Ce3+/Ce4+-Modified TiO2 Nanoflowers: Boosting Solar Photocatalytic Efficiency
Abstract
1. Introduction
2. Results and Discussion
2.1. Morphology and Structure
2.2. Optical Properties
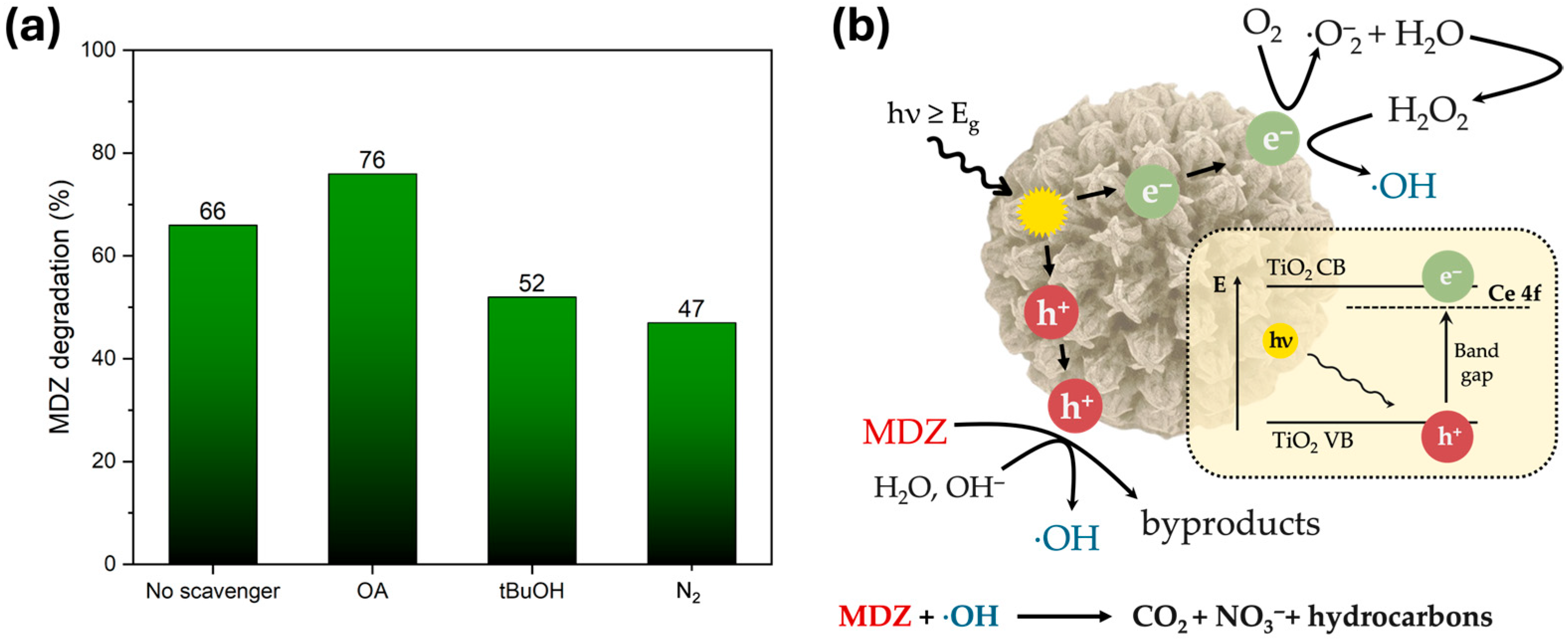
2.3. Photocatalytic Activity Evaluation
3. Materials and Methods
3.1. Materials
3.2. Synthesis of Bare and Ce-Doped TiO2 Nanoflowers
3.3. Material Characterization
3.4. Photocatalytic Evaluation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Salehi, M. Global Water Shortage and Potable Water Safety; Today’s Concern and Tomorrow’s Crisis. Environ. Int. 2022, 158, 106936. [Google Scholar] [CrossRef] [PubMed]
- Intergovernmental Panel on Climate Change (IPCC) Water. Climate Change 2022—Impacts, Adaptation and Vulnerability: Working Group II Contribution to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK, 2023; ISBN 978-1-009-32584-4. [Google Scholar]
- Hama Aziz, K.H.; Mustafa, F.S.; Karim, M.A.H.; Hama, S. Pharmaceutical Pollution in the Aquatic Environment: Advanced Oxidation Processes as Efficient Treatment Approaches: A Review. Mater. Adv. 2025, 6, 3433–3454. [Google Scholar] [CrossRef]
- Weir, C.B.; Le, J.K. Metronidazole. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Lushaj, E.; Bordin, M.; Akbar, K.; Liccardo, L.; Barroso-Martín, I.; Rodríguez-Castellón, E.; Vomiero, A.; Moretti, E.; Polo, F. Highly Efficient Solar-Light-Driven Photodegradation of Metronidazole by Nickel Hexacyanoferrate Nanocubes Showing Enhanced Catalytic Performances. Small Methods 2025, 9, 2301541. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-L.; Gómez-Avilés, A.; Zhang, S.; Rodriguez, J.J.; Bedia, J.; Belver, C. Metronidazole Photodegradation under Solar Light with UiO-66-NH2 Photocatalyst: Mechanisms, Pathway, and Toxicity Assessment. J. Environ. Chem. Eng. 2023, 11, 109744. [Google Scholar] [CrossRef]
- Ma, D.; Yi, H.; Lai, C.; Liu, X.; Huo, X.; An, Z.; Li, L.; Fu, Y.; Li, B.; Zhang, M.; et al. Critical Review of Advanced Oxidation Processes in Organic Wastewater Treatment. Chemosphere 2021, 275, 130104. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, S. Reactive Species in Advanced Oxidation Processes: Formation, Identification and Reaction Mechanism. Chem. Eng. J. 2020, 401, 126158. [Google Scholar] [CrossRef]
- Gupta, T.; Samriti; Cho, J.; Prakash, J. Hydrothermal Synthesis of TiO2 Nanorods: Formation Chemistry, Growth Mechanism, and Tailoring of Surface Properties for Photocatalytic Activities. Mater. Today Chem. 2021, 20, 100428. [Google Scholar] [CrossRef]
- Guo, Q.; Zhou, C.; Ma, Z.; Yang, X. Fundamentals of TiO2 Photocatalysis: Concepts, Mechanisms, and Challenges. Adv. Mater. 2019, 31, 1901997. [Google Scholar] [CrossRef] [PubMed]
- Shani, B.; Liccardo, L.; Bordin, M.; Martín, I.B.; Infantes-Molina, A.; Rodríguez-Castellón, E.; Ibrahim, K.B.; Vomiero, A.; Moretti, E. Ce3+/Ce4+–TiO2 Nano-Octahedra as Active Photocatalysts for Ciprofloxacin Photodegradation Under Solar Light. Adv. Sustain. Syst. 2024, 8, 2400375. [Google Scholar] [CrossRef]
- Liccardo, L.; Bordin, M.; Sheverdyaeva, P.M.; Belli, M.; Moras, P.; Vomiero, A.; Moretti, E. Surface Defect Engineering in Colored TiO2 Hollow Spheres Toward Efficient Photocatalysis. Adv. Funct. Mater. 2023, 33, 2212486. [Google Scholar] [CrossRef]
- Alberoni, C.; Barroso-Martín, I.; Infantes-Molina, A.; Rodríguez-Castellón, E.; Talon, A.; Zhao, H.; You, S.; Vomiero, A.; Moretti, E. Ceria Doping Boosts Methylene Blue Photodegradation in Titania Nanostructures. Mater. Chem. Front. 2021, 5, 4138–4152. [Google Scholar] [CrossRef]
- Li, F.B.; Li, X.Z.; Hou, M.F.; Cheah, K.W.; Choy, W.C.H. Enhanced Photocatalytic Activity of Ce3+–TiO2 for 2-Mercaptobenzothiazole Degradation in Aqueous Suspension for Odour Control. Appl. Catal. A Gen. 2005, 285, 181–189. [Google Scholar] [CrossRef]
- Kityakarn, S.; Worayingyong, A.; Suramitr, A.; Smith, M.F. Ce-Doped Nanoparticles of TiO2: Rutile-to-Brookite Phase Transition and Evolution of Ce Local-Structure Studied with XRD and XANES. Mater. Chem. Phys. 2013, 139, 543–549. [Google Scholar] [CrossRef]
- Khan, S.; Naushad, M.; Govarthanan, M.; Iqbal, J.; Alfadul, S.M. Emerging Contaminants of High Concern for the Environment: Current Trends and Future Research. Environ. Res. 2022, 207, 112609. [Google Scholar] [CrossRef] [PubMed]
- Eskandari, P.; Amarloo, E.; Zangeneh, H.; Rezakazemi, M.; Aminabhavi, T.M. Photocatalytic Degradation of Metronidazole and Oxytetracycline by Novel L-Arginine (C, N Codoped)-TiO2/g-C3N4: RSM Optimization, Photodegradation Mechanism, Biodegradability Evaluation. Chemosphere 2023, 337, 139282. [Google Scholar] [CrossRef] [PubMed]
- Farzadkia, M.; Bazrafshan, E.; Esrafili, A.; Yang, J.-K.; Shirzad-Siboni, M. Photocatalytic Degradation of Metronidazole with Illuminated TiO2 Nanoparticles. J. Environ. Health Sci. Eng. 2015, 13, 35. [Google Scholar] [CrossRef] [PubMed]
- Schlumberger, C.; Thommes, M. Characterization of Hierarchically Ordered Porous Materials by Physisorption and Mercury Porosimetry—A Tutorial Review. Adv. Mater. Interfaces 2021, 8, 2002181. [Google Scholar] [CrossRef]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of Gases in Multimolecular Layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Kubelka, P.; Munk, F. An Article on Optics of Paint Layers. Z. Tech. Phys. 1931, 12, 593. [Google Scholar]
- Makuła, P.; Pacia, M.; Macyk, W. How To Correctly Determine the Band Gap Energy of Modified Semiconductor Photocatalysts Based on UV–Vis Spectra. J. Phys. Chem. Lett. 2018, 9, 6814–6817. [Google Scholar] [CrossRef] [PubMed]
- Lakowicz, J.R. (Ed.) Instrumentation for Fluorescence Spectroscopy. In Principles of Fluorescence Spectroscopy; Springer: Boston, MA, USA, 2006; pp. 27–61. ISBN 978-0-387-31278-1. [Google Scholar]




| Sample | Intensity (a.u.) | ns-Lifetime <τ> (ns) |
|---|---|---|
| TNF150 | 2.0 × 10−6 ± 4.8 × 10−7 | 2.48 ± 0.09 |
| Ce03 | 4.1 × 10−7 ± 1.2 × 10−7 | -- |
| Ce05 | 4.6 × 10−7 ± 1.3 × 10−7 | -- |
| Ce1 | 6.2 × 10−7 ± 1.9 × 10−7 | 1.45 ± 0.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Polido, B.; Liccardo, L.; Cattaneo, B.; Rodríguez-Castellón, E.; Vomiero, A.; Moretti, E. Ce3+/Ce4+-Modified TiO2 Nanoflowers: Boosting Solar Photocatalytic Efficiency. Catalysts 2025, 15, 1069. https://doi.org/10.3390/catal15111069
Polido B, Liccardo L, Cattaneo B, Rodríguez-Castellón E, Vomiero A, Moretti E. Ce3+/Ce4+-Modified TiO2 Nanoflowers: Boosting Solar Photocatalytic Efficiency. Catalysts. 2025; 15(11):1069. https://doi.org/10.3390/catal15111069
Chicago/Turabian StylePolido, Beatrice, Letizia Liccardo, Benedetta Cattaneo, Enrique Rodríguez-Castellón, Alberto Vomiero, and Elisa Moretti. 2025. "Ce3+/Ce4+-Modified TiO2 Nanoflowers: Boosting Solar Photocatalytic Efficiency" Catalysts 15, no. 11: 1069. https://doi.org/10.3390/catal15111069
APA StylePolido, B., Liccardo, L., Cattaneo, B., Rodríguez-Castellón, E., Vomiero, A., & Moretti, E. (2025). Ce3+/Ce4+-Modified TiO2 Nanoflowers: Boosting Solar Photocatalytic Efficiency. Catalysts, 15(11), 1069. https://doi.org/10.3390/catal15111069









