y.; Jiao, B.; Wang, Q. Improving Enzyme Accessibility in the Aqueous Enzymatic Extraction Process by Microwave-Induced Porous Cell Walls to Increase Oil Body and Protein Yields. Food Hydrocoll. 2024, 147, 109407. [Google Scholar] [CrossRef]
- Soto, C.; Chamy, R.; Zúñiga, M.E. Enzymatic Hydrolysis and Pressing Conditions Effect on Borage Oil Extraction by Cold Pressing. Food Chem. 2007, 102, 834–840. [Google Scholar] [CrossRef]
- Arroyo, B.Y.N.; Chacón, S.L.O.; Tacias-Pascacio, V.G.; Chac, G.E.O.; Canseco, C.V.; Gordillo, R.M.; Quintero, A.R. Aqueous Enzymatic Extraction of Oil from Microwave-Pretreated Jicaro Seeds. Curr. Biochem. Eng. 2019, 5, 42–49. [Google Scholar] [CrossRef]
- Zhu, F.; Wu, R.; Chen, B.; Zhang, F.; Chen, Y.; Cao, F.; Yu, P.; Su, E. Development of an Efficient Procedure for Preparing High Quality Camellia oleifera Seed Oil by Enzymatic Extraction and Demulsification. Ind. Crops Prod. 2024, 212, 118392. [Google Scholar] [CrossRef]
- Hu, B.; Wang, H.; He, L.; Li, Y.; Li, C.; Zhang, Z.; Liu, Y.; Zhou, K.; Zhang, Q.; Liu, A.; et al. A Method for Extracting Oil from Cherry Seed by Ultrasonic-Microwave Assisted Aqueous Enzymatic Process and Evaluation of Its Quality. J. Chromatogr. A 2019, 1587, 50–60. [Google Scholar] [CrossRef] [PubMed]
- Topete-Betancourt, A.; Martínez-Flores, H.E.; Jiménez-Sandoval, S.J.; de Dios Figueroa-Cárdenas, J.; Virgen-Ortiz, J.J.; Valencia, J.E.; Garnica-Romo, M.G.; Gómez-Ayala, M.A. Ultrasound Assisted by Microwave during the Extraction of Avocado Oil: Quality Assessment by Chromatographic Techniques, Raman Spectroscopy, and Thermogravimetric Analysis. Pol. J. Chem. Technol. 2024, 26, 97–103. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).