The Role of Ga Promoter in Enhancing the Performance of Ni/ZrO2+SiO2 Catalysts for Dry Methane Reforming
Abstract
1. Introduction
2. Results
2.1. BET
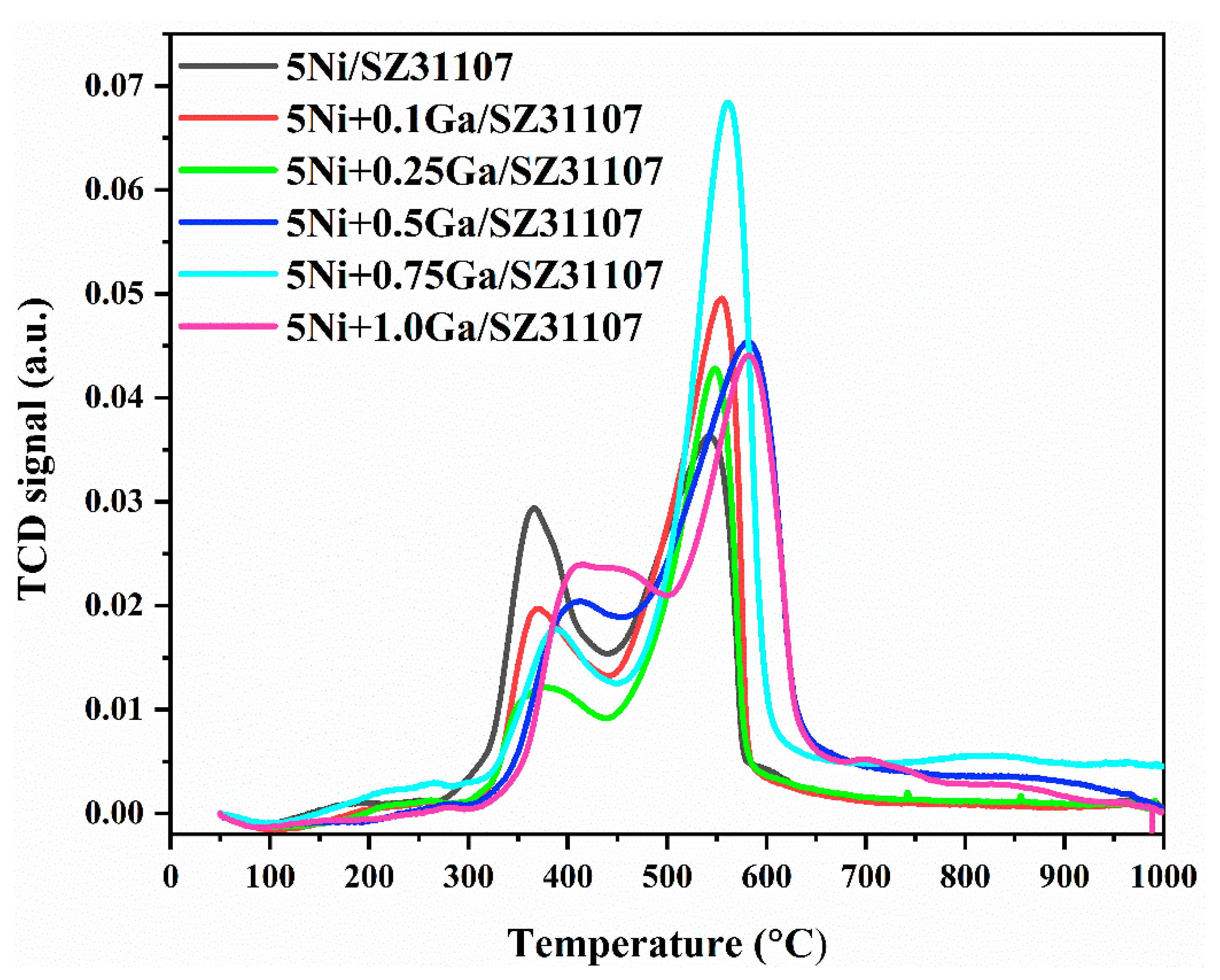
2.2. TPR
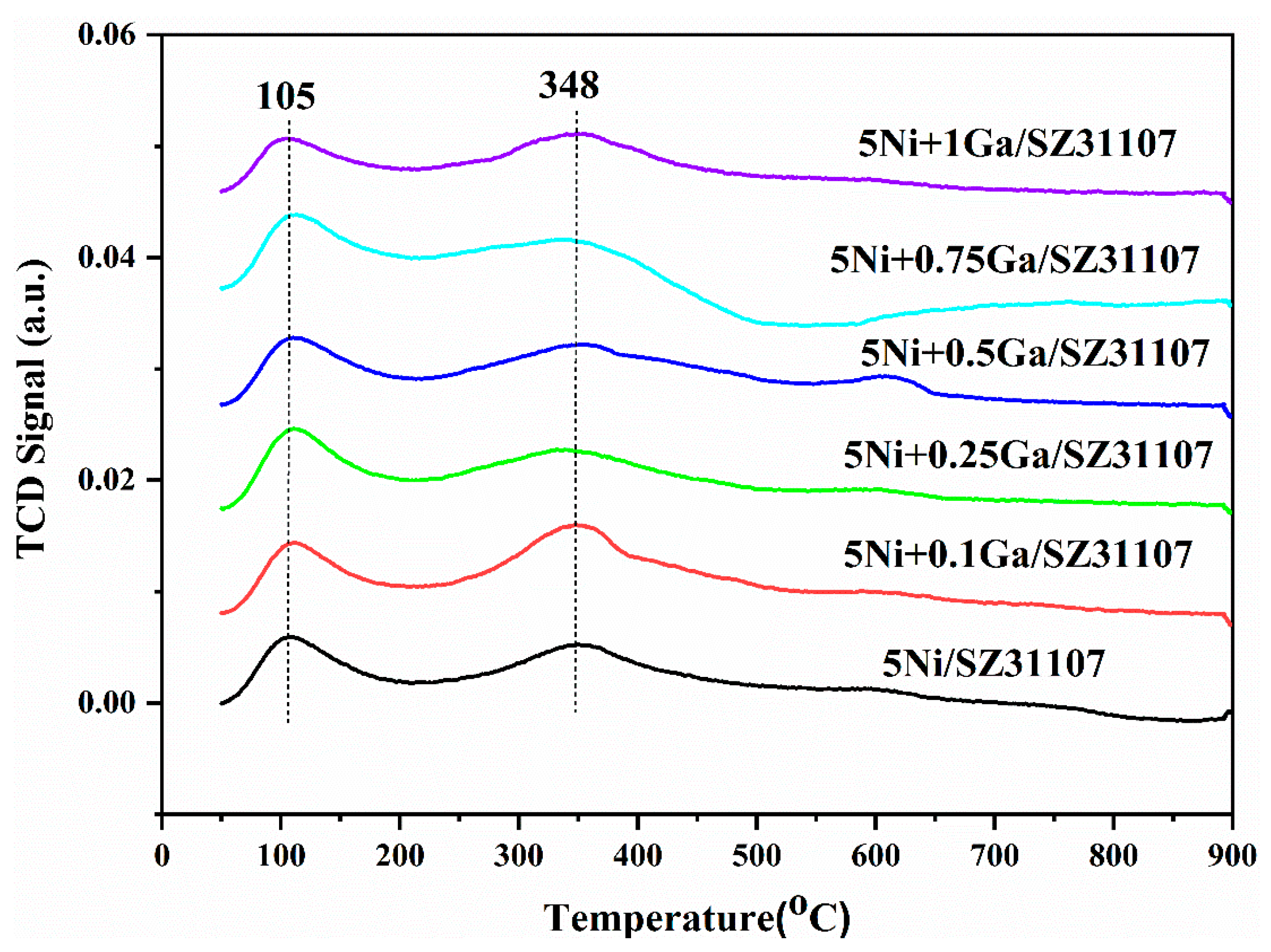
2.3. CO2-TPD
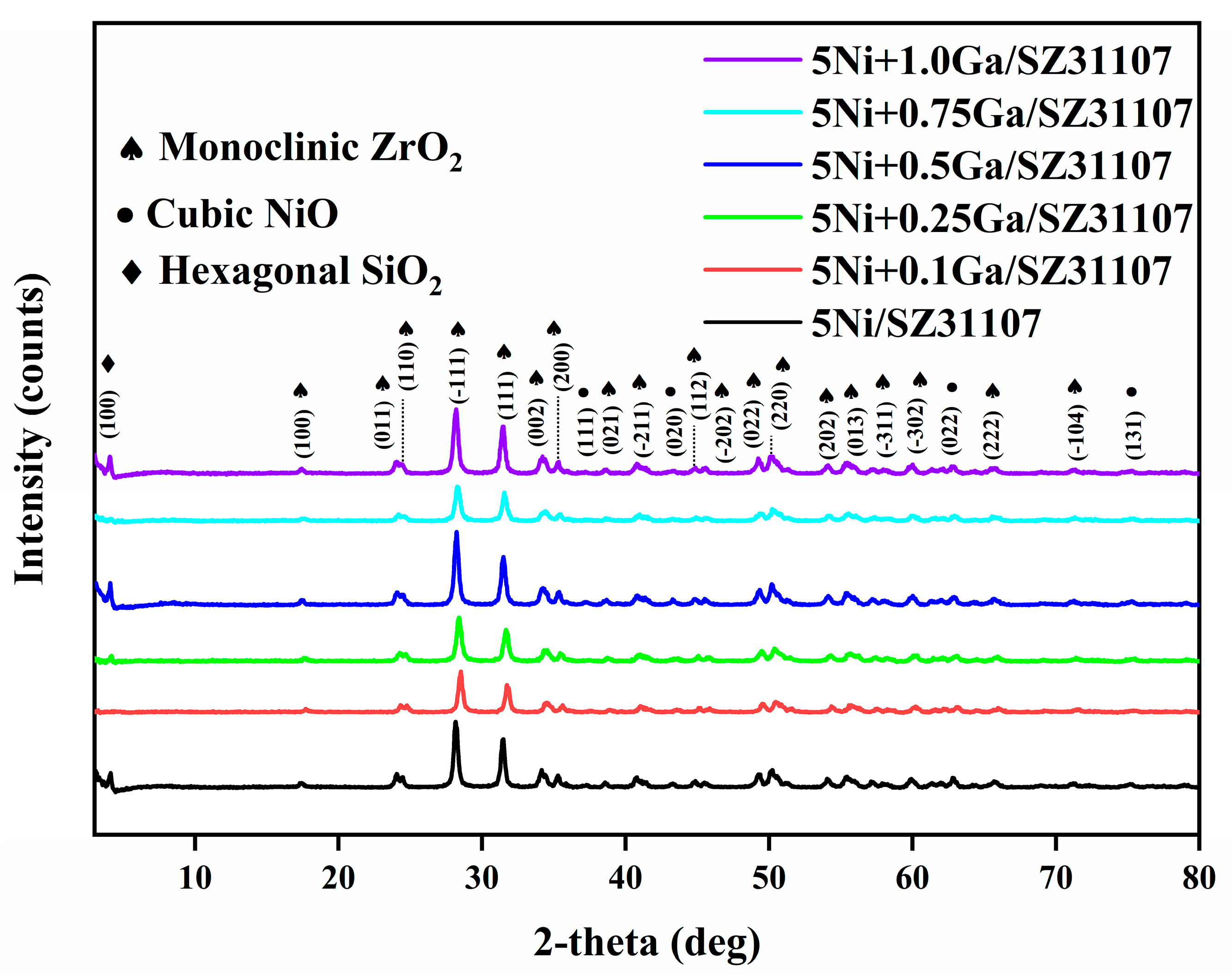
2.4. XRD
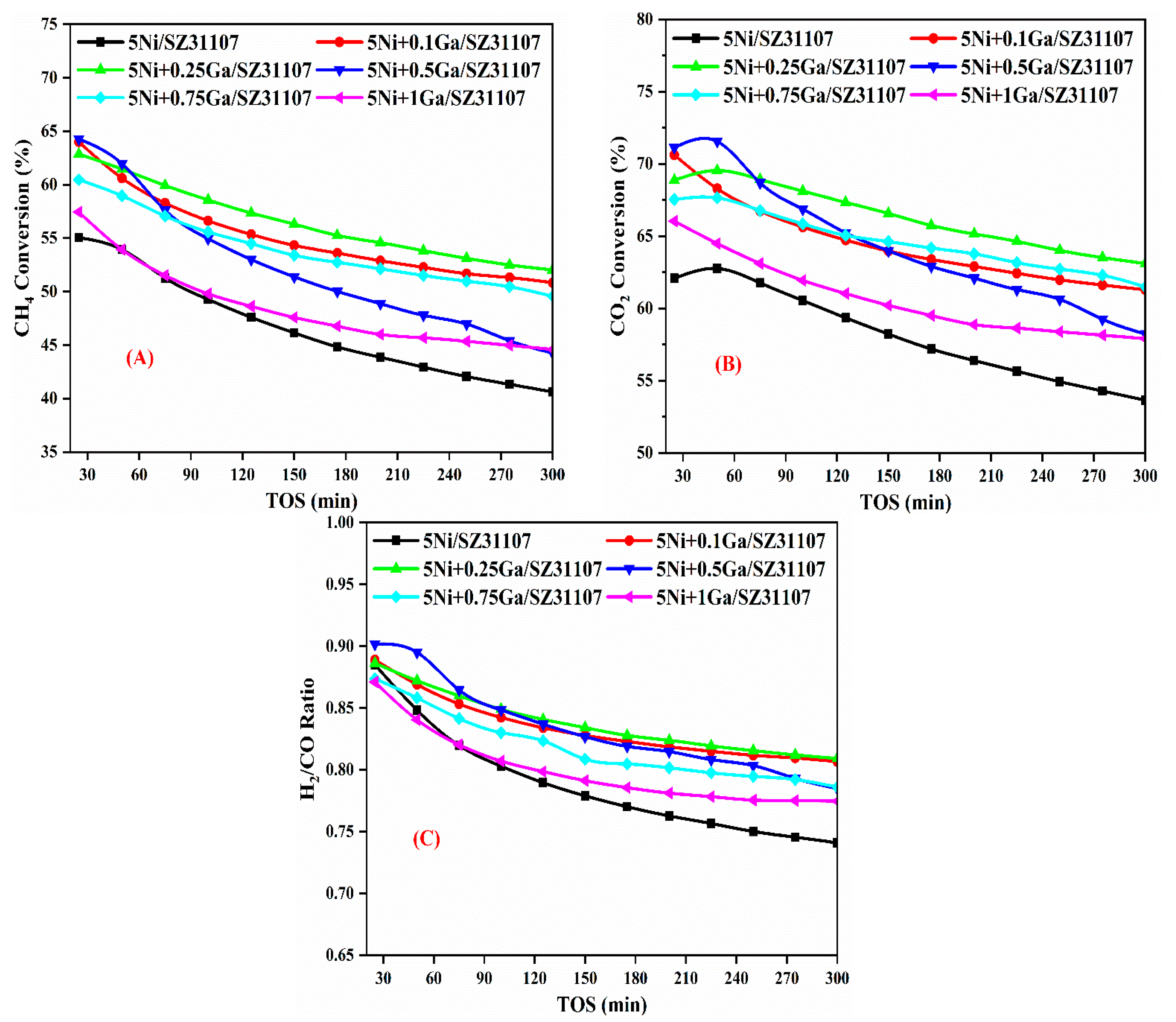
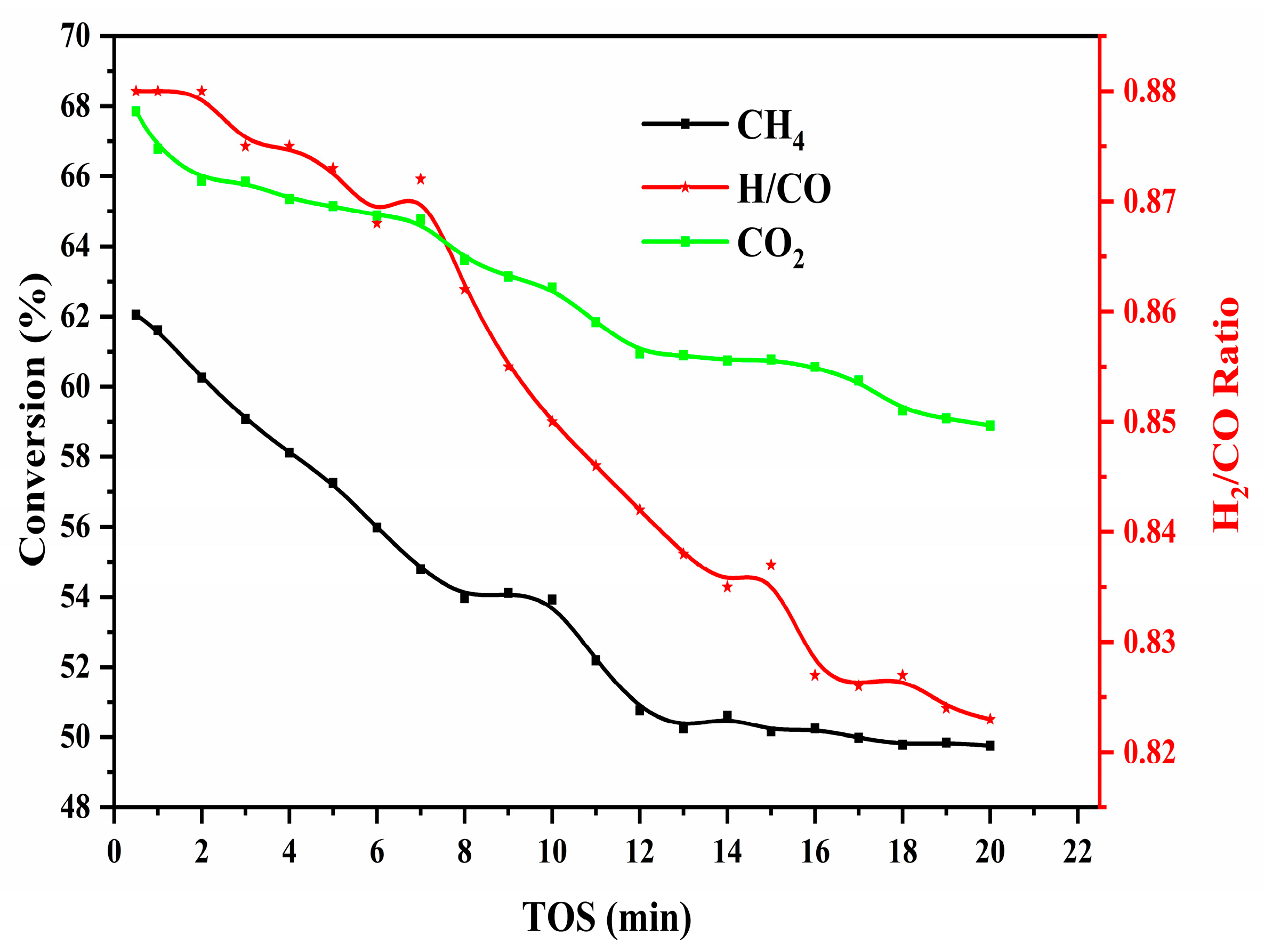
2.5. Catalytic Activity Result and Discussion
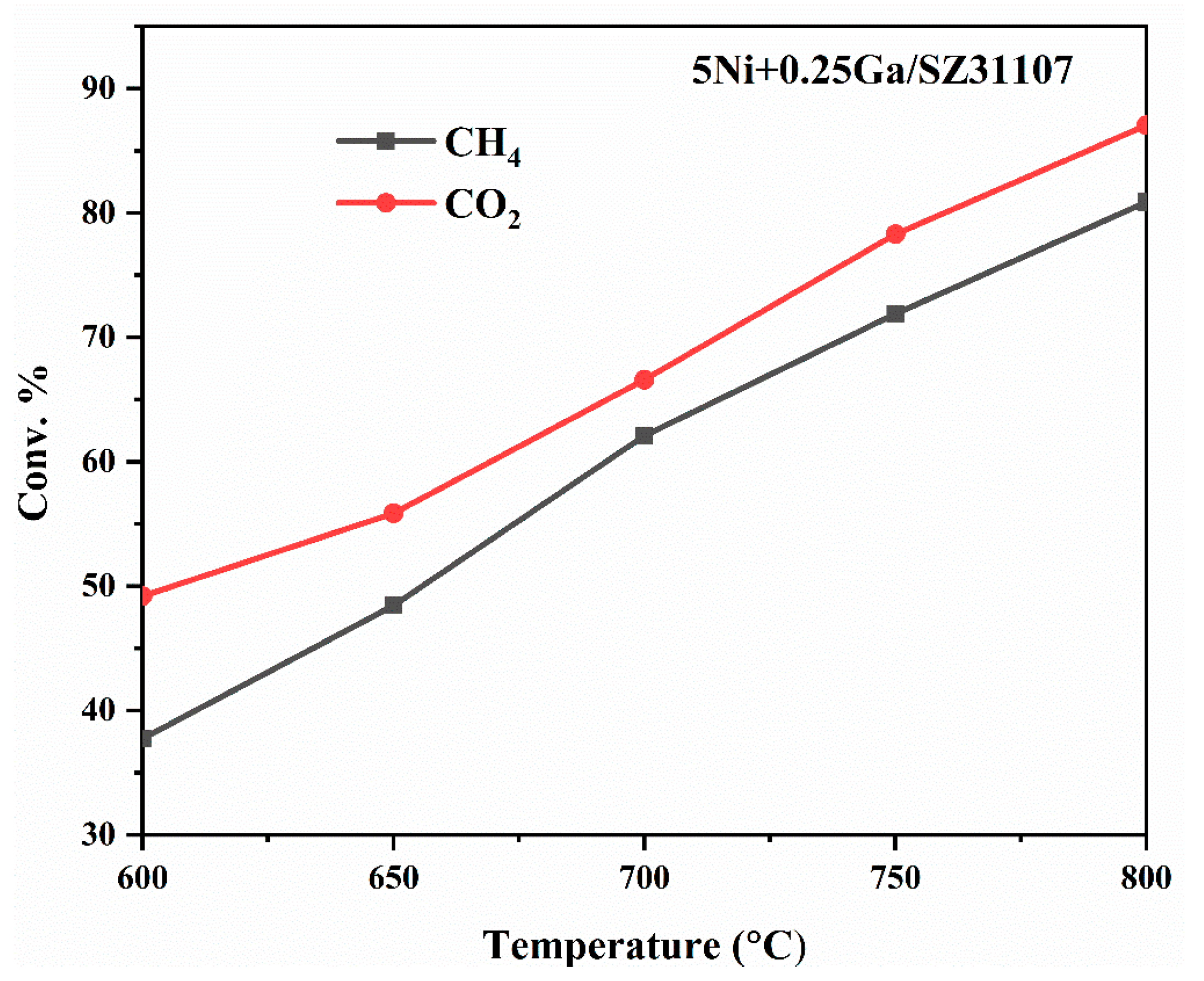
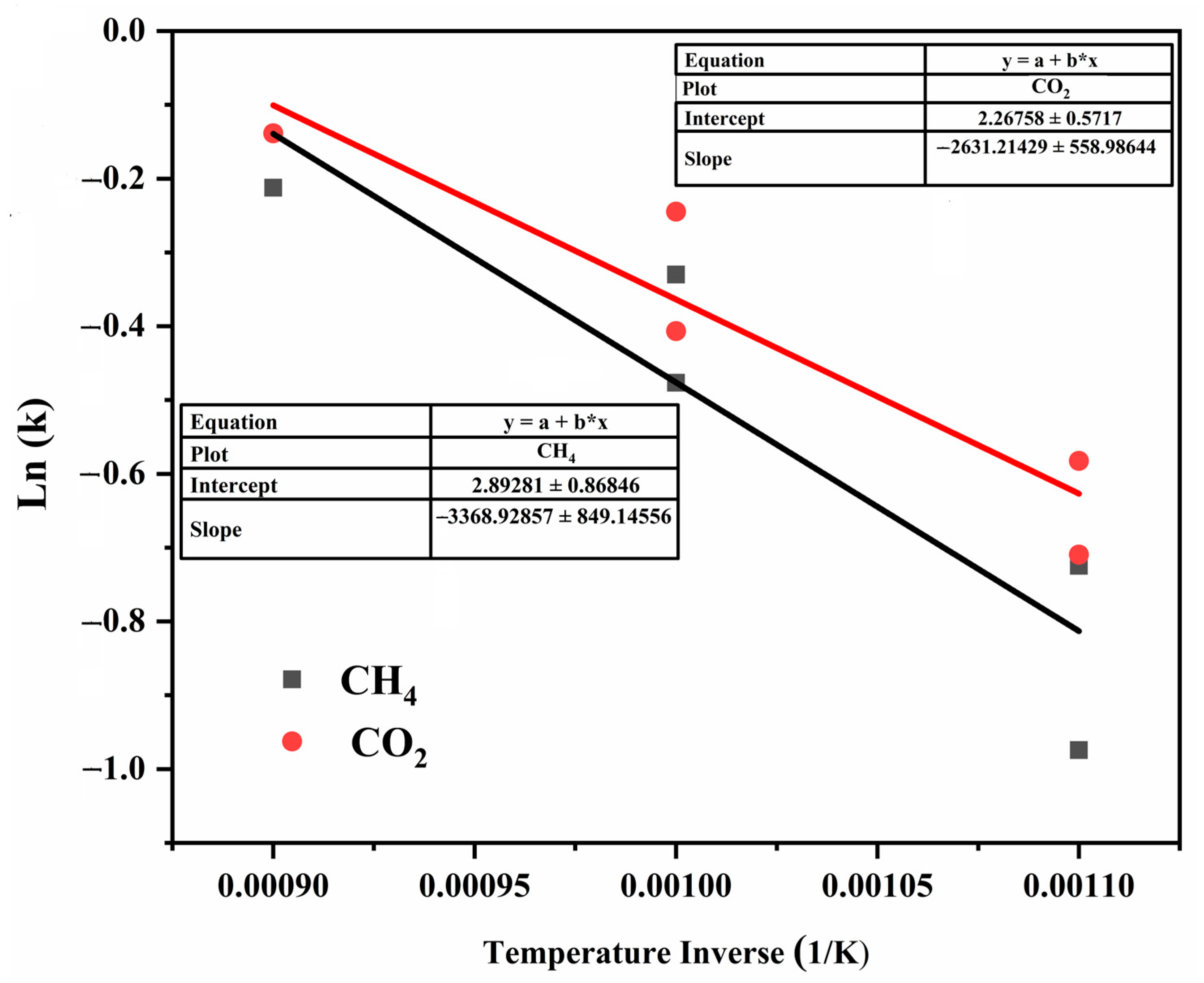
2.6. Impact of Reaction Temperature
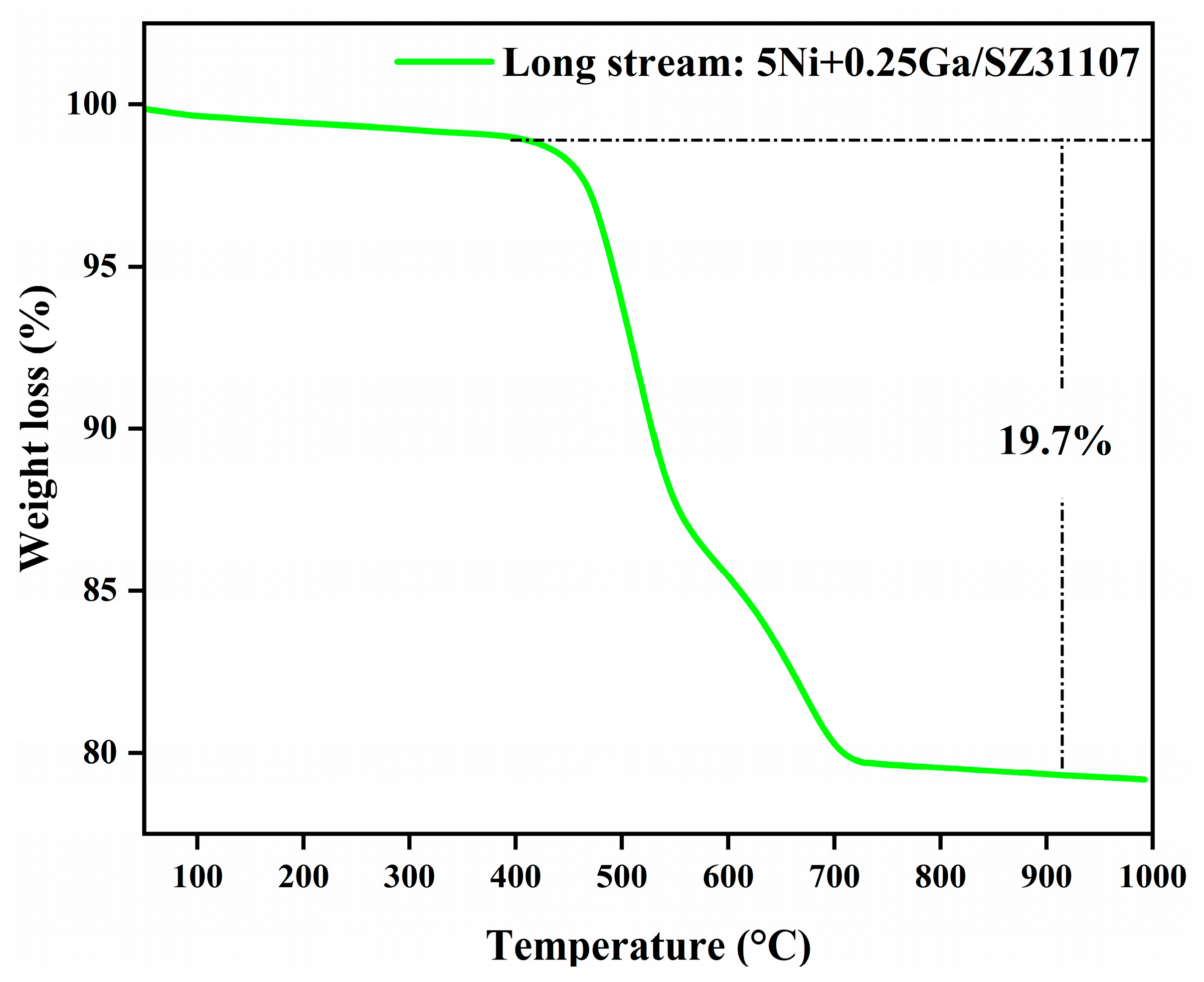
2.7. TGA
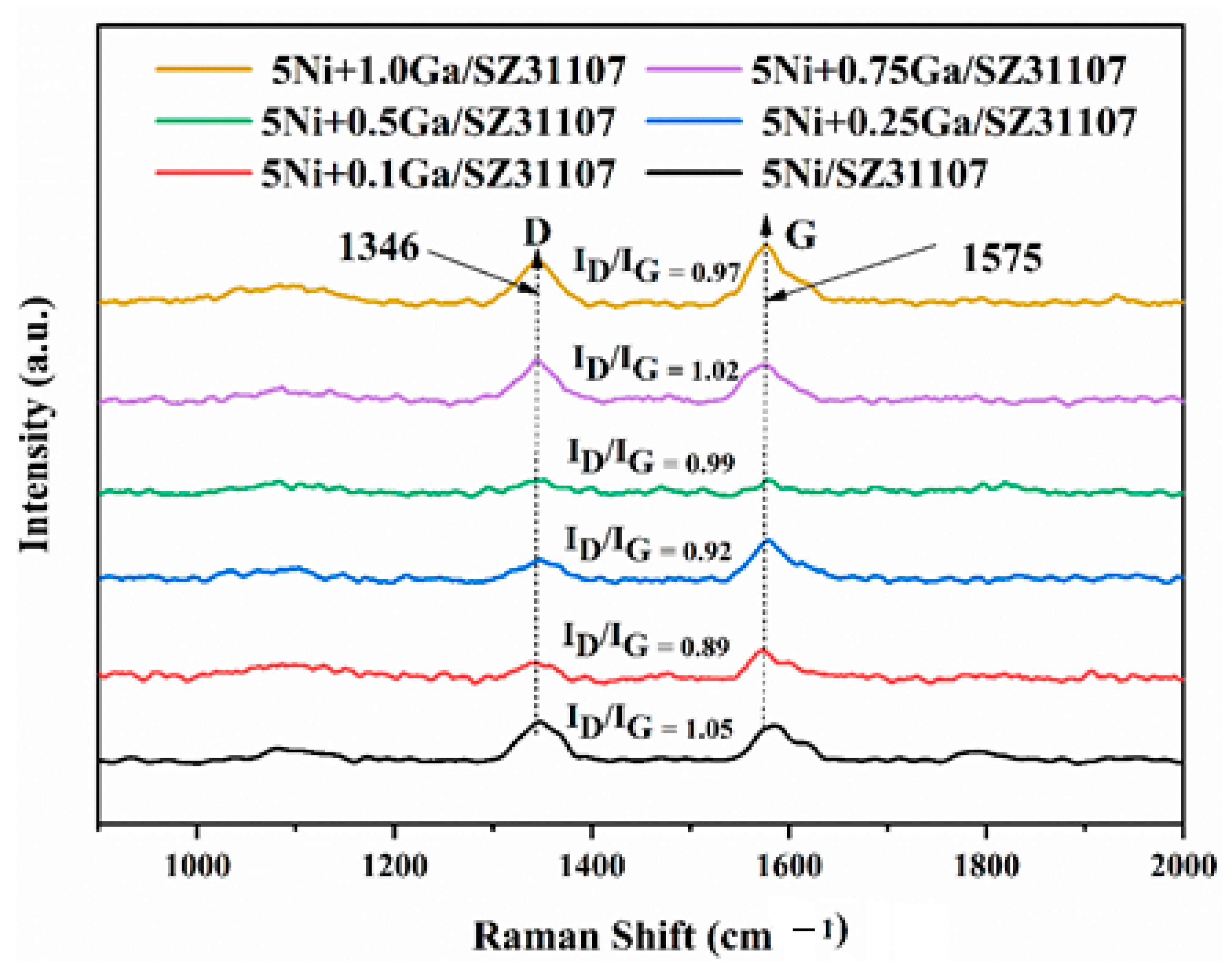
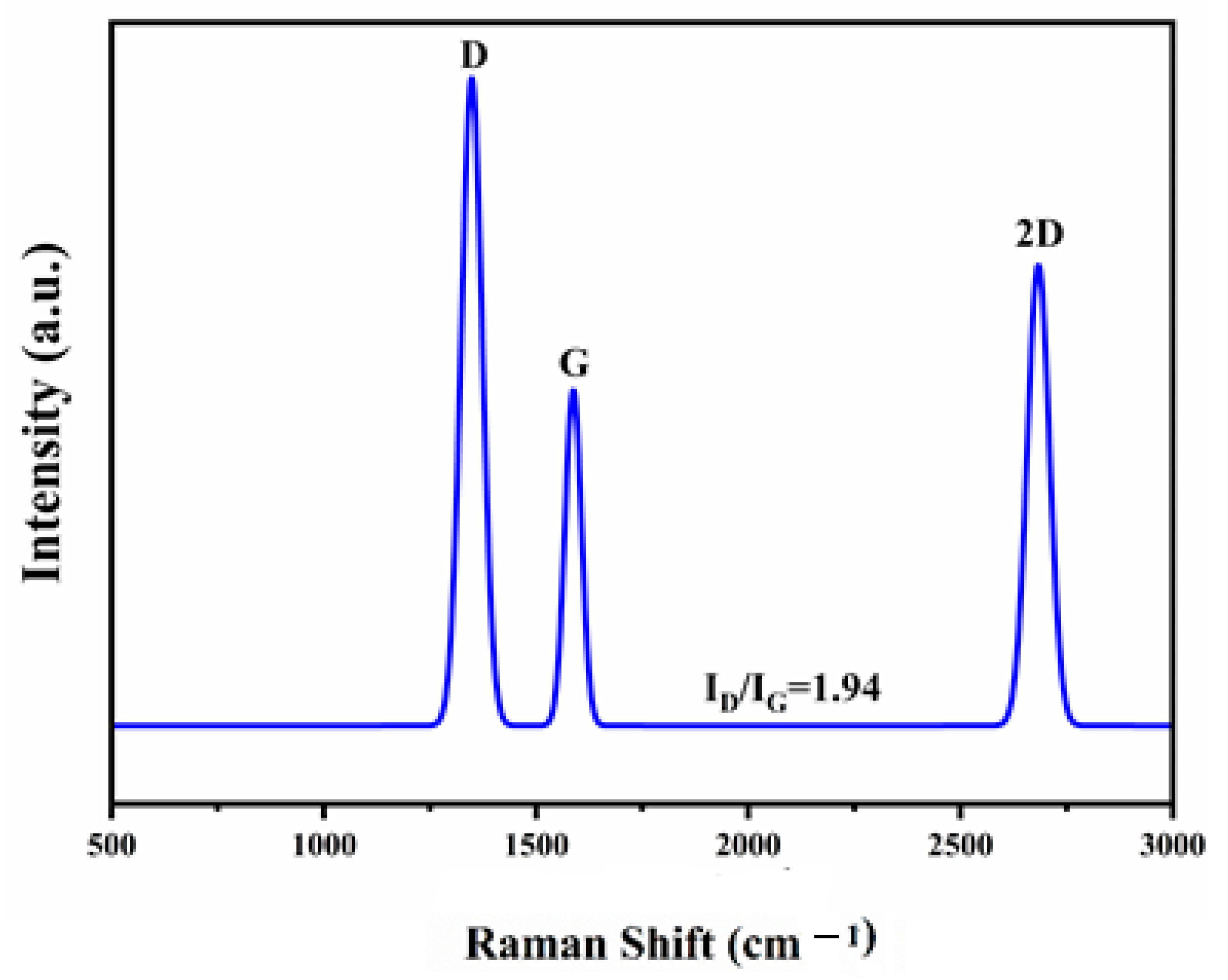
2.8. Raman Spectroscopic Analysis of Spent Catalysts
3. Materials and Methods
3.1. Materials Used
3.2. Catalysts Preparation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Botkin, D.B.; Saxe, H.; Araújo, M.B.; Betts, R.; Bradshaw, R.H.W.; Cedhagen, T.; Chesson, P.; Dawson, T.P.; Etterson, J.R.; Faith, D.P.; et al. Forecasting the Effects of Global Warming on Biodiversity. Bioscience 2007, 57, 227–236. [Google Scholar] [CrossRef]
- Chauhan, B.S.; Prabhjyot, K.; Mahajan, G.; Randhawa, R.K.; Singh, H.; Kang, M.S. Global Warming and Its Possible Impact on Agriculture in India. Adv. Agron. 2014, 123, 65–121. [Google Scholar] [CrossRef]
- Jafarbegloo, M.; Tarlani, A.; Mesbah, A.W.; Sahebdelfar, S. Thermodynamic Analysis of Carbon Dioxide Reforming of Methane and Its Practical Relevance. Int. J. Hydrogen Energy 2015, 40, 2445–2451. [Google Scholar] [CrossRef]
- Tasker, S.Z.; Standley, E.A.; Jamison, T.F. Recent Advances in Homogeneous Nickel Catalysis. Nature 2014, 509, 299–309. [Google Scholar] [CrossRef] [PubMed]
- Keim, W. Nickel: An Element with Wide Application in Industrial Homogeneous Catalysis. Angew. Chem. Int. Ed. Engl. 1990, 29, 235–244. [Google Scholar] [CrossRef]
- Li, F.; MacFarlane, D.R.; Zhang, J. Recent Advances in the Nanoengineering of Electrocatalysts for CO2 Reduction. Nanoscale 2018, 10, 6235–6260. [Google Scholar] [CrossRef]
- Xu, Z.; Park, E.D. Recent Advances in Coke Management for Dry Reforming of Methane over Ni-Based Catalysts. Catalysts 2024, 14, 176. [Google Scholar] [CrossRef]
- Mokrzycki, J.; Legutko, P.; Kaczmarczyk, O.; Fedyna, M. Investigating the Carbon Deposit Formation, Hysteresis Phenomenon and Stability of Ni-AlSBA-15 Catalysts in Dry Reforming of Methane. J. Environ. Chem. Eng. 2025, 13, 115040. [Google Scholar] [CrossRef]
- Guimarães, A.C.P.; Coelho, L.R.F.; Silva, A.A.A.; Xing, Y.; Colman, R.C.; Mattos, L.V.; Henriques, C.A. Dry Reforming of Methane over Ni Catalysts Supported on Hierarchical ZSM-5 and USY Zeolites. Catal. Today 2025, 447, 115159. [Google Scholar] [CrossRef]
- Zhang, G.; Liu, J.; Xu, Y.; Sun, Y. A Review of CH4–CO2 Reforming to Synthesis Gas over Ni-Based Catalysts in Recent Years (2010–2017). Int. J. Hydrogen Energy 2018, 43, 15030–15054. [Google Scholar] [CrossRef]
- Yentekakis, I.V.; Panagiotopoulou, P.; Artemakis, G. A Review of Recent Efforts to Promote Dry Reforming of Methane (DRM) to Syngas Production via Bimetallic Catalyst Formulations. Appl. Catal. B Environ. 2021, 296, 120210. [Google Scholar] [CrossRef]
- Charisiou, N.D.; Siakavelas, G.; Tzounis, L.; Sebastian, V.; Monzon, A.; Baker, M.A.; Hinder, S.J.; Polychronopoulou, K.; Yentekakis, I.V.; Goula, M.A. An in Depth Investigation of Deactivation through Carbon Formation during the Biogas Dry Reforming Reaction for Ni Supported on Modified with CeO2 and La2O3 Zirconia Catalysts. Int. J. Hydrogen Energy 2018, 43, 18955–18976. [Google Scholar] [CrossRef]
- Gao, X.; Ge, Z.; Zhu, G.; Wang, Z.; Ashok, J.; Kawi, S. Anti-Coking and Anti-Sintering Ni/Al2O3 Catalysts in the Dry Reforming of Methane: Recent Progress and Prospects. Catalysts 2021, 11, 1003. [Google Scholar] [CrossRef]
- bin Jumah, A.; Kaydouh, M.N.; Al-Fatesh, A.S.; Bayazed, M.O.; Fakeeha, A.H.; Ibrahim, A.A.; Abasaeed, A.E.; Chaudhary, K.J.; El Hassan, N. Cerium-Induced Modification of Acid-Base Sites in Ni-Zeolite Catalysts for Improved Dry Reforming of Methane. J. Energy Inst. 2025, 118, 101901. [Google Scholar] [CrossRef]
- Zimmerli, N.K.; Rochlitz, L.; Checchia, S.; Mu Ller, C.R.; Cope -Ret, C.; Abdala, P.M. Structure and Role of a Ga-Promoter in Ni-Based Catalysts for the Selective Hydrogenation of CO2 to Methanol. JACS Au 2023, 4, 237–252. [Google Scholar] [CrossRef] [PubMed]
- Häfele, M.; Reitzmann, A.; Roppelt, D.; Emig, G. Hydroxylation of Benzene with Nitrous Oxide on H-Ga-ZSM5 Zeolite. Appl. Catal. A Gen. 1997, 150, 153–164. [Google Scholar] [CrossRef]
- Al-Fatesh, A.S.; Ibrahim, A.A.; Abu-Dahrieh, J.K.; Al-Awadi, A.S.; El-Toni, A.M.; Fakeeha, A.H.; Abasaeed, A.E. Gallium-Promoted Ni Catalyst Supported on MCM-41 for Dry Reforming of Methane. Catalysts 2018, 8, 229. [Google Scholar] [CrossRef]
- Ochoa, J.V.; Malmusi, A.; Recchi, C.; Cavani, F. Understanding the Role of Gallium as a Promoter of Magnesium Silicate Catalysts for the Conversion of Ethanol into Butadiene. ChemCatChem 2017, 9, 2128–2135. [Google Scholar] [CrossRef]
- Medina, J.C.; Figueroa, M.; Manrique, R.; Rodríguez Pereira, J.; Srinivasan, P.D.; Bravo-Suárez, J.J.; Baldovino Medrano, V.G.; Jiménez, R.; Karelovic, A. Catalytic Consequences of Ga Promotion on Cu for CO2 Hydrogenation to Methanol. Catal. Sci. Technol. 2017, 7, 3375–3387. [Google Scholar] [CrossRef]
- Bai, Y.; Sun, K.; Wu, J.; Zhang, M.; Zhao, S.; Kim, Y.D.; Liu, Y.; Gao, J.; Liu, Z.; Peng, Z. The Ga-Promoted Ni/CeO2 Catalysts for Dry Reforming of Methane with High Stability Induced by the Enhanced CO2 Activation. Mol. Catal. 2022, 530, 112577. [Google Scholar] [CrossRef]
- Fakeeha, A.H.; Bagabas, A.A.; Lanre, M.S.; Osman, A.I.; Kasim, S.O.; Ibrahim, A.A.; Arasheed, R.; Alkhalifa, A.; Elnour, A.Y.; Abasaeed, A.E.; et al. Catalytic Performance of Metal Oxides Promoted Nickel Catalysts Supported on Mesoporous γ-Alumina in Dry Reforming of Methane. Processes 2020, 8, 522. [Google Scholar] [CrossRef]
- Abdullah, B.; Abd Ghani, N.A.; Vo, D.V.N. Recent Advances in Dry Reforming of Methane over Ni-Based Catalysts. J. Clean. Prod. 2017, 162, 170–185. [Google Scholar] [CrossRef]
- Li, W.; Liu, Y.; Mu, M.; Ding, F.; Liu, Z.; Guo, X.; Song, C. Organic Acid-Assisted Preparation of Highly Dispersed Co/ZrO2 Catalysts with Superior Activity for CO2 Methanation. Appl. Catal. B Environ. 2019, 254, 531–540. [Google Scholar] [CrossRef]
- Németh, M.; Srankó, D.; Károlyi, J.; Somodi, F.; Schay, Z.; Sáfrán, G.; Sajó, I.; Horváth, A. Na-Promoted Ni/ZrO2 Dry Reforming Catalyst with High Efficiency: Details of Na2O-ZrO2-Ni Interaction Controlling Activity and Coke Formation. Catal. Sci. Technol. 2017, 7, 5386–5401. [Google Scholar] [CrossRef]
- Roh, H.S.; Jun, K.W.; Dong, W.S.; Chang, J.S.; Park, S.E.; Joe, Y. Il Highly Active and Stable Ni/Ce–ZrO2 Catalyst for H2 Production from Methane. J. Mol. Catal. A Chem. 2002, 181, 137–142. [Google Scholar] [CrossRef]
- Li, S.; Li, M.; Zhang, C.; Wang, S.; Ma, X.; Gong, J. Steam Reforming of Ethanol over Ni/ZrO2 Catalysts: Effect of Support on Product Distribution. Int. J. Hydrogen Energy 2012, 37, 2940–2949. [Google Scholar] [CrossRef]
- Biswas, P.; Kunzru, D. Steam reforming of ethanol for production of hydrogen over Ni/CeO2-ZrO2 catalyst: Effect of support and metal loading. Int. J. Hydrogen Energy 2007, 32, 969–980. [Google Scholar] [CrossRef]
- Li, T.; Liu, J.; Zhang, Y.; Zhang, X.; Wang, Y.; Zhao, Y.; Li, G.; Zhang, G. Utilization of Coal Gasification Fine Slag-Derived Mesoporous Silica Supports for Enhanced Dry Reforming of Methane Catalysts. Fuel 2024, 375, 132619. [Google Scholar] [CrossRef]
- Al-Fatesh, A.S.; Kumar, R.; Kasim, S.O.; Ibrahim, A.A.; Fakeeha, A.H.; Abasaeed, A.E.; Alrasheed, R.; Bagabas, A.; Chsaudhary, M.L.; Frusteri, F.; et al. The Effect of Modifier Identity on the Performance of Ni-Based Catalyst Supported on γ-Al2O3 in Dry Reforming of Methane. Catal. Today 2020, 348, 236–242. [Google Scholar] [CrossRef]
- Zheng, B.; Hua, W.; Yue, Y.; Gao, Z. Dehydrogenation of Propane to Propene over Different Polymorphs of Gallium Oxide. J. Catal. 2005, 232, 143–151. [Google Scholar] [CrossRef]
- Gil, S.A.; Especel, C.; Passamonti, F.J.; Benítez, V.M.; Epron, F.; Gil, S.A.; Especel, C.; Passamonti, F.J.; Montouillout, V.; Viviana, M.; et al. Ga2O3/Al2O3 Catalysts for Propane Dehydrogenation: Modification of Surface Properties by H2 pretreatment or co-feeding. ChemCatChem, 2025; in press. [Google Scholar]
- Shao, C.T.; Lang, W.Z.; Yan, X.; Guo, Y.J. Catalytic Performance of Gallium Oxide Based-Catalysts for the Propane Dehydrogenation Reaction: Effects of Support and Loading Amount. RSC Adv. 2017, 7, 4710–4723. [Google Scholar] [CrossRef]
- Petre, A.L.; Auroux, A.; Gélin, P.; Caldararu, M.; Ionescu, N.I. Acid-Base Properties of Supported Gallium Oxide Catalysts. Thermochim. Acta 2001, 379, 177–185. [Google Scholar] [CrossRef]
- Patel, R.; Fakeeha, A.H.; Kasim, S.O.; Sofiu, M.L.; Ibrahim, A.A.; Abasaeed, A.E.; Kumar, R.; Al-Fatesh, A.S. Optimizing Yttria-Zirconia Proportions in Ni Supported Catalyst System for H2 Production through Dry Reforming of Methane. Mol. Catal. 2021, 510, 111676. [Google Scholar] [CrossRef]
- Busca, G.; Lorenzelli, V. Infrared Spectroscopic Identification of Species Arising from Reactive Adsorption of Carbon Oxides on Metal Oxide Surfaces. Mater. Chem. 1982, 7, 89–126. [Google Scholar] [CrossRef]
- Mohamed, A.T.; Ali, S.; Kumar, A.; Mondal, K.C.; El-Naas, M.H. Evaluation of Highly Active and Stable SiO2 Supported Fe-Based Catalysts for the Catalytic Methane Decomposition into COx Free Hydrogen and CNTs. Catal. Commun. 2023, 180, 106703. [Google Scholar] [CrossRef]
- Jorio, A.; Saito, R. Raman Spectroscopy for Carbon Nanotube Applications. J. Appl. Phys. 2021, 129, 021102. [Google Scholar] [CrossRef]
- La Parola, V.; Liotta, L.F.; Pantaleo, G.; Testa, M.L.; Venezia, A.M. CO2 Reforming of CH4 over Ni Supported on SiO2 Modified by TiO2 and ZrO2: Effect of the Support Synthesis Procedure. Appl. Catal. A Gen. 2022, 642, 118704. [Google Scholar] [CrossRef]
- Prasad, M.; Ray, K.; Sinhamahapatra, A.; Sengupta, S. Materials Ni/CexZr1−xO2 Catalyst Prepared via One-Step Co- Precipitation for CO2 Reforming of CH4 to Produce Syngas: Role of Oxygen Storage Capacity (OSC) and Oxygen Vacancy Formation Energy (OVFE). J. Mater. Sci. 2022, 57, 2839–2856. [Google Scholar] [CrossRef]
- Tengfei, L.; Cheng, J.; Li, D.; Xu, D.; Patel, B.; Guo, Y. Syngas Production from Photothermal Synergistic Dry Reforming of CH4 over a Core-Shell Structured Ni/CeO2–ZrO2@SiO2 Catalyst. Int. J. Hydrogen Energy 2024, 67, 1194–1205. [Google Scholar] [CrossRef]
- Bentalib, A.; Ali, D.A.; Alrashed, M.M.; Ibrahim, A.A.; Saeed, A.M.M.; Fakeeha, A.H.; Abasaeed, A.E.; Kumar, R.; Al-Fatesh, A.S. Tailoring Ni + Sr-MgO Catalysts for Efficient Dry Reforming of Methane: A Performance Study. Catal. Lett. 2025, 155, 128. [Google Scholar] [CrossRef]
- Min, H.; Ji, Y.J.; Kim, D.Y.; Ju, Y.; Yoo, C.G.; Kim, Y.J.; Kang, S.B. Steering Catalytic Property and Reactivity of Ni/SiO2 by Functionalized Silica for Dry Reforming of Methane. Appl. Surf. Sci. Adv. 2024, 24, 100663. [Google Scholar] [CrossRef]
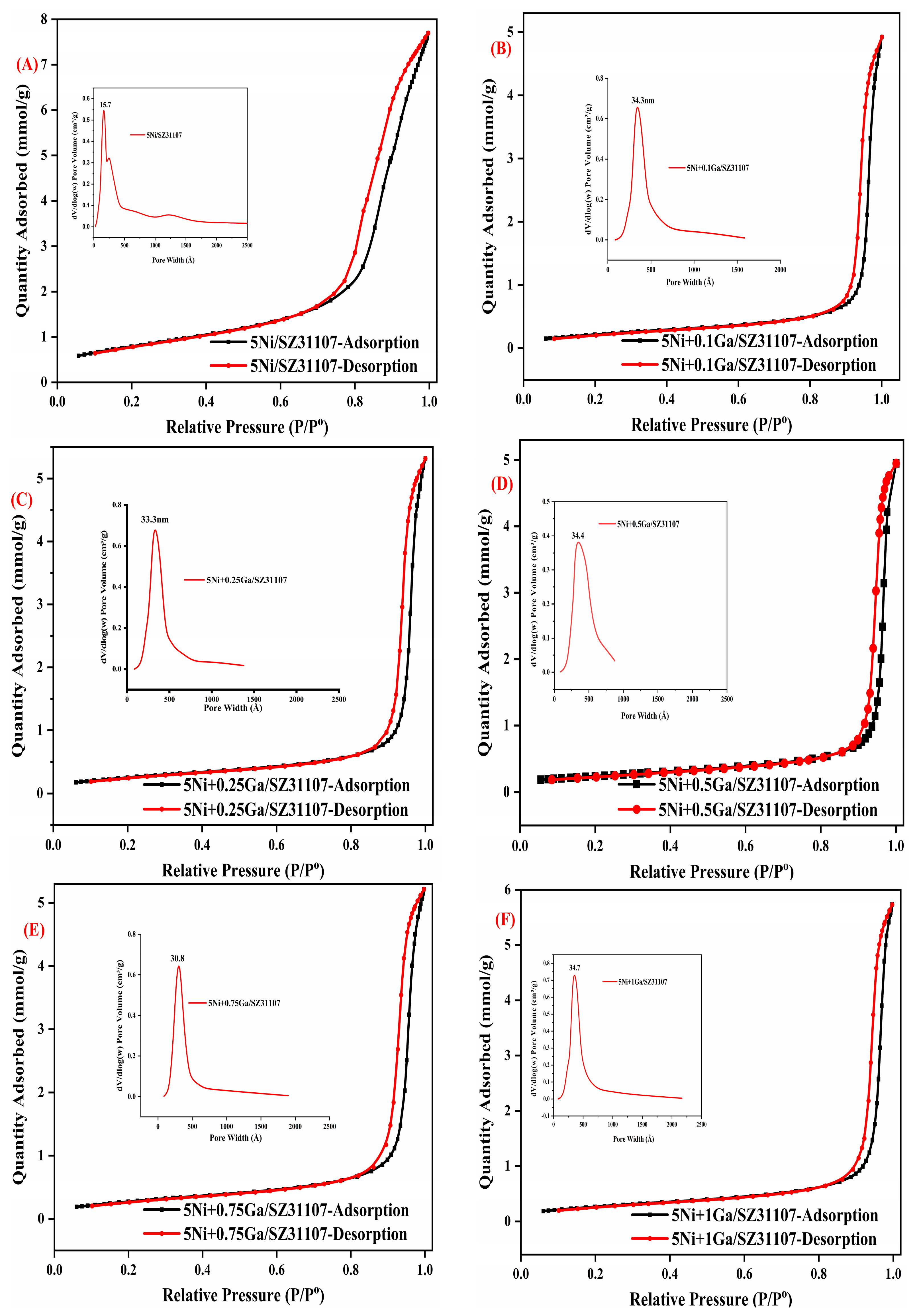
| Samples | BET (m2/g) | Pore Volume (cm3/g) | Pore Diameter (nm) |
|---|---|---|---|
| 5Ni/SZ31107 | 65.1 | 0.27 | 15.7 |
| 5Ni+0.1Ga/SZ31107 | 18.5 | 0.16 | 34.5 |
| 5Ni+0.25Ga/SZ31107 | 21.7 | 0.18 | 33.0 |
| 5Ni+0.5Ga/SZ31107 | 19.3 | 0.15 | 34.7 |
| 5Ni+0.75Ga/SZ31107 | 23.4 | 0.18 | 30.4 |
| 5Ni+1.0Ga/SZ31107 | 22.6 | 0.2 | 34.7 |
| Catalysts | Total Quantity (cm3/g STP) |
|---|---|
| 5Ni/SZ31107 | 19.70 |
| 5Ni+0.1Ga/SZ31107 | 12.11 |
| 5Ni+0.25Ga/SZ31107 | 16.53 |
| 5Ni+0.5Ga/SZ31107 | 11.28 |
| 5Ni+0.75Ga/SZ31107 | 14.51 |
| 5Ni+1.0Ga/SZ31107 | 26.60 |
| T (°C) | T(K) | 1/T(K) | Conversion CH4 | Ln(k) | Conversion CO2 | Ln(k) |
|---|---|---|---|---|---|---|
| 600 | 873 | 0.00114548 | 0.3774 | −0.9744 | 0.4920 | −0.7093 |
| 650 | 923 | 0.00108342 | 0.4845 | −0.7246 | 0.5586 | −0.5823 |
| 700 | 973 | 0.00102775 | 0.6210 | −0.4764 | 0.6660 | −0.4065 |
| 750 | 1023 | 0.00097752 | 0.7190 | −0.3299 | 0.7830 | −0.2446 |
| 800 | 1073 | 0.00093197 | 0.8088 | −0.2122 | 0.8706 | −0.1386 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Zahrani, S.A.; Ibrahim, A.A.; Almutairi, G.; Fakeeha, A.H.; Masood, N.; Rajeh, S.Y.; Otaib, A.A.; Al-Enazy, H.D.A.; Al-Fatesh, A.S. The Role of Ga Promoter in Enhancing the Performance of Ni/ZrO2+SiO2 Catalysts for Dry Methane Reforming. Catalysts 2025, 15, 627. https://doi.org/10.3390/catal15070627
Al-Zahrani SA, Ibrahim AA, Almutairi G, Fakeeha AH, Masood N, Rajeh SY, Otaib AA, Al-Enazy HDA, Al-Fatesh AS. The Role of Ga Promoter in Enhancing the Performance of Ni/ZrO2+SiO2 Catalysts for Dry Methane Reforming. Catalysts. 2025; 15(7):627. https://doi.org/10.3390/catal15070627
Chicago/Turabian StyleAl-Zahrani, Salma A., Ahmed A. Ibrahim, Ghzzai Almutairi, Anis Hamza Fakeeha, Najat Masood, Sahar Y. Rajeh, Ahmed Al Otaib, Hessah Difallah A. Al-Enazy, and Ahmed S. Al-Fatesh. 2025. "The Role of Ga Promoter in Enhancing the Performance of Ni/ZrO2+SiO2 Catalysts for Dry Methane Reforming" Catalysts 15, no. 7: 627. https://doi.org/10.3390/catal15070627
APA StyleAl-Zahrani, S. A., Ibrahim, A. A., Almutairi, G., Fakeeha, A. H., Masood, N., Rajeh, S. Y., Otaib, A. A., Al-Enazy, H. D. A., & Al-Fatesh, A. S. (2025). The Role of Ga Promoter in Enhancing the Performance of Ni/ZrO2+SiO2 Catalysts for Dry Methane Reforming. Catalysts, 15(7), 627. https://doi.org/10.3390/catal15070627









