Additive Manufacturing of CaO-Pt/Al2O3 Structured Catalysts for Cyclohexane Dehydrogenation
Abstract
1. Introduction
2. Results and Discussion
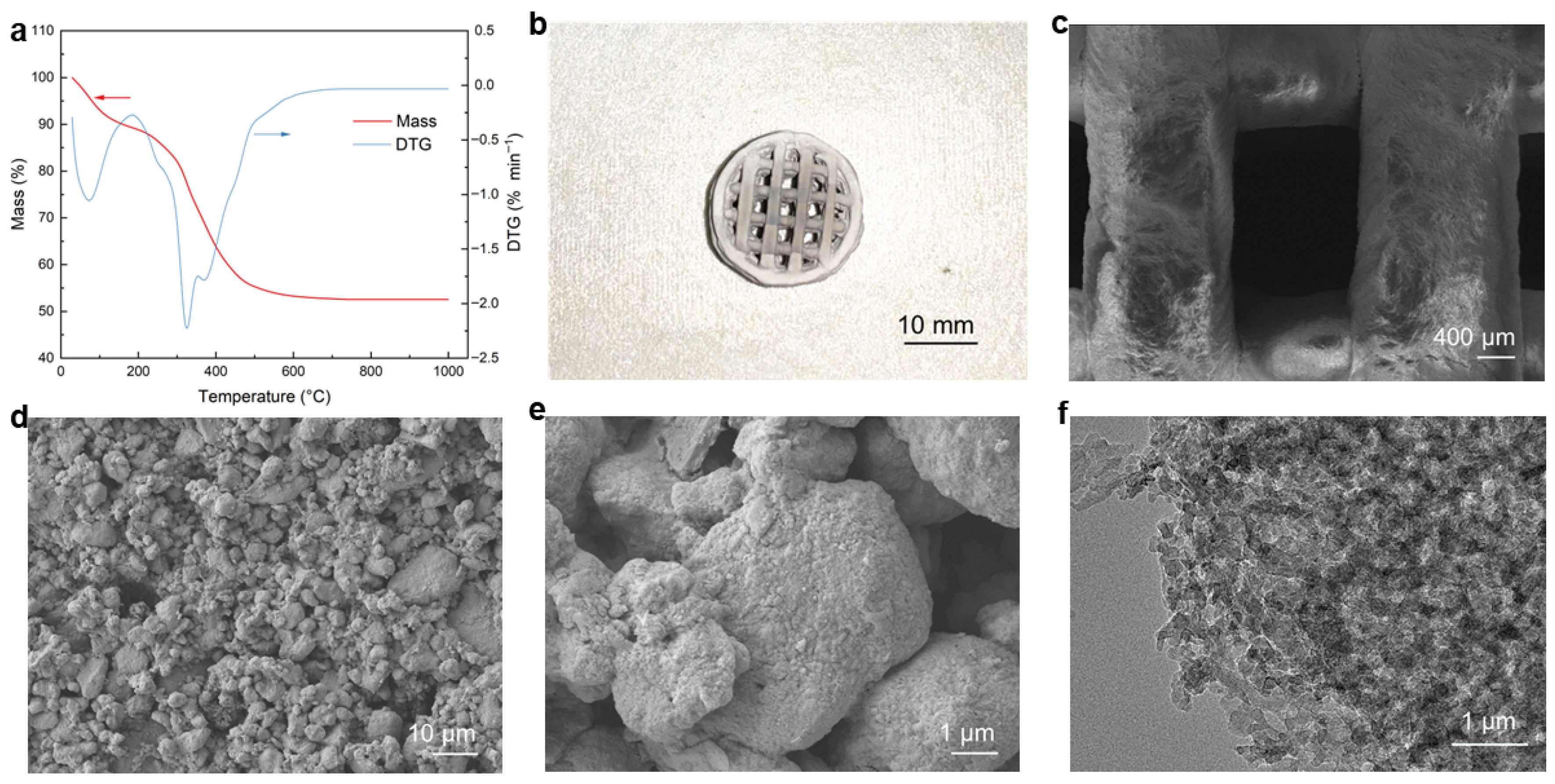
2.1. Three-Dimensional Printing, Debinding and Pore Analysis
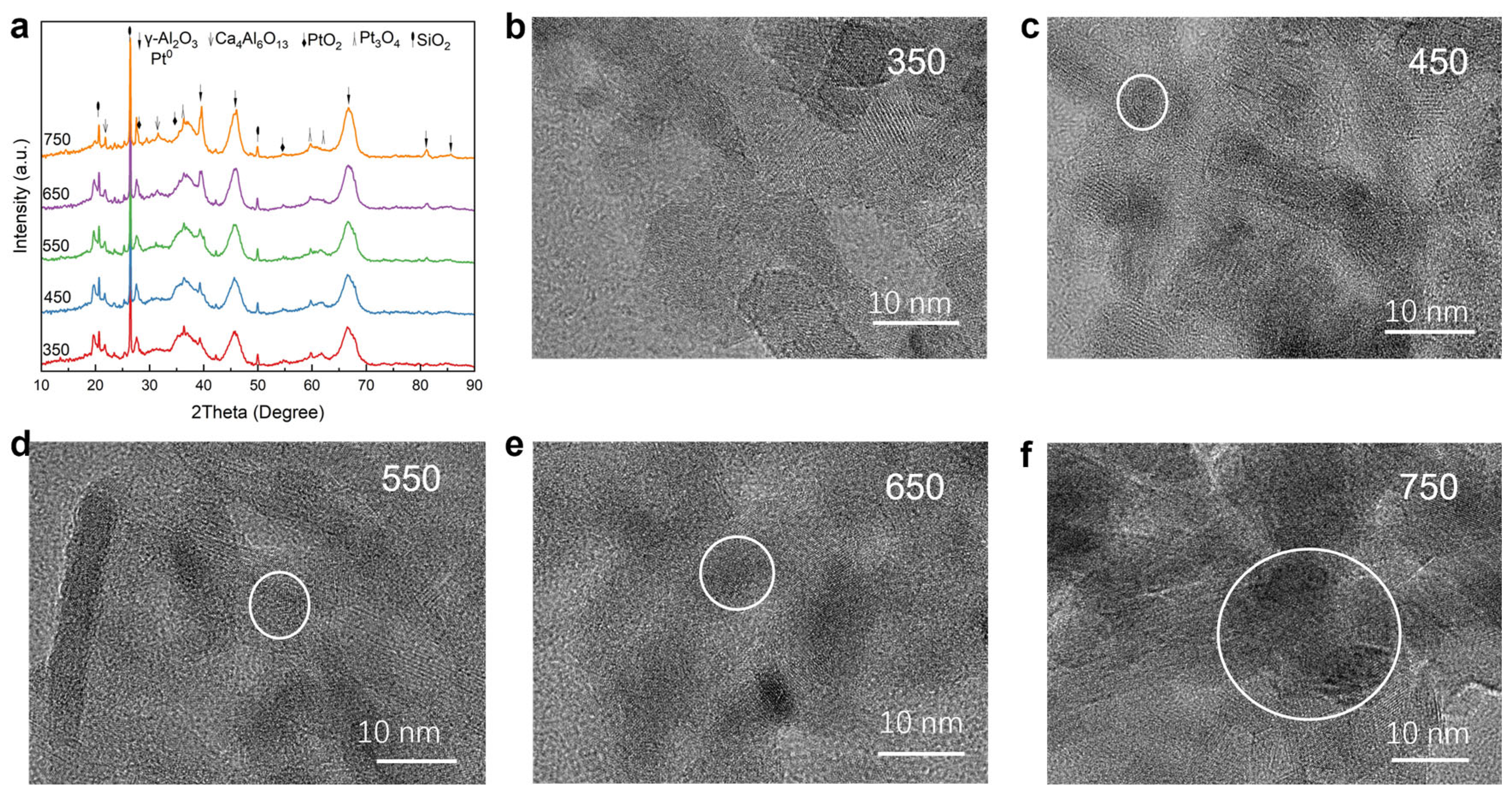
2.2. Analysis of Crystalline Structure
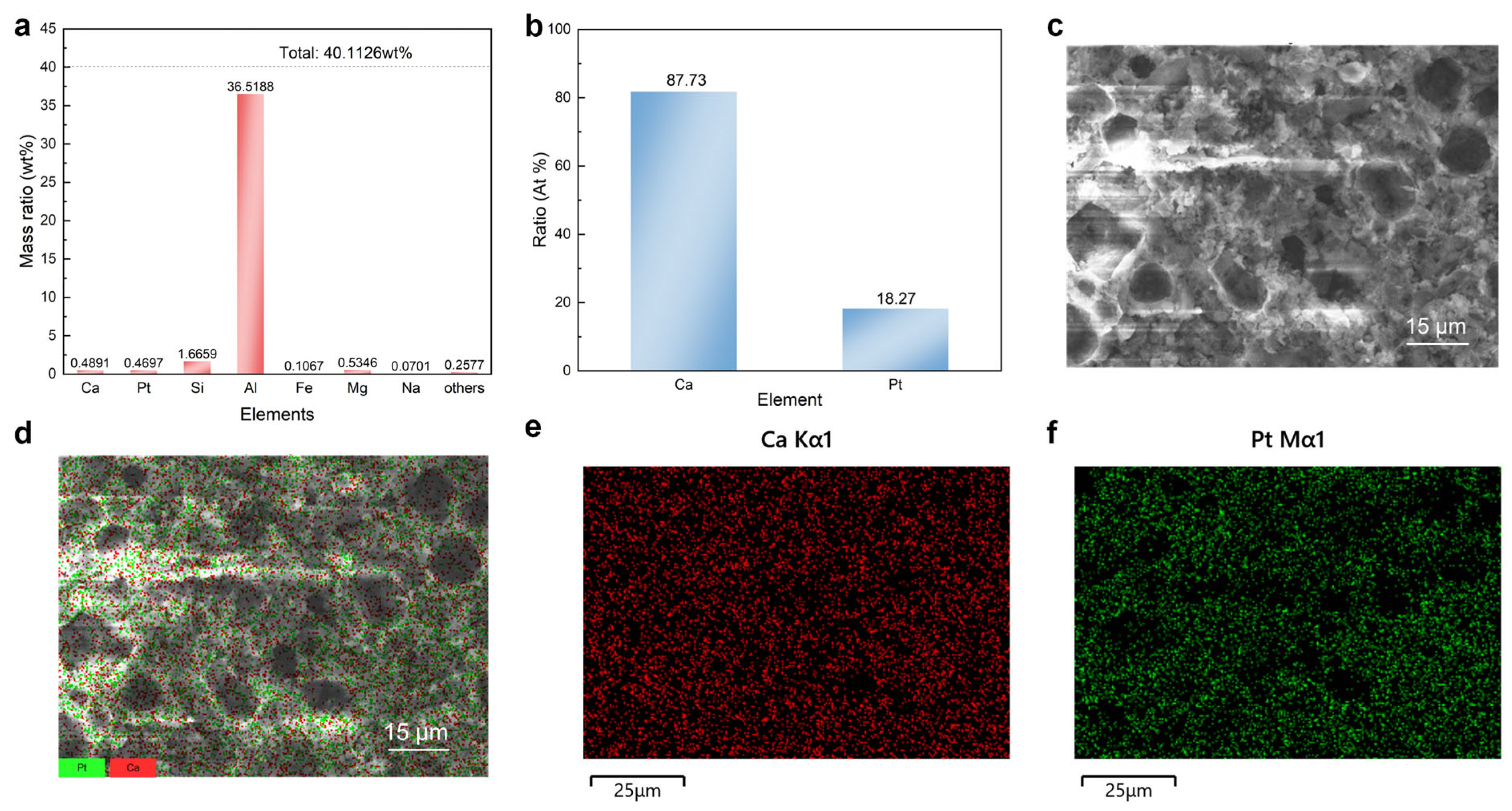
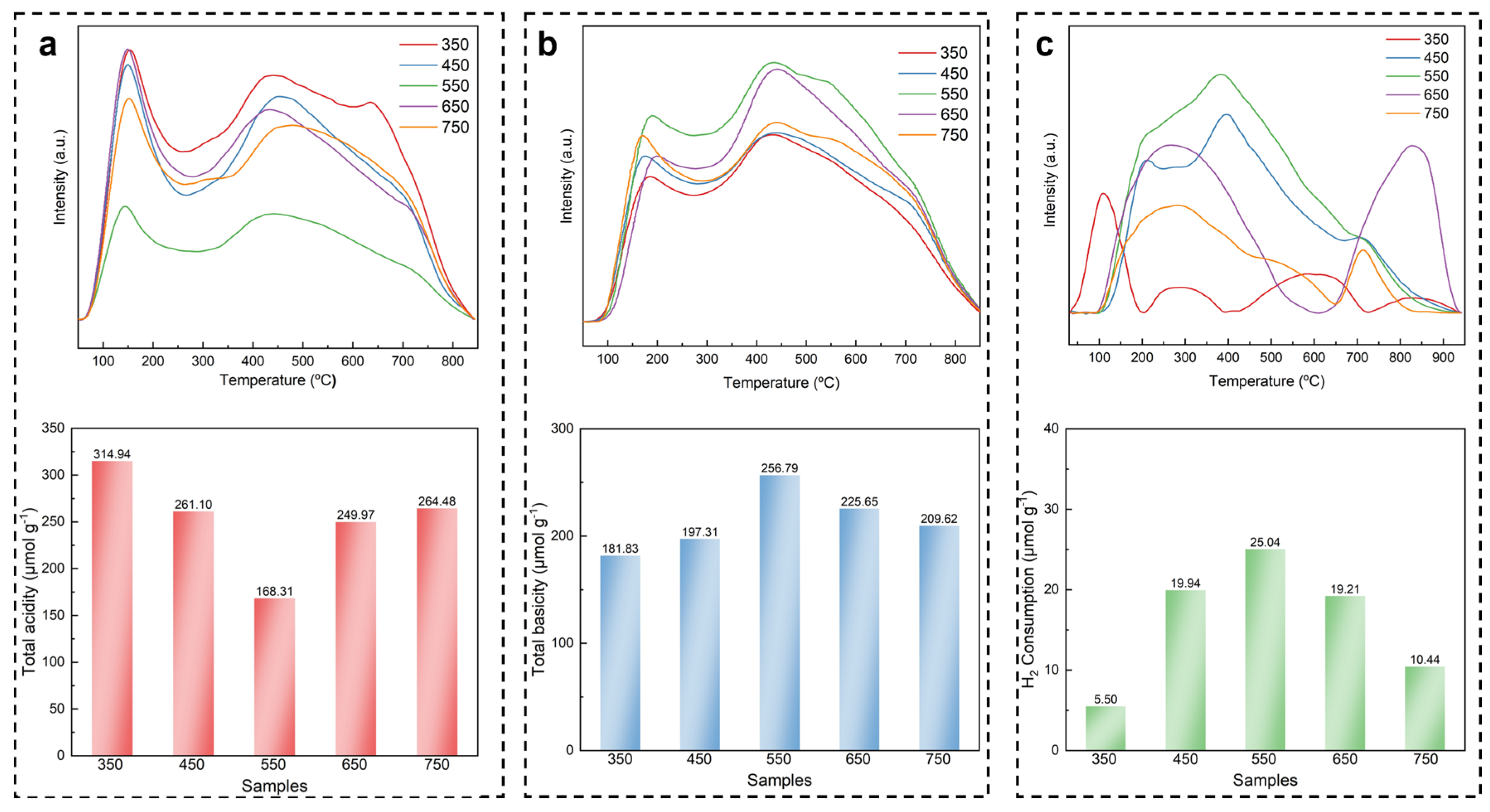
2.3. Analysis of Active Sites
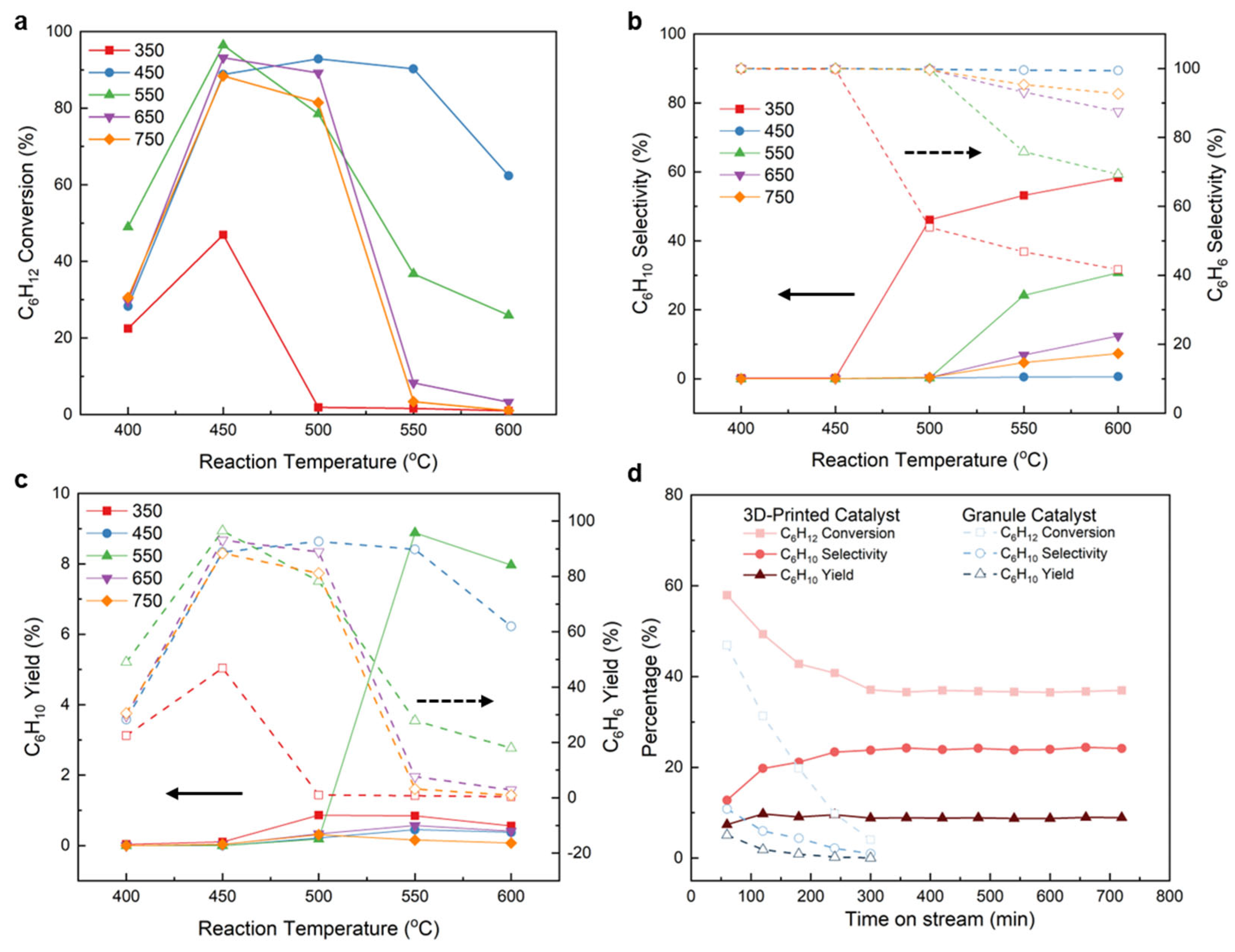
2.4. CDH Performances
3. Materials and Methods
3.1. Additively Manufacturing of Catalyst
3.2. Material Characterization
3.3. Evaluation of Dehydrogenation Performance
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wang, N.; Qiu, J.; Wu, J.; Yuan, X.; You, K.; Luo, H. Microwave assisted synthesis of Sn-modified MgAlO as support for platinum catalyst in cyclohexane dehydrogenation to cyclohexene. Appl. Catal. A Gen. 2016, 516, 9–16. [Google Scholar] [CrossRef]
- Yu, J.; Ge, Q.; Fang, W.; Xu, H. Enhanced performance of Ca-doped Pt/γ-Al2O3 catalyst for cyclohexane dehydrogenation. Int. J. Hydrogen Energy 2011, 36, 11536–11544. [Google Scholar] [CrossRef]
- Podila, S.; Al-Zahrani, A.A.; Daous, M.A.; Alhumade, H. Highly Efficient PtSn/Al2O3 and PtSnZnCa/Al2O3 Catalysts for Ethane Dehydrogenation: Influence of Catalyst Pretreatment Atmosphere. Catalysts 2024, 14, 312. [Google Scholar] [CrossRef]
- Miao, L.; Yan, J.; Wang, W.; Huang, Y.; Li, W.; Yang, Y. Dehydrogenation of methylcyclohexane over Pt supported on Mg–Al mixed oxides catalyst: The effect of promoter Ir. Chin. J. Chem. Eng. 2020, 28, 2337–2342. [Google Scholar] [CrossRef]
- Nakaya, Y.; Hayashida, E.; Asakura, H.; Takakusagi, S.; Yasumura, S.; Shimizu, K.-I.; Furukawa, S. High-Entropy Intermetallics Serve Ultrastable Single-Atom Pt for Propane Dehydrogenation. J. Am. Chem. Soc. 2022, 144, 15944–15953. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.-L.; Qiu, J.-E.; Wu, Z.-W.; Wu, J.; You, K.-Y.; Luo, H.-A. Effect of microwave calcination on catalytic properties of Pt/MgAl(Sn)Ox catalyst in cyclohexane dehydrogenation to cyclohexene. Appl. Catal. A Gen. 2015, 503, 62–68. [Google Scholar] [CrossRef]
- Yu, J.; Wang, R.; Ren, S.; Sun, X.; Chen, C.; Ge, Q.; Fang, W.; Zhang, J.; Xu, H.; Su, D.S. The Unique Role of CaO in Stabilizing the Pt/Al2O3 Catalyst for the Dehydrogenation of Cyclohexane. ChemCatChem 2012, 4, 1376–1381. [Google Scholar] [CrossRef]
- Mamivand, S.; Binazadeh, M.; Sohrabi, R. Applicability of membrane reactor technology in industrial hydrogen producing reactions: Current effort and future directions. J. Ind. Eng. Chem. 2021, 104, 212–230. [Google Scholar] [CrossRef]
- Li, X.; Shen, P.; Han, X.; Wang, Y.; Zhu, Y.; Wu, Z. Dehydrogenation mechanisms of liquid organic hydrogen carriers over Pt, Pd, Rh, and Ni surfaces: Cyclohexane as a model compound. Appl. Surf. Sci. 2021, 543, 148769. [Google Scholar] [CrossRef]
- Yadav, V.; Rosenberger, J.M.; Bolton, B.K.; Gounder, R.; Li, C.W. Calcium additives enhance coke migration and catalyst stability for Pt-based catalysts in ethane dehydrogenation. J. Catal. 2024, 432, 115446. [Google Scholar] [CrossRef]
- Yu, J.; Ge, Q.; Fang, W.; Xu, H. Influences of calcination temperature on the efficiency of CaO promotion over CaO modified Pt/γ-Al2O3 catalyst. Appl. Catal. A Gen. 2011, 395, 114–119. [Google Scholar] [CrossRef]
- Long, L.-L.; Lang, W.-Z.; Liu, X.; Hu, C.-L.; Chu, L.-F.; Guo, Y.-J. Improved catalytic stability of PtSnIn/xCa–Al catalysts for propane dehydrogenation to propylene. Chem. Eng. J. 2014, 257, 209–217. [Google Scholar] [CrossRef]
- Al-Zeghayer, Y.; Jibril, B. On the effects of calcination conditions on the surface and catalytic properties of γ-Al2O3-supported CoMo hydrodesulfurization catalysts. Appl. Catal. A Gen. 2005, 292, 287–294. [Google Scholar] [CrossRef]
- Zazo, J.; Fraile, A.; Rey, A.; Bahamonde, A.; Casas, J.; Rodriguez, J. Optimizing calcination temperature of Fe/activated carbon catalysts for CWPO. Catal. Today 2009, 143, 341–346. [Google Scholar] [CrossRef]
- Yang, J.; Zheng, H.-Y.; Zhu, Y.-L.; Zhao, G.-W.; Zhang, C.-H.; Teng, B.-T.; Xiang, H.-W.; Li, Y. Effects of calcination temperature on performance of Cu–Zn–Al catalyst for synthesizing γ-butyrolactone and 2-methylfuran through the coupling of dehydrogenation and hydrogenation. Catal. Commun. 2004, 5, 505–510. [Google Scholar] [CrossRef]
- Leboda, R.; Skubiszewska-Zięba, J.; Grzegorczyk, W. Effect of calcium catalyst loading procedure on the porous structure of active carbon from plum stones modified in the steam gasification process. Carbon 1998, 36, 417–425. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, G.; Zhang, X. Efficient and stable Pt/CaO-TiO2-Al2O3 for the catalytic dehydrogenation of cycloalkanes as an endothermic hydrocarbon fuel. Fuel 2023, 331, 125732. [Google Scholar] [CrossRef]
- Lawson, S.; Newport, K.A.; Axtell, A.; Boucher, C.; Grant, B.; Haas, M.; Lee, M.; Rezaei, F.; Rownaghi, A.A. Structured Bifunctional Catalysts for CO2 Activation and Oxidative Dehydrogenation of Propane. ACS Sustain. Chem. Eng. 2021, 9, 5716–5727. [Google Scholar] [CrossRef]
- Lawson, S.; Farsad, A.; Adebayo, B.; Newport, K.; Schueddig, K.; Lowrey, E.; Polo-Garzon, F.; Rezaei, F.; Rownaghi, A.A. A Novel Method of 3D Printing High-Loaded Oxide/H-ZSM-5 Catalyst Monoliths for Carbon Dioxide Reduction in Tandem with Propane Dehydrogenation. Adv. Sustain. Syst. 2021, 5, 2000257. [Google Scholar] [CrossRef]
- Lawson, S.; Baamran, K.; Newport, K.; Rezaei, F.; Rownaghi, A.A. Formulation and processing of dual functional Adsorbent/Catalyst structured monoliths using an additively manufactured contactor for direct Capture/Conversion of CO2 with cogeneration of ethylene. Chem. Eng. J. 2022, 431, 133224. [Google Scholar] [CrossRef]
- Baamran, K.; Lawson, S.; Rownaghi, A.A.; Rezaei, F. Process evaluation and kinetic analysis of 3D-printed monoliths comprised of CaO and Cr/H-ZSM-5 in combined CO2 Capture-C2H6 oxidative dehydrogenation to C2H4. Chem. Eng. J. 2022, 435, 134706. [Google Scholar] [CrossRef]
- Díaz-Herrezuelo, I.; Koller, M.; Quintanilla, A.; Vega, G.; Casas, J.A.; Pérez-Coll, D.; Seiner, H.; Osendi, M.I.; Miranzo, P.; Belmonte, M. 3D printing of cubic zirconia lattice supports for hydrogen production. Ceram. Int. 2023, 49, 22529–22536. [Google Scholar] [CrossRef]
- Mastroianni, L.; Russo, V.; Eränen, K.; Di Serio, M.; Murzin, D.Y.; Salmi, T. Towards unconstrained catalyst shaping: High accuracy DLP printing of porous γ-Al2O3-based catalysts. Catal. Sci. Technol. 2024, 14, 1336–1348. [Google Scholar] [CrossRef]
- Bui, H.M.; Großmann, P.F.; Berger, A.; Seidel, A.; Tonigold, M.; Szesni, N.; Fischer, R.; Rieger, B.; Hinrichsen, O. Comparison of Direct Ink Writing and Binder Jetting for additive manufacturing of Pt/Al2O3 catalysts for the dehydrogenation of perhydro-dibenzyltoluene. Chem. Eng. J. 2023, 458, 141361. [Google Scholar] [CrossRef]
- Großmann, P.F.; Tonigold, M.; Szesni, N.; Fischer, R.W.; Seidel, A.; Achterhold, K.; Pfeiffer, F.; Rieger, B. Influence of internal and external surface area on impregnation and activity of 3D printed catalyst carriers. Catal. Commun. 2023, 175, 106610. [Google Scholar] [CrossRef]
- Wu, S.; Xu, X.; Wang, Y.; Jiang, P.; Wu, J.; Jia, X.; Liu, D.; Wang, X.; Ji, Z. Coaxial 3D printed Al2O3 ceramic continuous-flow fixed-bed reactor with bionic core-shell structure. Ceram. Int. 2024, 50, 13662–13670. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, P.; Zhang, H.; Yang, H.; Wang, H.; Zhang, L. Structured Zeolite Monoliths with Ultrathin Framework for Fast CO2 Adsorption Enabled by 3D Printing. Ind. Eng. Chem. Res. 2020, 59, 8223–8229. [Google Scholar] [CrossRef]
- Chen, X.; Zhao, G.; Wu, Y.; Huang, Y.; Liu, Y.; He, J.; Wang, L.; Lian, Q.; Li, D. Cellular carbon microstructures developed by using stereolithography. Carbon 2017, 123, 34–44. [Google Scholar] [CrossRef]
- Wang, H.; Wang, P.; Wang, Q.; Zhang, R.; Zhang, L. Preparation of a High-Precision Gama-Al2O3 Structured Catalyst by DLP 3D Direct Printing for Hydrogen Production from Methanol. Ind. Eng. Chem. Res. 2021, 60, 13107–13114. [Google Scholar] [CrossRef]
- Wang, P.; Zhang, L.; Xing, W.; Lu, Z.; Qi, X.; Chai, Y.; Zhao, Z. 3D Printing of Monolithic Carbon Electrodes with Hierarchical Pores Enabled by Nucleated Resin. Energy Technol. 2025. [Google Scholar] [CrossRef]
- Baamran, K.; Rownaghi, A.A.; Rezaei, F. Direct Synthesis of Ethylene and Hydrogen from CO2 and Ethane over a Bifunctional Structured CaO/Cr2O3-V2O5/ZSM-5 Adsorbent/Catalyst Monolith. ACS Sustain. Chem. Eng. 2023, 11, 1006–1018. [Google Scholar] [CrossRef]
- Balla, P.; Shin, D.; Park, S.-J.; Kwak, G.; Kim, S. Investigating copper impregnated 3D printed Al2O3 catalyst for methanol steam reforming. Fuel 2025, 390, 134772. [Google Scholar] [CrossRef]
- Patra, A.K.; Dutta, A.; Bhaumik, A. Mesoporous Core–Shell Fenton Nanocatalyst: A Mild, Operationally Simple Approach to the Synthesis of Adipic Acid. Chem. Eur. J. 2013, 19, 12388–12395. [Google Scholar] [CrossRef] [PubMed]
- Naseri, M.; Tahriri Zangeneh, F.; Taeb, A. Effects of Mg, Ca, and K Addition on Pt-Sn/γ-Al2O3 for Propane Dehydrogenation. Iran. J. Chem. Chem. Eng. 2022, 41, 1921–1931. [Google Scholar]
- Li, N.; Hu, Z.; Zheng, M.; Lu, H.; Zhao, B.; Zhang, S.; Zheng, J.; Ji, G.; Cao, J. Formation of Pt nanoparticles in mesoporous silica channels via direct low-temperature decomposition of H2PtCl6·6H2O. Mater. Lett. 2013, 106, 193–196. [Google Scholar] [CrossRef]
- Wu, Z.; Yang, P.; Li, Q.; Xiao, W.; Li, Z.; Xu, G.; Liu, F.; Jia, B.; Ma, T.; Feng, S.; et al. Microwave Synthesis of Pt Clusters on Black TiO2 with Abundant Oxygen Vacancies for Efficient Acidic Electrocatalytic Hydrogen Evolution. Chem. Int. Ed. 2023, 62, e202300406. [Google Scholar] [CrossRef]
- Zhao, C.; You, R.; Jin, M.; Jin, X.; Wu, P.; He, M.; Liang, M. Purified CaO supported Pt nanoparticles for the selective hydrogenation of styrene oxide with enhanced selectivity of 1-phenylethanol. New J. Chem. 2024, 48, 10253–10261. [Google Scholar] [CrossRef]
- Chiang, K.-C.; Chen, K.-L.; Chen, C.-Y.; Huang, J.-J.; Shen, Y.-H.; Yeh, M.-Y.; Wong, F.F. Recovery of spent alumina-supported platinum catalyst and reduction of platinum oxide via plasma sintering technique. J. Taiwan Inst. Chem. Eng. 2011, 42, 158–165. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, C.; Chen, S.; Hu, Q.; Gao, Z.; Li, Y.; Qin, Y. Highly dispersed Pt nanoparticles supported on carbon nanotubes produced by atomic layer deposition for hydrogen generation from hydrolysis of ammonia borane. Catal. Sci. Technol. 2017, 7, 322–329. [Google Scholar] [CrossRef]
- Miller, J.T.; Schreier, M.; Kropf, A.J.; Regalbuto, J.R. A fundamental study of platinum tetraammine impregnation of silica: 2. The effect of method of preparation, loading, and calcination temperature on (reduced) particle size. J. Catal. 2004, 225, 203–212. [Google Scholar] [CrossRef]
- Liu, X.; Zhou, Y.; Liu, J.; Xia, H. The intrinsic enzyme mimetic activity of platinum oxide for biosensing of glucose. Spectrochim. Acta A 2021, 248, 119280. [Google Scholar] [CrossRef]
- Abe, Y.; Kawamura, M.; Sasaki, K. Preparation of PtO and α-PtO2 Thin Films by Reactive Sputtering and Their Electrical Properties. Jpn. J. Appl. Phys. 1999, 38, 2092. [Google Scholar] [CrossRef]
- Nichols, F.; Lu, J.E.; Mercado, R.; Dudschus, R.; Bridges, F.; Chen, S. Platinum Oxide Nanoparticles for Electrochemical Hydrogen Evolution: Influence of Platinum Valence State. Chem.—A Eur. J. 2020, 26, 4136–4142. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Jang, E.J.; Oh, D.G.; Szanyi, J.; Kwak, J.H. Morphology and size of Pt on Al2O3: The role of specific metal-support interactions between Pt and Al2O3. J. Catal. 2020, 385, 204–212. [Google Scholar] [CrossRef]
- Bai, L.; Zhou, Y.; Zhang, Y.; Liu, H.; Tang, M. Influence of Calcium Addition on Catalytic Properties of PtSn/ZSM-5 Catalyst for Propane Dehydrogenation. Catal. Lett. 2009, 129, 449–456. [Google Scholar] [CrossRef]
- Ding, H.; Qi, X.; Yun, X.; Ge, J.; Tian, Y.; Lei, X.; Zhang, F. Confined Ruthenium Nanoparticles as an Effective Catalyst for Aerobic Oxidation of Aqueous Ethanol to Acetic Acid. ACS Sustain. Chem. Eng. 2022, 10, 9687–9696. [Google Scholar] [CrossRef]
- Szanyi, J.; Kwak, J.H.; Hanson, J.; Wang, C.; Szailer, T.; Peden, C.H.F. Changing Morphology of BaO/Al2O3 during NO2 Uptake and Release. J. Phys. Chem. B 2005, 109, 7339–7344. [Google Scholar] [CrossRef]
- Palazov, A.; Bonev, C.; Shopov, D.; Lietz, G.; Sárkány, A.; Völter, J. Adsorption and hydrogenation of ethylene, 1-hexene, and benzene and CO adsorption on PtAl2O3 and PtSnAl2O3 catalysts. J. Catal. 1987, 103, 249–260. [Google Scholar] [CrossRef]
- Wang, F.; Ma, J.; He, G.; Chen, M.; Zhang, C.; He, H. Nanosize Effect of Al2O3 in Ag/Al2O3 Catalyst for the Selective Catalytic Oxidation of Ammonia. ACS Catal. 2018, 8, 2670–2682. [Google Scholar] [CrossRef]
- Cao, F.; Xiang, J.; Su, S.; Wang, P.; Hu, S.; Sun, L. Ag modified Mn–Ce/γ-Al2O3 catalyst for selective catalytic reduction of NO with NH3 at low-temperature. Fuel Process. Technol. 2015, 135, 66–72. [Google Scholar] [CrossRef]
- Zhao, A.; Xiong, B.; Han, Y.; Tong, H. Thermal decomposition paths of calcium nitrate tetrahydrate and calcium nitrite. Thermochim. Acta 2022, 714, 179264. [Google Scholar] [CrossRef]
- Xiong, X.; Ma, B.; Zhao, D.; Xia, K.; Shi, S.; Wang, C.; Chen, Y. Investigation of the thermal behavior of Ca(NO3)2·4H2O and its application for the regeneration of HNO3 and CaO. J. Anal. Appl. Pyrolysis 2023, 173, 106101. [Google Scholar] [CrossRef]
- Di Serio, M.; Ledda, M.; Cozzolino, M.; Minutillo, G.; Tesser, R.; Santacesaria, E. Transesterification of Soybean Oil to Biodiesel by Using Heterogeneous Basic Catalysts. Ind. Eng. Chem. Res. 2006, 45, 3009–3014. [Google Scholar] [CrossRef]
- Yang, W.; Feng, Y.; Chu, W. Promotion Effect of CaO Modification on Mesoporous Al2O3-Supported Ni Catalysts for CO2 Methanation. Int. J. Chem. Eng. 2016, 2016, 2041821. [Google Scholar] [CrossRef]
- Yu, C.; Xu, H.; Ge, Q.; Li, W. Properties of the metallic phase of zinc-doped platinum catalysts for propane dehydrogenation. J. Mol. Catal. A Chem. 2007, 266, 80–87. [Google Scholar] [CrossRef]
- Ivanova, A.S.; Slavinskaya, E.M.; Gulyaev, R.V.; Zaikovskii, V.I.; Stonkus, O.A.; Danilova, I.G.; Plyasova, L.M.; Polukhina, I.A.; Boronin, A.I. Metal–support interactions in Pt/Al2O3 and Pd/Al2O3 catalysts for CO oxidation. Appl. Catal. B Environ. 2010, 97, 57–71. [Google Scholar] [CrossRef]
- Beck, A.; Rzepka, P.; Marshall, K.P.; Stoian, D.; Willinger, M.G.; van Bokhoven, J.A. Hydrogen Interaction with Oxide Supports in the Presence and Absence of Platinum. J. Phys. Chem. C 2022, 126, 17589–17597. [Google Scholar] [CrossRef]
- Kugai, J.; Miller, J.T.; Guo, N.; Song, C. Oxygen-enhanced water gas shift on ceria-supported Pd–Cu and Pt–Cu bimetallic catalysts. J. Catal. 2011, 277, 46–53. [Google Scholar] [CrossRef]
- Rimaz, S.; Chen, L.; Monzón, A.; Kawi, S.; Borgna, A. Enhanced selectivity and stability of Pt-Ge/Al2O3 catalysts by Ca promotion in propane dehydrogenation. Chem. Eng. J. 2021, 405, 126656. [Google Scholar] [CrossRef]
- Li, Y.; Chen, S.; Cai, X.; Hong, J.; Wu, X.; Xu, Y.; Zou, J.; Chen, B.H. Rational design and preparation of hierarchical monoliths through 3D printing for syngas methanation. J. Mater. Chem. A 2018, 6, 5695–5702. [Google Scholar] [CrossRef]
- Lefevere, J.; Mullens, S.; Meynen, V. The impact of formulation and 3D-printing on the catalytic properties of ZSM-5 zeolite. Chem. Eng. J. 2018, 349, 260–268. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, P.; Lu, Z.; Qi, X.; Xing, W.; Shi, Y.; Yan, J. Additive Manufacturing of CaO-Pt/Al2O3 Structured Catalysts for Cyclohexane Dehydrogenation. Catalysts 2025, 15, 1064. https://doi.org/10.3390/catal15111064
Wang P, Lu Z, Qi X, Xing W, Shi Y, Yan J. Additive Manufacturing of CaO-Pt/Al2O3 Structured Catalysts for Cyclohexane Dehydrogenation. Catalysts. 2025; 15(11):1064. https://doi.org/10.3390/catal15111064
Chicago/Turabian StyleWang, Panfeng, Zhaoyang Lu, Xiang Qi, Wenting Xing, Yubo Shi, and Jiapo Yan. 2025. "Additive Manufacturing of CaO-Pt/Al2O3 Structured Catalysts for Cyclohexane Dehydrogenation" Catalysts 15, no. 11: 1064. https://doi.org/10.3390/catal15111064
APA StyleWang, P., Lu, Z., Qi, X., Xing, W., Shi, Y., & Yan, J. (2025). Additive Manufacturing of CaO-Pt/Al2O3 Structured Catalysts for Cyclohexane Dehydrogenation. Catalysts, 15(11), 1064. https://doi.org/10.3390/catal15111064





