Abstract
Zeolitic imidazolate frameworks (ZIFs) can provide fascinating stereo morphology and tunable metal active sites, which plays an important role in the synthesis of various catalytic materials. However, it is still a problem to make use of these advantages to design efficient hydrogen evolution reaction (HER) catalysts. Herein, we use covalent coordination strategy to synthesize bimetallic CoxZn1−x(2-MeIM)2 precursors with regular dodecahedral structures for providing uniform active sites and stable carbon skeleton. Furthermore, the ratio of Co and Zn atoms was optimized to balance the electron density and give full play to the synergistic catalytic effect. And then, the subsequent high temperature annealing process is used to construct the amorphous carbon layer, which can improve the overall stability of the material. The gas phase phosphating process realizes the transformation from ZIF material to metal phosphide resulting in enhanced hydrogen evolution activity. Finally, the optimized amorphous nitrogen-doped carbon (NC)-coated Zinc-doped cobalt phosphide (Zn0.17Co0.83P@NC) requires only 237.60 mV to reach the current density of 10 mA cm−2 in alkaline medium, which is 223.22 mV lower than that of CoP, and has a stability of up to 18 h. This work provides a reference for the rational design of efficient and stable compound electrocatalysts for alkaline hydrogen evolution based on the bimetallic ZIF as a precursor.
1. Introduction
Hydrogen energy is a promising renewable resource due to its high calorific value and clean combustion [1,2,3,4,5]. The “hydrogen economy” concept, introduced in the 1970s, offers significant benefits for sustainability and energy security [6,7,8]. A key challenge is transitioning from fossil fuel-based production to scalable, green alternatives [9,10]. Water electrolysis is a viable pathway for “green hydrogen” as it bypasses fossil fuels [11,12,13]. However, its practical application, particularly alkaline electrolysis, faces two major hurdles: the effective integration of intermittent renewable energy [14,15] and the development of efficient, low-cost catalysts to reduce overall expenses [16,17,18]. Because the catalyst can change the reaction rate by changing the reaction path, and alkaline water electrolysis, due to the existence of a multi-step reaction, has a high overpotential [19], through the design of a reasonable alkaline hydrogen precipitation reaction electrocatalyst to reduce the reaction overpotential for the realization of alkaline electrolysis of aquatic hydrogen in practical application is of great significance.
The ideal HER catalyst, rare metal Pt, has δGH* ≈ −0.09 eV [20]. Among non-rare metals, the research focuses on transition metal-based electrocatalysts. At present, phosphide [21,22,23], sulfide [24,25,26], selenide [27,28], nitride [29,30], and boride [31,32] have been explored to replace Pt-based catalysts. Among them, transition-metal phosphide has attracted extensive attention because of its many advantages [33]. Transition metal phosphide (TMPs) has obvious advantages in HER catalysis, in which the metal has a partial positive charge and the non-metallic phosphorus has a partial negative charge. Theory and experiment have confirmed that due to the difference in electronegativity between metal and phosphorus, phosphorus will bind protons and metal sites in the process of HER catalysis. Since H+ is the key intermediate of the HER reaction, P is expected to affect the catalytic process of HER [34].
As a special metal–organic compound, ZIFs have a 3D topological sequence. It has many advantages, which come from the ultra–high chemical stability of the metal-N bond. Non-metal’s incorporation plays a critical role beyond structural formation. It not only stabilizes the zeolitic imidazolate framework as a ligand but also electronically modulates the metal centers. This electronic regulation is key to optimizing the adsorption energetics of reaction intermediates [35,36]. Varying the type and connectivity of imidazole ligands enables directional pore-size control and enhanced surface adsorption through such fine-tuning [37]. When treated at high temperatures, the carbon and nitrogen contained in ZIF can be embedded in the product to speed up electron transfer.
Herein, we use a covalent coordination strategy to obtain bimetallic CoxZn1−x(2-MeIM)2 precursors and synthesize Zn0.17Co0.83P@NC for efficient hydrogen evolution reactions. Thanks to the domain–limited effect of the dodecahedron skeleton provided by ZIF, a large number of active sites are constructed to achieve “hydrogen spillover”. In order to weaken the electron density of the Co active site, the active metal Zn was introduced to accelerate the electron transfer from Co to P and reduce the overpotential. Electrochemical and physical characterization showed that Zn doping reduced the electron density around Co, increased the electrochemically active surface area, accelerated the interfacial charge transfer, and enhanced the catalytic activity of basic HER. This work provides a reference for the application of metal-doped cobalt phosphide electrocatalyst in alkaline electrolysis.
2. Results and Discussion
Bimetallic ZIFs were prepared by the co-coordination strategy of Zn(NO3)2·6H2O, Co(NO3)2·6H2O, and 2-methylimidazole in methanol solution. Its crystal structure is shown in Figure 1, where Co2+ and Zn2+ coordinate with nitrogen atoms in 2-methylimidazole to form ZnN4 and CoN4 tetrahedra, and the synthesized bimetallic ZIFs with different ratios are denoted as ZnxCo1−x(2-MeIM)2, where 1 − x/x represents the molar ratio of Co and Zn in synthesis. It is worth noting that ZIF-8, which contains only Zn2+, is white in color, and ZIF-67, which contains only Co2+, is purple in color. When Zn2+ and Co2+ coexist, the resulting ZnxCo1−x(2-MeIM)2 is lavender in color.
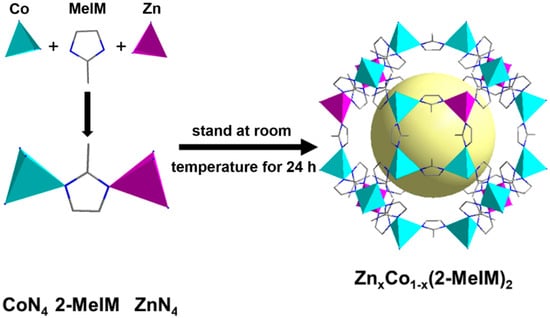
Figure 1.
Schematic representation of the crystal structure of bimetallic ZIFs (purple for Zn atoms, dark green for Co atoms, blue for nitrogen atoms, and gray for carbon atoms).
The Scanning Electron Microscope (SEM) of ZIF-67 prepared by standing at room temperature is shown in Figure 2a,b. Under low magnification SEM, it shows that the rhomboid dodecahedron is evenly distributed. Under high magnification SEM, most of them have a regular rhomboid dodecahedron appearance with clear edges and smooth surfaces. When partial Zn2+ is introduced into ZIF-67, ZnxCo1−x(2-MeIM)2 maintains the morphology of rhomboid dodecahedron, and the particles are uniformly distributed under a high-power microscope (Figure 2e,f). This suggests that the rhombodecahedral morphology of ZIF-67 can be maintained by adjusting the ratio of Co(NO3)2·6H2O to Zn(NO3)2·6H2O during synthesis, and the controlled, uniform introduction of Zn into ZIF-67 in situ can be achieved. The bimetallic MOF with special morphology synthesized by this method is more conducive to realizing the electronic regulation of the Co active site to play a synergistic catalytic effect.
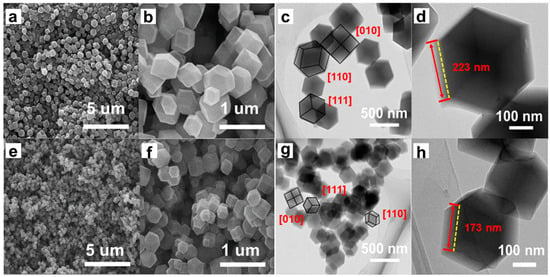
Figure 2.
The SEM of (a,b) ZIF-67 and (e,f) ZnxCo1−x(2-MeIM)2. The TEM of (c,d) ZIF-67 and (g,h) ZnxCo1−x(2-MeIM)2.
Figure 2c,d show Transmission Electron Microscope (TEM) of ZIF-67 at different magnifications. We observe a rhomboid dodecahedron with a regular shape, a smooth surface, and an edge length of 223 nm. At greater magnification, ZIF-67 is shown to grow along three different crystal directions [010], [110], and [111] (marked with black lines in Figure 2c). Figure 2g,h show the TEM of ZnxCo1−x(2-MeIM)2 at different magnifications, which also shows a rhomboid dodecahedron with regularly shaped rhombic dodecahedra, a smooth surface, and an edge length of 173 nm. Similarly, ZnxCo1−x(2-MeIM)2 also grows along three different crystal directions [010], [110], and [111], indicating that Zn introduction does not affect the crystal growth of ZIF-67. Compared with ZIF-67, Zn doping significantly shortened the edge length and reduced the size, but still maintained the original dodecahedron appearance, which may be more conducive to increasing the active site exposed at the edge. TEM analysis shows that we have successfully prepared bimetallic ZIF without damaging the crystal structure.
High-resolution transmission electron microscopy (HRTEM) was employed to further investigate the crystalline structure of ZnxCo1−x(2-MeIM)2. As shown in Figure 3a, the well-defined lattice fringes confirm the crystallinity of the synthesized material. The corresponding lattice spacing was measured and analyzed to identify specific crystallographic planes. In the higher magnification (Figure 3b), only the lattice fringes corresponding to the (311) plane of cobalt were distinctly resolved. The measured interplanar spacing aligned well with the expected value for the Co (311) plane, providing clear evidence of its presence in the crystal structure. This HRTEM analysis, revealing well-ordered lattice fringes and identifiable specific planes, conclusively demonstrates that the introduction of zinc into the framework successfully produced a bimetallic ZIF while preserving the intrinsic crystalline order of the parent ZIF-67 structure.
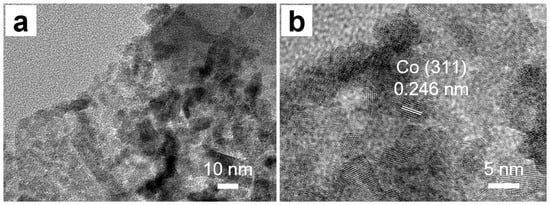
Figure 3.
(a,b) The HRTEM of ZnxCo1−x(2-MeIM)2.
After obtaining the precursors of the bimetallic complexes described above, the subsequent high-temperature annealing and phosphatization processes are of great significance for the formation of the amorphous carbon layer and the hydrogen evolution active site. All synthesis conditions were studied in detail, including high temperature calcination temperature, high temperature calcination time, gas phase phosphating temperature, gas phase phosphating time, Co/Zn atomic ratio, and sodium hypophosphite dosage. As shown in Figure 4a, the optimal temperature for high-temperature pyrolysis is 500 °C. The time of high-temperature pyrolysis may affect the formation of the amorphous carbon layer. Too short a pyrolysis time is not enough to complete the phase transition process, and too long a pyrolysis time will lead to the collapse of the catalyst structure. Therefore, the optimal pyrolysis time is 180 min (Figure 4b). In order to provide the best alkaline HER performance, the optimal conditions of gas phase phosphating treatment are: 300 °C, 60 min (Figure 4c,d). Figure 4e shows HER polarization curves obtained by pyrolysis of ZnxCo1−x(2-MeIM)2 containing different Co and Zn molar ratios (R = 0.5, 1, 3, 5, 18.79). It is shown that the best Co and Zn molar ratio is 5. In addition, the influence of the amount of NaH2PO2·H2O on HER performance was also explored, and the results showed that the optimal amount of NaH2PO2·H2O was 17 times that of the catalyst sample (Figure 4f). Based on the above experimental results, the optimal synthesis conditions for HER in 1 M KOH by using ZnxCo1−x(2-MeIM)2 as the precursor, pyrolysis and phosphatization to prepare ZnxCo1−xP@NC are as follows: The phosphating temperature was 300 °C, the phosphating time was 60 min, the pyrolysis temperature was 500 °C, the molar ratio of Co and Zn was 5, the pyrolysis time was 180 min, the mass ratio of NaH2PO2·H2O and ZnxCo1−x@NC was 17, and the synthesized catalyst was Zn0.17Co0.83P@NC according to the optimized synthesis conditions.
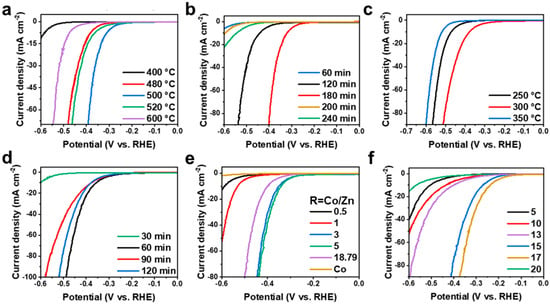
Figure 4.
Optimization of synthesis conditions in 1 M KOH: (a) high temperature calcination temperature; (b) high temperature calcination time; (c) gas phase phosphatizing temperature; (d) gas phase phosphatizing time; (e) Co/Zn atomic ratio of precursor; (f) NaH2PO2·H2O dosage (mass ratio to electrocatalyst).
There is no obvious characteristic peak in the X-ray diffraction (XRD) pattern of Zn0.17Co0.83P@NC in Figure 5a, indicating that the structure of the amorphous phase may be formed after calcination and phosphating. Due to the disorder and rich defect structure of amorphous phase materials, they can often provide unique physical and electrochemical characteristics [38], which is expected to improve the alkaline HER process. XPS was used to study the chemical composition and states of the catalyst surface. The full X-ray Photoelectron Spectroscopy (XPS) spectrum of Zn0.17Co0.83P@NC is shown in Figure 5b, showing the presence of Zn, Co, P, N, and C elements in the sample, as well as the presence of O due to surface oxidation. The valence state of the element was qualitatively analyzed by high-resolution X-ray photoelectron spectroscopy. Figure 5c shows the XPS spectrum of Zn 2p. The binding energy of 1045.3 eV is related to Zn 2p1/2, and the binding energy of 1022.2 eV is related to Zn 2p3/2. It is close to 1022.1 eV in the literature [39]. There is no characteristic peak related to ZnP in the region of Zn 2p. Figure 5d shows the Co 2p XPS spectrum, Co 2p1/2 spectrum deconvolved to Co2+ (797.6 eV) and satellite peak (803.6 eV), Co 2p3/2 spectrum deconvolved to Co2+ (781.6 eV) and satellite peak (785.0 eV), which indicates the presence of Co2+ on the sample surface. The peaks with binding energies of 130.3 and 129.3 eV on the X-ray photoelectron spectra of P 2p in Figure 5e are related to the spin states of P 2P1/2 and P 2p3/2 electrons. In addition, the peak at 133.4 eV is related to oxidized phosphatizing species, and the peak at 139.9 eV belongs to Zn 3S. Because the Zn element has a variety of inner electrons, which can generate a variety of In XPS signals. Figure 5f N 1s spectrum decoupled into two peaks, pyridinic-N (400.1 eV) and graphitic-N (398.6 eV), indicating the successful synthesis of N-doped carbon [40]. The C 1S spectrum is decoupled into four peaks, O=C-O (288.8 eV), C-O or C-N (286.4 eV), C-P or C-N (285.3 eV), and C-C (284.5 eV) (Figure 5g). O 1S deconvolved into three peaks, O-P (533.1 eV), O-C (532.0 eV), and O-CO (531.2 eV) (Figure 5h) [41].
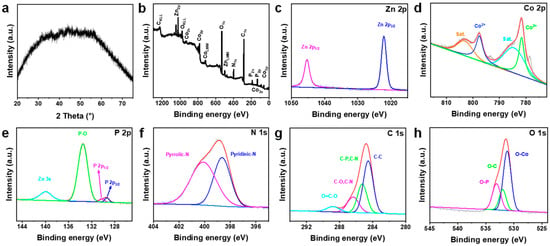
Figure 5.
(a) XRD of Zn0.17Co0.83P@NC. XPS of Zn0.17Co0.83P@NC: (b) Survey; (c) Zn 2p; (d) Co 2p; (e) P 2p; (f) N 1s; (g) C 1s and (h) O 1s.
The microstructure and element distribution of Zn0.17Co0.83P@NC were analyzed in detail by TEM. As shown in Figure 6a, the sample after calcination and phosphating treatment has an ultrathin lamellar structure. The formation of carbon nanotubes was also found at the edge of the nanosheet. This special structure can effectively expose more metal active sites and maintain a fast electron transport rate. TEM-Mapping results show that the constituent elements of Zn0.17Co0.83P@NC contain Co, Zn, P, C, N, and O (Figure 6b). As shown in Figure 6c–h, they are uniformly dispersed in the structure, which indicates that the subsequent high-temperature calcination treatment will not cause ion agglomeration. Especially for metal elements, an ideal dispersion effect is more conducive to their electronic interaction and synergistic catalytic effect.
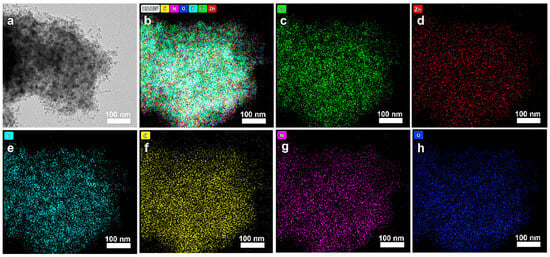
Figure 6.
(a) TEM of Zn0.17Co0.83P@NC. (b–h) TEM-Mapping of Zn0.17Co0.83P@NC (Chemical elements include: Co, Zn, P, C, N, and O).
All electrochemical tests were performed under a standard three-electrode system. As shown in Figure 7a, ZIF-8-5 had no hydrogen evolution activity. After high-temperature calcination and gas-phase phosphating, the CoP@NC obtained has certain hydrogen evolution activity. The optimized Zn0.17Co0.83P@NC has the best HER performance, and its overpotential is 223.22 mV lower than that of CoP@NC (Figure 7b). Such a huge performance improvement is due to the obvious electronic regulation brought by the introduction of Zn. However, the more Zn content in the precursor is not the better, and the most suitable Zn doping content is 2.17 at.%. The Tafel slopes of Zn0.17Co0.83P@NC and Zn0.17Co0.83@NC were between 40 and 120 mV dec−1, indicating that they performed the HER process with the Volmer–Heyrovsky mechanism. Zn doping optimizes the adsorption–desorption behavior of H* intermediates on the electrode surface [42,43]. Zn0.17Co0.83P@NC has the smallest Tafel slope, which indicates that it has the fastest hydrogen evolution reaction kinetics (Figure 7c). Then, cyclic voltammetry (CV) was performed on all the samples, and their double-layer capacitance (Cdl) images were obtained after data processing. In Figure 7d, Zn0.17Co0.83P@NC has the largest Cdl (153.65 uF cm−2), indicating that Zn doping and phosphating can provide a larger active area and expose more active sites [44]. At the same time, Zn0.17Co0.83P@NC also has the smallest interfacial charge transfer resistance (Rct), indicating that it has the fastest interfacial charge transfer rate to accelerate the HER process (Figure 7e). Thanks to the presence of an amorphous carbon layer, Zn0.17Co0.83P@NC has good stability. After 1000 cycles of voltammetry, there was no significant degradation of HER performance (Figure 7f). And after 18 h of constant voltage stability test, it can still provide a stable hydrogen evolution current. As shown in Table 1, the Zn0.17Co0.83P@NC in this work has a high-efficiency performance of alkaline HER electrocatalysis, compared with other Co/Zn-based electrocatalysts reported.
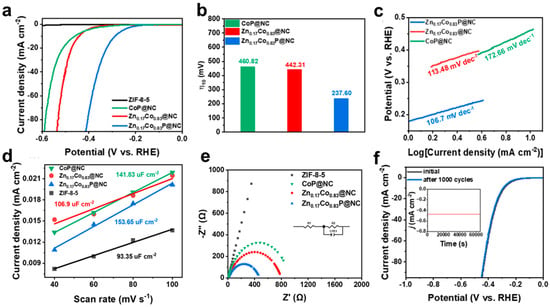
Figure 7.
Electrochemical test data in 1 M KOH:(a) LSV polarization curves, (b) overpotential (η10), (c) Tafel slope, (d) Cdl, (e) EIS of ZIF-8-5, CoP@NC, Zn0.17Co0.83@NC, Zn0.17Co0.83P@NC. (f) LSV comparison of Zn0.17Co0.83P@NC before and after 1000 cycles of CV. Chronoamperometry was performed at −0.48V vs. RHE for 18 h (insert).

Table 1.
Comparison of the HER activity of Zn0.17Co0.83P@NC with other Co/Zn-based electrocatalysts in alkaline electrolyte.
3. Experimental Section
3.1. Chemicals and Materials
Zinc nitrate hexahydrate (Zn(NO3)2·6H2O, 99%) Cobalt nitrate hexahydrate (Co(NO3)2·6H2O, 99%), Sodium hypophosphite (NaH2PO2·H2O), Methyl alcohol (CH3OH) and Anhydrous ethanol (C2H5OH, ≥99.7 wt%) were purchased from Sinopharm Chemical Reagent Co., Ltd., Shanghai, China. 2-Methylimidazole (2-MIM) was provided by Aladdin Scientific Corp. (Shanghai, China). All the experimental chemicals and materials used were of analytical purity and no further post-treatment was required before use.
3.2. CoxZn1−x(2-MeIM)2 Synthesis
0.5 mmol of Zn(NO3) 2·6H2O and 2.5 mmol of Co(NO3)2·6H2O were dissolved in 25 mL of methanol, and then 25 mL of 2-methylimidazole solution (0.48 M) was added to this solution, which was evenly dispersed by ultrasound for 20 min. It was placed on the experimental operating table without stirring at 25 °C for a day. About 25 mL of absolute ethanol was added to clean the purple substance, and centrifuged three times. The centrifuge parameters were set to 10,000 RPM, 6 min. The purple powder Co0.17Zn0.83(2-MeIM)2 was dried overnight in a vacuum drying oven at 60 °C. Co(NO3)2·6H2O was used to partially replace Zn(NO3)2·6H2O; the total molar number of the two was controlled to be 3 mmol, and the molar ratio of Co(NO3)2·6H2O/Zn(NO3)2 6H2O was changed to 1 − x/x = 0.5, 1, 5, 18.79.
3.3. CoxZn1−x@NC Synthesis
CoxZn1−x(2-MeIM)2 was raised to 500 °C in the tubular furnace in Ar atmosphere at the heating rate of 2 °C min−1 and kept at this temperature for 180 min to obtain CoxZn1−x@NC.
3.4. CoxZn1−xP@NC Synthesis
NaH2PO2·H2O is placed in the upstream, and CoxZn1−x@NC is placed in the downstream. Under the Ar atmosphere, the temperature is raised to 300 °C at the heating rate of 2 °C min−1, and the temperature is maintained for 60 min to obtain CoxZn1−xP@NC.
3.5. CoP@NC Synthesis
0.8730 g of Co(NO3)2·6H2O was dissolved in 25 mL of methanol, and then 25 mL of 2-methylimidazole solution (0.48 M) was added to this solution, which was dispersed evenly for 20 min by ultrasound, and placed on the experimental operating table for one day at 25 °C without stirring. Centrifuge parameters were set to (10,000 RPM, 6 min), about 25 mL of absolute ethanol was added to clean the purple substance, and centrifuged three times, and then dried overnight in a vacuum drying oven at 60 °C. Co(2-MeIM)2 was obtained, and then prepared according to the preparation method of ZnxCo1−xP@NC; CoP@NC was obtained.
3.6. Material Characterization
X-ray diffraction (XRD) was carried out on a Rigaku D/ Max-2500PC device (Rigaku Corporation, Tokyo, Japan) with Cu Kα radiation (λ = 1.54 A). The surface and internal morphology of the samples were investigated by scanning electron microscopy (SEM) using a Hitachi S-4800 microscope (Hitachi High-Technologies Corporation, Tokyo, Japan) and transmission electron microscopy (TEM) on an FEI Tecnai G20 instrument (FEI Company, Hillsboro, OR, USA) operating at 200 kV, respectively. X-ray photoelectron spectroscopy (XPS) was characterized using a Thermo Fisher Scientific K-Alpha spectrometer (Thermo Fisher Scientific, Waltham, MA, USA) with an Al Kα X-ray source (1486.6 eV).
3.7. Electrochemical Measurements
The HER performance of all catalysts was tested using a Gamry Reference 600 electrochemical device (Gamry Instruments, Warminster, PA, USA) in a standard three-electrode system. Specifically, a platinum sheet, a saturated calomel electrode, and a platinum carbon electrode dispersed with a catalyst were used as a counter electrode, a reference electrode, and a working electrode. All tests were performed in a standard alkaline electrolyte of 1 M KOH at room temperature. Linear sweep voltammetry (LSV) curves were obtained at a scan rate of 5 mV s−1. According to the Nernst equation:ERHE = ESCE + 0.059 pH + 0.242, all potentials with SCE were converted to standard reversible hydrogen electrode (RHE). Electrochemical impedance spectroscopy (EIS) was performed at a potential of −0.35 V (vs. SCE), and the frequency range was 105 to 0.1 Hz. Chronoamperometry was used to test the stability of the catalyst. Cyclic voltammetry (CV) tests were conducted at various scan rates, within a range devoid of faradaic processes, to determine the electrochemical double-layer capacitance (Cdl). The Cdl was calculated by plotting half the difference in current density (Δ j/2) against the scan rates.
4. Conclusions
In this study, a facile bimetallic covalent coordination strategy was reported to prepare nitrogen-doped carbon-coated Zn0.17Co0.83P@NC phosphide as a highly efficient alkaline HER electrocatalyst. When the current density reached 10 mA cm−2, the overpotential of Zn0.17Co0.83P@NC was 237.60 mV, 223.22 mV lower than that of CoP@NC. This well reflects that the electronic structure regulation effect caused by a small amount of Zn doping plays a key role in improving HER performance. In addition, the amorphous carbon layer obtained after high-temperature annealing can effectively avoid phosphorus loss and ensure excellent stability of the catalyst. This work provides a new design idea for the preparation of mixed-phase phosphide materials using bimetallic ZIF as the precursor as an efficient HER catalyst.
Author Contributions
G.-P.S.: Conceptualization, Formal analysis, Investigation, Writing—original draft, Visualization, Funding acquisition. X.-M.M.: data curation, Validation, Writing—review and editing. S.-J.G.: Formal analysis, Investigation, Writing—original draft, N.X.: data curation, Formal analysis, B.D.: Supervision, Project administration, Funding acquisition. All authors have read and agreed to the published version of the manuscript.
Funding
This work is financially supported by National Natural Science Foundation of China (22479161) and the Fundamental Research Funds for the Central Universities (24CX03012A).
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding authors.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Zhu, J.; Deng, C.; Xia, R.; Zhao, P.; Li, N.; Zhu, S.; Zhu, W.; Zhang, H.; Shen, D.; Lin, R. Biomimetic Engineering of Interfacial Hydrogen-Bond Network to Boost Proton Transfer for High-Performance Alkaline Water Electrolysis. Adv. Energy Mater. 2025, 15, e03403. [Google Scholar] [CrossRef]
- Zhong, X.; Hou, C.; Chen, Y.; Zhang, Z.; Li, Y.; Gan, T.; Liu, K.; Gao, Q.; Liu, B.; Huang, Y.; et al. Unraveling the Contrasting Dynamics of Reconstruction in Wolframite Cobalt Molybdate Polymorphs for Oxygen Evolution Reaction Electrocatalysis. ACS Catal. 2025, 15, 11958–11969. [Google Scholar] [CrossRef]
- Zhao, H.; Deng, K.; Liu, X.; Liu, P.; Lv, X.; Tian, W.; Ji, J. Promoted OH− Adsorption and Cl− Repulsion Ability of Ce-Decorated NiFe@CuO for Alkaline Seawater Electrolysis. Adv. Funct. Mater. 2025, 2508539. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, J.; Guan, P.; Ye, Q.; Zhao, Y.; Cheng, Y. Room-temperature synthesis of NiFe-hexamethylenetetramine as lattice oxygen involved electrocatalyst for efficient oxygen evolution reaction. J. Colloid Interface Sci. 2025, 690, 137287. [Google Scholar] [CrossRef]
- Zhu, K.; Yang, H.; Guo, G.; Wang, Y.; Tan, W.; Ma, F.; Zhang, H.; Peng, S. Pd Single Atoms/Clusters at the Oxygen Defect-Rich WOx_C Nanowire Structure Facilitate H* Adsorption and Desorption for Efficient and Stable Hydrogen Evolution Reaction. ACS Catal. 2025, 15, 9563–9573. [Google Scholar] [CrossRef]
- Zhu, A.; Qiao, L.; Liu, K.; Gan, G.; Luan, C.; Lin, D.; Zhou, Y.; Bu, S.; Zhang, T.; Liu, K.; et al. Rational design of precatalysts and controlled evolution of catalyst-electrolyte interface for efficient hydrogen production. Nat. Commun. 2025, 16, 1880. [Google Scholar] [CrossRef]
- Xue, L.; Wang, B.; Hu, J.; Hou, C.; Chen, C.; Zhu, Z.; Lv, X.; Dang, J. Dynamic Self-Optimizing Reconstruction of Stainless Steel Fe-Ni Dual Sites Accelerates Catalytic Intermediate Coupling for High-Efficiency Oxygen Evolution Reaction at Industrial Current Density. Adv. Funct. Mater. 2025, e16748. [Google Scholar] [CrossRef]
- Xia, L.; Gomes, B.F.; Jiang, W.; Escalera-López, D.; Wang, Y.; Hu, Y.; Faid, A.Y.; Wang, K.; Chen, T.; Zhao, K.; et al. Operando-informed precatalyst programming towards reliable high-current-density electrolysis. Nat. Mater. 2025, 24, 753–761. [Google Scholar] [CrossRef]
- Zhou, S.; Cao, W.; Shang, L.; Zhao, Y.; Xiong, X.; Sun, J.; Zhang, T.; Yuan, J. Facilitating alkaline hydrogen evolution kinetics via interfacial modulation of hydrogen-bond networks by porous amine cages. Nat. Commun. 2025, 16, 1849. [Google Scholar] [CrossRef]
- Zhang, Y.-Q.; Liu, M.; Wang, H.-Y.; Zhang, X.; Li, Z.-G.; Li, W.; Li, N.; Bu, X.-H. Interfacial Ni–O–Ru Bridges Manipulate d-Band Center of Dual-Metal Site Catalysts for Efficient Water Splitting. ACS Catal. 2025, 15, 11082–11092. [Google Scholar] [CrossRef]
- Zhang, H.; Li, N.; Gao, S.; Chen, A.; Qian, Q.; Kong, Q.; Xia, B.Y.; Hu, G. Quenching-induced atom-stepped bimetallic sulfide heterointerface catalysts for industrial hydrogen generation. eScience 2025, 5, 100311. [Google Scholar] [CrossRef]
- Wen, Q.; Liu, T.; Huang, D.; Lin, Y.; Yang, Z.; Yang, R.; Liu, Y.; Ai, X.; Fang, J.; Li, Y.; et al. Reversible Structural Oscillation Mediates Stable Oxygen Evolution Reaction. Angew. Chem. Int. Ed. 2025, 64, e202509915. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, M.; Wang, B.; Meng, X.; Dong, B.; Hojamberdiev, M.; Pan, Y.; Sun, G.; Zhang, H.; Jianrong, Z.; et al. Ultrafast reconstruction of Ni-based alloy precatalyst for bobust seawater oxidation. Adv. Funct. Mater. 2025, 35, 2509590. [Google Scholar] [CrossRef]
- Liu, T.; Chen, Y.; Wang, X.; Di, Y.; Müllen, K.; Zhang, Z.; Wang, F. Rare-Earth Oxychlorides as Promoters of Ruthenium Toward High-Performance Hydrogen Evolution Electrocatalysts for Alkaline Electrolyzers. Adv. Mater. 2025, 37, 2417621. [Google Scholar] [CrossRef]
- Liang, X.; Zhang, Z.; Wang, Z.; Hu, M.; Cheng, D.; Jiang, Y.; Ren, H.; Shen, F.; Yang, S.; Yang, X.; et al. Breaking the H2O dissociation-OH desorption scaling relationship in alkaline hydrogen evolution by oxophilic single atom M1–Run electrocatalysts. Energy Environ. Sci. 2025, 18, 4302–4311. [Google Scholar] [CrossRef]
- Nickel, C.; Troglauer, D.L.; Dallos, Z.; Abid, D.; Sowa, K.; Cichocka, M.O.; Kolb, U.; Mashtakov, B.; Mohazzab, B.F.; Han, S.; et al. Self-optimizing Cobalt Tungsten Oxide Electrocatalysts toward Enhanced Oxygen Evolution in Alkaline Media. Angew. Chem. Int. Ed. 2025, 64, e202424074. [Google Scholar] [CrossRef]
- Ma, Y.; Wang, J.-J.; Su, P.-j.; Gao, Z.-Y.; Li, X.; Wang, Y.-H.; Zhao, L.-M.; Chai, Y.-M.; Dong, B. Highly active FeNbO4/NiFeOOH heterojunction induced by coordination activation for efficient and stable industrial water oxidation. J. Colloid Interface Sci. 2025, 688, 67–78. [Google Scholar] [CrossRef]
- Ma, P.; Xue, J.; Li, J.; Cao, H.; Wang, R.; Zuo, M.; Zhang, Z.; Bao, J. Site-specific synergy in heterogeneous single atoms for efficient oxygen evolution. Nat. Commun. 2025, 16, 2573. [Google Scholar] [CrossRef]
- Li, W.; Ni, Z.; Akdim, O.; Liu, T.; Zhu, B.; Kuang, P.; Yu, J. Dual Active Site Engineering in Porous NiW Bimetallic Alloys for Enhanced Alkaline Hydrogen Evolution Reaction. Adv. Mater. 2025, 37, 2503742. [Google Scholar] [CrossRef]
- Dong, A.; Lin, G.; Li, Z.; Wu, W.; Cao, X.; Li, W.; Wang, L.; Zhao, Y.; Chen, D.; Sun, L. Interlayer-bonded Ni/MoO2 electrocatalyst for efficient hydrogen evolution reaction with stability over 6000 h at 1000 mA cm−2. Nat. Commun. 2025, 16, 4955. [Google Scholar] [CrossRef]
- Zha, D.; Wang, R.; Tian, S.; Jiang, Z.-J.; Xu, Z.; Qin, C.; Tian, X.; Jiang, Z. Defect engineering and carbon supporting to achieve Ni-doped CoP3 with high catalytic activities for overall water splitting. Nano-Micro Lett. 2024, 16, 250. [Google Scholar] [CrossRef]
- Zhang, W.; Yang, L.; Li, Z.; Nie, G.; Cao, X.; Fang, Z.; Wang, X.; Ramakrishna, S.; Long, Y.; Jiao, L. Regulating Hydrogen/Oxygen Species Adsorption via Built-in Electric Field -Driven Electron Transfer Behavior at the Heterointerface for Efficient Water Splitting. Angew. Chem. Int. Ed. 2024, 63, e202400888. [Google Scholar] [CrossRef]
- Zhang, Q.; Xiao, W.; Shi, J.X.; Lei, J.L.; Xiao, Q.; Luo, H.Q.; Li, N.B. Dynamic Molybdate Oxyanion Boosts Self-Optimization and Self-Healing on the NiMoFe Heterostructure for Water Splitting in Alkaline Media. ACS Catal. 2024, 14, 18003–18017. [Google Scholar] [CrossRef]
- Liu, Y.; Yao, R.-Q.; Chen, L.-B.; Dai, T.-Y.; Sun, X.-Y.; Zeng, S.-P.; Zhou, Z.-L.; Wang, Y.; Han, G.-F.; Wang, T.-H.; et al. Phase-Reconstruction of S-Doped (NiCo)6W6C for Efficient and Stable Oxygen Evolution Reaction Electrocatalysis. Nano Lett. 2025, 25, 13866–13874. [Google Scholar] [CrossRef]
- Ahmed, M.G.; Tay, Y.F.; Chi, X.; Razeen, A.S.; Fang, Y.; Zhang, M.; Sng, A.; Chiam, S.Y.; Rusydi, A.; Wong, L.H. Cation Migration-Induced Lattice Oxygen Oxidation in Spinel Oxide for Superior Oxygen Evolution Reaction. Angew. Chem. Int. Ed. 2024, 64, e202416757. [Google Scholar] [CrossRef]
- Zeng, S.-P.; Shi, H.; Dai, T.-Y.; Liu, Y.; Wen, Z.; Han, G.-F.; Wang, T.-H.; Zhang, W.; Lang, X.-Y.; Zheng, W.-T.; et al. Lamella-heterostructured nanoporous bimetallic iron-cobalt alloy/oxyhydroxide and cerium oxynitride electrodes as stable catalysts for oxygen evolution. Nat. Commun. 2023, 14, 1811. [Google Scholar] [CrossRef]
- Guo, Q.; Li, Y.; Xu, Z.; Liu, R. CeO2-Accelerated Surface Reconstruction of CoSe2 Nanoneedle Forms Active CeO2@CoOOH Interface to Boost Oxygen Evolution Reaction for Water Splitting. Adv. Energy Mater. 2024, 15, 2403744. [Google Scholar] [CrossRef]
- Zhang, J.; Cheng, C.; Xiao, L.; Han, C.; Zhao, X.; Yin, P.; Dong, C.; Liu, H.; Du, X.; Yang, J. Construction of Co–Se–W at Interfaces of Phase-Mixed Cobalt Selenide via Spontaneous Phase Transition for Platinum-Like Hydrogen Evolution Activity and Long-Term Durability in Alkaline and Acidic Media. Adv. Mater. 2024, 36, 2401880. [Google Scholar] [CrossRef]
- Yang, Q.; Li, Y.; Wu, Y.; Li, Y.; Yang, C.; Ban, L.; Zhao, Y.; Dai, B.; Wang, G.; Li, Y.; et al. A plasma-triggered N–Co–O–Fe motif in Co(OH)2 for efficient electrocatalytic oxygen evolution. EES Catal. 2025, 3, 407–419. [Google Scholar] [CrossRef]
- Li, C.; Ye, B.; Ouyang, B.; Zhang, T.; Tang, T.; Qiu, Z.; Li, S.; Li, Y.; Chen, R.; Wen, W.; et al. Dual Doping of N and F on Co3O4 to Activate the Lattice Oxygen for Efficient and Robust Oxygen Evolution Reaction. Adv. Mater. 2025, 37, 2501381. [Google Scholar] [CrossRef]
- Qu, M.-R.; Cheng, Y.-X.; Feng, S.-H.; Xu, J.; Yao, J.-K.; Yan, W.-S.; Zhu, S.; Cao, L.; Wu, R.; Yu, S.-H. Ordered interfacial domain expansion catalysis enhances hydrogen evolution for proton exchange membrane electrolysis. Energy Environ. Sci. 2025, 18, 5985–5997. [Google Scholar] [CrossRef]
- Li, Z.; Wang, Y.; Liu, H.; Feng, Y.; Du, X.; Xie, Z.; Zhou, J.; Liu, Y.; Song, Y.; Wang, F.; et al. Electroreduction-driven distorted nanotwins activate pure Cu for efficient hydrogen evolution. Nat. Mater. 2025, 24, 424–432. [Google Scholar] [CrossRef]
- Chen, W.; Xu, C.; Yu, H.; Huang, H.; Li, S.; Cao, Y.; Peng, W.; Li, Y.; Ke, H.; Xu, S.; et al. Hydroxyl Spillover Activated from the Strongly Coupled Ru@Mn3O4 Heterostructure to Promote Alkaline Hydrogen Evolution. Angew. Chem. Int. Ed. 2025, 64, e202504667. [Google Scholar] [CrossRef]
- Yue, J.; Li, Y.; Yang, C.; Luo, W. Hydroxyl-Binding Induced Hydrogen Bond Network Connectivity on Ru-based Catalysts for Efficient Alkaline Hydrogen Oxidation Electrocatalysis. Angew. Chem. Int. Ed. 2024, 64, e202415447. [Google Scholar] [CrossRef]
- Poudel, M.B.; Yu, C.; Kim, H.J. Synthesis of Conducting Bifunctional Polyaniline@Mn-TiO2 Nanocomposites for Supercapacitor Electrode and Visible Light Driven Photocatalysis. Catalysts 2020, 10, 546. [Google Scholar] [CrossRef]
- Perumal, S.; Chandra Kishore, S.; Atchudan, R.; Sundramoorthy, A.K.; Alagan, M.; Lee, Y.R. Sustainable Synthesis of N/S-Doped Porous Carbon from Waste-Biomass as Electroactive Material for Energy Harvesting. Catalysts 2022, 12, 436. [Google Scholar] [CrossRef]
- Yu, T.; Gao, P.; Du, H.; Dong, L. Promoting electrocatalytic water oxidation via crafting Co–O–W bridge bonds on an amorphous core/shell NiCo-ZIF@POM catalyst. Inorg. Chem. Front. 2024, 11, 6661–6670. [Google Scholar] [CrossRef]
- Yao, B.; Chen, Y.; Yan, Y.; Yang, Y.; Xing, H.; Xu, Y.; Jiao, D.; Xing, Z.; Wang, D.; Yang, X. Iron-Induced Localized Oxide Path Mechanism Enables Efficient and Stable Water Oxidation. Angew. Chem. Int. Ed. 2024, 64, e202416141. [Google Scholar] [CrossRef]
- Mei, Y.; Chen, J.; Wang, Q.; Guo, Y.; Liu, H.; Shi, W.; Lin, C.; Yuan, Y.; Wang, Y.; Xia, B.; et al. MoZn-based high entropy alloy catalysts enabled dual activation and stabilization in alkaline oxygen evolution. Sci. Adv. 2024, 10, eadq6758. [Google Scholar] [CrossRef]
- Wang, S.; Lv, H.; Bi, S.; Li, T.; Sun, Y.; Ji, W.; Feng, C.; Zhang, C. Defects tailoring IrO2@TiN1+x nano-heterojunctions for superior water oxidation activity and stability. Mater. Chem. Front. 2021, 5, 8047–8055. [Google Scholar] [CrossRef]
- Chen, H.; Yu, J.; Liu, L.; Gao, R.T.; Gao, Z.; Yang, Y.; Chen, Z.; Zhan, S.; Liu, X.; Zhang, X.; et al. Modulating Pt-N/O Bonds on Co-doped WO3 for Acid Electrocatalytic Hydrogen Evolution with Over 2000 h Operation. Adv. Energy Mater. 2024, 14, 2303635. [Google Scholar] [CrossRef]
- Zhang, W.; Xu, G.R.; Sun, T.; Li, J.; Miao, Y.; Wu, Z.; Zhang, Y.; Wang, L. Electron-Rich Ru Atoms in Loaded Ru4Fe Intermetallic Compounds/C Modulate the Kinetics of Neutral Hydrogen Evolution. Adv. Funct. Mater. 2025, 35, 2501508. [Google Scholar] [CrossRef]
- Wang, L.-L.; Wang, X.-R.; Wang, H.-J.; Zhang, C.; Li, J.-J.; Feng, G.-J.; Cheng, X.-X.; Qin, X.-R.; Yu, Z.-Y.; Lu, T.-B. Tailoring Lewis Acidity of Metal Oxides on Nickel to Boost Electrocatalytic Hydrogen Evolution in Neutral Electrolyte. J. Am. Chem. Soc. 2025, 147, 7555–7563. [Google Scholar] [CrossRef]
- Wan, L.; Wang, H.; Zeng, B.; Wang, W.; Liu, X.; Hu, Y.; Cao, L.; Cui, Z.; Dong, B. Surface-confined Growth of Ru Amorphous Sub-nanoclusters on Reductive Mn3O4: A Strongly Coupled Interface Engineering for Efficient Neutral Hydrogen Production. Energy Environ. Sci. 2025, 18, 4262–4275. [Google Scholar] [CrossRef]
- Wang, Y.; Pan, Y.; Zhu, L.; Yu, H.; Duan, B.; Wang, R.; Zhang, Z.; Qiu, S. Solvent-free assembly of Co/Fe-containing MOFs derived N-doped mesoporous carbon nanosheets for ORR and HER. Carbon 2019, 146, 671–679. [Google Scholar] [CrossRef]
- Lu, L.; Zhang, Y.; Chen, Z.; Feng, F.; Teng, K.; Zhang, S.; Zhuang, J.; An, Q. Synergistic promotion of HER and OER by alloying ternary Zn-Co-Ni nanoparticles in N-doped carbon interfacial structures. Chin. J. Catal. 2022, 43, 1316–1323. [Google Scholar] [CrossRef]
- Belhadj, H.; Messaoudi, Y.; Khelladi, M.R.; Azizi, A. A facile synthesis of metal ferrites (MFe2O4, M = Co, Ni, Zn, Cu) as effective electrocatalysts toward electrochemical hydrogen evolution reaction. Int. J. Hydrogen Energy 2022, 47, 20129–20137. [Google Scholar] [CrossRef]
- Cao, L.; Zhang, Y.; Feng, L.; He, D.; Liu, Q.; Gong, Y.; Li, G.; Huang, J. A three-dimensional coral-like Zn,O-codoped Ni3S2 electrocatalyst for efficient overall water splitting at a large current density. Sustain. Energy Fuels 2022, 6, 466–473. [Google Scholar] [CrossRef]
- Ibrahim, M.M.; Mezni, A.; Alsawat, M.; Kumeria, T.; Alrooqi, A.; Shaltout, A.A.; Ahmed, S.I.; Boukherroub, R.; Amin, M.A.; Altalhi, T. Crystalline ZnO and ZnO/TiO2 nanoparticles derived from tert-butyl N-(2 mercaptoethyl)carbamatozinc(II) chelate: Electrocatalytic studies for H2 generation in alkaline electrolytes. Int. J. Energy Res. 2020, 44, 6725–6744. [Google Scholar] [CrossRef]
- Wang, R.; Yuan, Q.; Sun, P.; Nie, R.; Wang, X. Tuning the active sites in the cobalt-based nitrogen-doped carbon by zinc for enhancing hydrogen evolution reaction. J. Alloys Compd. 2019, 789, 100–107. [Google Scholar] [CrossRef]
- Liu, W.; Zhou, Y.; Bao, J.; Wang, J.; Zhang, Y.; Sheng, X.; Xue, Y.; Guo, C.; Chen, X. Co-CoO/ZnFe2O4 encapsulated in carbon nanowires derived from MOFs as electrocatalysts for hydrogen evolution. J. Colloid Interface Sci. 2020, 561, 620–628. [Google Scholar] [CrossRef]
- Wang, Y.; He, Y.-C.; Zhao, F.-H.; Zhu, K.; Li, J.; Kan, W.-Q.; Jing, Z.; You, J. Synthesis, structure, fluorescence and electrochemical properties of a new Zn(ii)–organic framework constructed by a tricarboxylic acid ligand. New J. Chem. 2019, 43, 13635–13641. [Google Scholar] [CrossRef]
- Dong, B.; Xie, J.-Y.; Wang, N.; Gao, W.-K.; Ma, Y.; Chen, T.-S.; Yan, X.-T.; Li, Q.-Z.; Zhou, Y.-L.; Chai, Y.-M. Zinc ion induced three-dimensional Co9S8 nano-neuron network for efficient hydrogen evolution. Renew. Energy 2020, 157, 415–423. [Google Scholar] [CrossRef]
- Amin, M.A.; Ibrahim, M.M.; Gobouri, A.A.; Mersal, G.A.M.; Mostafa, N.Y.; Altalhi, T.; Al-Juaid, S. A newly synthesized single crystal zinc complex as molecular electrocatalyst for efficient hydrogen generation from neutral aqueous solutions. Int. J. Hydrogen Energy 2017, 42, 25980–25995. [Google Scholar] [CrossRef]
- Shi, W.; Liu, H.; Zhang, J.; Shen, S.; Wang, Y.; Guo, Y.; Yue, K.; Liang, Z.; Zhang, H.; Zhang, L.; et al. Roll-to-roll synthesis of multielement heterostructured catalysts. Nat. Synth. 2025, 4, 36–847. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).